Abstract
Several human MHC class II (HLA) molecules are strongly associated with high incidence of autoimmune diseases including type 1 diabetes (T1D). The HLA-humanized mice may thus represent valuable tools to test HLA-based vaccines and therapeutics for human autoimmune diseases. Herein, we have tested the therapeutic potential of a soluble HLA-DR4-GAD65271–280 (hu DEF-GAD65) chimera of human use in a newly-generated NOD/DR4/B7 double transgenic (dTg) mouse that develops spontaneously an accelerated T1D regardless the gender. The NOD/DR4/B7 dTg mice generated by a two-step crossing protocol express the HLA-DR*0401 molecules on 20% of antigen presenting cells, the human B7 molecules in pancreas, and HLA-DR4/GAD65-specific T-cells in the blood. Some 75% of pre-diabetic NOD/DR4/B7 dTg mice treated with hu DEF-GAD65 chimera remained euglycemic and showed a stabilized pancreatic insulitis 6 months after treatment. The 25% non responders developing hyperglycemia survived 3–4 months longer than their untreated littermates. T1D prevention by this reagent occurred by a Th2/TR-1 polarization in the pancreas. This study strongly suggests that the use of soluble pHLA reagents to suppress/stabilize the T1D progression and to extend the life expectancy in the absence of side effects is an efficient and safe therapeutic approach.
Introduction
Type 1 diabetes mellitus (T1D) is an organ-specific autoimmune disease induced by a polyclonal population of self-reactive T-cells that lead to the destruction of insulin-secreting pancreatic β-cells.Citation1 High incidence of T1D is strongly associated with the expression of particular human MHC class I and II alleles (i.e., HLA-DR4, HLA-DQ8, HLA-A2.1) and murine MHC class II (I-Ag7) in the NOD mice.Citation2,Citation3 Some 60% of the T1D patients in USA express HLA-DR4 alleles.Citation4
Several non-antigen specific immunosuppressive attempts to suppress T1D progression showed minimal beneficial effects in experimental conditions or clinical trials.Citation5-Citation8 Some of these approaches also raised safety concerns like induction of systemic immune suppressionCitation9 or pancreatic toxicity.Citation10 We and others reported that a new class of antigen-specific reagents, namely soluble peptide-MHC class II chimeras (DEF reagents, “Diabetes Eliminating Factor”) can delay the pre-clinical stage of diabetes, and more importantly reverse the early T1D onset in mouse models in the absence of side-effects and more efficiently than the synthetic peptide preparations.Citation11-Citation14 A murine DEF reagent was also able to protect grafted pancreatic islets against the re-emerging diabetes.Citation15 DEF reagents have a 2 to 3-d life-span in vivo,Citation16 do not require adjuvant to reach the therapeutic effect, and are devoid of side effects.Citation13-Citation16 On a molar basis, a soluble DEF dimer can deliver ≈1000-times more tolerogenic peptide than the APC-expressing MHC class II molecules can naturally load in vivo.Citation17 DEF anti-diabetogenicity was shown to rely mostly on the induction of IL-4-secreting Th2 cells and IL-10-secreting TR-1 suppressor cells in the pancreas.Citation13,Citation15,Citation17
We previously showed that a soluble dimeric HLA-DR*0401-GAD65271–285 chimera (huDEF-GAD65 reagent) of human use can induce IL-10-secreting TR-1 cells by GAD65271–285–specific T-cells in the peripheral blood lymphocytes of diabetic patients.Citation4 To test the therapeutic efficacy of this reagent, we have generated a suitable humanized NOD strain expressing on the APCs the human MHC class II HLA-DR*0401 molecules under the murine MHC class II I-Ed gene promoter, and at the same time the human B7.1 (CD80) costimulatory molecule under the rat insulin promoter in the pancreatic β-islets (NOD/DR4/B7 dTg mouse). While the NOD/DR4 Tg parental mouse used to generate our NOD/DR4/B7 dTg mouse does not develop diabetes, the NOD/DR4/B7 dTg mice develop an aggravated, spontaneous disease early in life and regardless the gender. These humanized NOD/DR4/B7 dTg mice were used in this study to test the preventive capacity of hu DEF-GAD65 reagent.
Results and Discussion
The humanized NOD/DR4/B7 dTg mouse is a suitable model for testing the therapeutic potential of hu DEF-GAD65 reagent
The NOD wt mouse is the closest model for human T1D.Citation2 Herein, we tested the therapeutic effect of a hu HLA-DR4-GAD65271–285 chimera of human use in a newly-generated, humanized NOD strain expressing the human MHC class II HLA-DR*0401 molecule on APCs under the I-Ed gene promoter and human B7.1 costimulatory molecule under the rat insulin promoter in pancreas (NOD/DR4/B7 dTg mouse). The soluble dimeric hu HLA-DR4-GAD65271–285 chimera is referred thereafter as to huDEF-GAD65 reagent.
Full recovery of the NOD diabetogenic background in the parental NOD/DR4 Tg mouse used to generate the NOD/DR4/B7 dTg mouse was confirmed by PCR and microsatellite analysis (). The HLA-DR*0401 requirement for the NOD/DR4/B7 dTg mouse was to present the GAD65271–285 self-peptide to CD4 T-cells, which in turn could be targeted by the hu DEF-GAD65271–285 reagent. The hu DEF-GAD65271–285 reagent has been previously shown to recognize and score by FACS the human GAD65271–285-specific CD4 T-cells from HLA-DR*0401+ diabetic patients.Citation4 FACS analysis confirmed the specific binding of hu DEF-GAD65271–285 reagent to the hu HLA-DR*0401 transgenic molecule on more than 20% of splenic cells (). The presence of GAD65271–285-specific CD4 T-cells in the spleen of NOD/DR4/B7 dTg mice was also detected in FACS by hu DEF-GAD65271–285 reagent for as much as 60 times higher (0.67%) than the signal-to-noise background detected in the NOD non Tg littermates (0.014%)().
Figure 1. Immunologic characterization of humanized NOD/DR4/B7 dTg mice. (A) microsatellite analysis of the NOD genetic background in the parental NOD/DR4 Tg strain (upper panel). Comparison between the genetic background in 2 representative NOD wt, C57BL/6 parental strains, and 8 out of 32 microsatelites in the F12 generation of parental NOD/DR4 Tg strain shows full transfer of the NOD background in the parental NOD/DR4 Tg strain. Lower panel, identification of human HLA-DR*0401 and B7.1 (CD80) transgenes by PCR using specific primers (forward: GTTTCTTGGA GCAGGTTAAA CA; reverse: CTGCACTGTG AAGCTCTCAC, and respectively: forward: GCTTACAACC TTTGGAGACC CAG; reverse: CGTCACTTCA GCCAGGTG). Internal control PCR primers for DNA quantification were specific for mouse IgG3 gene (forward: ACAACAGCCC CATCTGTCTA T; reverse: GTGGGCTACG TTGCAGATGA C). Lane 1, DNA size markers; lanes 2–5, NOD mice expressing both the human HLA-DR*0401 and B7.1 transgenes; lanes 6–11, NOD/DR4 littermates lacking the hu B7.1 transgene. (B) expression of HLA-DR4 molecules on splenic APCs from NOD/DR4/B7 dTg mice. Left panel, splenic cells stained with a rat IgG isotype control Ab-FITC conjugate; Right panel, splenic cells stained with a rat IgG anti-HLA-DR4-FITC conjugate. Shown is the mean ± SD values as determined in 4 NOD/DR4/B7 mice. (C), FACS detection of GAD65271–285-specific CD4+ T-cells in the blood of NOD/DR4/B7 dTg mice. Left panel, the signal-to-noise background of the secondary anti-human IgG1-FITC conjugate; right panel, the mean frequency of GAD65271–280-specific CD4+ T cells ± SD measured in 4 NOD/DR4/B7 dTg mice in spleen cells double-stained with hu DEF-GAD65 reagent and revealed by a goat anti-human IgG1-FITC conjugate, and anti-mouse CD4 Ab-APC conjugate (clone #GK1.5, ATCC, BD PharMingen). (D) immunohistochemical detection of human B7.1 (CD80) expression in the pancreatic β-islets of NOD/DR4/B7 dTg mice. Fresh pancreatic sections of 5μm in OCT from a NOD wt mouse (left panel) and NOD/DR4/B7dTg mouse (right panel) were stained with a rat IgG anti-human B7 molecule (BD PharMingen) and revealed by an anti-rat IgG-HRP conjugate (Jackson ImmunoResearch). Shown is the absence of B7 staining of a representative β-islet from a NOD wt mouse, and the positive B7 staining for a representative β-islet from a NOD/DR4/B7 dTg mouse. Dark arrows in each panel indicate the position of pancreatic β-islets.
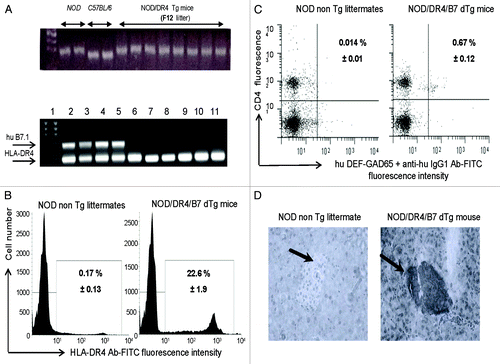
On the other hand, pancreatic B7.1 costimulation of diabetogenic T-cells was previously shown to accelerate the T1D onset, and to aggravate the disease progression in mouse models.Citation19,Citation20 PCR and imunohistochemical analyses confirmed the hu B7.1 expression in the pancreatic islets of NOD/DR4/B7 dTg mouse (). This mouse develops an aggressive, spontaneous diabetes by 3 to 4 mo after birth and regardless the gender (). Together, these data confirmed the suitability of our humanized NOD/DR4/B7 dTg mouse for testing the therapeutic potential of hu DEF-GAD65271–285 reagent.
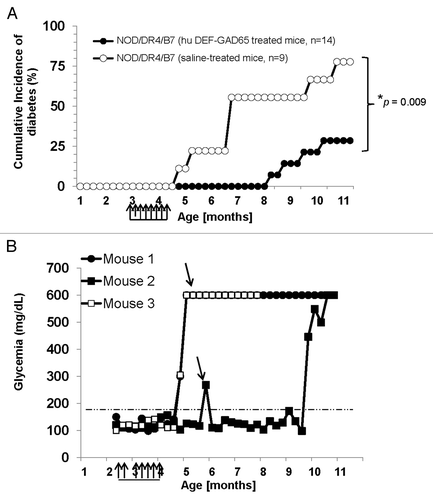
Figure 2. T1D incidence in the NOD/DR4/B7 dTg mice treated with hu DEF-GAD65 reagent. (A) Pre-diabetic, 2.5 mo-old NOD/DR4/B7 dTg mice were selected from 4 different litters (n = 14) and treated i.p. with 8 doses of 10 μg of hu DEF-GAD 65 reagent in saline (n = 14 mice) or with saline alone (control group, n = 9 mice) every other 4th day. Glycemia was measured bi-weekly from the tail vein. Y axis indicates the cumulative incidence of hyperglycemia calculated as a percent of mice developing hyperglycemia in each group at different time-points after treatment interruption. Grouped arrows on the X axis indicate the time-points and number of hu DEF-GAD65 i.p. injections. Shown is the significant relevance (* P value) between the two groups at the end of experiment. (B) hyperglycemia values in hu DEF-GAD65 non responders (NOD/DR4/B7 dTg mice) in two representative mice (Mouse #1 and #3) with stabilized hyperglycemia after one single dose of 20 μg hu DEF-GAD65 as followed up for 8 more months after injection. Arrows indicate the time of supplemental hu DEF-GAD65 injection (at 6 mo of age) that failed to reverse hyperglycemia in mice #1 and #3. Also shown is a NOD/DR4/B7 dTg mouse (mouse #2) treated with hu DEF-GAD65 reagent under the same regimen as in panel A, which developed mild hyperglycemia (250 and 270 mg/dL) some 1.5 mo after treatment interruption. Arrow indicates the time-point (6 mo of age) when mouse #2 received a supplemental 20 μg hu DEF-GAD65 i.p. injection that reversed hyperglycemia. Grouped arrows on the X axis indicate the time-points and number of hu DEF-GAD65 i.p. injections.
Human DEF-GAD65 reagent delays the T1D onset in NOD/DR4/B7 dTg mice
The rationale of using a different GAD65 peptide sequences expressed by human and murine DEF-GAD65 reagents is that the GAD65217–230 peptide expressed by the murine DEF-GAD65 reagent is recognized only by the MHC class II (I-Ag7) in NOD mouse, whereas the GAD65271–280 peptide expressed by the human DEF-GAD65 reagent is recognized only by the HLA-DR4 molecules in humans.
Treatment with pre-diabetic NOD/DR4/B7 dTg mice with hu DEF-GAD65 reagent in saline or saline alone (control group) was initiated 3 mo after birth when mice still show euglycemia and a light pancreatic insulitis. Some 75% of the NOD/DR4/B7 dTg mice (n = 14) treated i.p. twice a week with 8 small doses (10 μg/dose) of hu DEF-GAD65 reagent did not develop hyperglycemia for 6 mo after treatment (P = 0.009) (). In contrast, 75% of the NOD/DR4/B7 dTg littermates in the control group (n = 9) developed hyperglycemia shortly after the last i.p. injection of saline alone (P = 0.01). Treatment of pre-diabetic NOD/DR4/B7 dTg mice (n = 7) with hu DEF-OSPA control reagent that does not bind to human CD4 T-cellsCitation4 did not affect the T1D development (data not shown), indicating that the therapeutic effect is attributed only to the hu DEF-GAD65 reagent, but not the hu DEF molecule itself. It is very unlikely the endogenously expressed I-Ag7 murine molecules are responsible for the hu-DEF-GAD65 reagent used in this study, since the hu DEF-GAD65 expresses the peptide GAD65271–285 recognized only by the HLA-DR4 transgenic molecules but not the murine I-Ag7 molecules, Also, as shown in , the hu DEF-GAD65 reagent does not bind to I-Ag7 molecules in the NOD non Tg littermates, which rules out the possibility that I-Ag7 may contribute to T1D protection in this mouse model.
Together, these data demonstrated that, like the murine DEF-GAD65 reagent,Citation13,Citation14 the human DEF-GAD65 reagent exerted similar therapeutic effects in the humanized NOD/DR4/B7 dTg mouse model. The humanized NOD/DR4/B7 dTg mouse was an appropriate mouse model to test the hu DEF-GAD65 reagent, since it expresses the HLA-DR4 human molecules targeted specifically by the hu DEF-GAD65 reagent, and at the same time expresses a human B7.1 costimulatory in the pancreas, which was shown to accelerate the T1D development regardless the gender.Citation19,Citation20
Clinical trials in T1D patients using GAD protein or peptides as potential immunotherapeutic vaccine were so far unsuccessful (reviewed in ref. Citation20), though the hu DEF-GAD reagent showed promising results. This is likely to be the result of a different immune mechanism utilized by hu DEF-GAD655 reagent. Thus, while GAD65 protein immunization may lead to stimulation of T-cells including diabetogenic T-cells due to expression of various immunogenic GAD epitopes, the soluble hu DEF-GAD65 reagent utilizes an epitope that can selectively target the TR-1 protective cells.
Although our present focus was to test the T1D preventive ability of hu DEF-GAD65 reagent, this study suggests the likability of this reagent to reverse the early T1D onset. Thus, among the hu DEF-GAD65-treated mice with sustained euglycemic status, 1 mouse developed hyperglycemia 1 mo after last injection. This mouse responded well to an additional i.p. injection of 20 μg of DEF-GAD65 reagent when hyperglycemia scored 240 mg/dL and 270 mg/dL, as it returned next day to a stable euglycemic status for another 3 mo (). In contrast, several mice in the saline-treated group (control group) that have been also treated with a single dose of 20 μg hu DEF-GAD65 reagent when reaching higher sugar levels than 400 mg/dL, did not respond to the treatment. This led to the conclusion that, like previously shown in animal models treated with other murine DEF-like reagents, the hu DEF-GAD65 reagent may not be able to reverse the disease when hyperglycemia reaches higher levels than 300 mg/dL. Confirmatory therapeutic strategies using different protocols of immunization will be further required to determine if indeed the hu DEF-GAD65 reagent is beneficial in the late stages of disease.
Human DEF-GAD65 treatment improves the rate of survival in diabetic NOD/DR4/B7 dTg mice
Although ≈25% of NOD/DR4/B7 dTg mice did not respond to hu DEF-GAD65 therapy, their overall life expectancy was significantly increased. Two of the hyperglycemic non responders lived up to 4 more months after the interruption of treatment. Also, most of the hyperglycemic NOD/DR4/B7 dTg mice in the saline-treated group (control group) survived for 2 to 3 more months longer, and did not show a loss of weight (data not shown) when injected i.p. with a single dose of 20 μg of hu DEF-GAD65 reagent (). This is a significantly long rate of survival as compared with the untouched hyperglycemic littermates that succumb within 2 to 3 wk after the hyperglycemia onset. The non responder mice treated with a single dose of 20 μg hu DEF-GAD65 reagent maintained their sugar levels below 550 mg/dL and showed no body weight loss (data not shown). The results indicated that, although in a hyperglycemic stage, the mice survived longer with a limited progression of hyperglycemia when treated with hu DEF-GAD65 reagent at a late stage of disease.
Human DEF-GAD65 reagent stabilizes the lymphocyte infiltration in the pancreas of NOD/DR4/B7 dTg mice
We have previously reported that mice treated with murine DEF reagents show a significantly reduced number of pancreatic β-islets with destructive intra-islet infiltration, but rather a protective type of peri-insulitis.Citation13-Citation15 Indeed, prolonged survival observed in the non responder, hyperglycemic mice treated with hu DEF-GAD65 may be explained by the formation of a protective, stabilized peri-islet pancreatic infiltration, which in turn may provide a limited, but sufficient amount of insulin to stabilize glycemia.
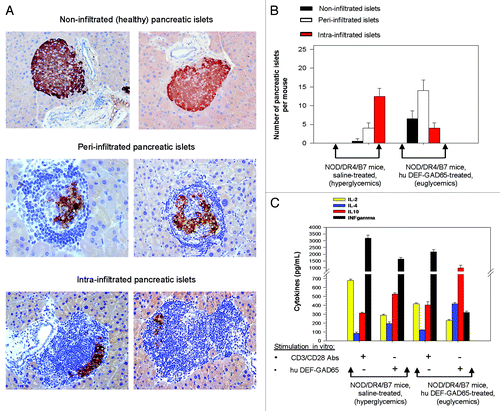
The NOD/DR4/B7 dTg mice protected by hu DEF-GAD65 treatment showed a significantly increased number of peri-infiltrated β-islets (P = 0.047), and a reduced number of intra-infiltrated β-islets (P = 0.042) than those in the control group (). Although not significantly increased, the number of non-infiltrated β-islets in euglycemic mice protected by hu DEF-GAD65 treatment was higher than in hyperglycemic mice from the control group (P = 0.063). This is because the overall number of infiltrated β-islets (intra- and per-infiltrated β−islets together) in both groups was similar, with the only difference that the ratio between the peri- vs. intra-infiltrated β−islets was higher in the euglycemic treated mice (ratio = 4:1) than in hyperglycemic mice from the control group (ratio = 1:3.5)(). The results indicated that, likewise the murine DEF-GAD65 reagent,Citation13-Citation15 the human DEF-GAD65 reagent was able to stabilize the pancreatic insulitis.
Figure 3. Pancreatic and T-cell analyses of NOD/DR4/B7 dTg mice treated with hu DEF-GAD65 reagent. (A) two representative, fully functional pancreatic β-islets lacking lymphocyte infiltration (upper panels), or peri-infiltrated β-islets (middle panels), or intra-infiltrated β-islets from the group of NOD/DR4/B7 dTg mice treated with hu DEF-GAD65 reagent as in , panel A. Pancreata from both groups of mice were analyzed after treatment interruption, when mice reached 11 mo of age. Shown in each panel are the HE staining of infiltrating lymphocytes (dark blue) and the presence of intra-islet insulin granules stained with a rabbit anti-Insulin-HRP conjugate. Of note, the peri-infiltrated islets show higher amount of insulin granules as compared with the intra-infiltrated islets. (B) Comparative morphologic analysis of pancreatic islet infiltration in NOD/DR4/B7 dTg mice treated or not with hu DEF-GAD65 reagent as in . The pancreata from both groups of mice were analyzed when mice reached 11 mo of age. Some 20–25 β-islets per pancreas were analyzed from individual mice (n = 5 mice/group). Of note, treated mice showed a significantly higher number of pancreatic peri-infiltrated islets than those in the control group. (C) cytokine analysis in stimulated spleen cultures from NOD/DR4/B7 dTg mice treated or not with hu DEF-GAD65 reagent. Of note, the CD4 T-cells from NOD/DR4/B7 dTg mice treated in vivo with hu DEF-GAD65 reagent secreted a significantly higher amount of IL-4 and IL-10, and lower amount of IFN-γ than those from saline-treated animals (control group). Shown are the mean values of cytokines ± SD for 4 individual mice in each group.
The therapeutic effects of hu DEF-GAD65 reagent rely on Th2/TR-1 polarization in pancreas
We have previously reported that the mechanism of T1D protection by murine DEF reagents relies on differentiation and expansion of IL-4+ Th2 cells and IL-10-secreting TR-1 suppressor cells in the pancreas.Citation14,Citation15,Citation17 Also, the hu DEF-GAD65 reagent induced a population of IL-4+ Th2 cells and IL-10-secreting TR-1 cells in the lymphocyte cultures of diabetic patients expressing HLA-DR*0401 molecules.Citation4 Herein, we tested the mechanism underlying T1D protection by the hu DEF-GAD65 reagent in NOD/DR4/B7 dTg mice relies also on Th2/TR-1 polarization. The Th2 response in stimulated cell cultures from the pancreatic lymph nodes of NOD/DR4/B7 dTg mice treated or not with hu DEF-GAD65 reagent was estimated based on IL-4 secretion, the TR-1 response based on IL-10 secretion, and the Th1 based on IFN-γ secretion.
CD4 T-cells from the pancreatic lymph nodes (pLN CD4 T-cells) mirror the phenotypic and functional profile of pancreatic infiltrated T-cells in hyperglycemic mice.Citation21 The pLN CD4 T-cells from treated euglycemic mice that were stimulated in vitro with hu DEF-GAD65 reagent 6 mo after treatment interruption showed a significant increase in IL-4 secretion (P = 0.0001) and IL-10 secretion (P = 0.018), while the IFN-γ secretion was drastically reduced (P = 4.5x10−5) as compared with the hyperglycemic mice in the control group. Also, the in vitro CD3 polyclonal stimulation of pLN CD4 T-cells from euglycemic treated mice showed a significantly reduced IFN-γ secretion (P = 0.039) and increased IL-10 secretion (P = 0.014) as compared with those from the hyperglycemic control mice ().
Together, these results showed that T1D prevention and stabilization in NOD/DR4/B7 dTg mice occurred in the context of Th2/TR-1 polarization of peripheral CD4 T-cells, much likely by a similar mechanism (single-epitope bystander suppression) described for the murine DEF-GAD65 reagent.Citation14 This mechanism refers as to the ability of a single peptide-MHC class II reagent (like DEF) to suppress a polyclonal population of autoreactive T-cells of various peptide specificities through the stimulation/expansion of non antigen-specific IL-10-secreting TR-1 suppressor cells at the site of inflammation.Citation14 In summary, this work suggests that human DEF-like reagents targeting diabetogenic T cells in the absence of systemic immune suppression may represent an efficient antigen-specific approach that can overcome the limitations of conventional antigen-specific reagents for T1D therapy.
Methods
The human DEF-GAD65 reagent
Genetic engineering of human HLA-DR*0401-GAD65271–285 soluble dimer (hu DEF-GAD65 reagent) has been previously described.Citation22 The hu DEF-GAD65 reagent is made of the extracellular domains of human HLA-DR*0401 (MHC class II) molecules on human Ig-Fcγ1 (hFcγ1) scaffold, and it expresses the human GAD65271–285 peptide covalently-linked at the N terminus of β−chain. The hu DEF-GAD65 reagent was produced in baculovirus-infected insect SF9 cells and purified from the cell culture supernatant by affinity chromatography using an anti-human IgG1-Sepharose 4B column, as described.Citation4,Citation22
The humanized NOD/HLA-DR4/B7 dTg mouse
The humanized NOD/DR4/B7 dTg strain was generated by a two-step crossing protocol. First, the C57BL/6 mice deficient for MHC class II molecules (H-2b+, IAβ-/IEα-) and transgenic for a human/murine chimeric HLA-DR4-IE molecule (HLA-DRA-IEdα/HLA-DRB1*0401-IEdβ) (Jackson Labs)Citation23 were backcrossed for 12 generations into the NOD diabetogenic background (IAg7+, IEdnull, H-2d+) to generate the NOD-DR4 Tg strain (HLA-DRA-IEdα/HLA-DRB1*0401-IEdβ+, IAg7+, IEdnull, H-2d+). The NOD/DR4 Tg mice were next crossed with NOD/RAG2−/−, hu B7.1+/+ Tg mice (Taconics) to generate the NOD/DR4/B7 dTg mice used in this study (HLA-DRA-IEdα/HLA-DRB1*0401-IEdβ+, huB7.1+, IAg7+, IEdnull, H-2d+, Rag2+). Selection of NOD/DR4/B7 dTg mice was performed by PCR using specific primers for HLA-DR4 and hu B7.1 genes ().
Therapeutic protocol
Pre-diabetic (euglycemic) NOD/DR4/B7 dTg mice were injected intraperitoneal (i.p.) every other 4th day with 8 doses of 10 μg of hu DEF-GAD65 reagent in saline (n = 14 mice) or saline alone (control group, n = 9 mice) every other 4th day, starting 3 mo after birth. Mice were followed weekly for the blood glucose levels using an Accu-Check glucose meter and glucose test strips (Roche). Non-responder hyperglycemic mice were also treated with a supplemental dose of 20 μg hu DEF-GAD65 reagent after the hyperglycemia onset, and monitored for the blood glucose levels and rate of survival. Experiments were performed at USUHS under the MED-11–655 and MED-11–805 IACUC protocols according to the federal, local regulations, and to EU Directive 2010/63/EU.
Flow cytometry
Single-cell suspension of splenocytes (106 cells) from individual mice were stained 30 min at 4 °C for various cell surface markers using specific Ab-dye conjugates or their isotype controls. Some 104 to10Citation5 cell events were acquired using a LSR II Becton-Dickinson flow cytometry instrument equipped with the WINLIST analysis software (Verity, Topsham, ME, USA), or with a BD FACS DIVA software (BD Biosciences).
T-cell stimulation and cytokine assays
Cells from the pancreatic lymph nodes of individual treated and saline-treated mice were harvested on 5 μm strainers and stimulated in vitro for 1 and 4 d with CD3 mAb (#2C11 clone, ATCC, 2.5 μg Ab/106 cells/well) or hu DEF-GAD65 reagent (5 μg reagent/106 cells/well). Cell viability scored microscopically in bromphenol stained cells was higher than 90%. The supernatant from triplicates cell cultures was measured by Luminex assay for cytokine secretion. IL-2 measurements were performed 1 d after stimulation, whereas other cytokines were measured 4 d after continuous stimulation.
Histology and Immunohistochemistry
Pancreata from individual mice responsive or not to hu DEF-GAD65 treatment were analyzed for the degree and morphology of insulitis among 20–25 paraffin-embedded 5μm sections stained with Hematoxilin-Eosin (HE). Serial 5µm sections of pancreata were also stained with a rabbit anti-insulin Ab (Santa Cruz Biotech) revealed by a goat anti-rabbit IgG-HRP conjugate (Southern Biotechnologies) to estimate the extend of insulin secretion and intra-islet distribution of insulin granules.
Biostatistics
Statistical significance for T1D incidence within the same group and between groups of hu DEF-GAD65-treated and saline-treated mice was performed by Log-rank (Mantel-Cox) test, SPSS software version 21.0.0 (IBM Corporation 1 New Orchard Road Armonk, New York 10504–1722 United States). The P values less than 0.05 were considered significant. Individual variations in the number of infiltrated islets and morphology (protective peri-insulitis vs. destructive intra-islet insulitis) between hu DEF-GAD65-treated and saline-treated mice were analyzed by the Student t test and expressed as mean ± standard deviation (SD) at 99% interval of confidence.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by an USUHS grant (#RO83ZK) to Brumeanu TD, and a JDRF grant to Casares S and Brumeanu TD. We thank Mrs Margaret Kehl and Jacqueline Surls for assistance with mice care and Luminex assays.
References
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001; 358:221 - 9; http://dx.doi.org/10.1016/S0140-6736(01)05415-0; PMID: 11476858
- Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol 2011; 33:67 - 87; http://dx.doi.org/10.1007/s00281-010-0204-1; PMID: 20424843
- Chao CC, Sytwu HK, Chen EL, Toma J, McDevitt HO. The role of MHC class II molecules in susceptibility to type I diabetes: identification of peptide epitopes and characterization of the T cell repertoire. Proc Natl Acad Sci U S A 1999; 96:9299 - 304; http://dx.doi.org/10.1073/pnas.96.16.9299; PMID: 10430937
- Preda I, McEvoy RC, Lin M, Bona CA, Rapaport R, Brumeanu TD, Casares S. Soluble, dimeric HLA DR4-peptide chimeras: an approach for detection and immunoregulation of human type-1 diabetes. Eur J Immunol 2005; 35:2762 - 75; http://dx.doi.org/10.1002/eji.200526158; PMID: 16106371
- Skyler JS. Immunomodulation for type 1 diabetes mellitus. Int J Clin Pract Suppl 2010; 166:59 - 63; http://dx.doi.org/10.1111/j.1742-1241.2009.02280.x; PMID: 20377666
- Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care 2009; 32:1244 - 9; http://dx.doi.org/10.2337/dc09-0054; PMID: 19366957
- Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simões BP, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009; 301:1573 - 9; http://dx.doi.org/10.1001/jama.2009.470; PMID: 19366777
- Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, Bluestone JA. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A 2008; 105:18895 - 900; http://dx.doi.org/10.1073/pnas.0810246105; PMID: 19015530
- Marzorati S, Bocca N, Molano RD, Hogan AR, Doni M, Cobianchi L, Inverardi L, Ricordi C, Pileggi A. Effects of systemic immunosuppression on islet engraftment and function into a subcutaneous biocompatible device. Transplant Proc 2009; 41:352 - 3; http://dx.doi.org/10.1016/j.transproceed.2008.09.057; PMID: 19249555
- Pedotti R, Sanna M, Tsai M, DeVoss J, Steinman L, McDevitt H, Galli SJ. Severe anaphylactic reactions to glutamic acid decarboxylase (GAD) self peptides in NOD mice that spontaneously develop autoimmune type 1 diabetes mellitus. BMC Immunol 2003; 4:2; http://dx.doi.org/10.1186/1471-2172-4-2; PMID: 12597780
- Li L, Yi Z, Wang B, Tisch R. Suppression of ongoing T cell-mediated autoimmunity by peptide-MHC class II dimer vaccination. J Immunol 2009; 183:4809 - 16; http://dx.doi.org/10.4049/jimmunol.0901616; PMID: 19752238
- Masteller EL, Warner MR, Ferlin W, Judkowski V, Wilson D, Glaichenhaus N, Bluestone JA. Peptide-MHC class II dimers as therapeutics to modulate antigen-specific T cell responses in autoimmune diabetes. J Immunol 2003; 171:5587 - 95; PMID: 14607967
- Casares S, Hurtado A, McEvoy RC, Sarukhan A, von Boehmer H, Brumeanu TD. Down-regulation of diabetogenic CD4+ T cells by a soluble dimeric peptide-MHC class II chimera. Nat Immunol 2002; 3:383 - 91; http://dx.doi.org/10.1038/ni770; PMID: 11862219
- Lin M, Stoica-Nazarov C, Surls J, Kehl M, Bona C, Olsen C, Brumeanu TD, Casares S. Reversal of type 1 diabetes by a new MHC II-peptide chimera: “Single-epitope-mediated suppression” to stabilize a polyclonal autoimmune T-cell process. Eur J Immunol 2010; 40:2277 - 88; http://dx.doi.org/10.1002/eji.200940094; PMID: 20540111
- Casares S, Lin M, Zhang N, Teijaro JR, Stoica C, McEvoy R, Farber DL, Bona C, Brumeanu TD. A peptide-major histocompatibility complex II chimera favors survival of pancreatic beta-islets grafted in type 1 diabetic mice. Transplantation 2008; 85:1717 - 25; http://dx.doi.org/10.1097/TP.0b013e31817752cc; PMID: 18580462
- Preda-Pais A, Stan AC, Casares S, Bona C, Brumeanu T-D. Efficacy of clonal deletion vs. anergy of self-reactive CD4 T-cells for the prevention and reversal of autoimmune diabetes. J Autoimmun 2005; 25:21 - 32; http://dx.doi.org/10.1016/j.jaut.2005.04.003; PMID: 16005609
- Casares S, Zong CS, Radu DL, Miller A, Bona CA, Brumeanu TD. Antigen-specific signaling by a soluble, dimeric peptide/major histocompatibility complex class II/Fc chimera leading to T helper cell type 2 differentiation. J Exp Med 1999; 190:543 - 53; http://dx.doi.org/10.1084/jem.190.4.543; PMID: 10449525
- Wong S, Guerder S, Visintin I, Reich E-P, Swenson KE, Flavell RA, Janeway CA Jr.. Expression of the co-stimulator molecule B7-1 in pancreatic β-cells accelerates diabetes in the NOD mouse. Diabetes 1995; 44:326 - 9; http://dx.doi.org/10.2337/diab.44.3.326; PMID: 7533734
- Rajagopalan G, Kudva YC, Chen L, Wen L, David CS. Autoimmune diabetes in HLA-DR3/DQ8 transgenic mice expressing the co-stimulatory molecule B7-1 in the beta cells of islets of Langerhans. Int Immunol 2003; 15:1035 - 44; http://dx.doi.org/10.1093/intimm/dxg103; PMID: 12917255
- Ludvigsson J, Krisky D, Casas R, Battelino T, Castaño L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med 2012; 366:433 - 42; http://dx.doi.org/10.1056/NEJMoa1107096; PMID: 22296077
- Casares S, Brumeanu TD. Insights into the pathogenesis of type 1 diabetes: a hint for novel immunospecific therapies. Curr Mol Med 2001; 1:357 - 78; http://dx.doi.org/10.2174/1566524013363753; PMID: 11899083
- Casares S, Bona CA, Brumeanu TD. Engineering and characterization of a murine MHC class II-immunoglobulin chimera expressing an immunodominant CD4 T viral epitope. Protein Eng 1997; 10:1295 - 301; http://dx.doi.org/10.1093/protein/10.11.1295; PMID: 9514118
- Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin DR, Arceo R, Campbell R, et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J Exp Med 1996; 183:2635 - 44; http://dx.doi.org/10.1084/jem.183.6.2635; PMID: 8676084
