Abstract
In a previous study, 3-dose primary vaccination of Nigerian infants with the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) was immunogenic for vaccine pneumococcal serotypes, with comparable tolerability between PHiD-CV and control groups. In an open-label study (ClinicalTrials.gov, NCT01153893), 68 primed children received a PHiD-CV booster dose co-administered with a diphtheria-tetanus-acellular pertussis (DTPa) booster dose at 15–21 months and 36 children unprimed for pneumococcal vaccination received two PHiD-CV catch-up doses (first dose co-administered with DTPa booster dose) at 15–21 and 17–23 months. Adverse events were recorded and immune responses were measured before and one month after vaccination. In both groups, pain was the most frequent solicited local symptom and fever was the most frequent solicited general symptom after the booster dose and each catch-up dose. Few grade 3 solicited symptoms and no vaccine-related serious adverse events were reported. After booster vaccination, for each vaccine serotype, at least 98.5% of children had an antibody concentration ≥0.2 µg/ml and at least 94.0% had an opsonophagocytic activity (OPA) titer ≥8. After 2-dose catch-up, for each vaccine serotype, at least 97.1% had an antibody concentration ≥0.2 µg/ml, except for serotypes 6B (82.9%) and 23F (88.6%), and at least 91.4% had an OPA titer ≥8, except for serotypes 6B (77.4%) and 19F (85.3%). PHiD-CV induced antibody responses against protein D in both groups. In conclusion, PHiD-CV administered to Nigerian toddlers as a booster dose or 2-dose catch-up was well tolerated and immunogenic for vaccine pneumococcal serotypes and protein D.
Introduction
Streptococcus pneumoniae infection, which is a major cause of bacterial pneumonia, meningitis and sepsis, is associated with significant morbidity and mortality in young children. Nigeria is one of the 10 countries with the greatest number of pneumococcal deaths in HIV-negative children aged less than 5 years, with a pneumococcal mortality rate in this age group of 300 to 500 per 100 000 children.Citation1
In young children, 10 to 15 pneumococcal serotypes account for at least 80% of invasive pneumococcal disease (IPD), with variations in the distribution of specific serotypes among global regions.Citation2 In Africa, pneumococcal serotypes 1 and 5 are estimated to cause almost one-quarter of IPD cases in children aged less than 5 y.Citation2 These serotypes are therefore part of the minimally acceptable target product profile for pneumococcal conjugate vaccines (PCVs) for African countries that are eligible for support from the Global Alliance for Vaccines and Immunization (GAVI).Citation3 Nigeria qualifies for GAVI supportCitation4 but PCV immunization is not yet used widely in this country and there are limited data on pneumococcal serotype distribution among young Nigerian children with pneumococcal disease.Citation5,Citation6 A recent analysis of children aged under 5 y with pneumococcal meningitis conducted in 8 West African countries including Nigeria found pneumococcal serotypes 1 and 5 were most common, accounting for approximately half of all pneumococcal isolates from cerebrospinal fluid samples.Citation6 These serotypes are however not frequently found in the nasopharynx of healthy Nigerian children,Citation7 a typical characteristic of these invasive serotypes.Citation8
PCV immunization schedules in Africa usually include 3 priming doses with no booster dose, as recommended by the World Health Organization (WHO).Citation9 In a randomized controlled study conducted in Mali and Nigeria, 3-dose primary vaccination with the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV; Synflorix™, GlaxoSmithKline Vaccines), according to the Expanded Program on Immunization (EPI) schedule at 6, 10, and 14 wk of age, was immunogenic for all vaccine pneumococcal serotypes and protein D.Citation10 In addition, tolerability was generally comparable between the PHiD-CV and control groups, both of which received diphtheria-tetanus-whole-cell pertussis-hepatitis B/Haemophilus influenzae type b (DTPw-HBV/Hib) and oral live attenuated poliovirus vaccines.Citation10 PHiD-CV contains pneumococcal serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F, and protein D as carrier protein for 8 of the 10 serotypes and tetanus or diphtheria toxoid for the remaining two serotypes (serotypes 18C and 19F). Protein D is a recombinant non-lipidated form of a cell surface lipoprotein of nontypeable H. influenzae (NTHi) that is highly conserved in both capsulated and non-capsulated strains.Citation11
As administration of a booster dose of PHiD-CV is recommended in other parts of the world,Citation12 and to comprehensively assess the vaccine’s safety and immunogenicity profile in Nigeria, the National Agency for Food and Drug Administration and Control (NAFDAC) in Nigeria requested a booster vaccination study with PHiD-CV. The primary vaccination study was therefore followed by the present study in which children previously primed with PHiD-CV were invited to receive a booster dose of PHiD-CV co-administered with a diphtheria-tetanus-acellular pertussis (DTPa; Infanrix™, GlaxoSmithKline Vaccines) booster dose at 15 to 21 mo of age. Moreover, to evaluate PHiD-CV administration among children who had not received the vaccine in infancy, the unprimed control group was offered 2-dose PHiD-CV catch-up vaccination (first dose co-administered with a DTPa booster dose) in the second year of life. In studies conducted in Europe and South America, this PHiD-CV regimen had an acceptable safety profileCitation13,Citation14 and provided adequate primingCitation13-Citation15 in children aged up to the fourth year of life.
We report results from the booster/catch-up vaccination study conducted in Nigeria. A similar study was also conducted in Mali (ClinicalTrials.gov identifier, NCT00985465) and will be reported separately.
Results
Study population
The primary vaccination study included 79 children in the PHiD-CV group and 40 in the control group; 69 and 36, respectively, were vaccinated in the PHiD-CV booster/catch-up vaccination study and of these, 68 and 36 were included in the total vaccinated cohort, which was the primary cohort for safety analysis (). The study was completed by all children, apart from 1 child in the PHiD-CV booster group who died due to drowning. The groups were comparable in terms of mean age, gender ratio and mean weight at booster dose or first catch-up dose (). All children were of African heritage.
Figure 1. Trial profile. Note: An issue was identified with the informed consent obtained for one child and the child’s parents didn’t permit GlaxoSmithKline Vaccines to use the child’s data. As a result, the data of the child, who had an SAE that was not considered to be related to the study medication by the investigator, are not detailed and were not used in the analysis.
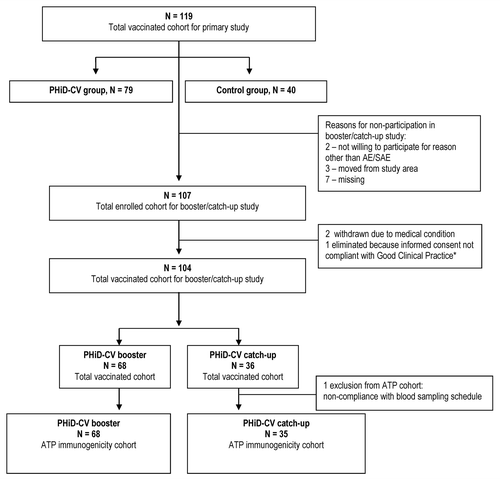
Table 1. Demographic characteristics (total vaccinated cohort)
The according-to-protocol (ATP) immunogenicity cohort included 68 children in the PHiD-CV booster group and 35 in the PHiD-CV catch-up group ().
Safety and reactogenicity
The analysis of safety was performed on the total vaccinated cohort. Pain was the most frequently reported solicited local symptom after the PHiD-CV booster dose and both of the catch-up doses (). The incidence of pain was in the same range after the booster dose (36.8% [95% confidence interval: 25.4–49.3]) and first catch-up dose (30.6% [95% CI: 16.3–48.1]) at the PHiD-CV injection sites and tended to be lower after the second PHiD-CV catch-up dose (13.9% [95% CI: 4.7–29.5]) than after the first dose (). Similarly, after the DTPa dose, the incidence of pain at the injection site was 37.3% (95% CI: 25.8–50.0) in the PHiD-CV booster group and 30.6% (95% CI: 16.3–48.1) in the PHiD-CV catch-up group. There were no reports of grade 3 pain in either group and reports of other grade 3 solicited local symptoms were infrequent after vaccination with PHiD-CV (no more than 2.9%, ) or DTPa (no reports). There was 1 report of a large swelling reaction in the PHiD-CV booster group (local swelling around the PHiD-CV injection site, of diameter 60 mm, that lasted 2 d and resolved without sequel) and no reports in the PHiD-CV catch-up group. After DTPa vaccination, except for 3.0% (95% CI: 0.4–10.4) of subjects reporting swelling around the DTPa injection site, no other solicited local AE was reported.
Table 2. Incidences of solicited local and general symptoms per dose (total vaccinated cohort)
Fever (axillary temperature ≥37.5 °C) was the most common solicited general symptom after the booster dose and each of the catch-up doses (). There were no grade 3 solicited general symptoms in the PHiD-CV booster group and only 1 report (grade 3 fever after dose 1) in the PHiD-CV catch-up group. Use of antipyretic medication during the 4-d post-vaccination period was frequent in both groups, taken by 85.3% (95% [CI]: 74.6–92.7%) of children after the PHiD-CV booster dose, 86.1% (95% CI: 70.5–95.3%) after the first PHiD-CV catch-up dose and 72.2% (95% CI: 54.8–85.8%) after the second catch-up dose. Administration was mostly prophylactic (prevalences 77.9% [95% CI: 66.2–87.1%], 75.0% [95% CI: 57.8–87.9%] and 66.7% [95% CI: 49.0–81.4%], respectively).
At least 1 unsolicited adverse event (AE) was reported by 45.6% of subjects (95% CI: 33.5–58.1%) in the PHiD-CV booster group and 50.0% of subjects (95% CI: 32.9–67.1%) in the PHiD-CV catch-up group. The most common unsolicited AEs were respiratory tract infection (reported by 19.1% [95% CI: 10.6–30.5%] and 19.4% [95% CI: 8.2–36.0%] of subjects, respectively, and following 9.7% [95% CI: 4.0–19.0%] of PHiD-CV catch-up doses) and malaria (11.8% [95% CI: 5.2–21.9%] and 27.8% [95% CI: 14.2–45.2%] of subjects, respectively; following 15.3% [95% CI: 7.9–25.7%] of PHiD-CV catch-up doses). None of the unsolicited AEs were considered to be causally related to vaccination.
One fatal serious AE (SAE) was reported in the PHiD-CV booster group (drowning) and no SAEs were reported in the PHiD-CV catch-up group. For one vaccinated child, who was not included in the total vaccinated cohort because of an informed consent issue (), a SAE was reported, which was not considered by the investigator to be related to vaccination. The event cannot be detailed because the child’s parents did not permit use of the child's data by GlaxoSmithKline Vaccines.
Immune responses
PHiD-CV booster vaccination
Vaccine pneumococcal serotype geometric mean antibody concentrations (GMCs) declined between the post-primary vaccination and pre-booster vaccination time points (). Before booster vaccination, for each of the vaccine pneumococcal serotypes, at least 91.2% of subjects had detectable antibody concentrations ≥0.05 µg/ml and at least 82.4% of subjects had antibody concentrations ≥0.2 µg/ml for all serotypes except serotypes 1 (61.8%), 4 (76.5%) and 23F (70.6%) (). Even with the decline since primary vaccination, antibody concentrations against each serotype were significantly higher before booster vaccination than in the control group before the first catch-up dose ().
Figure 2. (A) Geometric mean antibody concentrations (GlaxoSmithKline’s 22F-inhibition ELISA, binary logarithmic scale, ATP cohort for immunogenicity) and (B) opsonophagocytic geometric mean titers against individual pneumococcal serotypes (decimal logarithmic scale, ATP cohort for immunogenicity). Note: Post-pri, 1 mo after 3-dose priming (at approximately 5 mo of age) with PHiD-CV in PHiD-CV booster group and control vaccine in PHiD-CV catch-up group; Pre-bst/catch-up, before booster dose in PHiD-CV booster group or before first catch-up dose in PHiD-CV catch-up group (15 to 21 mo of age); Post-bst/catch-up, 1 mo after booster dose in PHiD-CV booster group (16 to 22 mo of age) or 1 mo after second catch-up dose in PHiD-CV catch-up group (18 to 24 mo of age). Error bars represent 95% confidence intervals.
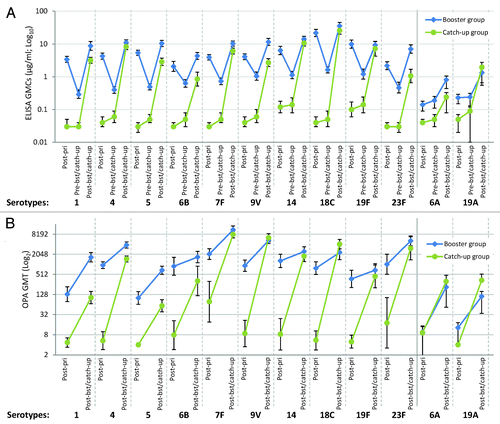
After PHiD-CV booster vaccination, antibody GMCs for each of the vaccine pneumococcal serotypes were 6.8- to 30.1-fold higher compared with pre-booster levels and 1.6- to 3.2-fold higher compared with post-priming levels, apart from serotype 19F, which had similar GMCs at both time points (). For each of the vaccine serotypes, an antibody concentration ≥0.2 µg/ml was measured in at least 98.5% of children and opsonophagocytic activity (OPA) titer ≥8 was measured in at least 94.0% (). Post-booster seropositivity rates for cross-reactive serotypes 6A and 19A were high; 80.6% and 85.1% of children, respectively, had an antibody concentration ≥0.2 µg/ml, and 82.0% and 77.3%, respectively, had an OPA titer ≥8 ().
Table 3. 22F-ELISA antibody and opsonophagocytic responses against individual pneumococcal serotypes 1 mo after vaccination (ATP cohort for immunogenicity)
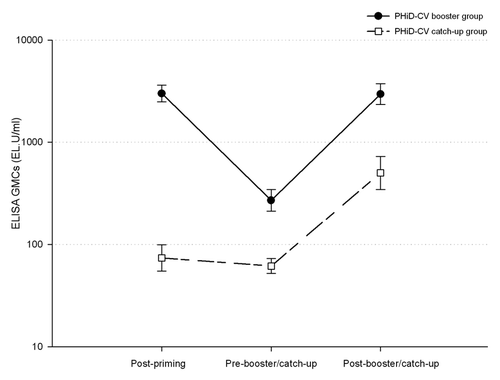
The anti-protein D antibody GMC was higher in the PHiD-CV group before booster vaccination than in the control group before the first catch-up dose (). After PHiD-CV booster vaccination, the anti-protein D antibody GMC increased 10.9-fold from pre-booster GMC.
Figure 3. ELISA antibody responses (with 95% confidence intervals) against protein D (logarithmic scale, ATP cohort for immunogenicity). Note: Post-priming, 1 mo after 3-dose priming (at approximately 5 mo of age) with PHiD-CV in PHiD-CV booster group and control vaccine in PHiD-CV catch-up group; Pre-booster/catch-up, before booster dose in PHiD-CV booster group or before first catch-up dose in PHiD-CV catch-up group (15 to 21 mo of age); Post-booster/catch-up, 1 mo after booster dose in PHiD-CV booster group (16 to 22 mo of age) or 1 mo after second catch-up dose in PHiD-CV catch-up group (18 to 24 mo of age).
PHiD-CV 2-dose catch-up
One month after 2-dose catch-up in the second year of life, for each vaccine pneumococcal serotype, antibody GMCs increased by 17.0- to 512.2-fold from pre-vaccination values. Antibody GMCs were in the same ranges as the immune responses following PHiD-CV booster vaccination for serotypes 4, 14, 18C, and 19F while a trend for lower antibody GMCs was observed in the catch-up group for the other 6 vaccine serotypes (). OPA geometric mean titers (GMTs) were in the same range as after the PHiD-CV booster for serotypes 7F, 9V, 14, 18C, 19F, and 23F and tended to be lower for the remaining 4 serotypes ().
For each vaccine serotype, percentages of children with an antibody concentration ≥0.2 µg/ml or OPA titer ≥8 were within the same ranges between the booster and catch-up groups, although the 95% CI overlap was borderline for the percentage with OPA titer ≥8 for serotype 6B, with a tendency toward a lower percentage in the catch-up group (). For each vaccine serotype, at least 97.1% of children had an antibody concentration ≥0.2 µg/ml, except for serotypes 6B (82.9%) and 23F (88.6%), and at least 91.4% of children had an OPA titer ≥8, except for serotypes 6B (77.4%) and 19F (85.3%).
The percentages of children with an antibody concentration ≥0.2 µg/ml were in the same range between groups for cross-reactive serotype 19A, but tended to be lower in the PHiD-CV catch-up group for cross-reactive serotype 6A (). Percentages with an OPA titer ≥8 for cross-reactive serotypes 6A and 19A were in the same ranges as those in the PHiD-CV booster group ().
After 2-dose catch-up vaccination, the anti-protein D antibody GMC was 501.1 EL.U/ml, an 8.1-fold increase from the pre-vaccination value ().
Discussion
This study evaluated the safety and immunogenicity of PHiD-CV when administered as a booster dose to children primed in infancy or as a 2-dose catch-up regimen to unprimed children in the second year of life. The results show both schedules were well tolerated and immunogenic for all 10 vaccine serotypes and NTHi protein D in Nigerian children.
In both groups, pain was the most common solicited local symptom, with fever as the most common solicited general symptom. Reports of grade 3 solicited symptoms were infrequent. Antipyretic medications were not prescribed for prophylactic use by the study investigators, but reported prophylactic antipyretic use was high after booster and catch-up vaccination (for 78% of PHiD-CV booster doses, 75% of first catch-up doses and 67% of second catch-up doses). This might have contributed to low incidences of fever in both groups (12% after booster dose; 6% and 3% after first and second catch-up doses, respectively). Prophylactic administration of antipyretic drugs at the time of primary vaccination was shown to result in lower antibody responses to several vaccine antigens.Citation16,Citation17 In our study, immune responses were good for all vaccine serotypes, despite the high rate of prophylactic antipyretic medication use.
No SAEs or unsolicited AEs related to vaccination were reported during the booster/catch-up study. Incidences of malaria reported as an unsolicited AE were 12% in the booster group and 28% (following 15% of doses) in the catch-up group. However, the small number of subjects in each group must be taken into account, along with the different AE follow-up reporting durations in each group (1 mo after each dose: one month in the PHiD-CV booster group vs. 2 mo in the PHiD-CV catch-up group), as well as other possible confounding factors. Also, as there was no case definition for the clinical diagnosis of malaria or guidelines for laboratory confirmation in the study protocol, the possibility of misdiagnoses of febrile illnesses cannot be excluded.
Anti-pneumococcal IgG responses after 2-dose PHiD-CV catch-up vaccination were consistent with those measured after 3-dose PHiD-CV priming in infancy, with antibody GMCs within the same range or higher after catch-up immunization for all vaccine serotypes except serotypes 5 and 6B. Antibody GMCs were lower for 6 vaccine serotypes and within the same range for the remaining 4 after the catch-up regimen in comparison to those observed after booster vaccination, although percentages of children with an antibody concentration ≥0.2 µg/ml were consistent between the groups for each vaccine serotype. OPA GMTs were within the same range or higher after 2-dose catch-up compared with GMTs after priming in infancy, and were consistent with post-booster GMTs for 6 vaccine serotypes and lower for the remaining 4 serotypes. Percentages of children with an OPA titer ≥8 for each serotype were within the same range after booster and catch-up immunization. The antibody GMC and OPA GMT results illustrate the benefit of including a booster dose in the immunization schedule. Moreover, these data suggest that immunological memory induced by primary vaccination permitted anamnestic post-booster responses with a single dose that resulted in comparable or higher responses than observed after 2-dose catch-up immunization. In a European trial that included groups of children who received 3 primary doses of PHiD-CV and a booster dose at 12 to 15 mo or 2 catch-up doses in the second year of life,Citation15 2 doses seemed to provide adequate priming although the results suggested that a booster dose might confer further benefit.
Immune responses in our study in terms of antibody GMCs and OPA GMTs in the PHiD-CV booster group were in line with or higher than those observed in a Latin American study population (manuscript submitted and results publicly available via ClinicalTrial.gov: http://clinicaltrials.gov/ct2/show/results/NCT00466947) in which PHiD-CV showed clinical efficacy against community-acquired pneumonia, IPD and acute otitis media following administration as 3 priming doses in infancy and booster vaccination in the second year of life.Citation18-Citation20 A tendency toward higher immune responses in countries outside of Europe or North America has been reported in previous PCV trials.Citation13,Citation21-Citation23 However, the clinical implications of these differences are unclear, particularly as comparisons between studies are complicated by differences in study population and immunization schedule.
PHiD-CV induced immune responses to cross-reactive serotypes 6A and 19A, and high percentages of children (77% to 86%) had a functional OPA response ≥8 against each of these serotypes in both groups. In the analysis of pneumococcal nasopharyngeal carriage in 14 peri-urban Nigerian communities, serotypes 6A and 19A were among the top 10 serotypes isolated in children aged less than 5 y.Citation7 However, it is yet to be established if these serotypes are common causes of pneumococcal disease in young Nigerian children.
Potential limitations of this study were its open design and the inability to exclude the possibility of immunologic interference between co-administered vaccines. However, it is unlikely that a PHiD-CV booster dose would be administered alone in routine pediatric vaccination schedules. Also, although 3-dose primary PCV vaccination with no booster dose is currently use in most African countries introducing PCV, there is clear value in the comprehensive assessment of a vaccine in countries where it was previously untested. Finally, the small sample sizes in both groups of this study must be taken into consideration when interpreting its results.
In conclusion, PHiD-CV was well tolerated in Nigerian toddlers when administered as a booster dose at 15 to 21 mo of age following 3-dose priming in infancy, and as 2-dose catch-up immunization administered at 15 to 21 and 17 to 23 mo of age. PHiD-CV was immunogenic for all 10 vaccine serotypes and NTHi protein D in both immunization schedules. This demonstration of immunogenicity and good tolerability suggests that PHiD-CV booster dose administration and 2-dose catch-up vaccination are clinically acceptable for Nigerian toddlers.
Materials and Methods
Study design and participants
This phase III, open study (ClinicalTrials.gov, NCT01153893) was conducted between October 2010 and February 2011 at the same purpose-built research suite at the Lagos State University Teaching Hospital, Ikeja, as used in the primary vaccination study.Citation10 It was conducted according to the Somerset West 1996 version of the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines, apart from a deviation from GCP in the informed consent process for one child (described in the legend Note to ). For all other children, written informed consent was obtained from a parent or legal guardian or the thumb print of an illiterate parent/guardian was obtained on the consent form, countersigned by an independent, literate witness. The protocol was reviewed and approved by the Health Research and Ethics Committee of the hospital and authorization for the study was provided by NAFDAC.
In the previous open, randomized controlled primary vaccination study, healthy infants received DTPw-HBV/Hib (Zilbrix™ Hib, GlaxoSmithKline Vaccines) and oral live attenuated poliovirus (Polio Sabin™, GlaxoSmithKline Vaccines) vaccines with (PHiD-CV group) or without (control group) PHiD-CV at 6–10–14 wk of age.Citation10 In Nigeria, all children in the PHiD-CV group who received 3-dose priming with PHiD-CV were invited to receive a booster dose of PHiD-CV co-administered with a booster dose of DTPa vaccine (Infanrix™, GlaxoSmithKline Vaccines) at 15 to 21 mo of age; Zilbrix™ Hib was no longer available at the time of the booster/catch-up study. Children in the control group were offered 2-dose catch-up vaccination with PHiD-CV administered at 15 to 21 mo of age and 17 to 23 mo of age. A booster dose of DTPa vaccine was co-administered with the first PHiD-CV catch-up dose. Both vaccines were administered by intramuscular injection using standard vaccination techniques.
The primary objective of the study was to assess the safety and reactogenicity of PHiD-CV booster dose in relation to the occurrence of grade 3 AEs. Other safety and reactogenicity measures and the immunogenicity of PHiD-CV booster dose and 2-dose catch-up vaccination were evaluated as secondary objectives.
Safety assessment
Study field workers, who were trained in study procedures before and during the trial, made contact with children’s parents via regular telephone calls and daily home visits for 4 d after each vaccine dose. Parents were encouraged to contact study personnel if they had any concerns related to their child’s participation in the study. Safety observations were recorded by the field workers on diary cards that were returned to the study team for review.
Local (pain, redness, swelling at the injection site) and general (fever [axillary temperature ≥37.5 °C], drowsiness, irritability, loss of appetite) symptoms were actively solicited during the 4-d period after the booster dose and each catch-up dose. Large swelling reactions (defined as any local injection site swelling with diameter >50 mm, noticeable diffuse swelling or noticeable increase in circumference of injected limb) were also recorded.
The intensity of each solicited AE was graded on a scale from 0 to 3. Pain at the injection site was considered to have a grade 3 intensity if the child cried when the limb was moved/was spontaneously painful, redness and swelling at the injection site if the diameter was >30 mm and fever if axillary temperature was >39.5 °C. Irritability/fussiness was considered of grade 3 intensity if the child cried and could not be comforted/prevented normal activity, and loss of appetite was considered grade 3 if the child did not eat at all. Grade 3 intensity for all other symptoms and AEs was defined as preventing normal everyday activity and/or causing parents/guardians to seek medical advice.
Other AEs were recorded within a 31-d follow-up period after each vaccine dose and SAEs (defined as any medical event resulting in death, any life-threatening event or any event causing disability, or requiring hospitalization or prolongation of hospitalization) were recorded during the entire study period. All solicited local symptoms were defined in the protocol to be considered causally related to vaccination. For all other AEs, assessment of causal relationship to vaccination was based on the investigator’s clinical judgment. Use of therapeutic and prophylactic antipyretic medication was recorded within 4 d following each vaccine dose. An antipyretic medication was considered to be prophylactic when given in the absence of fever and any other symptom to prevent the occurrence of fever.
Immunogenicity assessment
Blood specimens were collected before the PHiD-CV booster dose and first PHiD-CV catch-up dose and 1 mo after the booster dose and second catch-up dose. Serum aliquots were stored at –20 °C until analysis at GlaxoSmithKline Vaccines’ laboratory in Rixensart, Belgium, or (for OPA analysis) at SGS Lab Simon SA, Wavre, Belgium.
Serum anti-pneumococcal, serotype-specific IgG antibodies were measured using GlaxoSmithKline’s 22F-inhibition enzyme-linked immunosorbent assay (22F-ELISA) at a cut-off (defined as the lower limit of quantification of antibodies) value of 0.05 µg/ml, as described before.Citation24,Citation25 Antibody concentrations of 0.2 µg/ml measured with this 22F-ELISA have been shown to be equivalent to 0.35 µg/ml with the WHO reference laboratory non-inhibition assay.Citation26 OPA was measured by a pneumococcal killing assay in HL60 phagocyte cellsCitation27 with a cut-off titer of 8.Citation28 The results were presented as reciprocal of maximum serum dilution (opsonic titer) able to sustain 50% killing of live pneumococci under the assay conditions. IgG antibody and OPA responses were also determined for the pneumococcal cross-reactive serotypes 6A and 19A and antibodies against NTHi protein D with a cut-off of 100 EL.U/ml were measured by a GlaxoSmithKline ELISA in all subjects.
Statistical analysis
Analyses of safety were performed on the total vaccinated cohort. Incidences of AEs and the prevalence of concomitant medication were calculated with exact 95% CIs.
Immunogenicity analyses were performed on the ATP immunogenicity cohort, defined as vaccinated subjects who complied with all protocol-defined procedures and for whom antibody assay results were available. ELISA antibody GMCs and OPA GMTs calculations were performed by taking the anti-log of the mean of the log titer/concentration transformations. Antibody titers/concentrations below the cut-off of the assay were given an arbitrary value of half the cut-off for the purpose of GMT/GMC calculation. GMCs and GMTs were calculated with 95% CIs and percentages of children with an antibody concentration ≥0.2 µg/ml or OPA titer ≥8 against pneumococcal serotypes were calculated with exact 95% CIs. All comparisons of immune responses were exploratory. Non-overlapping 95% CI boundaries were considered indicative of possible differences between groups.
The statistical analyses were performed using SDD (Statistical Analysis System [SAS] Drug and Development) web portal version 3.5 and SAS version 9.2.
| Abbreviations: | ||
| AE | = | adverse event |
| ATP | = | according-to-protocol |
| CI | = | confidence interval |
| DTPa | = | diphtheria-tetanus-acellular pertussis |
| DTPw-HBV/Hib | = | diphtheria-tetanus-whole-cell pertussis-hepatitis B/Haemophilus influenzae type b |
| 22F-ELISA | = | 22F-inhibition enzyme-linked immunosorbent assay |
| EPI | = | Expanded Program on Immunization |
| GAVI | = | Global Alliance for Vaccines and Immunization |
| GCP | = | Good clinical practices |
| GMC | = | geometric mean antibody concentration |
| GMT | = | geometric mean titer |
| IPD | = | invasive pneumococcal disease |
| NAFDAC | = | National Agency for Food and Drug Administration and Control |
| NTHi | = | nontypeable Haemophilus influenzae |
| OPA | = | opsonophagocytic activity |
| PCV | = | pneumococcal conjugate vaccine |
| PHiD-CV | = | 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine |
| SAE | = | serious adverse event |
| SAS | = | Statistical Analysis System |
| WHO | = | World Health Organization |
Disclosure of Potential Conflicts of Interest
O.O.O., Y.A.K., and O.A.K. declare having received honoraria and/or consulting fees from the GlaxoSmithKline group of companies. J.U.R. was employed by the GlaxoSmithKline group of companies at the time of study conduct. F.S., N.F., J.P.Y., K.D., D.B., and L.S. are employed by the GlaxoSmithKline group of companies; J.U.R., D.B., L.S., J.P.Y., and K.D. declare owning stocks.
Acknowledgments
The authors thank the children and their families who participated in this trial. For their contributions to the study we thank: the staff members of the study center; medical officer T. Idowu,; the clinical and serological laboratory teams of GlaxoSmithKline Vaccines, in particular: Candice Collin (Clinical Trial assistant); Meera Chandregowda (Data Manager); Jan Cleerbout (Central Safety Manager); Aurelia Le Prince (Clinical Data Coordinator); Nele Martens, Mireille Venken and Syed Yasin (Scientific Writers); Sudheer Ravula (statistical analyses); Joanne Knowles (freelance, UK, for GlaxoSmithKline Vaccines, for medical writing support) for drafting the manuscript and Aneta Skwarek-Maruszewska, and Thomas Déplanque (XPE Pharma and Science for GlaxoSmithKline Vaccines, manuscript coordination and editing). GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript.
Contributions
O.O.O., Y.A.K., O.A.K., F.S., N.F., J.P.Y., J.U.R., K.D., and L.S. participated in the design and implementation of the study, including gathering of data. All authors participated in the analysis and interpretation of the data. All authors had full access to the data and had final responsibility to submit for publication. Drafts were developed by a professional publication writer according to the recommendations, documentations and outline provided by all authors. There were agreements concerning confidentiality of the data between the industry sponsor and the academic authors.
Trademark Statement
Synflorix, Zilbrix Hib, Polio Sabin, and Infanrix are trademarks of the GlaxoSmithKline group of companies.
References
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893 - 902; http://dx.doi.org/10.1016/S0140-6736(09)61204-6; PMID: 19748398
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O’Brien KL. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med 2010; 7:7; http://dx.doi.org/10.1371/journal.pmed.1000348; PMID: 20957191
- Alliance GAVI. Target Product Profile (TPP) for the pneumococcal AMC: master table [Internet]. GAVI Alliance; 2008 Dec 18 [cited 2013 July 31]. Available from: http://www.gavialliance.org/funding/pneumococcal-amc/about/.
- Alliance GAVI. Countries approved for support - results & evidence [Internet]. GAVI Alliance; 2013 March [cited 2013 July 31]. Available from: http://www.gavialliance.org/results/countries-approved-for-support/.
- Falade AG, Lagunju IA, Bakare RA, Odekanmi AA, Adegbola RA. Invasive pneumococcal disease in children aged <5 years admitted to 3 urban hospitals in Ibadan, Nigeria. Clin Infect Dis 2009; 48:Suppl 2 S190 - 6; http://dx.doi.org/10.1086/596500; PMID: 19191615
- Jarju S, Baldeh FB, Dione MM, Kwambana B, Ba M, Sonko MA, Hama KM, Aminata GA, Francois H, Basile AG, et al. Potential serotype coverage of PCV-13 against pneumococcal meningitis in West Africa. Poster session presented at 8th International Symposium on Pneumococci and Pneumococcal Diseases; 2012 Mar 11-15; Iguaçu Falls, Brazil.
- Adetifa IM, Antonio M, Okoromah CA, Ebruke C, Inem V, Nsekpong D, Bojang A, Adegbola RA. Pre-vaccination nasopharyngeal pneumococcal carriage in a Nigerian population: epidemiology and population biology. PLoS One 2012; 7:e30548; http://dx.doi.org/10.1371/journal.pone.0030548; PMID: 22291984
- Greenberg D, Givon-Lavi N, Newman N, Bar-Ziv J, Dagan R. Nasopharyngeal carriage of individual Streptococcus pneumoniae serotypes during pediatric pneumonia as a means to estimate serotype disease potential. Pediatr Infect Dis J 2011; 30:227 - 33; http://dx.doi.org/10.1097/INF.0b013e3181f87802; PMID: 20861756
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, November 2011 – conclusions and recommendations. Wkly Epidemiol Rec 2012; 87:1 - 16; PMID: 22242233
- Dicko A, Odusanya OO, Diallo AI, Santara G, Barry A, Dolo A, Diallo A, Kuyinu YA, Kehinde OA, François N, et al. Primary vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in infants in Mali and Nigeria: a randomized controlled trial. BMC Public Health 2011; 11:882; http://dx.doi.org/10.1186/1471-2458-11-882; PMID: 22112189
- Forsgren A, Riesbeck K, Janson H. Protein D of Haemophilus influenzae: a protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin Infect Dis 2008; 46:726 - 31; http://dx.doi.org/10.1086/527396; PMID: 18230042
- Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines 2009; 8:1479 - 500; http://dx.doi.org/10.1586/erv.09.113; PMID: 19863240
- Lagos RE, Muñoz AE, Levine MM, Lepetic A, François N, Yarzabal JP, Schuerman L. Safety and immunogenicity of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Chilean children. Hum Vaccin 2011; 7:511 - 22; http://dx.doi.org/10.4161/hv.7.5.14634; PMID: 21441782
- Silfverdal SA, Prymula R, Traskine M, Habib A, Borys D, Schuerman L. Immunogenicity/reactogenicity of 2-dose catch-up vaccination with 10-valent pneumococcal non-typeable Haemophilus influenzae protein-D conjugate vaccine (PHiD-CV) during fourth year of life. Poster session presented at: 8th International Symposium on Pneumococci and Pneumococcal Diseases; 2012 Mar 11-15; Iguaçu Falls, Brazil.
- Vesikari T, Karvonen A, Korhonen T, Karppa T, Sadeharju K, Fanic A, Dieussaert I, Schuerman L. Immunogenicity of 10-valent pneumococcal nontypeable Haemophilus Influenzae Protein D Conjugate Vaccine when administered as catch-up vaccination to children 7 months to 5 years of age. Pediatr Infect Dis J 2011; 30:e130 - 41; http://dx.doi.org/10.1097/INF.0b013e31821d1790; PMID: 21540760
- Prymula R, Habib A, François N, Borys D, Schuerman L. Immunological memory and nasopharyngeal carriage in 4-year-old children previously primed and boosted with 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) with or without concomitant prophylactic paracetamol. Vaccine 2013; 31:2080 - 8; http://dx.doi.org/10.1016/j.vaccine.2013.01.044; PMID: 23391599
- Prymula R, Siegrist CA, Chlibek R, Zemlickova H, Vackova M, Smetana J, Lommel P, Kaliskova E, Borys D, Schuerman L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet 2009; 374:1339 - 50; http://dx.doi.org/10.1016/S0140-6736(09)61208-3; PMID: 19837254
- Tregnaghi MW, Saez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, Cortes-Barbosa C, Ceballos A, Tregnaghi M, et al. Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) against invasive pneumococcal disease in Latin America. Poster Session presented at 9th International Symposium on Antimicrobial Agents and Resistance (ISAAR); 2013 Mar 13-15; Kuala Lumpur, Malaysia.
- Saez-Llorens X, Castrejon MM, Rowley S, Wong D, Calvo A, Rodriguez M, Troitino M, Lommel P, Hausdorff WP, Borys D, et al. Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) against acute otitis media in children in Panama. Paper presented at 9th International Symposium on Antimicrobial Agents and Resistance (ISAAR); 2013 Mar 13-15; Kuala Lumpur, Malaysia.
- Tregnaghi MW, Sáez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, et al. Efficacy of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) against community-acquired pneumonia in children from Latin America: a randomized controlled trial. Paper presented at XV Congresso Latino Americano Infectologia Pediátrica – SLIPE; 2013 Jun 26-29; Sao Paulo, Brazil.
- Bermal N, Szenborn L, Edison A, Hernandez M, Pejcz J, Majda-Stanislawska E, Gatchalian S, Fanic A, Dieussaert I, Schuerman L. Safety and immunogenicity of a booster dose of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine coadministered with DTPw-HBV/Hib and poliovirus vaccines. Pediatr Infect Dis J 2011; 30:69 - 72; http://dx.doi.org/10.1097/INF.0b013e3181f2da06; PMID: 20980933
- Nunes MC, Madhi SA. Review on the immunogenicity and safety of PCV-13 in infants and toddlers. Expert Rev Vaccines 2011; 10:951 - 80; http://dx.doi.org/10.1586/erv.11.76; PMID: 21806394
- Lalwani S, Chatterjee S, Chhatwal J, Verghese VP, Mehta S, Shafi F, Borys D, Moreira M, Schuerman L. Immunogenicity, safety, and reactogenicity of the 10-valent pneumococcal non-typeable Hemophilus influenzae protein D conjugate vaccine (PHiD-CV) when co-administered with the DTPw-HBV/Hib vaccine in Indian infants: a single-blind, randomized, controlled study. Hum Vaccin Immunother 2012; 8:612 - 22; http://dx.doi.org/10.4161/hv.19287; PMID: 22634448
- Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2001; 8:266 - 72; PMID: 11238206
- Henckaerts I, Goldblatt D, Ashton L, Poolman J. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin Vaccine Immunol 2006; 13:356 - 60; http://dx.doi.org/10.1128/CVI.13.3.356-360.2006; PMID: 16522777
- Poolman JT, Frasch CE, Käyhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol 2010; 17:134 - 42; http://dx.doi.org/10.1128/CVI.00289-09; PMID: 19889940
- Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997; 4:415 - 22; PMID: 9220157
- Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 2007; 25:2518 - 27; http://dx.doi.org/10.1016/j.vaccine.2006.09.029; PMID: 17034907
