Abstract
Psoriasis is a chronic, immune skin disease associated with significant morbidity. Development of psoriasis is influenced by numerous genes, one allele is HLA-CW*0602. Other genes and single nucleotide polymorphisms affect immunologic pathways and antimicrobial peptide synthesis. Dendritic cells initiate psoriasis by activating T-cells toward a Th1 and Th17 response, with increased cytokines including TNF-α, IL-6, -12, -17, -22, and -23. IL-22 appears to promote keratinocyte dedifferentiation and increased antimicrobial peptide synthesis while TNF-α and IL-17 induce leukocyte localization within the psoriatic plaque. These recent insights identifying key cytokine pathways have led to the development of inhibitors with significant efficacy in the treatment of psoriasis. While a strategy for vaccine modulation of the immune response in psoriasis is in progress, with new technology they may provide a cost-effective long-term treatment that may induce tolerance or targeted self-inhibition for patients with autoimmune disorders, such as psoriasis.
Impact of Psoriasis
Psoriasis is a chronic inflammatory skin disease characterized by thick, erythematous, scaly, plaques with preferential involvement of extensor extremities, which in some patients can be generalized to affect greater than 90% of the skin. Because psoriasis affects patients appearance, social stigmatization and sexual dysfunction are impacted,Citation1 and unfortunately, such patients have increased rates of depression and suicide.Citation2,Citation3 There is a substantial psychologic burden from psoriasis, and patients commonly note flares associated with stress.Citation4 The inflammation extends beyond the skin and joints and it is now accepted that the risk of cardiovascular disease is increased.Citation5 Psoriasis is a highly prevalent condition with around 2–3% of the US and UK populace affected.Citation6,Citation7 In addition, psoriatic arthritis may affect around 11% of patients with psoriasis.Citation8
Patients with severe psoriasis may find employment prospects more challenging and patients with more severe disease have been found to have less income compared with patients with mild disease.Citation9 Economic total and out-of-pocket costs, and time lost from work due to psoriasis symptoms also increase based on severity.Citation10 The incremental cost associated with the diagnosis of psoriasis, not including out-of-pocket expenses or loss of productivity, was estimated to be $1500 per patient in the United States.Citation11 The estimated cost to the entire US health system is estimated at over $1.3 billion.Citation12 The impact of psoriasis is substantial with skin, psychosocial, arthritic, and cardiovascular implications.
Genetic Basis of Psoriasis
In the recent years, major strides have been made in elucidating the genetic basis of psoriasis, with 36 genes linked to the development of psoriasis.Citation13 The exact inheritance is complex and clinical phenotype depends on variables from the environment, genetics, and adaptive and innate immunity.Citation14 Results from twin studies show a concordance rate for monozygotic twins between 35–70% and there is a 2-fold increased risk of psoriasis in monozygotic twins compared with dizygotic twins.Citation15,Citation16 Other family analyses demonstrated a 10-fold increased risk ratio for developing the disease in first-degree relatives of patients with juvenile-onset psoriasis.Citation17 The strong association of HLA type, observed over 40 y ago, suggested 2 different psoriasis patterns: type I with more severe disease, family history, and expression of HLA-CW*0602; and type II with less severe disease and less genetic predisposistion.Citation17,Citation18 The extended haplotype of this HLA association showed a 26-fold increased risk of developing early onset psoriasis.Citation19 However, tight linkage disequilibrium made it uncertain whether the most strongly associated risk factor was HLA-CW*0602 or a colocalized gene.Citation20 This region was therefore called PSORS1 until more accurate sequence specific locus could be identified. Studies continued to be conducted implicating the primary association with psoriasis to the HLA-CW*0602 locus on chromosome 6p21 with a 16-fold increased risk of developing the disease compared with the general population.Citation21 However, it was not until 2006 that the problem of linkage disequilibrium was elucidated and researchers confirmed that this HLA type was indeed the primary gene associated with the PSORS1 locus predisposing to psoriasis.Citation22,Citation23
As psoriasis is a clinically heterogeneous disease with several distinct subtypes- chronic plaque type, pustular, inverse, guttate, and palmoplantar, each may have a different genetic predispositions. While the genetic region of the guttate form has been found to be associated with the PSORS1 region, the palmoplantar form of psoriasis has been shown to be distinct.Citation24
With the advent of the ability to rapidly sequence the genome, numerous new genes and single nucleotide polymorphisms associated with psoriasis have been discovered.Citation13,Citation25,Citation26 Most of these genes encode proteins such as those involved in skin barrier function, antimicrobial peptides (AMP), and immunologic signaling pathways including IL-23, TNF-α, NFκB, and interferons (IFN).Citation26-Citation28 One susceptibility gene, endoplasmic reticulum aminopeptidase 1 (ERAP1), encodes a peptidase that cleaves proteins that are then displayed on MHC class I proteins, and is only associated with psoriasis if the HLA-Cw6 risk allele is present.Citation29 These GWAS studies have also identified another gene that is located within the PSORS1 region that is independently associated with psoriasis, and encodes for an integrated human endogeneous retrovirus K (HERV-K) dUTPase.Citation30,Citation31 Although little is known about the regulation and pattern of expression of this retrovirus gene in psoriasis, similar to many other susceptibility genes, it does appear to have immunogenic properties.Citation31,Citation32
Vast strides have been made in the field of the genetics of psoriasis and GWAS has allowed for identification of additional new genes linked to the susceptibility of psoriasis.Citation13 These genes appear to function primarily within immunologic pathways, skin barrier function, and AMPs.Citation13
Role of Dendritic Cells
The rapid production of thick adherent scales from keratinocytes in psoriasis suggested that the pathogenesis of psoriasis was fundamentally initiated by hyperproliferation of keratinocytes, but studies using a mouse xenotransplanation model demonstrated that human peripheral blood cells were necessary for recreating psoriasis histopathology in transplanted normal skin from a psoriasis patient, indicating the primary driver of psoriasis to have an immune basis.Citation33 This concept has been supported by the transfer of psoriasis from human donors to previously psoriasis-free recipients in the process of blood and marrow transplantation.Citation34
Dendritic cells (DC) are professional antigen presenting cells that play an important role in immune regulation and function to connect innate and adaptive immunity. Several types of dendritic cells have been identified based on surface markers, including Langerhans cells in the epidermis, plasmacytoid dendritic cells, and dermal DC, with both a resident and inflammatory phenotype. These subsets have been reviewed in relation to psoriasis by Zaba et al.Citation35 The plasmacytoid and dermal DC are believed to be integral in the immunopathogenesis of psoriasis. Plasmacytoid DC, normally part of the host anti-viral response, can produce a large amount of interferon-α (IFN-α) to initiate the immune cascade, followed by differentiation to myeloid DC, and stimulate naïve T cells into Th1 and Th17 polarized effector T cells ().Citation36,Citation37 Evidence suggests plasmacytoid DC are initially recruited to the psoriatic skin by fibroblast, endothelial cell, and mast cell-released chemerin that is expressed in the perilesional psoriasis skin.Citation38 Chemerin functions as an AMP.Citation39 Plasmacytoid DC are then triggered to produce IFN-α by self-DNA/RNA from damaged keratinocytes coupled with the AMP, LL37, which activates Toll-like receptor (TLR)7/Toll-like receptor(TLR)9.Citation37,Citation40,Citation41 The inflammatory cytokines released by plasmacytoid DC can then activate or induce monocyte differentiation to dermal myeloid DC that are important in the chronic phase of the disease.Citation36,Citation42 Heat shock proteins(HSP) may also play a role based on increased expression in patients with guttate psoriasis.Citation43 HSP60 has been shown to induce TNF-α, IL-12, IL-15, and IL-23 from macrophage cell lines and dendritic cells.Citation44,Citation45 These proteins and others may interact with DC through TLRs. Inflammatory DC of chronic psoriasis skin show differential TLR activation such as TLR1, and 2, in addition to upregulation of TNF-α signaling, and inducible nitric oxide synthetase (iNOS) pathways.Citation46,Citation47
Figure 1. Development of psoriasis plaque. An initial insult activates TLR-pathways in plasmocytoid DC. Dermal DC differentiate and are activated and secrete cytokines IL-12, TNF-α, TGF-β, and IL-6 that stimulate naïve T-cell to mobilize transcription factors that induces genes to polarize T cells into Th1 and Th17 subtypes, with suppression of Tregs. The corresponding cytokines, IL-17A, IL-17F, and IL-22 from Th17 T cells stimulate keratinocyte proliferation and result in the skin microenvironment with leukocyte infiltration, keratinocyte dedifferentiation, and increased AMP to form the clinical phenotype of a scaly lesion of psoriasis.
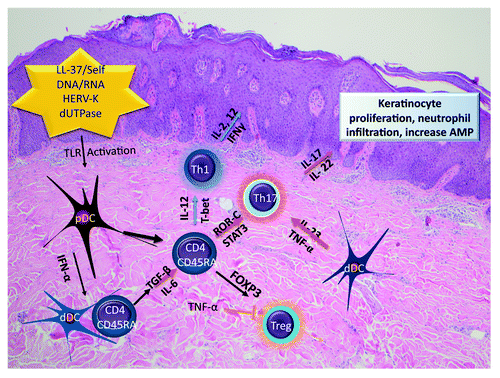
TLRs function as pattern recognition receptors of the innate immune system, are present on dendritic cells, and likely are the initial receptor that becomes activated in psoriasis, leading to activation of plasmacytoid DC through TLR7 and 9 and expression of high levels of interferon.Citation37,Citation40 Synthetic stimulation of TLR7, by imiquimod also produces high levels of interferon and can induce clinical progression of psoriasis.Citation48 TLR8 and self-complexed RNA may also be involved in DC stimulation.Citation41 Dendritic cells have also been shown to be activated via TLR2, by the HERV-K dUTPase that is associated with genetic susceptibility to psoriasis.Citation32,Citation46
After the initial appearance, established psoriasis plaques have an increased population of DC with the potential to activate T-cells and polarize cytokine expression characteristic of psoriasis, TNF-α, and IL-17.Citation36,Citation49 DC also play a role in the initial activation and proliferation of the Th17 helper cells and potentially γ/δ T-cells that express IL-17.Citation50 Dendritic cells appear to be the link between AMPs, skin damage, and the initiation of the cytokine cascade that activates T-cells resulting in the Th1 and Th17 response of psoriasis.
Cytokine Expression and T-Cells in Psoriasis
Psoriasis is a chronic autoimmune disease associated with the activation of an inappropriate cytokine cascade in the skin that propagates inflammation. In psoriasis, the plasmacytoid DC normally critical in anti-viral responses appear to initiate the imbalance with high levels of IFN-α production before differentiating into tissue resident or dermal DC.Citation37,Citation42 IL-20, IL-23, iNOS, and other inflammatory products are produced by these dermal DC, of which IL-23 correlates well with the development of the psoriatic plaque.Citation51-Citation53 TNF-α, which is also critical in the development of the psoriasis phenotype, is secreted from resident dermal DC and activates the resident T-cells.Citation54,Citation55
Early studies into T-cell function in the immune response revealed 2 major phenotypic subsets based on cytokine expression: Th1 cytokine expression including IL-2, IFN-γ, IL-12, and TNF-α regulated by the transcription factor T-bet; and Th2 cytokine expression including IL-4, IL-5, IL-10, and IL13 regulated by the transcription factor GATA3.Citation56,Citation57 This paradigm provided the conceptual framework for understanding the immune response, Th1 regulating cell mediated immunity and Th2 regulating humoral immunity. With the clinical efficacy of therapeutic agents such as cyclosporine in psoriasis, a pivotal role of T-cells in the psoriasis pathogenesis was demonstrated.Citation58,Citation59 Furthermore, from analysis of the cytokine pattern detected in psoriasis with an increase in TNF-α, and IFN-γ, this was initially classified as a strongly Th1 dependent disease.
With continued research into T-cell function, the Th1/Th2 model was revised to include the role of additional T-cell subsets that have distinct functional properties to more accurately account for the murine disease model Experimental Autoimmune Encephalitis (EAE), used in studying Th1 mediated neurologic disease. In studies to explore the specific role of Th1 cytokines, it was observed that knockout of IL-12 receptor subunit, IL-12R-β2, worsened EAE rather than prevented the disease as was expected for a Th1 dominant disease.Citation60 On the other hand, the absence of the IL-12p40 subunit led to resistance in EAE and suggested the role of T cells regulated by IL-23 in EAE.Citation61 Thus this observation supported the role of additional signaling pathways in T cell regulation and led to the discovery of the role of IL-17 in immune regulation and the identification of the Th17 cell subset, that is mediated by the transcription factor RORγt or ROR-C and gene RORC.Citation62,Citation63 Since the identification of this additional T cell subset, it has now been shown that Th17 cells are critical in the pathogenesis of psoriasis.Citation54,Citation64,Citation65
In the presence of polarizing cytokines, CD4+ T-cell subsets, are stimulated to differentiate into effector subsets that play a role in psoriasis, such as Th1, Th17, Th22, and regulatory T-cells (Tregs).Citation49,Citation66-Citation68 Beyond the Th1/Th2 dichotomy of T-cell subsets, the previous beliefs of a stable phenotype regulated by specific transcription factors that orchestrate terminal differentiation in T-cell development has also been subjected to reanalysis. There is evidence from recent results to suggest that there is plasticity in the polarity of T-cell phenotypes, and that under certain conditions, cytokines have the ability to modulate gene expression of T-cell subset specific transcription factors and stimulate expression of genes of other T-cell subsets.Citation69,Citation70
With regard to the key pathogenic cells important in psoriasis, emerging evidence has helped to highlight the role of different T cell subsets in human psoriasis. Th1 T-cells have been implicated in the pathogenesis with the ability to secrete TNF-α, but are not sufficient alone. Th17 cells, which were initially described in 2005,Citation71,Citation72 are characterized by IL-17A and IL-17F cytokine production and normally stimulate neutrophils in the immune response to several microorganisms.Citation71,Citation73 The cytokine subtypes IL17A, C, and F have been shown to be strikingly elevated in lesional psoriasis skin.Citation74 Upstream cytokines that allow Th17 differentiation include TGF-β, IL-6, and IL-21.Citation75,Citation76 TGF-β upregulates the gene expression of ROR-C, a nuclear hormone transcription factor that is a marker of human Th17 cells. Activation of STAT3, a transcription factor activated by IL-6 receptor ligation is also important in differentiation of the Th17 phenotype. IL-23 is also increased in psoriatic plaques and promotes the expansion and survival of Th17 cells.Citation52,Citation77,Citation78
IL-17A and IL-17F directly affect gene regulation in keratinocytes involved in the innate immune response.Citation79-Citation82 These cytokines also stimulate the expression of CCL20 which binds to CCR6 present on Th17 cells to promote leukocyte infiltration of the affected tissue,Citation72,Citation83,Citation84 Combined with TNF-α, it produces a synergistic inflammatory effect on the keratinocytes.Citation85 IL-22, expressed by both Th17 and Th22 cells, promotes abnormal differentiation of keratinocytes, AMP response, and hyperproliferation of the keratinocytes.Citation86-Citation89
Regulatory T cells (Tregs) are a subset of T cells that act as brakes to downregulate the immune responses and these cells also have a role in psoriasis. These cells can be further subdivided into natural and induced Tregs. The former are derived directly from the thymus, while the latter arise as a response to inflammatory process or diseases. The cells were characterized initially by surface marker CD4+CD25+, but the identification of the transcription factor Foxp3 lead to a more specific marker to follow the Treg population.Citation90,Citation91 Mice having mutations in Foxp3 typically demonstrate the scurfy phenotype, characterized by lymphoproliferation and robust inflammatory process involving the skin. In humans with Foxp3 mutations, a condition known as immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) manifests at an early age and is characterized by numerous autoimmune diseases.Citation92,Citation93 The intracellular nature of Foxp3 has led to a search for specific surface markers to facilitate the study of Tregs, and markers such as high expression of CD25 (IL2Rα) and LRRC32 (GARP) as well as the low expression or absence of CD127 (IL-Rα) have all been proposed as markers to improve identification of potent Tregs.Citation94-Citation96
The function of Tregs is important in immunoregulation and loss of activity or cell number can lead to a dysregulated inflammatory response. Indeed, decreased Tregs have been demonstrated in autoimmune diseases such as diabetes,Citation95 and dysfunction has been described in psoriasis, autoimmune hepatitis, Crohn disease, diabetes, and autoimmune thyroid disease.Citation97-Citation101 Until recently, it was unclear as to how exactly Treg dysfunction contributed to psoriasis, beyond being less capable of inhibiting the growth of potentially autoimmune T cells. Studies by Sugiyama et al. demonstrated that psoriatic Tregs could not suppress the growth of lymphocytes in a mixed lymphocyte assay as well as a comparable number or Tregs derived from normal individuals.Citation98 However recent studies have now linked dysfunctional Tregs directly to the production of IL-17 via their differentiation into Th17 cells.Citation102 In addition, elevated IL-6 produced by dermal DC and endothelial cells in psoriatic lesions has been shown in a murine model to reverse Treg suppressive activity resulting in loss of inhibition of cellular differentiation to the IL-17 pathway.Citation103
Highlighting the altered role of Tregs in autoimmune disease, in rheumatoid arthritis (RA), which is associated with decreased Treg function and increased TNF-α, treatment with anti-TNF therapy can restore Treg function.Citation104,Citation105
It was recently shown that elevated IL-6 produced by CD11c+ dermal dendritic cells and CD31+ endothelial cells in psoriatic lesions in a murine model can reduce Treg suppressive activity via STAT3 phosphorylation in Tregs and also effector T (Teff) cells, resulting in bias toward Th17 differentiation.Citation103 This failed to show a direct link between Tregs and Th17 cell development in patients with psoriasis. Under certain situations, Tregs may actually change into IL-17 producing Tregs.Citation102 Increased numbers of these IL-17 producing Tregs were seen in psoriatic lesions, thereby giving credence to the idea that such cells can become Th17 cells.Citation102 In addition, increases in the level of the transcription factor RORC, induced by inflammatory cytokines such as IL-1β, IL-23, IL-2, and Il-15, in psoriatic Tregs, along with stimulation and costimulation via the TCR and CD28, resulted in a decrease in Foxp3 and a corresponding decrease in Treg activity.Citation102,Citation106 IL-17 production was inversely correlated with this finding.Citation102,Citation106
There is now marked evidence for a direct linkage between dysfunctional Tregs and the development of pathogenic Th17 cells in psoriasis.Citation106 Further studies into precisely how these IL-17 producing Tregs differentiate into the pathogenic Th17 cells will likely aid our understanding of how to better regulate the immune system in patients with psoriasis.
Viral Role in Psoriasis
Human endogenous retroviruses (HERVs) are a family of retroviruses incorporated previously into the human genome during the course of evolution. The retroviral sequences constitute 8% of human DNA.Citation107 These endogenous retroviruses integrated throughout the human DNA and can be detected at sites of long-terminal repeats (LTRs). Over millions of years, mutations have accumulated in these genes, but transcription is still maintained in up to 1/3 ofknown sequences and encode products that have been inactivated functionally by point and frame shift mutations.Citation108 Human endogenous retrovirus K (HERV-K) is the youngest of the family of endogenous retroviruses and the only one with open reading frames for all genes to encode the major proteins. Like other ERVs, it is named for the tRNA specificity of the primer binding site to initiate reverse transcription, hence HERV-K family uses lysine.Citation109
Virus transcription has been specifically detected among HERV-W, K, and E families in psoriatic skin while it is rarely detected in atopic dermatitis and normal skin samples.Citation110 HERV-E glycoprotein envelope has been visualized via immunohistochemistry in both psoriatic and atopic skin, although only rarely in normal skin.Citation111 mRNA encoding for a putative deoxyuridine triphosphate nucleotidohydrolase (dUTPase) encoded by HERV-K has been demonstrated in peripheral blood mononuclear cells and skin in psoriasis patients.Citation30 This HERV-K dUTPase gene, although closely linked and just telomeric to the HLA-C allele, is independently associated with psoriasis.Citation31 There is a significant humoral response to the HERV-K dUTPase in patients with psoriasis when compared with controlsCitation31 and the HERV-K dUTPase product induces a pro-inflammatory Th1and Th17 cytokine environment through interactions with TLR2 on dendritic cells and a lesser extent keratinocytes in vitro.Citation32 Multiple questions concerning the possible role(s) of these viruses in psoriasis persists but the genetic association in conjunction with the relevant cytokine response remain intriguing for the HERV-K encoded dUTPase. Additional research is needed concerning the prevalence, expression, and role in psoriasis of this dUTPase. Eventually this product may become a target in the management of psoriasis.
Targeted Pathways in the Treatment of Psoriasis
Advances in understanding the molecular and cellular pathways in psoriasis have led to the development of highly targeted therapies for the treatment of psoriasis. There are currently 5 available TNF-α inhibitors, of which 3 (etanercept, infliximab, and adalimumab) are FDA approved in the treatment of psoriasis.Citation112 All 3 bind to either membrane bound and/or soluble TNF-α. Etanercept is a recombinant human TNF-α receptor protein (p75) fused with the Fc portion of immunoglobulin IgG1. Infliximab is a chimeric antibody generated with human and murine sequences in the hypervariable region recognizing TNF-α. Adalimumab is a fully human monoclonal antibody(mAb). Certolizumab pegol, which is pegylated for increased half-life, and golimumab are human TNF-α MAbs with FDA approval for the treatment of psoriatic arthritis, but also have clinical efficacy in cutaneous psoriasis.Citation113,Citation114 All of the biologic monoclonal antibodies inhibit the inflammatory cascade by binding and preventing TNF-α from activating TNF receptors. Downstream effects from this inhibition include decreased IL-1, -8, -23, and iNOS, with a decrease in dermal DC resulting in blockage of the signaling for downstream Th17 induced infiltration and Th22 induced acanthosis.Citation53,Citation85,Citation115 While the class as a whole is highly effective, (), there are some differences with infliximab having the most efficacy based on clinical response as measured by the psoriasis activity severity index (PASI). PASI is a validated scoring system for psoriasis that incorporates morphologic features of psoriasis lesions such as the thickness, degree of redness and severity of scaliness in addition to extent of involvement.Citation116 The PASI scale ranges from 1 to 72. Moderate to severe psoriasis have a PASI score that is typically greater than 12. However, infliximab has the highest rate of discontinuation due to serious events, particularly infusion reactions.Citation117
Table 1. Summary of FDA approved and selected late stage development treatments for psoriasis
Ustekinumab, a MAb against the shared protein subunit of IL-12 and IL-23 receptor known as p40, blocks IL-12-driven differentiation of naïve T cells to a Th1 phenotype and IL-23-driven survival signal to the Th17 phenotype.Citation118,Citation119 Targeting IL-12 and IL-23 is an upstream method of blocking Th17 activity, and results in significant clinical improvement.Citation120 A similar antibody molecule, briakinumab is not currently being pursued for FDA approval after an increased number of cardiovascular events was seen in trials.Citation121 However, it is unclear whether this represents more than sampling error. Overall, it does not appear to be a class risk against the IL-12/23 pathway based on the analysis of the 3 y safety data of ustekinumab having a similar risk of cardiovascular events compared with expected frequencies, no skewing toward a Th2 response (asthma exacerbation), and no increased salmonella infections.Citation122-Citation124 Another option in IL-23 inhibition is targeting the opposing subunit of the molecule, the p19 subunit. Two such MAbs, CNTO1959 and SCH 900222, are currently undergoing clinical trials.Citation125
Targeting the Th-17 pathway from at a step that is further downstream, IL-17 directed biologics are the most recent agents and are currently in late-phase clinical development. IL17 is produced by Th17 cells which are activated by a cytokine mileu that includes IL-23.Citation78 There are several MAbs that target the IL-17 pathway in clinical studies. Brodalumab is an anti-IL-17 receptor MAb, and secukinumab and ixekizumab are MAbs to IL-17A.Citation125-Citation127 Preliminary reports show these drugs have extensive efficacy, and appear to have better efficacy than the TNF-antagonists currently available in the treatment of psoriasis.Citation65,Citation126-Citation128 However, phase 3 studies and long-term safety studies will be needed to ensure long-term efficacy and safety of this class of medications.
Janus-associated kinase (JAK) –and Signal Transducer and Activator of Transcription (STAT) proteins are involved in signal transduction from various cytokines involved in psoriasis, including IL6, IL-12, and IL-23.Citation125,Citation129,Citation130 Inhibiting the activation of JAK/STATs may alter signals needed in the differentiation of Th-17 T cells. Therefore targeting this intracellular signal transduction pathway offers the advantage of a small molecule that can be given as an oral therapy as oppose to subcutaneous injections. There are 4 subtypes of JAK/STAT molecules that contribute to different pathways. that can be inhibited. JAK3 is specific to immune cells, and targeting this kinase may theoretically have a better therapeutic index. Preliminary trials of both topical and systemic JAK inhibitors have been promising, especially an oral JAK1/3 inhibitor called tofacitinib.Citation129,Citation131,Citation132 Several clinical trials are ongoing with regards to small molecule inhibitors of JAKs and the resultant cytokine signaling.Citation125 The STAT pathway may also be a future target but it does not possess enzymatic activity and is not as easily targeted. However, STAT3 inhibitors have been developed and may eventually be translated to studies for the treatment of psoriasis.Citation133,Citation134
Another small molecule therapy that is in advanced trials for psoriasis is apremilast. This molecule targets phosphodiesterase-4 that is the primary phosphodiesterase in leukocytes. Blockage of this enzyme decreases cAMP in these cells and prevents activation including cytokine production of IL-12, -23, and TNF-α.Citation135 Apremilast has completed phase III studies and has shown efficacy for therapy of both psoriasis and psoriatic arthritis.Citation136-Citation138
Finally, fezakinumab (ILV-094), a MAb directed against IL-22, which is upregulated in response to IL-17, is currently undergoing clinical trials.Citation125 The inhibition of IL-22 would be suspected to block the final step of stimulation of the keratinocytes causing hyperproliferation and increased antimicrobial peptide synthesis.Citation87 Additional small molecules and biologics that are in early stages of development have recently been reviewed.Citation139
Potential for Vaccine and Immunotherapy in Psoriasis
Several groups have published on potential and early phase clinical studies on the use of vaccines in psoriasis, first using Mycobacterium vaccae after observation of improvement in psoriasis during treatment of leprosy. In a preliminary study, a formulation of heat-killed, deglycolipidated, delipidated M. vaccae underwent rigid trials and an IND application was filed with the FDA for further testing. With intradermal injections, 3 wk apart, 65% of patients developed marked improvement in the PASI score at 12 wk after injection, although no placebo evaluation was performed.Citation140 An early comparison randomized patients to heat-killed M. vaccae vs. tetanus toxoid showed a decreased in PASI from baseline in intradermal injections although the placebo group decreased similarly.Citation141 The results of this trial was compromised by high levels of drop-out, responder bias, and comparison to baseline rather than the placebo tetanus toxoid.Citation141 The most rigidly conducted phase 2 study comparison testing of the same M. vaccae formulation ended disappointingly after subjecting patients to a high dose, low dose, and placebo immunotherapy injection 3 wk apart and did not demonstrate a PASI-75 improvement different between groups, with the highest response of 18% seen in the placebo group.Citation142 The same vaccine did not show a benefit in psoriatic arthritis.Citation143 Two recent studies, one placebo controlled, have evaluated the use of Mycobacterium w, another non-pathogenic, rapid growing mycobacterial strain in psoriasis and these studies suggested potential benefit with some improvement, but unfortunately the studies lacked rigorous methodology and intergroup comparisons.Citation144,Citation145
Another group has published on the use of a live attenuated varicella zoster virus strain for treatment of psoriasis, again after empiric improvement in a patient with severe psoriasis after infection with the live virus. This study was not double blind but did show improvement in comparison with a saline placebo while patients were on cyclosporine.Citation146
A third potential microbial based vaccination strategy developed in the third world was empirically developed based on improvement in psoriasis patients vaccinated for leishmania. A study of 2770 participants demonstrated up to 68% of patients treated with a leishmania AS100 vaccine achieved a PASI-75 response.Citation147 However, this study suffered from non-rigorous planning, inclusion, lack of clarity in reporting concommitant medications, baseline, and completion data between study groups. A secondary analysis of these patients found miraculous improvement using a non-validated arthritis scoring system by tender joint counts with this vaccine.Citation148 Both of these studies present an extensive quantity of data in a large number of patients, but serious methodologic flaws, lack of clarity of data, inclusion criteria, lack of placebo control, and lack of prospective nature make the results suspect.
The mechanism for the efficacy of the mycobacterium vaccae is unclear. However, a possible reason is that patients with psoriasis have an associated reduced T cell responsiveness to mycobacterium antigens.Citation149 Furthermore, dendritic cells and macrophages have been shown to be increased in the skin of patients with psoriasis underoing M. vaccae therapy, with an increased production of IL-10.Citation143 Since IL-10 is an immunosuppressing cytokine, stimulating immunity to mycobacterium with increased IL-10 may alter the immune balance to favor psoriasis clearance.Citation150
Lastly, a non-microbial based vaccination strategy has been performed in targeting soluble receptors such as TNF-α by conjugating human cytokines to an immunogenic virus like particle.Citation151 However, this is in very early stages of development. This approach, while potentially long-lasting, will require careful experimental design and titration to reduce excessive TNF-α responsible for clinical disease. It also must be monitored to ensure that that immune suppression does not result in insufficient TNF-α needed for intact immune functions against mycobacterium and other bacterial infections.
Overall, vaccines studies that have been conducted have suffered from serious methodologic flaws and have been primarily pursued in third world countries. The most rigorous studies have not shown any benefit in psoriasis.Citation142,Citation143 The most promising development may be immunization against human soluble receptors that are involved in psoriatic diathesis such as TNF-α, IL-12, -17, -22, and -23, however, this technology is far from ready for human use. The selection of cytokines to target may be based on the clinical experience with MAbs to these cytokine pathways. Ideally, cytokines that are highly specific for psoriasis, validated from clinical experience with MAbs against these cytokines would serve as early targets for vaccine development.
Summary
Psoriasis is a prevalent, chronic, and potentially incapacitating disease. Remarkable strides have been made in uncovering the immune pathways dysregulated in psoriasis, such as a Th1 and Th17 pathways. These discoveries have allowed for the development of highly targeted treatments with unparalleled efficacy. However, numerous challenges remain including how to maintain response in long-term treatment with biologic agents, how to ensure cost-effective treatments to all patients, and ensuring the long-term safety of these treatments. While vaccines are far from clinical use, they may eventually provide a “cure” to psoriasis by introducing tolerance or targeted self-inhibition to the immune system of the psoriasis patient with resolution of the maladaptive Th17 response.
| AMP | = | anti-microbial peptide |
| DC | = | dendritic cell |
| ERV | = | endogeneous retrovirus |
| GWAS | = | genome wide association study |
| HLA | = | human leukocyte antigen |
| HERV-K | = | human endogeneous retrovirus K |
| HSP | = | heat shock protein |
| IFN | = | interferon |
| IL | = | interleukin |
| iNOS | = | inducible nitric oxide synthetase |
| JAK | = | Janus kinase |
| LTR | = | long terminal repeat |
| Mab | = | monoclonal antibody |
| PASI | = | psoriasis area and severity index |
| STAT | = | signal transducer and activator of transcription |
| Teff | = | T-effector cell |
| Th1 | = | T-helper 1 cell |
| Th17 | = | T-helper 17 cell |
| Th22 | = | T-helper 22 cell |
| TLR | = | toll-like receptor |
| TNF-α | = | tumor necrosis factor α |
| Treg | = | regulatory T-cell |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Financial Disclosure
H.K.W. honoraria from Amgen. In addition, he is an investigator for Amgen, Janssen, Abbvie, and Celgene. All other authors report no conflicts of interest.
Funding
National Psoriasis Foundation grants to M.E.A., B.H.K., and H.K.W.
Acknowledgments
The authors would like to acknowledge the support of the National Psoriasis Foundation Grants to M.E.A., B.H.K., and H.K.W.
References
- Choi J, Koo JY. Quality of life issues in psoriasis. J Am Acad Dermatol 2003; 49:Suppl S57 - 61; http://dx.doi.org/10.1016/S0190-9622(03)01136-8; PMID: 12894127
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 2010; 146:891 - 5; PMID: 20713823
- Picardi A, Lega I, Tarolla E. Suicide risk in skin disorders. Clin Dermatol 2013; 31:47 - 56; http://dx.doi.org/10.1016/j.clindermatol.2011.11.006; PMID: 23245973
- Seville RH. Stress and psoriasis: the importance of insight and empathy in prognosis. J Am Acad Dermatol 1989; 20:97 - 100; http://dx.doi.org/10.1016/S0190-9622(89)70015-3; PMID: 2643644
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006; 296:1735 - 41; http://dx.doi.org/10.1001/jama.296.14.1735; PMID: 17032986
- Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc 2004; 9:136 - 9; http://dx.doi.org/10.1046/j.1087-0024.2003.09102.x; PMID: 15083780
- Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol 2005; 141:1537 - 41; http://dx.doi.org/10.1001/archderm.141.12.1537; PMID: 16365254
- Gelfand JM, Gladman DD, Mease PJ, Smith N, Margolis DJ, Nijsten T, Stern RS, Feldman SR, Rolstad T. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol 2005; 53:573 - 7; http://dx.doi.org/10.1016/j.jaad.2005.03.046; PMID: 16198775
- Horn EJ, Fox KM, Patel V, Chiou C-F, Dann F, Lebwohl M. Association of patient-reported psoriasis severity with income and employment. J Am Acad Dermatol 2007; 57:963 - 71; http://dx.doi.org/10.1016/j.jaad.2007.07.023; PMID: 17761358
- Feldman SR Jr., Fleischer AB Jr., Reboussin DM, Rapp SR, Bradham DD, Exum ML, Clark AR. The economic impact of psoriasis increases with psoriasis severity. J Am Acad Dermatol 1997; 37:564 - 9; http://dx.doi.org/10.1016/S0190-9622(97)70172-5; PMID: 9344194
- Fowler JF, Duh MS, Rovba L, Buteau S, Pinheiro L, Lobo F, Sung J, Doyle JJ, Swensen A, Mallett DA, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol 2008; 59:772 - 80; http://dx.doi.org/10.1016/j.jaad.2008.06.043; PMID: 19119095
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, Gould C, Gemmen E, Dall T, American Academy of Dermatology Association, Society for Investigative Dermatology. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 2006; 55:490 - 500; http://dx.doi.org/10.1016/j.jaad.2006.05.048; PMID: 16908356
- Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, et al, Collaborative Association Study of Psoriasis (CASP), Genetic Analysis of Psoriasis Consortium, Psoriasis Association Genetics Extension, Wellcome Trust Case Control Consortium 2. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet 2012; 44:1341 - 8; http://dx.doi.org/10.1038/ng.2467; PMID: 23143594
- Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol 2005; 5:699 - 711; http://dx.doi.org/10.1038/nri1689; PMID: 16138103
- Brandrup F, Hauge M, Henningsen K, Eriksen B. Psoriasis in an unselected series of twins. Arch Dermatol 1978; 114:874 - 8; http://dx.doi.org/10.1001/archderm.1978.01640180008002; PMID: 566529
- Duffy DL, Spelman LS, Martin NG. Psoriasis in Australian twins. J Am Acad Dermatol 1993; 29:428 - 34; http://dx.doi.org/10.1016/0190-9622(93)70206-9; PMID: 8349859
- Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol 1985; 13:450 - 6; http://dx.doi.org/10.1016/S0190-9622(85)70188-0; PMID: 4056119
- Russell TJ, Schultes LM, Kuban DJ. Histocompatibility (HL-A) antigens associated with psoriasis. N Engl J Med 1972; 287:738 - 40; http://dx.doi.org/10.1056/NEJM197210122871503; PMID: 5056734
- Schmitt-Egenolf M, Eiermann TH, Boehncke WH, Ständer M, Sterry W. Familial juvenile onset psoriasis is associated with the human leukocyte antigen (HLA) class I side of the extended haplotype Cw6-B57-DRB1*0701-DQA1*0201-DQB1*0303: a population- and family-based study. J Invest Dermatol 1996; 106:711 - 4; http://dx.doi.org/10.1111/1523-1747.ep12345600; PMID: 8618009
- Elder JT, Nair RP, Henseler T, Jenisch S, Stuart P, Chia N, Christophers E, Voorhees JJ. The genetics of psoriasis 2001: the odyssey continues. Arch Dermatol 2001; 137:1447 - 54; http://dx.doi.org/10.1001/archderm.137.11.1447; PMID: 11708947
- Mallon E, Newson R, Bunker CB. HLA-Cw6 and the genetic predisposition to psoriasis: a meta-analysis of published serologic studies. J Invest Dermatol 1999; 113:693 - 5; http://dx.doi.org/10.1046/j.1523-1747.1999.00724.x; PMID: 10504461
- Elder JT. PSORS1: linking genetics and immunology. J Invest Dermatol 2006; 126:1205 - 6; http://dx.doi.org/10.1038/sj.jid.5700357; PMID: 16702966
- Nair RP, Stuart PE, Nistor I, Hiremagalore R, Chia NVC, Jenisch S, Weichenthal M, Abecasis GR, Lim HW, Christophers E, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet 2006; 78:827 - 51; http://dx.doi.org/10.1086/503821; PMID: 16642438
- Asumalahti K, Ameen M, Suomela S, Hagforsen E, Michaëlsson G, Evans J, Munro M, Veal C, Allen M, Leman J, et al. Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. J Invest Dermatol 2003; 120:627 - 32; http://dx.doi.org/10.1046/j.1523-1747.2003.12094.x; PMID: 12648227
- Stuart PE, Nair RP, Ellinghaus E, Ding J, Tejasvi T, Gudjonsson JE, Li Y, Weidinger S, Eberlein B, Gieger C, et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat Genet 2010; 42:1000 - 4; http://dx.doi.org/10.1038/ng.693; PMID: 20953189
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng B-J, et al, Collaborative Association Study of Psoriasis. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet 2009; 41:199 - 204; http://dx.doi.org/10.1038/ng.311; PMID: 19169254
- Riveira-Munoz E, He S-M, Escaramís G, Stuart PE, Hüffmeier U, Lee C, Kirby B, Oka A, Giardina E, Liao W, et al. Meta-analysis confirms the LCE3C_LCE3B deletion as a risk factor for psoriasis in several ethnic groups and finds interaction with HLA-Cw6. J Invest Dermatol 2011; 131:1105 - 9; http://dx.doi.org/10.1038/jid.2010.350; PMID: 21107349
- de Cid R, Riveira-Munoz E, Zeeuwen PLJM, Robarge J, Liao W, Dannhauser EN, Giardina E, Stuart PE, Nair R, Helms C, et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet 2009; 41:211 - 5; http://dx.doi.org/10.1038/ng.313; PMID: 19169253
- York IA, Chang S-C, Saric T, Keys JA, Favreau JM, Goldberg AL, Rock KL. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8-9 residues. Nat Immunol 2002; 3:1177 - 84; http://dx.doi.org/10.1038/ni860; PMID: 12436110
- Foerster J, Nolte I, Junge J, Bruinenberg M, Schweiger S, Spaar K, van der Steege G, Ehlert C, Mulder M, Kalscheuer V, et al. Haplotype sharing analysis identifies a retroviral dUTPase as candidate susceptibility gene for psoriasis. J Invest Dermatol 2005; 124:99 - 102; http://dx.doi.org/10.1111/j.0022-202X.2004.23504.x; PMID: 15654959
- Lai OY, Chen H, Michaud HA, Hayashi G, Kuebler PJ, Hultman GK, Ariza ME, Williams MV, Batista MD, Nixon DF, et al. Protective effect of human endogenous retrovirus K dUTPase variants on psoriasis susceptibility. J Invest Dermatol 2012; 132:1833 - 40; http://dx.doi.org/10.1038/jid.2012.69; PMID: 22437317
- Ariza M-E, Williams MV. A human endogenous retrovirus K dUTPase triggers a TH1, TH17 cytokine response: does it have a role in psoriasis?. J Invest Dermatol 2011; 131:2419 - 27; http://dx.doi.org/10.1038/jid.2011.217; PMID: 21776007
- Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest 1996; 98:1878 - 87; http://dx.doi.org/10.1172/JCI118989; PMID: 8878440
- Kaffenberger BH, Wong HK, Jarjour W, Andritsos LA. Remission of psoriasis after allogeneic, but not autologous, hematopoietic stem-cell transplantation. J Am Acad Dermatol 2013; 68:489 - 92; http://dx.doi.org/10.1016/j.jaad.2012.08.021; PMID: 22981608
- Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol 2009; 129:302 - 8; http://dx.doi.org/10.1038/jid.2008.225; PMID: 18685620
- Chu C-C, Di Meglio P, Nestle FO. Harnessing dendritic cells in inflammatory skin diseases. Semin Immunol 2011; 23:28 - 41; http://dx.doi.org/10.1016/j.smim.2011.01.006; PMID: 21295490
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu Y-J, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med 2005; 202:135 - 43; http://dx.doi.org/10.1084/jem.20050500; PMID: 15998792
- Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S, Fortugno P, Gonzalvo-Feo S, Franssen J-D, Parmentier M, et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med 2009; 206:249 - 58; http://dx.doi.org/10.1084/jem.20080129; PMID: 19114666
- Banas M, Zabieglo K, Kasetty G, Kapinska-Mrowiecka M, Borowczyk J, Drukala J, Murzyn K, Zabel BA, Butcher EC, Schroeder JM, et al. Chemerin is an antimicrobial agent in human epidermis. PLoS One 2013; 8:e58709; http://dx.doi.org/10.1371/journal.pone.0058709; PMID: 23527010
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang Y-H, Homey B, Cao W, Wang Y-H, Su B, Nestle FO, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007; 449:564 - 9; http://dx.doi.org/10.1038/nature06116; PMID: 17873860
- Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, Homey B, Barrat FJ, Zal T, Gilliet M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med 2009; 206:1983 - 94; http://dx.doi.org/10.1084/jem.20090480; PMID: 19703986
- Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science 2001; 294:1540 - 3; http://dx.doi.org/10.1126/science.1064890; PMID: 11711679
- Seung NR, Park EJ, Kim CW, Kim KH, Kim KJ, Cho HJ, Park HR. Comparison of expression of heat-shock protein 60, Toll-like receptors 2 and 4, and T-cell receptor gammadelta in plaque and guttate psoriasis. J Cutan Pathol 2007; 34:903 - 11; http://dx.doi.org/10.1111/j.1600-0560.2007.00756.x; PMID: 18001412
- Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol 1999; 162:3212 - 9; PMID: 10092772
- Ausiello CM, Fedele G, Palazzo R, Spensieri F, Ciervo A, Cassone A. 60-kDa heat shock protein of Chlamydia pneumoniae promotes a T helper type 1 immune response through IL-12/IL-23 production in monocyte-derived dendritic cells. Microbes Infect 2006; 8:714 - 20; http://dx.doi.org/10.1016/j.micinf.2005.09.007; PMID: 16460983
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Johnson-Huang LM, Nograles KE, White TR, Pierson KC, Lentini T, Suárez-Fariñas M, Lowes MA, et al. Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J Allergy Clin Immunol 2010; 125:1261 - , e98; http://dx.doi.org/10.1016/j.jaci.2010.03.018; PMID: 20471070
- Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin S-L, Nussbaum R, Novitskaya I, Carbonaro H, Cardinale I, Kikuchi T, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci U S A 2005; 102:19057 - 62; http://dx.doi.org/10.1073/pnas.0509736102; PMID: 16380428
- Gilliet M, Conrad C, Geiges M, Cozzio A, Thürlimann W, Burg G, Nestle FO, Dummer R. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol 2004; 140:1490 - 5; http://dx.doi.org/10.1001/archderm.140.12.1490; PMID: 15611427
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, Gonzalez J, Krueger JG, Lowes MA. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol 2009; 129:79 - 88; http://dx.doi.org/10.1038/jid.2008.194; PMID: 18633443
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 2011; 35:596 - 610; http://dx.doi.org/10.1016/j.immuni.2011.08.001; PMID: 21982596
- Tonel G, Conrad C, Laggner U, Di Meglio P, Grys K, McClanahan TK, Blumenschein WM, Qin J-Z, Xin H, Oldham E, et al. Cutting edge: A critical functional role for IL-23 in psoriasis. J Immunol 2010; 185:5688 - 91; http://dx.doi.org/10.4049/jimmunol.1001538; PMID: 20956338
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med 2004; 199:125 - 30; http://dx.doi.org/10.1084/jem.20030451; PMID: 14707118
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suárez-Fariñas M, Fuentes-Duculan J, Novitskaya I, Khatcherian A, Bluth MJ, Lowes MA, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 2007; 204:3183 - 94; http://dx.doi.org/10.1084/jem.20071094; PMID: 18039949
- Ariza M-E, Williams MV, Wong HK. Targeting IL-17 in psoriasis: from cutaneous immunobiology to clinical application. Clin Immunol 2013; 146:131 - 9; http://dx.doi.org/10.1016/j.clim.2012.12.004; PMID: 23314273
- Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med 2004; 199:731 - 6; http://dx.doi.org/10.1084/jem.20031482; PMID: 14981113
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010; 28:445 - 89; http://dx.doi.org/10.1146/annurev-immunol-030409-101212; PMID: 20192806
- Kanhere A, Hertweck A, Bhatia U, Gökmen MR, Perucha E, Jackson I, Lord GM, Jenner RG. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun 2012; 3:1268; http://dx.doi.org/10.1038/ncomms2260; PMID: 23232398
- Heydendael VMR, Spuls PI, Opmeer BC, de Borgie CA, Reitsma JB, Goldschmidt WF, Bossuyt PM, Bos JD, de Rie MA. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med 2003; 349:658 - 65; http://dx.doi.org/10.1056/NEJMoa021359; PMID: 12917302
- Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol 2008; 158:116 - 21; PMID: 17986302
- Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol 2003; 170:2153 - 60; PMID: 12574388
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003; 421:744 - 8; http://dx.doi.org/10.1038/nature01355; PMID: 12610626
- Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol 2007; 19:409 - 17; http://dx.doi.org/10.1016/j.smim.2007.10.011; PMID: 18053739
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006; 126:1121 - 33; http://dx.doi.org/10.1016/j.cell.2006.07.035; PMID: 16990136
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 2009; 129:1339 - 50; http://dx.doi.org/10.1038/jid.2009.59; PMID: 19322214
- Elloso MM, Gomez-Angelats M, Fourie AM. Targeting the Th17 pathway in psoriasis. J Leukoc Biol 2012; 92:1187 - 97; http://dx.doi.org/10.1189/jlb.0212101; PMID: 22962689
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 2008; 128:1207 - 11; http://dx.doi.org/10.1038/sj.jid.5701213; PMID: 18200064
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, et al. Induction of IL-17+ T cell trafficking and development by IFN-γ: mechanism and pathological relevance in psoriasis. J Immunol 2008; 181:4733 - 41; PMID: 18802076
- Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 2010; 130:1373 - 83; http://dx.doi.org/10.1038/jid.2009.399; PMID: 20032993
- Kato H, Fox DA. Are Th17 cells an appropriate new target in the treatment of rheumatoid arthritis?. Clin Transl Sci 2010; 3:319 - 26; http://dx.doi.org/10.1111/j.1752-8062.2010.00233.x; PMID: 21167010
- Krawczyk CM, Shen H, Pearce EJ. Functional plasticity in memory T helper cell responses. J Immunol 2007; 178:4080 - 8; PMID: 17371962
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6:1123 - 32; http://dx.doi.org/10.1038/ni1254; PMID: 16200070
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y-H, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6:1133 - 41; http://dx.doi.org/10.1038/ni1261; PMID: 16200068
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 2007; 8:950 - 7; http://dx.doi.org/10.1038/ni1497; PMID: 17676044
- Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol 2009; 160:319 - 24; http://dx.doi.org/10.1111/j.1365-2133.2008.08902.x; PMID: 19016708
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 2007; 8:942 - 9; http://dx.doi.org/10.1038/ni1496; PMID: 17676045
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-β are required for differentiation of human T(H)17 cells. Nature 2008; 454:350 - 2; http://dx.doi.org/10.1038/nature07021; PMID: 18469800
- Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol 2006; 176:1908 - 15; PMID: 16424222
- Awasthi A, Riol-Blanco L, Jäger A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol 2009; 182:5904 - 8; http://dx.doi.org/10.4049/jimmunol.0900732; PMID: 19414740
- Kao C-Y, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol 2004; 173:3482 - 91; PMID: 15322213
- Liang SC, Tan X-Y, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006; 203:2271 - 9; http://dx.doi.org/10.1084/jem.20061308; PMID: 16982811
- Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, Khatcherian A, Novitskaya I, Carucci JA, Bergman R, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol 2008; 181:7420 - 7; PMID: 18981165
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005; 22:285 - 94; http://dx.doi.org/10.1016/j.immuni.2005.01.011; PMID: 15780986
- Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, Zhang HH, Grinberg A, Love PE, Hwang ST, et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest 2009; 119:2317 - 29; http://dx.doi.org/10.1172/JCI37378; PMID: 19662682
- Mabuchi T, Chang TW, Quinter S, Hwang ST. Chemokine receptors in the pathogenesis and therapy of psoriasis. J Dermatol Sci 2012; 65:4 - 11; http://dx.doi.org/10.1016/j.jdermsci.2011.11.007; PMID: 22177422
- Chiricozzi A, Guttman-Yassky E, Suárez-Fariñas M, Nograles KE, Tian S, Cardinale I, Chimenti S, Krueger JG. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 2011; 131:677 - 87; http://dx.doi.org/10.1038/jid.2010.340; PMID: 21085185
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity 2004; 21:241 - 54; http://dx.doi.org/10.1016/j.immuni.2004.07.007; PMID: 15308104
- Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005; 174:3695 - 702; PMID: 15749908
- Wolk K, Witte E, Wallace E, Döcke W-D, Kunz S, Asadullah K, Volk H-D, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 2006; 36:1309 - 23; http://dx.doi.org/10.1002/eji.200535503; PMID: 16619290
- Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, Kunz S, Döcke W-D, Asadullah K, Volk H-D, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol 2009; 39:3570 - 81; http://dx.doi.org/10.1002/eji.200939687; PMID: 19830738
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330 - 6; http://dx.doi.org/10.1038/ni904; PMID: 12612578
- Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4(+)CD25(+)T cells: multiple pathways on the road. J Cell Physiol 2007; 211:590 - 7; http://dx.doi.org/10.1002/jcp.21001; PMID: 17311282
- Chang X, Gao JX, Jiang Q, Wen J, Seifers N, Su L, Godfrey VL, Zuo T, Zheng P, Liu Y. The Scurfy mutation of FoxP3 in the thymus stroma leads to defective thymopoiesis. J Exp Med 2005; 202:1141 - 51; http://dx.doi.org/10.1084/jem.20050157; PMID: 16230479
- Wildin RS, Freitas A. IPEX and FOXP3: clinical and research perspectives. J Autoimmun 2005; 25:Suppl 56 - 62; http://dx.doi.org/10.1016/j.jaut.2005.04.008; PMID: 16243487
- Su H, Longhi MS, Wang P, Vergani D, Ma Y. Human CD4+CD25(high)CD127 (low/neg) regulatory T cells. Methods Mol Biol 2012; 806:287 - 99; http://dx.doi.org/10.1007/978-1-61779-367-7_20; PMID: 22057460
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701 - 11; http://dx.doi.org/10.1084/jem.20060772; PMID: 16818678
- Chan DV, Somani A-K, Young AB, Massari JV, Ohtola J, Sugiyama H, Garaczi E, Babineau D, Cooper KD, McCormick TS. Signal peptide cleavage is essential for surface expression of a regulatory T cell surface protein, leucine rich repeat containing 32 (LRRC32). BMC Biochem 2011; 12:27; http://dx.doi.org/10.1186/1471-2091-12-27; PMID: 21615933
- Ishikawa D, Okazawa A, Corridoni D, Jia LG, Wang XM, Guanzon M, Xin W, Arseneau KO, Pizarro TT, Cominelli F. Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn’s disease. Mucosal Immunol 2013; 6:267 - 75; http://dx.doi.org/10.1038/mi.2012.67; PMID: 22785225
- Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol 2005; 174:164 - 73; PMID: 15611238
- Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC, Mieli-Vergani G, Vergani D, Longhi MS. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology 2013; Forthcoming http://dx.doi.org/10.1002/hep.26583; PMID: 23787765
- Glick AB, Wodzinski A, Fu P, Levine AD, Wald DN. Impairment of regulatory T-cell function in autoimmune thyroid disease. Thyroid 2013; 23:871 - 8; http://dx.doi.org/10.1089/thy.2012.0514; PMID: 23379353
- Garg G, Tyler JR, Yang JH, Cutler AJ, Downes K, Pekalski M, Bell GL, Nutland S, Peakman M, Todd JA, et al. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. J Immunol 2012; 188:4644 - 53; http://dx.doi.org/10.4049/jimmunol.1100272; PMID: 22461703
- Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJPM. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol 2011; 131:1853 - 60; http://dx.doi.org/10.1038/jid.2011.139; PMID: 21654831
- Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol 2009; 183:3170 - 6; http://dx.doi.org/10.4049/jimmunol.0803721; PMID: 19648274
- Huang Z, Yang B, Shi Y, Cai B, Li Y, Feng W, Fu Y, Luo L, Wang L. Anti-TNF-α therapy improves Treg and suppresses Teff in patients with rheumatoid arthritis. Cell Immunol 2012; 279:25 - 9; http://dx.doi.org/10.1016/j.cellimm.2012.09.001; PMID: 23059810
- Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J Exp Med 2007; 204:33 - 9; http://dx.doi.org/10.1084/jem.20061531; PMID: 17200409
- Soler DCD, McCormick TS. The dark side of regulatory T cells in psoriasis. J Invest Dermatol 2011; 131:1785 - 6; http://dx.doi.org/10.1038/jid.2011.200; PMID: 21844929
- Li WH, Gu Z, Wang H, Nekrutenko A. Evolutionary analyses of the human genome. Nature 2001; 409:847 - 9; http://dx.doi.org/10.1038/35057039; PMID: 11237007
- Pérot P, Mugnier N, Montgiraud C, Gimenez J, Jaillard M, Bonnaud B, Mallet F. Microarray-based sketches of the HERV transcriptome landscape. PLoS One 2012; 7:e40194; http://dx.doi.org/10.1371/journal.pone.0040194; PMID: 22761958
- Tristem M. Identification and characterization of novel human endogenous retrovirus families by phylogenetic screening of the human genome mapping project database. J Virol 2000; 74:3715 - 30; http://dx.doi.org/10.1128/JVI.74.8.3715-3730.2000; PMID: 10729147
- Molès J-P, Tesniere A, Guilhou J-J. A new endogenous retroviral sequence is expressed in skin of patients with psoriasis. Br J Dermatol 2005; 153:83 - 9; http://dx.doi.org/10.1111/j.1365-2133.2005.06555.x; PMID: 16029331
- Bessis D, Molès JP, Basset-Séguin N, Tesniere A, Arpin C, Guilhou JJ. Differential expression of a human endogenous retrovirus E transmembrane envelope glycoprotein in normal, psoriatic and atopic dermatitis human skin. Br J Dermatol 2004; 151:737 - 45; http://dx.doi.org/10.1111/j.1365-2133.2004.06116.x; PMID: 15491412
- Lee SJ, Chinen J, Kavanaugh A. Immunomodulator therapy: monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins. J Allergy Clin Immunol 2010; 125:Suppl 2 S314 - 23; http://dx.doi.org/10.1016/j.jaci.2009.08.018; PMID: 20036416
- Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, Papp K, Zrubek J, Mudivarthy S, Mack M, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009; 60:976 - 86; http://dx.doi.org/10.1002/art.24403; PMID: 19333944
- Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, Woltering F, Stach C, Hoepken B, Arledge T, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014; 73:48 - 55; http://dx.doi.org/10.1136/annrheumdis-2013-203696; PMID: 23942868
- Gottlieb ABA, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, Chen F, Magliocco M, Krueger JG. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol 2005; 175:2721 - 9; PMID: 16081850
- Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica 1978; 157:238 - 44; http://dx.doi.org/10.1159/000250839; PMID: 357213
- Yeung H, Wan J, Van Voorhees AS, Callis Duffin K, Krueger GG, Kalb RE, Weisman JD, Sperber BR, Brod BA, Schleicher SM, et al. Patient-reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasis. J Am Acad Dermatol 2013; 68:64 - 72; http://dx.doi.org/10.1016/j.jaad.2012.06.035; PMID: 22846688
- Robertson MJ, Ritz J. Interleukin 12: Basic Biology and Potential Applications in Cancer Treatment. Oncologist 1996; 1:88 - 97; PMID: 10387973
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med 2009; 361:888 - 98; http://dx.doi.org/10.1056/NEJMra0707449; PMID: 19710487
- Blauvelt A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J Invest Dermatol 2008; 128:1064 - 7; http://dx.doi.org/10.1038/jid.2008.85; PMID: 18408745
- Gordon KB, Langley RG, Gottlieb AB, Papp KA, Krueger GG, Strober BE, Williams DA, Gu Y, Valdes JM. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol 2012; 132:304 - 14; http://dx.doi.org/10.1038/jid.2011.304; PMID: 22011907
- Lebwohl M, Leonardi C, Griffiths CEM, Prinz JC, Szapary PO, Yeilding N, Guzzo C, Li S, Hsu M-C, Strober B. Long-term safety experience of ustekinumab in patients with moderate-to-severe psoriasis (Part I of II): results from analyses of general safety parameters from pooled Phase 2 and 3 clinical trials. J Am Acad Dermatol 2012; 66:731 - 41; http://dx.doi.org/10.1016/j.jaad.2011.06.011; PMID: 21930328
- Gordon KB, Papp KA, Langley RG, Ho V, Kimball AB, Guzzo C, Yeilding N, Szapary PO, Fakharzadeh S, Li S, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol 2012; 66:742 - 51; http://dx.doi.org/10.1016/j.jaad.2011.06.041; PMID: 21978572
- Reich K, Langley RG, Lebwohl M, Szapary P, Guzzo C, Yeilding N, Li S, Hsu M-C, Griffiths CEM. Cardiovascular safety of ustekinumab in patients with moderate to severe psoriasis: results of integrated analyses of data from phase II and III clinical studies. Br J Dermatol 2011; 164:862 - 72; http://dx.doi.org/10.1111/j.1365-2133.2011.10257.x; PMID: 21332467
- Laws PM, Young HS. Current and emerging systemic treatment strategies for psoriasis. Drugs 2012; 72:1867 - 80; http://dx.doi.org/10.2165/11634980-000000000-00000; PMID: 22938141
- Papp KAK, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 2012; 366:1181 - 9; http://dx.doi.org/10.1056/NEJMoa1109017; PMID: 22455412
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012; 366:1190 - 9; http://dx.doi.org/10.1056/NEJMoa1109997; PMID: 22455413
- Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, Haemmerle S, Thurston HJ, Papavassilis C, Richards HB. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol 2013; 168:412 - 21; http://dx.doi.org/10.1111/bjd.12110; PMID: 23106107
- Punwani N, Scherle P, Flores R, Shi J, Liang J, Yeleswaram S, Levy R, Williams W, Gottlieb A. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol 2012; 67:658 - 64; http://dx.doi.org/10.1016/j.jaad.2011.12.018; PMID: 22281165
- O’shea JJ. Targeting the Jak/STAT pathway for immunosuppression. Ann Rheum Dis 2004; 63:Suppl 2 ii67 - 71; PMID: 15479876
- Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, Gupta P, Krishnaswami S, Tan H, Harness JA. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol 2012; 167:668 - 77; http://dx.doi.org/10.1111/j.1365-2133.2012.11168.x; PMID: 22924949
- Mamolo C, Harness J, Tan H, Menter A. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-blind, placebo-controlled study in patients with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol 2013; Forthcoming PMID: 23294276
- Andrés RM, Montesinos MC, Navalón P, Payá M, Terencio MCNF. NF-κB and STAT3 inhibition as a therapeutic strategy in psoriasis: in vitro and in vivo effects of BTH. J Invest Dermatol 2013; 133:2362 - 71; http://dx.doi.org/10.1038/jid.2013.182; PMID: 23594598
- Zhang S, Suvannasankha A, Crean CD, White VL, Johnson A, Chen C-S, Farag SS. OSU-03012, a novel celecoxib derivative, is cytotoxic to myeloma cells and acts through multiple mechanisms. Clin Cancer Res 2007; 13:4750 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-07-0136; PMID: 17699852
- Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L, Bartlett JB, Loveland MA, Gilhar A, Cheung YF, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol 2010; 159:842 - 55; http://dx.doi.org/10.1111/j.1476-5381.2009.00559.x; PMID: 20050849
- Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, Hu C, Day RM. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet 2012; 380:738 - 46; http://dx.doi.org/10.1016/S0140-6736(12)60642-4; PMID: 22748702
- Papp KA, Kaufmann R, Thaçi D, Hu C, Sutherland D, Rohane P. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. J Eur Acad Dermatol Venereol 2013; 27:e376 - 83; http://dx.doi.org/10.1111/j.1468-3083.2012.04716.x; PMID: 23030767
- Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, Hu C, Stevens R, de Vlam KL. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2012; 64:3156 - 67; http://dx.doi.org/10.1002/art.34627; PMID: 22806399
- Gudjonsson JE, Johnston A, Ellis CN. Novel systemic drugs under investigation for the treatment of psoriasis. J Am Acad Dermatol 2012; 67:139 - 47; http://dx.doi.org/10.1016/j.jaad.2011.06.037; PMID: 22305044
- Balagon MV, Tan PL, Prestidge R, Cellona RV, Abalos RM, Tan EV, Walsh GP, Watson JD, Walsh DS. Improvement in psoriasis after intradermal administration of delipidated, deglycolipidated Mycobacterium vaccae (PVAC): results of an open-label trial. Clin Exp Dermatol 2001; 26:233 - 41; http://dx.doi.org/10.1046/j.1365-2230.2001.00804.x; PMID: 11422164
- Lehrer A, Bressanelli A, Wachsmann V, Bottasso O, Bay ML, Singh M, Stanford C, Stanford J. Immunotherapy with Mycobacterium vaccae in the treatment of psoriasis. FEMS Immunol Med Microbiol 1998; 21:71 - 7; http://dx.doi.org/10.1111/j.1574-695X.1998.tb01151.x; PMID: 9657323
- Netto EM, Takahashi D, de Fátima Paim de Oliveira M, Barbosa P, Ferraz N, Paixão A, Oyafuso LK, Bortoletto C, Matos D, Paixão M, et al. Phase II randomized, placebo-controlled trial of M. vaccae-derived protein (PVAC) for the treatment of psoriasis. Vaccine 2006; 24:5056 - 63; http://dx.doi.org/10.1016/j.vaccine.2006.03.047; PMID: 16621200
- Dalbeth N, Yeoman S, Dockerty JL, Highton J, Robinson E, Tan PL, Herman D, McQueen FM. A randomised placebo controlled trial of delipidated, deglycolipidated Mycobacterium vaccae as immunotherapy for psoriatic arthritis. Ann Rheum Dis 2004; 63:718 - 22; http://dx.doi.org/10.1136/ard.2003.007104; PMID: 15140780
- Rath N, Kar HK. Efficacy of intradermal heat-killed Mycobacterium w in psoriasis: a pilot study. Int J Dermatol 2003; 42:756 - 7; PMID: 12956699
- Kumar B, Sandhu K, Kaur I. Role of Mycobacterium w vaccine in the management of psoriasis. Br J Dermatol 2005; 152:380 - 2; http://dx.doi.org/10.1111/j.1365-2133.2005.06343.x; PMID: 15727665
- El-Darouti MA, Hegazy RA, Abdel Hay RM, Abdel Halim DM. Live attenuated varicella vaccine: a new effective adjuvant weapon in the battlefield against severe resistant psoriasis, a pilot randomized controlled trial. J Am Acad Dermatol 2012; 66:511 - 3; http://dx.doi.org/10.1016/j.jaad.2011.07.032; PMID: 22342019
- O’Daly JA, Lezama R, Rodriguez PJ, Silva E, Indriago NR, Peña G, Colorado I, Gleason J, Rodríguez B, Acuña L, et al. Antigens from Leishmania amastigotes induced clinical remission of psoriasis. Arch Dermatol Res 2009; 301:1 - 13; http://dx.doi.org/10.1007/s00403-008-0883-9; PMID: 18777031
- O’Daly JA, Gleason J, Lezama R, Rodriguez PJ, Silva E, Indriago NR. Antigens from Leishmania amastigotes inducing clinical remission of psoriatic arthritis. Arch Dermatol Res 2011; 303:399 - 415; http://dx.doi.org/10.1007/s00403-011-1133-0; PMID: 21328087
- Bay ML, Lehrer A, Bressanelli A, Morini J, Bottasso O, Stanford J. Psoriasis patients have T-cells with reduced responsiveness to common mycobacterial antigens. FEMS Immunol Med Microbiol 1998; 21:65 - 70; http://dx.doi.org/10.1111/j.1574-695X.1998.tb01150.x; PMID: 9657322
- Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, Volk HD, Döcke WD. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest 1998; 101:783 - 94; http://dx.doi.org/10.1172/JCI1476; PMID: 9466973
- Spohn G, Guler R, Johansen P, Keller I, Jacobs M, Beck M, Rohner F, Bauer M, Dietmeier K, Kündig TM, et al. A virus-like particle-based vaccine selectively targeting soluble TNF-alpha protects from arthritis without inducing reactivation of latent tuberculosis. J Immunol 2007; 178:7450 - 7; PMID: 17513796
- Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, Gottlieb AB, Etanercept Psoriasis Study Group. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003; 349:2014 - 22; http://dx.doi.org/10.1056/NEJMoa030409; PMID: 14627786
- Reich K, Nestle FO, Papp K, Ortonne J-P, Evans R, Guzzo C, Li S, Dooley LT, Griffiths CEM, EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005; 366:1367 - 74; http://dx.doi.org/10.1016/S0140-6736(05)67566-6; PMID: 16226614
- Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, Strober BE, Kaul M, Gu Y, Okun M, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58:106 - 15; http://dx.doi.org/10.1016/j.jaad.2007.09.010; PMID: 17936411
- Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB, PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665 - 74; http://dx.doi.org/10.1016/S0140-6736(08)60725-4; PMID: 18486739
