Abstract
Cellular immunotherapy can be an effective adjuvant treatment for multiple myeloma (MM), as demonstrated by induction of durable remissions after allogeneic stem cell transplantation. However, anti-myeloma immunity is often hampered by suppressive mechanisms in the tumor micro-environment resulting in relapse or disease progression. To overcome this immunosuppression, new cellular immunotherapies have been developed, based on the important effector cells in anti-myeloma immunity, namely T cells and natural killer cells. These effectors can be modulated to improve their functionality, activated by dendritic cell vaccines, or combined with immune stimulating antibodies or immunomodulatory drugs to enhance their efficacy. In this review, we discuss promising pre-clinical and clinical data in the field of cellular immunotherapy in MM. In addition, we address the potential of combining these strategies with other therapies to maximize clinical effects without increasing toxicity. The reviewed therapies might pave the way to effective personalized treatments for MM patients.
Introduction
Multiple myeloma (MM) is an aggressive plasma cell disease, which accounts for approximately 10% of all hematologic neoplasms.Citation1 In Western countries, the annual incidence is 5.4 cases per 10 000 persons.Citation2 MM is characterized by clonal proliferation and accumulation of malignant plasma cells in the bone marrow and a high concentration of monoclonal immunoglobulin light and/or heavy chains in the blood or urine. This results in organ damage and clinical symptoms including anemia, bone pain, renal insufficiency, hypercalcemia, and infections.Citation3 Over the past decade overall survival (OS) has improved significantly, especially in younger patients, because of the introduction of novel therapies like immunomodulatory drugs and proteasome inhibitors before and after autologous hematopoietic stem cell transplantation (SCT). Currently, the average 10-y OS is approximately 17% for all ages, and in patients younger than 60 y the 10-y OS is about 30%.Citation4
Current treatment of young patients, generally defined as 65 y of age or younger, consists of induction therapy followed by autologous SCT. The induction therapy can reduce tumor mass and create a state of minimal residual disease (MRD). Initially, it consisted of conventional chemotherapy, however, nowadays immunomodulatory derivates (IMiDs), like thalidomide and lenalidomide and the proteasome inhibitor bortezomib are combined with chemotherapy. These new combination therapies improved the complete response (CR) rates, which are correlated with improved progression-free survival.Citation5 After induction therapy, patients are treated with high dose melphalan in order to destroy residual tumor cells, followed by autologous stem cell rescueCitation6
Older patients more often have a poor performance status and suffer from co-morbidities. Although it has been shown that autologous SCT is feasible in patients up to 75 y of age with good performance status,Citation7 benefits for older patients have not been demonstrated in clinical trials as these patients are often excluded.Citation8 Therefore, patients above 65 y of age are generally excluded from autologous SCT.Citation7 Traditionally, older patients were treated with melphalan and prednisone (MP). In recent years, phase III randomized trials in patients not eligible for SCT have investigated the combination of MP plus thalidomide or lenalidomide, or proteasome inhibitors, like bortezomib and recently carfilzomib, on outcome. These studies, demonstrated improved progression-free survival (PFS) in patients with combined therapy.Citation9 MP was compared with the combination of MP and thalidomide (MPT) in a meta-analysis using pooled data of 1682 patients treated in 6 different trials. Median OS was 32.7 (95% CI 30.4–36.5) months in the MP arm and 39.3 (95% CI 35.6–39) months in the MPT arm. Median PFS was 14.9 (14.0–16.6) in the MP arm and 20.4 (18.8–21.6) months in the MPT arm.Citation10 The largest MP-based phase III study so far, the VISTA (Velcade as Initial Standard Therapy) trial, investigated combination of MP with bortezomib (VMP) in 682 patients. Time to progression in the VMP group was 24.0 mo, as compared with 16.6 mo in the MP group (P < 0.001). Furthermore CR rates were 30% and 4%, respectively (P < 0.001).Citation11 After 3 y, OS rates were 68.5% in the VMP group vs. 54.0% in the MP group.Citation12 However, despite these improvements in MM treatment, OS is still poor and most patients eventually experience relapse of the disease. Therefore, additional potent therapeutic strategies are urgently needed.
In this review, we will discuss promising novel cellular immunotherapeutic therapies, which could improve outcome in MM patients with reduced side effects. We will first describe how allogeneic SCT, which is the oldest immunotherapeutic strategy in MM, indicated the importance of the immune system in targeting MM. Second, we will explain how MM can progress or relapse by evasion of the immune system. Finally, we will address how different cellular immunotherapeutic strategies, alone or in combination with other therapies, can circumvent immune evasion and thereby improve anti-myeloma immune responses.
Lessons from Allogeneic SCT
Hematopoietic SCT is a well-established treatment for MM patients. In autologous SCT, stem cells are isolated from the patients themselves and may contain residual tumor cells, which can cause relapse of the disease. Additionally, malignant plasma cells that survive the high dose melphalan may cause relapse of the original disease. In allogeneic SCT, stem cells are derived from a Human Leukocyte Antigen (HLA)-matched healthy donor and a potent graft-vs.-myeloma (GVM) response can be induced. This response can eliminate residual tumor cells in the patient, thereby resulting in long-term remission and potentially even cure of the disease. However, allogeneic SCT is curative only in a minority of MM patients, and treatment-related mortality (TRM) is generally high.
Important immune effectors involved in the GVM response are T cells and Natural Killer (NK) cells. T cells can recognize specific antigens presented by HLA molecules via their T cell receptor (TCR). When T cells encounter their cognate antigens and receive appropriate co-stimulation, they become activated and acquire effector functions. In MM, T cell responses can be induced toward the tumor specific immunoglobulin idiotype (Id) protein and/or tumor-associated antigens (TAAs). These latter are antigens expressed at high levels by the tumor cells, but generally also at low levels by normal cells which limits their immunogenicity.Citation13 Important TAAs in MM are cancer germline antigens (CGAGs), like Mage, Gage, Lage and NY-ESO-1,Citation14 Survivin,Citation15 BCMA,Citation16 and MUC1.Citation17 Moreover, in the allogeneic SCT setting potent immune responses can be generated against recipient-specific allo-antigens, known as minor histocompatibility antigens (MiHAs). MiHAs are polymorphic peptides derived from intracellular proteins that are presented by HLA molecules, and differ between donors and recipients. Numerous MiHAs have been identified in the past decades and T cell responses against these MiHAs have been associated with improved relapse-free survival. While in some studies the induction of MiHA-specific T cell responses was associated with an increase in the incidence of GVHD and improved relapse-free survival,Citation18-Citation21 other studies could not confirm these results.Citation22,Citation23 Importantly, boosting of T cell responses against MiHAs with a hematopoietic-restricted expression pattern, e.g., HA1,Citation24 LRH1,Citation25 ARHGDIB,Citation26 and UTA2–1Citation27 has the potential to induce a selective GVM effect with only limited risk of eliciting GVHD. Therefore, these MiHAs are interesting candidates for targeted immunotherapy.
The other important immune effectors are NK cells, which are part of the innate immune system. Their activation is regulated by the balance in expression levels of numerous inhibitory and activating receptors. The most well characterized inhibitory receptors are the killer immunoglobulin-like receptors (KIR) and NKG2A. KIR receptors can bind to HLA-A, -B, and -C molecules, while NKG2A binds to HLA-E. Examples of activating receptors are CD16, which is involved in antibody-dependent cytotoxicity (ADCC), activating KIRs (e.g., KIR2DS, KIR3DS), NKG2D, DNAX accessory molecule-1 (DNAM-1), and the natural cytotoxicity receptors (NCRs). These latter receptors can interact with ligands, like UL16-binding protein (ULBP)1–4, MHC class I chain-related protein A (MIC-A) and Nectin-2, that are expressed during infections or stress. In homeostasis, NK cells are inhibited by their inhibitory receptors recognizing self HLA class I molecules. On the other hand, GVM effect can be induced by upregulation of activating ligands or downregulation of MHC class I molecules. In addition, in the setting of allogeneic SCT, donor NK cells may lack expression of inhibitory KIRs for recipient MHC class I molecules and hence be activated. This phenomenon is called missing-self recognition and can contribute to the GVM effect.Citation28,Citation29 Nevertheless, this effect is usually limited because in allogeneic SCT donor and recipient are matched for their HLA molecules. This is essential to prevent induction of severe alloreactive T cell responses against healthy tissues expressing foreign HLA molecules causing graft-vs.-host disease (GVHD).Citation30
Despite the immune susceptibility of MM, the OS after allogeneic SCT was not improved compared with autologous SCT. This was mainly due to high TRM after allogeneic SCT consisting of conditioning-related toxicity, infections, and GVHD.Citation31,Citation32 Furthermore, relapse rates were still high. In recent years, transplant-related toxicity decreased significantly due to the introduction of reduced intensity conditioning (RIC) chemotherapy regimens, but unfortunately OS did not improve due to higher relapse rates.Citation33 In order to reduce the incidence and severity of GVHD, complete and partial T cell-depleted allogeneic SCT programs have been developed. After six months, when the treatment-related inflammation has resolved, GVM responses can be boosted by giving donor lymphocyte infusions (DLI).Citation34 Furthermore, DLI can also be an effective therapy in relapsed MM.Citation35
RIC allogeneic SCT has been compared with autologous SCT in six large trials.Citation36-Citation41 Two trials reported a survival benefit for patients receiving allogeneic SCT. The first was performed by the Italian GIMEMA group and found a median OS of 80 mo in 80 patients in the allogeneic SCT group compared with 54 mo in 82 patients in the autologous SCT group.Citation37 In addition the EBMT observed improved OS after 6 y in 108 patients who underwent autologous/allogeneic SCT (auto/alloSCT) compared with 249 patients receiving only autologous SCT (49% vs. 36%, P = 0.030).Citation40 Four trials did not show improved OS after allogeneic SCT. The first study was performed by Garban et al.Citation36 who observed a trend toward better OS in the autologous group at 56 mo (OS 48 vs. 34 mo, P = 0.07),Citation42 however high dose anti-thymocyte globulin (ATG) was part of the conditioning regimen. The PETHEMA study included patients not achieving a CR after a first autologous SCT and found no difference in OS after 5 y (autologous vs. allogeneic = 60% [95% CI 48.3–73%] vs. 61.8% [95% CI 40.6–82%]).Citation38 The BMT CTN study compared auto/alloSCT in 226 patients with double autologous SCT in 484 patients. OS survival was 77% in the auto/alloSCT-group and 80% in the double autologous SCT-group after three years, however this follow-up period is still relatively short, as the EBMT trial only observed improved OS after six years.Citation41 In a study by the Dutch HOVON Group 115 patients who underwent a single autologous SCT followed by maintenance therapy consisting of α-interferon or thalidomide were compared with 99 patients with an auto/alloSCT with a follow-up of 6 y. After 6 y, PFS was prolonged in patients who underwent allogeneic SCT compared with autologous SCT (HR 0.75, 95% CI = 0.55–1.03, P = 0.07), however OS was not different.Citation39 Remarkably, survival rates of the autologous SCT-group in this study were better than those in other studies. An explanation for this could be that one of the novel agents, thalidomide, bortezomib, or lenalidomide could have been given to patients with relapsed disease. These compounds can significantly prolong survival of relapsed patients, therefore the survival benefit for allogeneic SCT will only become clear very long time after transplantation. Therefore, allogeneic SCT is currently not considered first line treatment for MM patients.Citation43
Immune Escape in Multiple Myeloma
Despite the high immunogenicity of MM, as demonstrated with allogeneic SCT, still too many patients relapse after initial therapy. This might be the result of immune evasion mechanisms exploited by the tumor cells, including intrinsic alterations and the establishment of an immunosuppressive milieu thereby limiting the efficacy of immune effector cells.Citation44 Here, we will discuss which mechanisms can be involved in immune escape of MM ().
Figure 1. Mechanisms involved in immune escape in Multiple Myeloma. Multiple myeloma can inhibit efficient immune recognition and destruction via multiple direct and indirect mechanisms. These include impaired antigen presentation, signaling via co-inhibitory molecules, secretion of immunosuppressive factors and recruitment of suppressive immune cells. HLA, human leukocyte antigen; Th2 cell, T helper 2 cell; IDO, indoleamine 2,3-dioxygenase; TGF-β, transforming growth factor-β; sMIC-a, soluble major histocompatibility antigen class I polypeptide-related sequence A; IL-6, Interleukin-6; NK cell, natural killer cell; CTLA-4, cytotoxic T lymphocyte associated antigen-4; PD-1, programmed death-1; BTLA, B, and T lymphocyte attenuator.
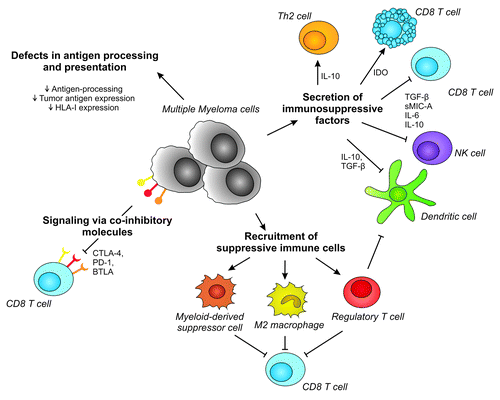
Defects in antigen processing and presentation
Presentation of the tumor antigen is essential for the induction of tumor-reactive T cell responses. In order to activate T cells, the TCR:CD3 complex should interact with the HLA-antigen complex presented on antigen presenting cells (APCs).Citation44 It has been described that antigen expression can be downregulated by tumor cells. Furthermore, defects in components of the antigen presentation machinery can occur, including the transporter associated with antigen processing-1 (TAP-1) and subunits of the immunoproteasome (LMP-2, LMP-7).Citation45 In addition, tumor cells can downregulate or even lose expression of HLA class I proteins, due to deletions in chromosome 6, where HLA alleles are located, or due to mutations in the β2-microglobulin, an essential molecule for stable HLA expression on the cell surface.Citation46 All these modifications contribute to invisibility of the tumor cells, thereby hampering efficient recognition by tumor-reactive T cells.
Signaling via co-inhibitory molecules
In addition to presentation of the antigen by an APC, T cells need a second signal provided by co-stimulatory molecules of the B7/CD28 family in order to become activated. Together, these two signals trigger expansion and differentiation of the T cells, and induce acquisition of effector functions. Following T cell activation, expression levels of co-inhibitory molecules like Cytotoxic T lymphocyte associated antigen-4 (CTLA-4), B and T lymphocyte attenuator (BTLA), and Programmed death-1 (PD-1) are upregulated. Ligation of these receptors to their corresponding ligands on APCs results in functional inhibition of the T cells. Via this natural feedback loop sustained T cell activation is prevented and the effector T cell response is resolved.Citation47 However, tumor cells can upregulate co-inhibitory molecules and downregulate co-stimulatory molecules to acquire an immune inhibitory phenotype and thereby prevent productive tumor-reactive T cell responses. In MM increased expression levels of the co-inhibitory ligands of PD-1, BTLA, and CD200R have been observed on MM cells.Citation47-Citation50 Moreover, interference with BTLA signaling using blocking antibodies can augment proliferation of tumor-reactive CD8+ T cells stimulated with peptide-loaded HVEM+ DCsCitation49 and small interfering RNA (siRNA) mediated silencing of PD-L1 and PD-L2 on DCs enhances their potential to stimulate T cell proliferation and cytokine production.Citation51 In addition to the B7/CD28 family members, MM cells can also express other inhibitory molecules, like carcinoembryonic antigen-related cell adhesion molecule-6 (CEACAM-6), an immunoglobulin-like receptor. It has been shown that increased expression of CEACAM-6 in MM resulted in inhibition of anti-myeloma T cell responses.Citation52
Secretion of immunosuppressive factors
Another way to evade the immune system is creation of an immunosuppressive microenvironment by secretion of immune inhibitory factors like soluble MIC-A, interleukins (IL), transforming growth factor (TGF)-β and indoleamine 2,3-dioxygenase (IDO). Increased levels of these factors have been detected in serum of MM patients.Citation53-Citation56 Secretion of soluble MIC-A by MM cells can mediate inhibition of NK cells and CD8+ T cells by downregulation of NKG2D, and is associated with poor survival.Citation54,Citation55 Moreover, IL-6 producing tumors can directly impair NK cell cytotoxicity and can stimulate IL-10 production in MM cells.Citation57,Citation58 This IL-10 can suppress dendritic cell (DC) function and induce the development of Th2 T cells, which are less effective in supporting tumor-reactive cytotoxic T cell formation and function than the Th1 counterparts.Citation44 Furthermore, interferon (IFN)-γ production by NK cells, which is important for their killing capacity, is inhibited by IL-10.Citation59 In addition, TGF-β can downregulate expression of activating NK cell receptors, such as NCRs and NKG2D, and also inhibits IFN-γ production by NK cells, as well as T cells. Additionally, other effector molecules like perforin are downregulated and DC activation can be blocked by TGF-β.Citation60 Finally, IDO is an enzyme that catalyzes metabolization of the essential amino acid tryptophan resulting in tryptophan depletion in the tumor microenvironment. This causes cell cycle arrest and apoptosis of effector T cells.Citation61
Recruitment of suppressive immune cells
Beside apoptosis of T cells, IDO and also IL-10 and TGF-β can recruit or induce suppressive immune cells like M2 macrophages, regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Tregs are characterized by expression of CD25 and transcription factor FOXP3 and are capable of inducing expression of co-inhibitory molecules on APCs and IDO production by APCs. In addition, they produce IL-10 and TGF-β themselves, and have the potential to kill APCs and cytotoxic T cells via perforin- and granzyme-dependent pathways.Citation62,Citation63 Increased levels of Treg subsets have been observed in blood of MM patients and were associated with increased disease burden and poor survival.Citation56,Citation64 Other suppressive cell types are MDSCs and M2 macrophages. MDSCs are a heterogeneous subset of immature myeloid progenitor cells, characterized by expression of CD33 and CD11b, and lack of CD14 and HLA-DR expression. M2 macrophages have an IL12loIL-10hiIL-1decoyRhiIL-1RAhi phenotype and express scavenger receptors such as the mannose receptor (CD206) and hemoglobin/haptoglobin scavenger receptor (CD163) on their cell surface.Citation65 MDSCs and M2 macrophages can suppress both T cell and NK cell mediated immune responses by arginine depletion due to catabolism by arginase-1, and nitric oxide production by inducible nitric oxygen synthase.Citation66,Citation67 Increased numbers of MDSCs have been observed in the blood and bone marrow of MM patients. Notably, it has been demonstrated that MM cells can induce development of MDSCs from healthy donor peripheral blood mononuclear cells (PBMCs).Citation68,Citation69 In one study in 68 patients, increased numbers of M2 macrophages, characterized by expression of CD163 and CD68, in the bone marrow of MM patients, were associated with an unfavorable prognostic impact on 6-y OS.Citation70
T-cell Based Immunotherapy
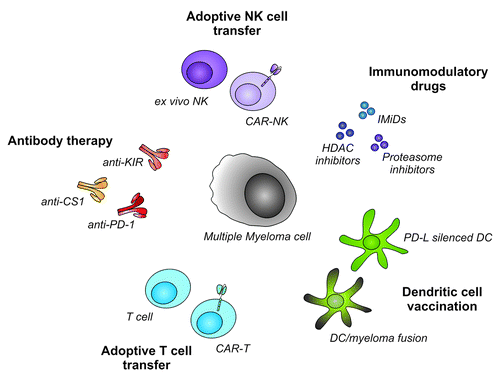
To overcome immune escape, resulting in improved PFS and OS in MM patients, potent cellular immunotherapies boosting T or NK cell-mediated immunity can be exploited. These highly potent immune effectors can be given either as single therapy, or in combination with immune stimulating antibodies or immunomodulatory drugs (). First, we will address promising T cell based therapies, and in the next part we will discuss interesting NK cell based therapies. An overview of the reported and ongoing clinical trials in MM patients is given in .
Figure 2. Cellular immunotherapeutic approaches in multiple myeloma. To improve survival in multiple myeloma patients, several cellular immunotherapies boosting NK cell and T cell mediated immunity, can be exploited. NK cells can be isolated or generated ex vivo, and can subsequently be used for adoptive transfer. The efficacy of NK cell-based therapy can be enhanced by the introduction of tumor-targeting CARs. Furthermore, combination with antibodies like anti-KIR or anti-CS1, or anti-myeloma drugs further boost anti-myeloma NK cell immunity. Adoptive T cell transfer after allogeneic stem cell transplantation can induce complete remission in MM patients. In addition adoptive transfer CAR-modified T cells might be even more specific and effective. Tumor specific T cells can also be expanded in vivo following DC vaccination. Silencing of co-inhibitory molecules like PD-L by silencing RNA, can further increase efficacy of DC vaccination. Finally, interference with co-inhibitory pathways using blocking antibodies like anti-PD1 is a promising strategy to increase the therapeutic effect. NK cell, Natural Killer cell; CAR, Chimeric Antigen Receptor; HDAC, Histone Deacetylase; PD-L, Programmed Death-ligand; DC, Dendritic Cell; anti-PD-1, anti-Programmed Death-1; anti-KIR, anti-Killer Immunoglobulin-like Receptor.
Tabel 1. Clinical trials in the field of immunotherapy in MM
Chimeric antigen receptor (CAR)-modified T cells
The presence of circulating T cells specific for myeloma-associated antigens has been correlated with increased OS in MM patients, indicating the importance of exploiting myeloma-reactive T cells to further improve outcome.Citation71 However, the isolation and expansion of these low frequent T cells for adoptive transfer is challenging. Therefore, genetic modification of T cells by induction of CARs is a promising alternative. These CARs consist of a single chain variable fragment, derived from a monoclonal antibody specific for a tumor specific protein, fused to native human T cell receptor CD3ζ signaling domains. The 2nd and 3rd generation CARs have one or more additional activating signaling domains derived from co-stimulatory molecules such as CD28, 4–1BB, and OX40. Very promising results have been obtained in pilot studies using CD19-reactive CAR-T cells in CD19 expressing B cell leukemias and lymphomas.Citation72-Citation74 Kochenderfer et al.Citation74 infused 8 patients with either advanced chronic lymphocytic leukemia or B cell lymphoma with 0.3 to 3 × 107 CAR-T cells/kg in combination with IL-2, after conditioning with cyclophosphamide and fludarabine. Importantly, six patients obtained remission showing the potent anti-tumor efficacy of this CAR-T cell therapy. However, 4/8 patients showed long-term depletion of normal polyclonal CD19+ B-lineage cells. Furthermore, 4 patients had prominent elevations in serum levels of IFNγ and TNFα resulting in acute toxicity. Other studies also reported chronic hypogammaglobulinemiaCitation72,Citation73 and delayed tumor lysis syndrome.Citation72 In order to improve safety, new methods to ablate CAR-T cells are being developed, like the incorporation of a suicide gene that encodes human caspase-9 fused to a modified human FK-binding protein that confers sensitivity to a synthetic small molecule.Citation75
For the treatment of MM various CARs have been developed. For instance, T cells can be transfected with a chimeric NKG2D receptor. MM cells express the corresponding ligands, which enhance NK cell and CD8+ T cell mediated lysis. Infusion of these NKG2D-expressing CAR-T cells induced tumor reduction in a mouse model.Citation76 Even though most healthy tissues do not express NKG2D ligands, low levels of NKG2D ligands could potentially induce toxicity in patients. Therefore, more specific targets are probably warranted. In this regard, expression of the carbohydrate antigen Lewis(Y) has been detected on 52% of plasma cells in bone marrow samples of MM patients, while it is expressed in very low levels on healthy tissues.Citation77 In a mouse model delayed growth of myeloma xenografts was observed in NOD/SCID mice treated with CAR-T cells directed against Lewis(Y).Citation78 Safety of the Lewis Y CAR is currently investigated in a phase I trial in AML, myelodysplastic syndrome (MDS), and MM patients (NCT01716364). Notably, due to the relatively low expression levels of Lewis(Y) on MM cells there is a potential risk of immune escape. Hence, B cell maturation antigen (BCMA) might be a more suitable target, as it is uniformly and highly expressed on MM cells and expression is absent on normal tissues, except for normal plasma cells. Preclinical studies have shown that CAR-T cells specific for BCMA are able to eradicate human MM cell line tumors in immunodeficient mice. These data indicate that adoptive transfer of CAR-T cells directed against BCMA might be an interesting approach for the treatment of MM.Citation79
Dendritic cell vaccination
Another strategy to boost anti-myeloma T cell immunity in MM patients is DC vaccination. DCs are the most potent professional APCs, and therefore they an attractive way to expand myeloma-reactive T cells in vivo. Reichardt et al. were the first to explore DC vaccination in MM patients.Citation80 In this pioneering study, 12 patients were treated with blood-derived precursor DCs, cultured for 36–48 h in the presence of the Id protein. Two out of 12 patients developed an Id-specific proliferative T cell response and remained in complete remission. Moreover, one patient developed a transient Id-specific cytotoxic T cell response. Following this first report, multiple clinical studies have investigated Id-pulsed DCs and showed that these DCs are capable of inducing humoral and cellular immune responses against the Id protein with limited toxicity.Citation81-Citation83 However, clinical efficacy was disappointing. A possible explanation could be that most of these studies were performed in patients with advanced disease. Nevertheless, one study in stage-I myeloma also showed a decrease in M-protein in only 3 out of 9 patients.Citation84 Furthermore, most studies have been performed using relatively immature DCs, while it has been shown that mature DCs are superior in the induction of anti-tumor immunity.Citation85 Another explanation could be the lack of Id-specific T cell precursors in MM patients, due to tolerance and deletion as a result of high amounts of secreted free protein.Citation86 Consequently, other TAAs can be targeted to improve DC vaccination.
Recently, we performed a phase 1 clinical trial in which we evaluated safety and immunological effects of mature DCs pulsed with TAA mRNA, specific for MAGE3, Survivin, and BCMA, in 12 MM patients with a CR or partial response (PR) after high-dose chemotherapy and autologous SCT.Citation87 Loading with TAA RNA results in processing of both class I and II epitopes, and hence has the potential to induce a broad TAA-reactive T cell repertoire.Citation88 In all patients vaccination was well tolerated with limited toxicity. Importantly, in two patients vaccine-specific T cells were detected, illustrating that TAA-mRNA-loaded mature DCs are capable of inducing TAA-reactive T cell responses in MM patients after autologous SCT. Another strategy to achieve presentation of a broad panel of MM antigens is the use of autologous DC/MM cell fusion vaccines. These vaccines expanded circulating CD4+ and CD8+ cells reactive to autologous MM cells, and were demonstrated to stabilize disease in 11 out of 16 MM patients with advanced disease in a phase I study.Citation89 In a phase II study, where patients were vaccinated with DC/MM cell fusion vaccines after autologous SCT, 24% of the patients who achieved a PR upon transplant converted to complete remission/near complete remission (CR/nCR) following vaccination.Citation90
In order to further augment the activation and expansion of anti-myeloma T cells, DC vaccination may be combined with strategies that interfere with immunosuppressive mechanisms exploited by MM cells. We recently showed that siRNA mediated silencing of programmed death ligand-1 (PD-L1) and PD-L2 on monocyte-derived DCs resulted in generation of DCs with superior potential to stimulate tumor-reactive T cell responses ex vivo.Citation51,Citation91
Anti-PD-1
Besides using PD-L siRNAs, DC vaccine efficacy can also be improved by combination with blocking antibodies targeting the PD-1/PD-L co-inhibition pathway. Incubation of DC/MM cell fusions with anti-PD-1 antibody (CT-011) and subsequent co-culture with autologous T cells, resulted in reduced numbers of regulatory T cells, increased levels of Th1 T cells and augmented tumor killing in ex vivo cytotoxicity assays.Citation92 Currently, the combination therapy of DC/MM cell fusions with CT-011 after autologous SCT is being investigated in MM patients (NCT01067287). Recently, a phase I clinical trial investigating CT-011 in patients with varying hematological malignancies, including one patient with MM, has been finished. CT-011 proved to be safe, and a clinical benefit was observed in 33% of 17 patients. Patients showed increased CD4+ T cell counts without further evidence of T cell activation.Citation93 In addition to improved T cell immunity, Benson et al.Citation94 showed that NK cell-mediated killing of MM cells can also be enhanced by CT-011, suggesting a role for CT-011 in NK cell-based therapies. Another potent anti-PD-1 antibody, nivolumab, has shown durable tumor regression in a phase 1 trial in patients with melanoma, renal-cell cancer, and non-small-cell lung cancer, and is currently investigated in patients with hematological malignancies in a phase I trial (NCT01592370).
Natural Killer Cell Based Immunotherapy
NK cell based monotherapy
Besides T cells, NK cells are important effector cells involved in anti-myeloma immunity. They are activated upon increased expression of activating ligands or decreased expression of inhibitory ligands. In vitro studies indicated that cytokines, like IL-2 and IL-15 can enhance the killing capacity of NK cells.Citation95 Therefore, the effect of in vivo administration of these cytokines on NK expansion and activation has been investigated in patients.Citation96 It has been shown that IL-2 administration mediated expansion of the NK cells. In addition, IFN-α increased NK cell activity, however the therapy induced toxicity and clinical benefits in MM patients were limited.Citation97-Citation99
Importantly, various studies indicate an anti-tumor effect mediated by NK cells. For example, after allogeneic SCT early NK cell repopulation has been associated with decreased relapse rates, without increasing GVHD incidence.Citation100 In haplo-identical SCT, where patient and donor are not fully matched for HLA-type, improved disease-free survival and lower relapse rates, without increased incidence of GVHD, have been observed if the patient and donor were mismatched for their KIR-ligands.Citation29 Those results have been confirmed in different studies,Citation101,Citation102 though other groups could not reproduce these findings.Citation103,Citation104 These differences can probably be attributed to variance in conditioning and transplantation regimens.Citation96 The KIR gene-gene model, which uses KIR genotype, is another method used to predict NK reactivity and clinical outcome after allogeneic SCT. KIR genes are located apart from HLA genes on chromosome 16. Two KIR genotypes can be distinguished; genotype A, which contains mostly inhibitory KIR receptors, and group B, which comprises more activating receptors. Consequently, donors and recipients can be categorized as having one of two KIR genotypes: AA which is homozygous for group A KIR haplotypes, or Bx, which contains either one (AB) or two (BB) group B haplotypes.Citation105 After non-myeloablative HLA-haploidentical SCT, it was observed that patients with KIR group A haplotypes showed improved overall survival, event-free survival, and non-relapse mortality in case the donor had a KIR group B haplotype. For patients with KIR group B haplotypes no benefit was demonstrated.Citation106 Furthermore, improved PFS and OS was observed in acute myeloid leukemia (AML) patients and relapsed MM patients after HLA-matched SCT when the donor had a group B haplotype. This was independent of the patients haplotype. In AML patients no effect on the incidence of GVHD was found, however in MM patients a donor with a group B haplotype was associated with an increased incidence of chronic GVHD, but not acute GVHD.Citation107,Citation108
Nevertheless, allogeneic SCT is not standard treatment for MM, because of the high TRM.Citation43 Therefore, novel strategies exploiting the anti-myeloma activity of NK cells in MM with less side effects are being explored. One approach is the adoptive transfer of NK cells. This provides the opportunity to infuse alloreactive NK cells, that are optimally mismatched, without the toxicity associated with allogeneic SCT. Shi et al.Citation109 investigated infusion of haplo-identical KIR mismatched NK cells in relapsed MM patients following conditioning with melphalan and fludarabine. Furthermore, patients were treated daily with IL-2 injections for 11 d, and received a delayed autograft at day 14. Although donor NK cells could be detected, they disappeared by day 9 – 14. Moreover, the response rate was 50% compared with 40% in the control group not receiving NK cells (P = 0.32). An explanation for the quick disappearance of NK cells could be the early repopulation of T cells. Furthermore, the number of infused NK cells was low (1x106/kg). In a study by Miller et al.Citation110 in poor risk AML patients a complete hematologic remission was induced in 5 out of 19 patients by infusion of ex vivo expanded alloreactive NK cells combined with a high dose immunosuppressive regimen and low dose IL-2. However, in this study low levels of T cells in the graft could have contributed to the observed effect.Citation110,Citation111 In order to infuse higher numbers of NK cells without T cell contamination a new GMP-grade culture protocol has been developed by our group. In this procedure CD34+ hematopoietic stem cells can be expanded ex vivo 2000-fold, and a NK cell product with a purity >90% and without B or T cell-contamination can be generated.Citation112 Currently, these NK cells are investigated in a phase I clinical trial in older AML patients who are not eligible for allogeneic SCT (NTR2818). In the future these NK cells, combined with autologous SCT, could also be a promising therapy in relapsed MM patients.
NK cell based combination therapy
Beside adoptive transfer of NK cells, several other therapies exist that aim to improve NK cell mediated anti-myeloma immunity. New anti-myeloma drugs introduced into the clinic have shown to influence NK cell mediated killing, therefore combination therapy with these drugs can be a rational therapeutic strategy. Furthermore, in the past decade increasing numbers of clinical grade antibodies have been developed for the treatment of cancer. These antibodies can mediate tumor clearance via various mechanisms.Citation113 Upon binding to the target molecule, the Fc-tail of the antibody can be recognized by Fc-receptors on NK cells resulting in NK cell degranulation and lysis of the target cell. Besides this ADCC, cytolysis can be mediated by activation of the complement system upon binding of complement components to the Fc-tail of the antibody. In addition, the antibody itself may directly affect signal transduction of the targeted molecule, or induce apoptosis. Furthermore, cytotoxic drugs or radionuclides can be coupled to the antibodies, thereby the targeted cells are specifically damaged and patients will encounter less side effects than with regular treatment. At the moment, several monoclonal antibodies are being developed for MM.Citation114 Some of these antibodies are promising candidates for combination therapy, together with adoptive transfer of NK cells, to enhance NK cell mediated killing.
IPH2101
One of these interesting antibodies, is the anti-KIR antibody IPH2101. NK cells initiate cytotoxicity against target cells through a positive balance of signals received via activating and inhibitory receptors. Binding of inhibitory KIRs to MHC class I molecules on target cells prevents NK cell activation.Citation96 IPH2101 is a human IgG4 monoclonal antibody against common inhibitory KIR2DL-1, KIR2DL-2, and KIR2DL-3, which blocks the KIR-ligand interaction and thereby augments killing of autologous tumor cells.Citation115,Citation116 In a phase I clinical trial in 32 relapsed/refractory MM patients, IPH2101 was well tolerated and no evidence of autoimmunity was observed. In the highest dose > 90% KIR occupancy was achieved and enhanced ex vivo patient-derived NK cell cytoxicity against MM was observed.Citation117 IPH2101 is currently investigated in a phase II trial in smoldering MM patients (NCT01248455, NCT01222286). Besides, it is investigated in patients in stable partial response after first line therapy (NCT00999830) and in combination with lenalidomide in relapsed MM. (NCT01217203).
Elotuzumab
Another interesting antibody which can augment NK cell-mediated immunity against MM is elotuzumab. Elotuzomab (i.e. HuLuc63) is a humanized antibody against CS1 (i.e. CD2 subset-1, CRACC, SLAMF7, or CD319), a cell surface glycoprotein that is highly expressed on MM cells and normal plasma cells.Citation118 In addition to expression on MM cells, CS1 is also expressed on NK cells, NKT cells, CD8+ T cells, activated monocytes and DCs, but to a lesser extent. Notably, CS1 is not expressed by healthy hematopoietic stem cells, other hematological malignancies or non-lymphoid tissues. Although the function of CS1 is not fully known, on MM cell is seems to interact with cell adhesion molecules on bone marrow stromal cells.Citation118,Citation119 On NK cells, CS1 appears to serve as an activator via the adaptor protein Ewing’s sarcoma-activated transcript-2 (EAT-2).Citation120 Elotuzumab performs its anti-MM effects mainly via induction of NK cell-mediated ADCC.Citation118 In ex vivo experiments ADCC against primary MM cells, resistant to conventional or novel therapies like bortezomib, could be enhanced using elotuzumab.Citation119 A phase I study in relapsed/refractory MM patients showed acceptable toxicity and disease stabilization in 26.5% of patients.Citation121 Furthermore, two phase I studies investigating elotuzumab, in combination with either lenalidomide and low-dose dexamethason, or bortezomib, in relapsed or refractory MM patients showed objective response rates of 82% and 48%, including a response in 2 out of 3 bortezomib resistant patients in the study combining elotuzumab with bortezomib.Citation122,Citation123 Currently, phase I/II trials combining elotuzumab with bortezomib/dexamethasone in newly diagnosed and relapsed MM patients are being performed (NCT01668719, NCT01478048). Additionally, phase III trials are performed investigating elotuzumab in combination with lenalidomide and dexamethasone in newly diagnosed patients (ELOQUENT-1, NCT018916430) and relapsed/refractory patients (ELOQUENT-2, NCT01239797).
Anti-myeloma drugs
As described earlier novel anti-myeloma drugs including the IMiDs and proteasome inhibitors have improved outcome of MM patients in the last decade.Citation5 Interestingly, these drugs seem to confer their effects, at least partly, trough stimulation of NK cell-mediated killing.
Thalidomide was the first drug of the IMiD group that was used, though later more potent and less toxic IMiDs like lenalidomide have been introduced.Citation124 IMiDs mediate their anti-myeloma effects via several mechanisms. They can directly kill MM cells by induction of cell cycle arrest and caspase-dependent apoptosis,Citation125 decrease binding of MM cells to bone marrow stromal cells,Citation126 block angiogenesisCitation127 and inhibit production of cytokines (IL-6, TNF-α).Citation128 Furthermore NK cell-mediated cytotoxicity can be augmented by these drugs.Citation129 Hayashi et al.Citation130 reported that IMiDs stimulate NK cells via increased IL-2 production by T cells. Additionally in vitro studies have shown that pretreatment of NK cells with lenalidomide can enhance ADCC against CD40-expressing MM cells if NK cells are combined with SGN-40, an anti-CD40 monoclonal antibody.Citation131 Pomalidomide, a novel IMiD, which has been approved by the FDA recently, significantly increases serum IL-2 receptor and IL-12 levels.Citation132 These cytokines are important for NK cell activation and could contribute to increased NK cell-mediated killing.
Proteasome inhibitors are a second group of anti-myeloma drugs. These drugs interact with the ubiquitin-proteasome pathway, which is responsible for the degradation of the majority of regulatory proteins in eukaryotic cells, including proteins that control cell-cycle progression, apoptosis, and DNA repair.Citation133 Inhibition of proteasome activity results in growth arrest and cell death due to the induction of an apoptotic cascade as a result of rapid accumulation of incompatible regulatory proteins within the cell.Citation134 Malignant cells show increased sensitivity to proteasome inhibition, probably as a result of higher proteasome activity. Therefore, the proteasome is one of the most interesting therapeutic targets in oncology, and it is currently an important component of MM treatment.Citation135 Beside direct apoptotic effects on MM cells, treatment of MM cells with bortezomib can induce sensitization to NK cell-mediated lysis via several mechanisms. By downregulation of cell-surface expression of HLA class I molecules on MM cells, NK cell activation is no longer inhibited, since HLA class I is an important inhibitory ligand for NK cells.Citation136 In addition, bortezomib can induce expression of NK cell activating ligands like Tumor Necrosis Factor-related apoptosis-inducing ligand (TRIAL)-R2,Citation137,Citation138 DNAM-1 ligands (poliovirus receptor, Nectin-2, CD112, CD155) and NKG2D ligands (MICA/B, ULBPs).Citation139,Citation140 On the downside a dose-dependent suppression of NKp46-mediated and TRAIL-mediated cytotoxicity, as well as induction of apoptosis in NK cells treated with bortezomib has been described.Citation141,Citation142 These findings indicate that low dose bortezomib may support NK cell-based immunotherapy, while high dose bortezomib may disturb NK cell-mediated cytotoxicity.
A third group of anti-myeloma drugs are the histone deacetylase (HDAC) inhibitors. HDACs play an important role in epigenetic modulation of gene expression and alterations in HDAC expression have been found in many types of cancers, making these enzymes a attractive targets for cancer therapy.Citation143 Clinical phase II and phase III studies using the HDAC inhibitor vorinostat in relapsed MM patients have gained promising results.Citation144,Citation145 Another HDAC inhibitor, panobinostat, is currently investigated in a large phase III clinical trial (NCT01023308). In vitro and in vivo studies have reported NK cell-activating effects of HDAC inhibitors. Upon exposure to HDAC inhibitors, increased expression of MICA/B on lymphoma, leukemia, and hepatocellular carcinoma cells was observed.Citation146-Citation148 Another in vitro study reported increased DNAM-1- and NKG2D-dependent NK cytotoxicity.Citation149 However, decreased expression of the activating NKp30 ligand B7-H6 has been observed in cell lines treated with HDAC inhibitors, reducing NKp30-dependent tumor cell recognition by NK cells.Citation150 Additionally, in mice treated with HDAC inhibitors, diminished expression of NKG2D and NKp46 on, and decreased IFN-γ production by NK cells was observed. Therefore, it is essential to obtain data on the influence of HDAC inhibitors on NK cell-mediated killing in clinical studies in order to draw definite conclusions on the benefit of these drugs in NK cell-mediated myeloma therapy.
Future Prospects
Survival of MM patients has improved significantly due to the introduction of new therapies like bortezomib and lenalidomide, however OS survival is still poor. Therefore additional potent therapeutic strategies are urgently needed. Currently, several cellular immunotherapeutic strategies are being developed to improve anti-myeloma immunity with reduced side effects. NK cell-based adoptive immunotherapy has shown promising clinical effects, without induction of severe adverse effects. Notably combination of NK cell adoptive transfer with antibodies like elotuzumab and KIR-blocking antibodies, anti-myeloma drugs, or even anti-PD1 antibodies may further enhance anti-myeloma immunity. Furthermore, new strategies like genetic engineering hold great potential for the development of NK cells with superior tumor-reactive functionality. For instance, inhibitory KIR receptors might be specifically downregulated in NK cells by the use of siRNAs. Another strategy would be the introduction of CARs on NK cells. Recently, Chu et al.Citation151 generated a CS1-specific CAR which could be expressed by a human NK cell line. These NK cells showed improved killing of CS1-expressing MM cells in vitro and in vivo. Therefore, it would be highly interesting to explore such MM-specific CAR expressing NK cells in a clinical trial.
Another interesting strategy, which can improve T cell-mediated killing of MM is the application of bispecific antibodies. Blinatumomab, a CD3/CD19 bispecific single chain antibody, can redirect T cells to CD19 expressing B-lineage acute lymphoblastic leukemia (ALL) cells. In a phase II study, 16 out of 21 patients with MRD or relapse after therapy were MRD negative after blinatumomab treatment.Citation152 Based on the successes in B-ALL, development of bispecific antibodies against MM-specific molecules, like CS1 or CD138, would be an interesting strategy to explore for MM treatment.
Taken together, ongoing efforts to improve therapy for MM have resulted in the development of several promising therapies, these therapies will potentially reach the clinic in the coming years. An important question is how to combine and sequence these new therapies in the initial treatment plan of patients with MM. The combination of cellular immunotherapy with other therapies has the potential to maximize the anti-myeloma effect without significant increased toxicity. Further research will have to elucidate prognostic factors predicting therapeutic responses and side effects in patients, enabling more effective personalized treatments and better survival and quality of life for future MM patients.
| Abbrevations | = | ADCC, antibody-dependent cytotoxicity |
| ALL | = | acute lymphoblastic leukemia |
| AML | = | acute myeloid leukemia |
| APC | = | antigen presenting cell |
| ATG | = | anti-thymocyte globulin |
| Auto/alloSCT | = | autologous stem cell transplantation followed by allogeneic stem cell transplantation |
| BCMA | = | B cell maturation antigen |
| BTLA | = | B and T lymphocyte attenuator |
| CAR | = | chimeric antigen receptor |
| CARs | = | chimeric antigen receptor modified T cells |
| CEACAM-6 | = | carcinoembryonic antigen-related cell adhesion molecule-6 |
| CGAG | = | cancer germline antigens |
| CR | = | comlete response |
| CR/nCR | = | complete remission/near complete remission |
| CTLA-4 | = | cytotoxic T lymphocyte associated antigen-4 |
| DC | = | dendritic Cell |
| DLI | = | donor lymphocyte infusion |
| DNAM-1 | = | DNAX-accessory molecule-1 |
| EAT-2 | = | Ewing’s sarcoma-activated transcript-2 |
| GVHD | = | graft-versus-host disease |
| GVM | = | graft-versus-myeloma |
| HDAC | = | histone deacetylase |
| HLA | = | human leukocyte antigen |
| Id | = | idiotype |
| IDO | = | indoleamine 2,3-dioxygenase |
| IFN-γ | = | interferon-γ |
| IL | = | interleukin |
| IMiDs | = | immunomodulatory derivates |
| KIR | = | killer immunoglobulin-like receptor |
| MDS | = | myelodysplastic syndrome |
| MDSC | = | myeloid-derived suppressor cell |
| MIC-A | = | major histocomplatibility antigen class I polypeptide-related sequence A |
| MiHA | = | minor histocompatibility antigen |
| MM | = | multiple myeloma |
| MP | = | Melphalan and Prednisone |
| MPT | = | Melphalan, Prednisone, and Thalidomide |
| MRD | = | minimal residual disease |
| NCR | = | natural cytotoxicity receptor |
| NK cell | = | natural killer cell |
| OS | = | overall survival |
| PBMC | = | peripheral blood mononuclear cell |
| PD-1 | = | programmed death-1 |
| PD-L1 | = | programmed death ligand-1 |
| PFS | = | progression free survival |
| PR | = | partial response |
| RIC | = | reduced intensity conditioning |
| SCT | = | stem cell transplantation |
| siRNA | = | small interfering RNA |
| TAA | = | tumor associated antigen |
| TAP-1 | = | transporter associated with antigen processing-1 |
| TCR | = | T cell receptor |
| TGF-β | = | transforming growth factor-β |
| TRAIL | = | tumor necrosis factor-related apoptosis-inducing ligand |
| Treg | = | regulatory T cell |
| TRM | = | treatment-related mortality |
| ULBP | = | UL16-binding protein |
| VMP | = | Velcade, Melphalan, and Prednisone |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer 2011; 105:1684 - 92; http://dx.doi.org/10.1038/bjc.2011.450; PMID: 22045184
- Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, Marcos-Gragera R, Maynadié M, Simonetti A, Lutz JM, et al, HAEMACARE Working Group. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 2010; 116:3724 - 34; http://dx.doi.org/10.1182/blood-2010-05-282632; PMID: 20664057
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364:1046 - 60; http://dx.doi.org/10.1056/NEJMra1011442; PMID: 21410373
- Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood 2008; 111:2521 - 6; http://dx.doi.org/10.1182/blood-2007-08-104984; PMID: 17901246
- van de Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica 2007; 92:1399 - 406; http://dx.doi.org/10.3324/haematol.11534; PMID: 18024376
- Cavo M, Rajkumar SV, Palumbo A, Moreau P, Orlowski R, Bladé J, Sezer O, Ludwig H, Dimopoulos MA, Attal M, et al, International Myeloma Working Group. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood 2011; 117:6063 - 73; http://dx.doi.org/10.1182/blood-2011-02-297325; PMID: 21447828
- Kumar SK, Dingli D, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, Rajkumar SV, Litzow MR, Gertz MA. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: Results from a matched pair analysis. Am J Hematol 2008; 83:614 - 7; http://dx.doi.org/10.1002/ajh.21191; PMID: 18429054
- Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC, Richardson PG, Anderson KC, Soiffer RJ, Alyea EP 3rd. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant 2007; 13:183 - 96; http://dx.doi.org/10.1016/j.bbmt.2006.09.010; PMID: 17241924
- Gay F, Palumbo A. Management of older patients with multiple myeloma. Blood Rev 2011; 25:65 - 73; http://dx.doi.org/10.1016/j.blre.2010.10.003; PMID: 21295387
- Waage A, Palumbo A, Hulin C, Beksac M, Fayers P, Mary JY, et al. Mp Versus Mpt for Previously Untreated Elderly Patients with Multiple Myeloma: A Meta Analysis of Survival of 1682 Individual Patient Data from 6 Randomized Clinical Trials. Haematol-Hematol J 2010; 95:235
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, et al, VISTA Trial Investigators. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008; 359:906 - 17; http://dx.doi.org/10.1056/NEJMoa0801479; PMID: 18753647
- Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol 2010; 28:2259 - 66; http://dx.doi.org/10.1200/JCO.2009.26.0638; PMID: 20368561
- Riddell SR, Berger C, Murata M, Randolph S, Warren EH. The graft versus leukemia response after allogeneic hematopoietic stem cell transplantation. Blood Rev 2003; 17:153 - 62; http://dx.doi.org/10.1016/S0268-960X(03)00007-9; PMID: 12818225
- Goodyear O, Piper K, Khan N, Starczynski J, Mahendra P, Pratt G, Moss P. CD8+ T cells specific for cancer germline gene antigens are found in many patients with multiple myeloma, and their frequency correlates with disease burden. Blood 2005; 106:4217 - 24; http://dx.doi.org/10.1182/blood-2005-02-0563; PMID: 16144804
- Grube M, Moritz S, Obermann EC, Rezvani K, Mackensen A, Andreesen R, Holler E. CD8+ T cells reactive to survivin antigen in patients with multiple myeloma. Clin Cancer Res 2007; 13:1053 - 60; http://dx.doi.org/10.1158/1078-0432.CCR-06-1722; PMID: 17289902
- Bellucci R, Alyea EP, Chiaretti S, Wu CJ, Zorn E, Weller E, Wu B, Canning C, Schlossman R, Munshi NC, et al. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood 2005; 105:3945 - 50; http://dx.doi.org/10.1182/blood-2004-11-4463; PMID: 15692072
- Choi C, Witzens M, Bucur M, Feuerer M, Sommerfeldt N, Trojan A, Ho A, Schirrmacher V, Goldschmidt H, Beckhove P. Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood 2005; 105:2132 - 4; http://dx.doi.org/10.1182/blood-2004-01-0366; PMID: 15561890
- Pérez-García A, De la Cámara R, Torres A, González M, Jiménez A, Gallardo D. Minor histocompatibility antigen HA-8 mismatch and clinical outcome after HLA-identical sibling donor allogeneic stem cell transplantation. Haematologica 2005; 90:1723 - 4; PMID: 16330460
- Goulmy E, Schipper R, Pool J, Blokland E, Falkenburg JH, Vossen J, Gratwohl A, Vogelsang GB, van Houwelingen HC, van Rood JJ. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med 1996; 334:281 - 5; http://dx.doi.org/10.1056/NEJM199602013340501; PMID: 8532022
- Hobo W, Broen K, van der Velden WJ, Greupink-Draaisma A, Adisty N, Wouters Y, Kester M, Fredrix H, Jansen JH, van der Reijden B, et al. Association of disparities in known minor histocompatibility antigens with relapse-free survival and graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2013; 19:274 - 82; http://dx.doi.org/10.1016/j.bbmt.2012.09.008; PMID: 23022467
- Markiewicz M, Siekiera U, Karolczyk A, Szymszal J, Helbig G, Wojnar J, Dzierzak-Mietla M, Kyrcz-Krzemien S. Immunogenic disparities of 11 minor histocompatibility antigens (mHAs) in HLA-matched unrelated allogeneic hematopoietic SCT. Bone Marrow Transplant 2009; 43:293 - 300; http://dx.doi.org/10.1038/bmt.2008.326; PMID: 18850018
- Spellman S, Warden MB, Haagenson M, Pietz BC, Goulmy E, Warren EH, Wang T, Ellis TM. Effects of mismatching for minor histocompatibility antigens on clinical outcomes in HLA-matched, unrelated hematopoietic stem cell transplants. Biol Blood Marrow Transplant 2009; 15:856 - 63; http://dx.doi.org/10.1016/j.bbmt.2009.03.018; PMID: 19539218
- Lin MT, Gooley T, Hansen JA, Tseng LH, Martin EG, Singleton K, Smith AG, Mickelson E, Petersdorf EW, Martin PJ. Absence of statistically significant correlation between disparity for the minor histocompatibility antigen-HA-1 and outcome after allogeneic hematopoietic cell transplantation. Blood 2001; 98:3172 - 3; http://dx.doi.org/10.1182/blood.V98.10.3172; PMID: 11721683
- den Haan JM, Meadows LM, Wang W, Pool J, Blokland E, Bishop TL, Reinhardus C, Shabanowitz J, Offringa R, Hunt DF, et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science 1998; 279:1054 - 7; http://dx.doi.org/10.1126/science.279.5353.1054; PMID: 9461441
- de Rijke B, van Horssen-Zoetbrood A, Beekman JM, Otterud B, Maas F, Woestenenk R, Kester M, Leppert M, Schattenberg AV, de Witte T, et al. A frameshift polymorphism in P2X5 elicits an allogeneic cytotoxic T lymphocyte response associated with remission of chronic myeloid leukemia. J Clin Invest 2005; 115:3506 - 16; http://dx.doi.org/10.1172/JCI24832; PMID: 16322791
- Van Bergen CA, Rutten CE, Van Der Meijden ED, Van Luxemburg-Heijs SA, Lurvink EG, Houwing-Duistermaat JJ, Kester MG, Mulder A, Willemze R, Falkenburg JH, et al. High-throughput characterization of 10 new minor histocompatibility antigens by whole genome association scanning. Cancer Res 2010; 70:9073 - 83; http://dx.doi.org/10.1158/0008-5472.CAN-10-1832; PMID: 21062987
- Oostvogels R, Minnema MC, van Elk M, Spaapen RM, te Raa GD, Giovannone B, et al. Towards effective and safe immunotherapy after allogeneic stem cell transplantation: identification of hematopoietic-specific minor histocompatibility antigen UTA2-1. Leukemia: official journal of the Leukemia Society of America. Leukemia Research Fund, UK 2013; 27:642 - 9; http://dx.doi.org/10.1038/leu.2012.277
- Godfrey J, Benson DM Jr.. The role of natural killer cells in immunity against multiple myeloma. Leuk Lymphoma 2012; 53:1666 - 76; http://dx.doi.org/10.3109/10428194.2012.676175; PMID: 22423650
- Ruggeri L, Mancusi A, Burchielli E, Aversa F, Martelli MF, Velardi A. Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr Opin Oncol 2007; 19:142 - 7; http://dx.doi.org/10.1097/CCO.0b013e3280148a1a; PMID: 17272987
- Petersdorf EW. Optimal HLA matching in hematopoietic cell transplantation. Curr Opin Immunol 2008; 20:588 - 93; http://dx.doi.org/10.1016/j.coi.2008.06.014; PMID: 18674615
- Alyea E, Weller E, Schlossman R, Canning C, Mauch P, Ng A, Fisher D, Gribben J, Freeman A, Parikh B, et al. Outcome after autologous and allogeneic stem cell transplantation for patients with multiple myeloma: impact of graft-versus-myeloma effect. Bone Marrow Transplant 2003; 32:1145 - 51; http://dx.doi.org/10.1038/sj.bmt.1704289; PMID: 14647268
- Reynolds C, Ratanatharathorn V, Adams P, Braun T, Silver S, Ayash L, Carson E, Eisbruch A, Dawson LA, McDonagh K, et al. Allogeneic stem cell transplantation reduces disease progression compared to autologous transplantation in patients with multiple myeloma. Bone Marrow Transplant 2001; 27:801 - 7; http://dx.doi.org/10.1038/sj.bmt.1703006; PMID: 11477436
- Crawley C, Iacobelli S, Björkstrand B, Apperley JF, Niederwieser D, Gahrton G. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood 2007; 109:3588 - 94; http://dx.doi.org/10.1182/blood-2006-07-036848; PMID: 17158231
- Levenga H, Schaap N, Maas F, Esendam B, Fredrix H, Greupink-Draaisma A, de Witte T, Dolstra H, Raymakers R. Partial T cell-depleted allogeneic stem cell transplantation following reduced-intensity conditioning creates a platform for immunotherapy with donor lymphocyte infusion and recipient dendritic cell vaccination in multiple myeloma. Biol Blood Marrow Transplant 2010; 16:320 - 32; http://dx.doi.org/10.1016/j.bbmt.2009.10.006; PMID: 19835972
- Lokhorst HM, Schattenberg A, Cornelissen JJ, Thomas LL, Verdonck LF. Donor leukocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation. Blood 1997; 90:4206 - 11; PMID: 9354693
- Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, Lamy T, Marit G, Maloisel F, Berthou C, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood 2006; 107:3474 - 80; http://dx.doi.org/10.1182/blood-2005-09-3869; PMID: 16397129
- Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, Giaccone L, Sorasio R, Omedè P, Baldi I, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med 2007; 356:1110 - 20; http://dx.doi.org/10.1056/NEJMoa065464; PMID: 17360989
- Rosiñol L, Pérez-Simón JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, González JD, Díaz-Mediavilla J, Hernández B, García-Frade J, et al, Programa para el Estudio y la Terapéutica de las Hemopatías Malignas y Grupo Español de Mieloma (PETHEMA/GEM). A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood 2008; 112:3591 - 3; http://dx.doi.org/10.1182/blood-2008-02-141598; PMID: 18612103
- Lokhorst HM, van der Holt B, Cornelissen JJ, Kersten MJ, van Oers M, Raymakers R, Minnema MC, Zweegman S, Janssen JJ, Zijlmans M, et al. Donor versus no-donor comparison of newly diagnosed myeloma patients included in the HOVON-50 multiple myeloma study. Blood 2012; 119:6219 - 25, quiz 6399; http://dx.doi.org/10.1182/blood-2011-11-393801; PMID: 22442350
- Gahrton G, Iacobelli S, Björkstrand B, Hegenbart U, Gruber A, Greinix H, Volin L, Narni F, Carella AM, Beksac M, et al, EBMT Chronic Malignancies Working Party Plasma Cell Disorders Subcommittee. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood 2013; 121:5055 - 63; http://dx.doi.org/10.1182/blood-2012-11-469452; PMID: 23482933
- Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E 3rd, Antin JH, Comenzo R, Goodman S, Hari P, et al, Blood Marrow Transplant Clinical Trials Network (BMT CTN). Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol 2011; 12:1195 - 203; http://dx.doi.org/10.1016/S1470-2045(11)70243-1; PMID: 21962393
- Moreau P, Garban F, Attal M, Michallet M, Marit G, Hulin C, Benboubker L, Doyen C, Mohty M, Yakoub-Agha I, et al, IFM Group. Long-term follow-up results of IFM99-03 and IFM99-04 trials comparing nonmyeloablative allotransplantation with autologous transplantation in high-risk de novo multiple myeloma. Blood 2008; 112:3914 - 5; http://dx.doi.org/10.1182/blood-2008-07-168823; PMID: 18948589
- Lokhorst H, Einsele H, Vesole D, Bruno B, San Miguel J, Pérez-Simon JA, Kröger N, Moreau P, Gahrton G, Gasparetto C, et al, International Myeloma Working Group. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol 2010; 28:4521 - 30; http://dx.doi.org/10.1200/JCO.2010.29.7929; PMID: 20697091
- Croci DO, Zacarías Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol Immunother 2007; 56:1687 - 700; http://dx.doi.org/10.1007/s00262-007-0343-y; PMID: 17571260
- Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother 2008; 57:1719 - 26; http://dx.doi.org/10.1007/s00262-008-0515-4; PMID: 18408926
- Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, Perrelli NF, Cosentino C, Torri F, Angius A, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med 2009; 361:478 - 88; http://dx.doi.org/10.1056/NEJMoa0811036; PMID: 19641204
- Norde WJ, Hobo W, van der Voort R, Dolstra H. Coinhibitory molecules in hematologic malignancies: targets for therapeutic intervention. Blood 2012; 120:728 - 36; http://dx.doi.org/10.1182/blood-2012-02-412510; PMID: 22563087
- Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-gamma and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 2007; 110:296 - 304; http://dx.doi.org/10.1182/blood-2006-10-051482; PMID: 17363736
- Hobo W, Norde WJ, Schaap N, Fredrix H, Maas F, Schellens K, Falkenburg JH, Korman AJ, Olive D, van der Voort R, et al. B and T lymphocyte attenuator mediates inhibition of tumor-reactive CD8+ T cells in patients after allogeneic stem cell transplantation. J Immunol 2012; 189:39 - 49; http://dx.doi.org/10.4049/jimmunol.1102807; PMID: 22634623
- Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E, Moine P, Bourin P, Moos M, Corre J, et al. CD200 is a new prognostic factor in multiple myeloma. Blood 2006; 108:4194 - 7; http://dx.doi.org/10.1182/blood-2006-06-029355; PMID: 16946299
- Hobo W, Maas F, Adisty N, de Witte T, Schaap N, van der Voort R, Dolstra H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8+ T cells. Blood 2010; 116:4501 - 11; http://dx.doi.org/10.1182/blood-2010-04-278739; PMID: 20682852
- Witzens-Harig M, Hose D, Jünger S, Pfirschke C, Khandelwal N, Umansky L, Seckinger A, Conrad H, Brackertz B, Rème T, et al. Tumor cells in multiple myeloma patients inhibit myeloma-reactive T cells through carcinoembryonic antigen-related cell adhesion molecule-6. Blood 2013; 121:4493 - 503; http://dx.doi.org/10.1182/blood-2012-05-429415; PMID: 23603913
- Bonanno G, Mariotti A, Procoli A, Folgiero V, Natale D, De Rosa L, Majolino I, Novarese L, Rocci A, Gambella M, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity correlates with immune system abnormalities in multiple myeloma. J Transl Med 2012; 10:247; http://dx.doi.org/10.1186/1479-5876-10-247; PMID: 23232072
- Rebmann V, Schütt P, Brandhorst D, Opalka B, Moritz T, Nowrousian MR, Grosse-Wilde H. Soluble MICA as an independent prognostic factor for the overall survival and progression-free survival of multiple myeloma patients. Clin Immunol 2007; 123:114 - 20; http://dx.doi.org/10.1016/j.clim.2006.11.007; PMID: 17218152
- Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR, Dranoff G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A 2008; 105:1285 - 90; http://dx.doi.org/10.1073/pnas.0711293105; PMID: 18202175
- Giannopoulos K, Kaminska W, Hus I, Dmoszynska A. The frequency of T regulatory cells modulates the survival of multiple myeloma patients: detailed characterisation of immune status in multiple myeloma. Br J Cancer 2012; 106:546 - 52; http://dx.doi.org/10.1038/bjc.2011.575; PMID: 22223085
- Klein B, Lu ZY, Gu ZJ, Costes V, Jourdan M, Rossi JF. Interleukin-10 and Gp130 cytokines in human multiple myeloma. Leuk Lymphoma 1999; 34:63 - 70; PMID: 10350333
- Kovacs E. Interleukin-6 leads to interleukin-10 production in several human multiple myeloma cell lines. Does interleukin-10 enhance the proliferation of these cells?. Leuk Res 2010; 34:912 - 6; http://dx.doi.org/10.1016/j.leukres.2009.08.012; PMID: 19762082
- D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med 1993; 178:1041 - 8; http://dx.doi.org/10.1084/jem.178.3.1041; PMID: 8102388
- Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res 2007; 13:5262 - 70; http://dx.doi.org/10.1158/1078-0432.CCR-07-1157; PMID: 17875754
- Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev 2008; 222:206 - 21; http://dx.doi.org/10.1111/j.1600-065X.2008.00610.x; PMID: 18364004
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006; 6:295 - 307; http://dx.doi.org/10.1038/nri1806; PMID: 16557261
- Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004; 21:589 - 601; http://dx.doi.org/10.1016/j.immuni.2004.09.002; PMID: 15485635
- Feyler S, von Lilienfeld-Toal M, Jarmin S, Marles L, Rawstron A, Ashcroft AJ, Owen RG, Selby PJ, Cook G. CD4(+)CD25(+)FoxP3(+) regulatory T cells are increased whilst CD3(+)CD4(-)CD8(-)alphabetaTCR(+) Double Negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br J Haematol 2009; 144:686 - 95; http://dx.doi.org/10.1111/j.1365-2141.2008.07530.x; PMID: 19133978
- De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013; 23:277 - 86; http://dx.doi.org/10.1016/j.ccr.2013.02.013; PMID: 23518347
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162 - 74; http://dx.doi.org/10.1038/nri2506; PMID: 19197294
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122:787 - 95; http://dx.doi.org/10.1172/JCI59643; PMID: 22378047
- Görgün GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, Raje N, Munshi NC, Richardson PG, Anderson KC. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 2013; 121:2975 - 87; http://dx.doi.org/10.1182/blood-2012-08-448548; PMID: 23321256
- Ramachandran IR, Martner A, Pisklakova A, Condamine T, Chase T, Vogl T, Roth J, Gabrilovich D, Nefedova Y. Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J Immunol 2013; 190:3815 - 23; http://dx.doi.org/10.4049/jimmunol.1203373; PMID: 23460744
- Suyanı E, Sucak GT, Akyürek N, Sahin S, Baysal NA, Yağcı M, Haznedar R. Tumor-associated macrophages as a prognostic parameter in multiple myeloma. Ann Hematol 2013; 92:669 - 77; http://dx.doi.org/10.1007/s00277-012-1652-6; PMID: 23334187
- Raitakari M, Brown RD, Gibson J, Joshua DE. T cells in myeloma. Hematol Oncol 2003; 21:33 - 42; http://dx.doi.org/10.1002/hon.704; PMID: 12605421
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365:725 - 33; http://dx.doi.org/10.1056/NEJMoa1103849; PMID: 21830940
- Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118:4817 - 28; http://dx.doi.org/10.1182/blood-2011-04-348540; PMID: 21849486
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119:2709 - 20; http://dx.doi.org/10.1182/blood-2011-10-384388; PMID: 22160384
- Riddell SR, Jensen MC, June CH. Chimeric antigen receptor--modified T cells: clinical translation in stem cell transplantation and beyond. Biol Blood Marrow Transplant 2013; 19:Suppl S2 - 5; http://dx.doi.org/10.1016/j.bbmt.2012.10.021; PMID: 23085599
- Barber A, Meehan KR, Sentman CL. Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Ther 2011; 18:509 - 16; http://dx.doi.org/10.1038/gt.2010.174; PMID: 21209626
- Westwood JA, Murray WK, Trivett M, Haynes NM, Solomon B, Mileshkin L, Ball D, Michael M, Burman A, Mayura-Guru P, et al. The Lewis-Y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected T cells despite the presence of soluble antigen in serum. J Immunother 2009; 32:292 - 301; http://dx.doi.org/10.1097/CJI.0b013e31819b7c8e; PMID: 19242371
- Peinert S, Prince HM, Guru PM, Kershaw MH, Smyth MJ, Trapani JA, Gambell P, Harrison S, Scott AM, Smyth FE, et al. Gene-modified T cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the Lewis Y antigen. Gene Ther 2010; 17:678 - 86; http://dx.doi.org/10.1038/gt.2010.21; PMID: 20200563
- Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, Gress RE, Hakim FT, Kochenderfer JN. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 2013; 19:2048 - 60; http://dx.doi.org/10.1158/1078-0432.CCR-12-2422; PMID: 23344265
- Reichardt VL, Okada CY, Liso A, Benike CJ, Stockerl-Goldstein KE, Engleman EG, Blume KG, Levy R. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma--a feasibility study. Blood 1999; 93:2411 - 9; PMID: 10090953
- Liso A, Stockerl-Goldstein KE, Auffermann-Gretzinger S, Benike CJ, Reichardt V, van Beckhoven A, Rajapaksa R, Engleman EG, Blume KG, Levy R. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant 2000; 6:621 - 7; http://dx.doi.org/10.1016/S1083-8791(00)70027-9; PMID: 11128812
- Titzer S, Christensen O, Manzke O, Tesch H, Wolf J, Emmerich B, Carsten C, Diehl V, Bohlen H. Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: immunological and clinical aspects. Br J Haematol 2000; 108:805 - 16; http://dx.doi.org/10.1046/j.1365-2141.2000.01958.x; PMID: 10792287
- Yi Q, Szmania S, Freeman J, Qian J, Rosen NA, Viswamitra S, Cottler-Fox M, Barlogie B, Tricot G, van Rhee F. Optimizing dendritic cell-based immunotherapy in multiple myeloma: intranodal injections of idiotype-pulsed CD40 ligand-matured vaccines led to induction of type-1 and cytotoxic T-cell immune responses in patients. Br J Haematol 2010; 150:554 - 64; http://dx.doi.org/10.1111/j.1365-2141.2010.08286.x; PMID: 20618329
- Röllig C, Schmidt C, Bornhäuser M, Ehninger G, Schmitz M, Auffermann-Gretzinger S. Induction of cellular immune responses in patients with stage-I multiple myeloma after vaccination with autologous idiotype-pulsed dendritic cells. J Immunother 2011; 34:100 - 6; http://dx.doi.org/10.1097/CJI.0b013e3181facf48; PMID: 21150718
- de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res 2003; 9:5091 - 100; PMID: 14613986
- Turtle CJ, Brown RD, Joshua DE, Hart DN. DC in multiple myeloma immunotherapy. Cytotherapy 2004; 6:128 - 37; http://dx.doi.org/10.1080/14653240410005357; PMID: 15203989
- Hobo W, Strobbe L, Maas F, Fredrix H, Greupink-Draaisma A, Esendam B, de Witte T, Preijers F, Levenga H, van Rees B, et al. Immunogenicity of dendritic cells pulsed with MAGE3, Survivin and B-cell maturation antigen mRNA for vaccination of multiple myeloma patients. Cancer Immunol Immunother 2013; 62:1381 - 92; http://dx.doi.org/10.1007/s00262-013-1438-2; PMID: 23728352
- Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med 2000; 6:1011 - 7; http://dx.doi.org/10.1038/79519; PMID: 10973321
- Rosenblatt J, Vasir B, Uhl L, Blotta S, Macnamara C, Somaiya P, Wu Z, Joyce R, Levine JD, Dombagoda D, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood 2011; 117:393 - 402; http://dx.doi.org/10.1182/blood-2010-04-277137; PMID: 21030562
- Rosenblatt J, Avivi I, Vasir B, Uhl L, Munshi NC, Katz T, Dey BR, Somaiya P, Mills H, Campigotto F, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res 2013; 19:3640 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-13-0282; PMID: 23685836
- Hobo W, Novobrantseva TI, Fredrix H, Wong J, Milstein S, Epstein-Barash H, Liu J, Schaap N, van der Voort R, Dolstra H. Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol Immunother 2013; 62:285 - 97; http://dx.doi.org/10.1007/s00262-012-1334-1; PMID: 22903385
- Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, Joyce RM, Wellenstein K, Keefe W, Schickler M, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother 2011; 34:409 - 18; http://dx.doi.org/10.1097/CJI.0b013e31821ca6ce; PMID: 21577144
- Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 2008; 14:3044 - 51; http://dx.doi.org/10.1158/1078-0432.CCR-07-4079; PMID: 18483370
- Benson DM Jr., Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010; 116:2286 - 94; http://dx.doi.org/10.1182/blood-2010-02-271874; PMID: 20460501
- Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol 2005; 86:209 - 39; http://dx.doi.org/10.1016/S0065-2776(04)86006-1; PMID: 15705423
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007; 7:329 - 39; http://dx.doi.org/10.1038/nri2073; PMID: 17438573
- Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 1987; 316:889 - 97; http://dx.doi.org/10.1056/NEJM198704093161501; PMID: 3493432
- Peest D, Leo R, Bloche S, Hein R, Stannat-Kiessling S, Tschechne B, Fett W, Harms P, Hoffmann L, Bartl R, et al. Low-dose recombinant interleukin-2 therapy in advanced multiple myeloma. Br J Haematol 1995; 89:328 - 37; http://dx.doi.org/10.1111/j.1365-2141.1995.tb03308.x; PMID: 7873383
- Einhorn S, Ahre A, Blomgren H, Johansson B, Mellstedt H, Strander H. Interferon and natural killer activity in multiple myeloma. Lack of correlation between interferon-induced enhancement of natural killer activity and clinical response to human interferon-alpha. Int J Cancer 1982; 30:167 - 72; http://dx.doi.org/10.1002/ijc.2910300207; PMID: 6182111
- Baron F, Petersdorf EW, Gooley T, Sandmaier BM, Malkki M, Chauncey TR, Maloney DG, Storb R. What is the role for donor natural killer cells after nonmyeloablative conditioning?. Biol Blood Marrow Transplant 2009; 15:580 - 8; http://dx.doi.org/10.1016/j.bbmt.2009.01.018; PMID: 19361750
- Hsu KC, Gooley T, Malkki M, Pinto-Agnello C, Dupont B, Bignon JD, Bornhäuser M, Christiansen F, Gratwohl A, Morishima Y, et al, International Histocompatibility Working Group. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Marrow Transplant 2006; 12:828 - 36; http://dx.doi.org/10.1016/j.bbmt.2006.04.008; PMID: 16864053
- Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, Maccario R, Bonetti F, Wojnar J, Martinetti M, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 2003; 102:814 - 9; http://dx.doi.org/10.1182/blood-2003-01-0091; PMID: 12689936
- Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, Boudreau C, Nelson G, Oudshoorn M, van Rood J, et al, KIR Study Group, Center for International Blood and Marrow Transplantation Research. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant 2006; 12:876 - 84; http://dx.doi.org/10.1016/j.bbmt.2006.05.007; PMID: 16864058
- Morishima Y, Yabe T, Matsuo K, Kashiwase K, Inoko H, Saji H, Yamamoto K, Maruya E, Akatsuka Y, Onizuka M, et al, Japan Marrow Donor Program. Effects of HLA allele and killer immunoglobulin-like receptor ligand matching on clinical outcome in leukemia patients undergoing transplantation with T-cell-replete marrow from an unrelated donor. Biol Blood Marrow Transplant 2007; 13:315 - 28; http://dx.doi.org/10.1016/j.bbmt.2006.10.027; PMID: 17317585
- Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, Corliss B, Tyan D, Lanier LL, Parham P. Human diversity in killer cell inhibitory receptor genes. Immunity 1997; 7:753 - 63; http://dx.doi.org/10.1016/S1074-7613(00)80394-5; PMID: 9430221
- Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant 2010; 16:533 - 42; http://dx.doi.org/10.1016/j.bbmt.2009.11.022; PMID: 19961944
- Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, Marsh SG, Guethlein LA, Parham P, Miller JS, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 2009; 113:726 - 32; http://dx.doi.org/10.1182/blood-2008-07-171926; PMID: 18945962
- Kroger N, Zabelina T, Berger J, Duske H, Klyuchnikov E, Binder T, et al. Donor KIR haplotype B improves progression-free and overall survival after allogeneic hematopoietic stem cell transplantation for multiple myeloma. Leukemia: official journal of the Leukemia Society of America. Leukemia Research Fund, UK 2011; 25:1657 - 61; http://dx.doi.org/10.1038/leu.2011.138
- Shi J, Tricot G, Szmania S, Rosen N, Garg TK, Malaviarachchi PA, Moreno A, Dupont B, Hsu KC, Baxter-Lowe LA, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol 2008; 143:641 - 53; http://dx.doi.org/10.1111/j.1365-2141.2008.07340.x; PMID: 18950462
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105:3051 - 7; http://dx.doi.org/10.1182/blood-2004-07-2974; PMID: 15632206
- Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 2011; 118:3273 - 9; http://dx.doi.org/10.1182/blood-2011-01-329508; PMID: 21791425
- Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, Schaap N, Dolstra H. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One 2011; 6:e20740; http://dx.doi.org/10.1371/journal.pone.0020740; PMID: 21698239
- Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell 2012; 148:1081 - 4; http://dx.doi.org/10.1016/j.cell.2012.02.034; PMID: 22424219
- van de Donk NW, Kamps S, Mutis T, Lokhorst HM. Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma. Leukemia: official journal of the Leukemia Society of America. Leukemia Research Fund, UK 2012; 26:199 - 213; http://dx.doi.org/10.1038/leu.2011.214
- Romagné F, André P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Benson DM Jr., Blaser BW, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 2009; 114:2667 - 77; http://dx.doi.org/10.1182/blood-2009-02-206532; PMID: 19553639
- Benson DM Jr., Bakan CE, Zhang S, Collins SM, Liang J, Srivastava S, Hofmeister CC, Efebera Y, Andre P, Romagne F, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood 2011; 118:6387 - 91; http://dx.doi.org/10.1182/blood-2011-06-360255; PMID: 22031859
- Benson DM Jr., Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, Bakan C, Andre P, Efebera Y, Tiollier J, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012; 120:4324 - 33; http://dx.doi.org/10.1182/blood-2012-06-438028; PMID: 23033266
- Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 2008; 14:2775 - 84; http://dx.doi.org/10.1158/1078-0432.CCR-07-4246; PMID: 18451245
- Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008; 112:1329 - 37; http://dx.doi.org/10.1182/blood-2007-08-107292; PMID: 17906076
- Cruz-Munoz ME, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol 2009; 10:297 - 305; http://dx.doi.org/10.1038/ni.1693; PMID: 19151721
- Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, Fry J, Afar DE, Singhal AK. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012; 120:552 - 9; http://dx.doi.org/10.1182/blood-2011-06-360552; PMID: 22184404
- Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, Kaufman JL, Leleu X, Tsao LC, Westland C, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 2012; 30:1953 - 9; http://dx.doi.org/10.1200/JCO.2011.37.2649; PMID: 22547589
- Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A, Richardson PG, Afar DE, Singhal AK, Anderson KC. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol 2012; 30:1960 - 5; http://dx.doi.org/10.1200/JCO.2011.37.7069; PMID: 22291084
- Andhavarapu S, Roy V. Immunomodulatory drugs in multiple myeloma. Expert Rev Hematol 2013; 6:69 - 82; http://dx.doi.org/10.1586/ehm.12.62; PMID: 23373782
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP, Anderson KC. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood 2002; 99:4525 - 30; http://dx.doi.org/10.1182/blood.V99.12.4525; PMID: 12036884
- Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene 2001; 20:4519 - 27; http://dx.doi.org/10.1038/sj.onc.1204623; PMID: 11494147
- Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia: official journal of the Leukemia Society of America. Leukemia Research Fund, UK 2001; 15:1950 - 61; http://dx.doi.org/10.1038/sj.leu.2402295
- Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI, Kaplan G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 1999; 163:380 - 6; PMID: 10384139
- Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 2001; 98:210 - 6; http://dx.doi.org/10.1182/blood.V98.1.210; PMID: 11418482
- Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, Kumar S, Chauhan D, Treon SP, Richardson P, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol 2005; 128:192 - 203; http://dx.doi.org/10.1111/j.1365-2141.2004.05286.x; PMID: 15638853
- Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, Song W, Podar K, Hideshima T, Chauhan D, et al. Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer Res 2005; 65:11712 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-05-1657; PMID: 16357183
- Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, Streetly M, Dalgleish AG. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol 2004; 22:3269 - 76; http://dx.doi.org/10.1200/JCO.2004.10.052; PMID: 15249589
- Adams J. The proteasome: structure, function, and role in the cell. Cancer Treat Rev 2003; 29:Suppl 1 3 - 9; http://dx.doi.org/10.1016/S0305-7372(03)00081-1; PMID: 12738238
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006; 107:4907 - 16; http://dx.doi.org/10.1182/blood-2005-08-3531; PMID: 16507771
- Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, Harousseau JL. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012; 120:947 - 59; http://dx.doi.org/10.1182/blood-2012-04-403733; PMID: 22645181
- Shi J, Tricot GJ, Garg TK, Malaviarachchi PA, Szmania SM, Kellum RE, Storrie B, Mulder A, Shaughnessy JD Jr., Barlogie B, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood 2008; 111:1309 - 17; http://dx.doi.org/10.1182/blood-2007-03-078535; PMID: 17947507
- Lundqvist A, Abrams SI, Schrump DS, Alvarez G, Suffredini D, Berg M, Childs R. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res 2006; 66:7317 - 25; http://dx.doi.org/10.1158/0008-5472.CAN-06-0680; PMID: 16849582
- Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood 2009; 113:6120 - 7; http://dx.doi.org/10.1182/blood-2008-11-190421; PMID: 19202127
- Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood 2009; 113:3503 - 11; http://dx.doi.org/10.1182/blood-2008-08-173914; PMID: 19098271
- Jardine L, Hambleton S, Bigley V, Pagan S, Wang XN, Collin M. Sensitizing primary acute lymphoblastic leukemia to natural killer cell recognition by induction of NKG2D ligands. Leuk Lymphoma 2013; 54:167 - 73; http://dx.doi.org/10.3109/10428194.2012.708026; PMID: 22742576
- Wang X, Ottosson A, Ji C, Feng X, Nordenskjöld M, Henter JI, Fadeel B, Zheng C. Proteasome inhibition induces apoptosis in primary human natural killer cells and suppresses NKp46-mediated cytotoxicity. Haematologica 2009; 94:470 - 8; http://dx.doi.org/10.3324/haematol.13783; PMID: 19229052
- Feng X, Yan J, Wang Y, Zierath JR, Nordenskjöld M, Henter JI, Fadeel B, Zheng C. The proteasome inhibitor bortezomib disrupts tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression and natural killer (NK) cell killing of TRAIL receptor-positive multiple myeloma cells. Mol Immunol 2010; 47:2388 - 96; http://dx.doi.org/10.1016/j.molimm.2010.05.003; PMID: 20542572
- Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene 2007; 26:5420 - 32; http://dx.doi.org/10.1038/sj.onc.1210610; PMID: 17694083
- Siegel DS, Dimopoulos MA, Yoon S-S, Laubach JP, Kaufman JL, Goldschmidt H, Reece DE, Leleu X, Durrant S, Offner FC, et al. Vantage 095: vorinostat in combination with bortezomib in salvage multiple myeloma patients: final study results of a global phase 2b trial. [abstract 480]. Blood 2011; 114
- Dimopoulos MA, Jagannath S, Yoon S-S, Siegel DS, Lonial S, Hajek R, et al. Vantage 088: vorinostat in combination with Bortezomib in patients with relapsed/refractory multiple myeloma: results of a global, randomized phase 3 trial. ASH Annu Meeting Abstr 2011; 118:811
- Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res 2005; 65:11136 - 45; http://dx.doi.org/10.1158/0008-5472.CAN-05-0599; PMID: 16322264
- Kato N, Tanaka J, Sugita J, Toubai T, Miura Y, Ibata M, et al. Regulation of the expression of MHC class I-related chain A, B (MICA, MICB) via chromatin remodeling and its impact on the susceptibility of leukemic cells to the cytotoxicity of NKG2D-expressing cells. Leukemia: official journal of the Leukemia Society of America. Leukemia Research Fund, UK 2007; 21:2103 - 8; http://dx.doi.org/10.1038/sj.leu.2404862
- Zhang C, Wang Y, Zhou Z, Zhang J, Tian Z. Sodium butyrate upregulates expression of NKG2D ligand MICA/B in HeLa and HepG2 cell lines and increases their susceptibility to NK lysis. Cancer Immunol Immunother 2009; 58:1275 - 85; http://dx.doi.org/10.1007/s00262-008-0645-8; PMID: 19139882
- Schmudde M, Braun A, Pende D, Sonnemann J, Klier U, Beck JF, Moretta L, Bröker BM. Histone deacetylase inhibitors sensitize tumour cells for cytotoxic effects of natural killer cells. Cancer Lett 2008; 272:110 - 21; http://dx.doi.org/10.1016/j.canlet.2008.06.027; PMID: 18718708
- Fiegler N, Textor S, Arnold A, Rölle A, Oehme I, Breuhahn K, Moldenhauer G, Witzens-Harig M, Cerwenka A. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood 2013; 122:684 - 93; http://dx.doi.org/10.1182/blood-2013-02-482513; PMID: 23801635
- Chu J, Deng Y, Benson DM Jr., He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance In Vitro and In Vivo anti-tumor activity against human multiple myeloma. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK 2013.
- Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29:2493 - 8; http://dx.doi.org/10.1200/JCO.2010.32.7270; PMID: 21576633
