Abstract
Long-term safety is critical for the development and later use of a vaccine to prevent HIV/AIDS. Likewise, the persistence of vaccine-induced antibodies and their impact on HIV testing must be established. IAVI has sponsored several Phase I and IIA HIV vaccine trials enrolling healthy, HIV-seronegative African volunteers. Plasmid DNA and viral vector based vaccines were tested. No vaccine-related serious adverse events were reported. After completion of vaccine trials conducted between 2001–2007, both vaccine and placebo recipients were offered enrolment into an observational long-term follow-up study (LTFU) to monitor potential late health effects and persistence of immune responses. At scheduled 6-monthly clinic visits, a health questionnaire was administered; clinical events were recorded and graded for severity. Blood was drawn for HIV testing and cellular immune assays. 287 volunteers were enrolled; total follow-up after last vaccination was 1463 person years (median: 5.2 years). Ninety-three (93)% of volunteers reported good health at their last LTFU visit. Infectious diseases and injuries accounted for almost 50% of the 175 reported clinical events, of which over 95% were mild or moderate in severity. There were 30 six pregnancies, six incident HIV infections and 14 volunteers reported cases of social harm. Persistence of immune responses was rare. No safety signal was identified. No potentially vaccine-related medical condition, no immune mediated disease, or malignancy was reported. HIV vaccines studied in these trials had a low potential of induction of persisting HIV antibodies.
Introduction
Between 2001 and 2007, the International AIDS Vaccine Initiative (IAVI) sponsored 7 Phase I and IIA single- and multi-center HIV preventive vaccine clinical trials enrolling healthy HIV-uninfected African adults in East and South African countries. Two different recombinant plasmid DNA vaccines and three vaccines based on viral vectors (Modified vaccinia Ankara [MVA], Adeno-associated virus type 2 [AAV-2], and Adenovirus type 5 [Ad5]) were tested and found to be generally safe and well tolerated. No vaccine-related serious adverse events were reported.Citation1-Citation4
Once follow up in the respective vaccine trial ended, enrollment into a prospective, observational long-term follow-up (LTFU) study designed to monitor potential late health effects and persistence of humoral and cellular immune responses was offered to both vaccine and placebo recipients who previously participated in an IAVI-sponsored HIV vaccine study and had received at least one dose of investigational product.
Although IAVI supported the conduct of HIV vaccine clinical trials in Africa, Europe, India, and the USA, the LTFU study was implemented at African clinical research centers (CRCs) only, since local ethics and research committees had been supportive of following up study participants beyond the actual study period.
Here we present descriptive clinical data collected during LTFU, including types of clinical events and their severity, pregnancies and outcomes, reasons for hospitalization, frequency of new HIV infections, performance of HIV test kits to detect persisting vaccine-induced antibodies in HIV-uninfected volunteers who had a positive HIV test result at the final visit of the previous vaccine trial and data on persisting cellular immune responses. Frequency of events was compared between vaccine and placebo recipients. These data contribute to the long-term safety profile of HIV candidate vaccines and to the impact of vaccine-induced antibodies on HIV testing.
Results
From May 2007 through March 2010, 287 individuals (males: n = 196 [68.3%]; vaccine recipients = 222 [77.4%]) were enrolled into the LTFU study, which represents 83% of all participants from previous HIV vaccine trials conducted at the respective CRCs. ( and , ). There was no difference in vaccine to placebo ratio of volunteers enrolled into HIV vaccine trials and LTFU.
Table 1. Potential and Actual Enrollments, Study Identification Numbers and Vaccines Tested
Table 2. Demographics
Figure 1. CONSORT diagram showing the flow of participants from HIV vaccine trial participation through LTFU.
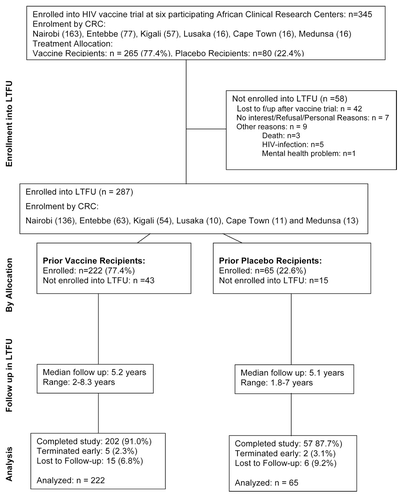
Reasons for non-enrolment of 58 participants were: (1) acquisition of HIV and follow-up in other prospective studies evaluating acute or recently acquired HIV-infection and/or referral for treatment and care provided by other in-country programs (n = 5); (2) relocation or loss to follow-up after end of vaccine trial (n = 42); (3) no interest (n = 7); (4) mental health problem (n = 1); (5) deaths (n = 3).
Two volunteers died during the vaccine trials (one was killed during riots, the other died of encephalitis, which was judged as unrelated to study vaccine and previously reportedCitation3). One volunteer (vaccine recipient) committed suicide approximately 1 y after his final vaccine study visit. It was later found out that he had suffered from severe depression since prior to enrolment into the HIV vaccine trial, but had not disclosed it during screening.
Two-hundred fifty-nine (259) volunteers completed the LTFU study; 7 terminated early and 21 were lost to follow. Total follow-up was 1463 person years since last vaccination (range: 1.8–8.3 y, median 5.2 y) (Table S1). Follow-up of study participants ended in March 2012. On average, volunteers completed 5 visits in LTFU (range: 1–8 visits, median: 4 visits). There was no difference in length of follow-up between vaccine and placebo recipients. ().
Results generated from health assessment questionnaire
93% of volunteers reported good health at their final visit in LTFU (no difference between vaccine and placebo recipients). ()
Table 3. Volunteers in Good Health at Final LTFU Visit
Clinical events and reasons for “not being in good health”
One hundred and 70 five (175) clinical events (CLE) were reported. Thirty-two (32) percent of vaccine and 35% of placebo recipients reported at least one CLE (P = 0.656 Chi-Square). More than 40% of all CLE were assigned to the Medical Dictionary for Regulatory Activities (MedDRA) System Organ class (SOC) Infections and Infestations. The frequency of clinical diagnoses or events assigned to other SOCs accounted for <10% each ().
Table 4. Clinical Events (CLE), by SOC
Of the 175 events, five were graded as severe and two as very severe; all others were mild (63.4%) or moderate (32.6%) (). One of the severe and both very severe events were due to trauma. The other 4 severe events were assigned to SOCs: (1) Infections and infestations (1 malaria, 1 HIV-infection); (2) Nervous system disorders (sciatic neuropathy); (3) Vascular disorders (gestational hypertension resulting in hospitalization). Of the 7 severe and very severe events, 5 occurred among the 222 vaccine recipients and two (trauma, vascular disorder) was among the 65 placebo recipients.
Table 5. Severity of Clinical Events (CLE), by SOC
The most common diagnoses (MedDRA Preferred Term, PT) under the SOC “Infections and infestations” were malaria, body tinea, and urinary tract infections (UTI). (Table S2: Clinical Events (CLE), by SOC and PT)
Hospitalizations
There were 17 non-pregnancy related hospitalizations, most were due to trauma or malaria. One female volunteer was hospitalized and subsequently diagnosed with Type II diabetes 3.5 y post last vaccination. (Table S3: Summary of Hospitalizations (non-pregnancy related), by SOC)
Pregnancies and congenital anomalies
Ninety one (91) women were enrolled in the LTFU study, of whom 34 (24 vaccine, 10 placebo recipients) became pregnant (36 pregnancies in total). There were 15 pregnancy-related hospitalizations reported for: (1) caesarian section (n = 8); (2) spontaneous abortion (n = 2); (3) ectopic pregnancy (n = 1); (4) gestational hypertension (n = 1); (5) normal vaginal delivery (n = 3). A placebo recipient delivered twins 4 y after last injection; one of the twins died at age 1 y due to severe malaria. Congenital anomalies were reported in 3 children born to women who had received different HIV vaccines: 2 of the 3 children had extra digits; one child was diagnosed at the age of 13 mo with arachnoid cysts and agenesis of corpus callosum. The latter event - judged as unrelated to study vaccine – was reported previously.Citation4
New/Incident HIV infections
Six volunteers acquired HIV after their last vaccination: One of 65 placebo recipients (1.54%, exact 95% CI: 0.04% to 8.28%) and 5/222 vaccine recipients (2.3%, exact 95% CI: 0.74% to 5.18%). Time from last vaccination to diagnosis of HIV infection ranged from 11–64 mo. Four volunteers had participated in study A002 (3 vaccine and 1 placebo recipient, all from a different CRC), one in study 009 and one in study 010. (For study identification numbers and vaccines tested, please see .)
Medication prescribed by physician and reported to be taken for >4 wk
The 3 most commonly prescribed medications belonged to the following categories: (1) antibiotics/anti-infectives/anti-parasite/anti-fungal/anti-inflammartory/anti-microbial; (2) analgesics/ NSAID; (3) antacids.
HIV test results and persistence of vaccine-induced antibodies
Follow-up of participants who received DNA.HIVA, MVA.HIVA, or AAV-2 HIV vaccines
Three vaccine recipients who had no detectable vaccine-induced antibodies during the respective vaccine trial had a onetime positive HIV test result during LTFU: (1) One MVA.HIVA recipient had a positive HIV test result by Abbot Determine approximately 4.5 y after last vaccination; (2) One AAV-2 HIV recipient had a positive HIV test result by Capillus 2.5 y after final vaccination; (3) Another AAV-2 HIV recipient had a positive HIV test results by Capillus (Rapid) and Murex (ELISA) 3 y after final vaccination. None of the three individuals was found to be HIV infected.
Follow-up of vaccine recipients from the V001 trial (VRC Ad5 alone or DNA prime-Ad5 boost)
During LTFU, there was no positive HIV test result by Rapid test kits in any vaccine recipient except one after a single dose of Ad5. He had a onetime positive HIV test result by Abbot Determine approximately 2.5 y after study vaccination; confirmatory tests were negative. He had no positive HIV test result at any time point during the HIV vaccine study.
There was no positive HIV test result by a 4th generation HIV ELISA test kit (used at one CRC only) in any recipient of Ad5 alone, while three of 29 recipients of DNA prime-Ad5 boost had a persistently positive HIV test result by Vironostika Uniform II Plus O until 4–4.5 y after the final study vaccination.
Persistence of cellular immune responses
Persistence of cellular immune responses was not evaluated in recipients of DNA.HIVA, MVA.HIVA, or AAV-2 vaccines due to moderate or poor immune responses at the final visit in the respective vaccine trial.
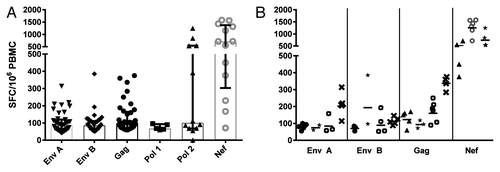
Sixty-seven vaccine recipients from the V001 trial (VRC Ad5 alone or DNA prime-Ad5 boost) provided PBMCs for assessment of IFN-ɣ ELISPOT responses. Twenty nine (29) of the 67 individuals had 4 or more visits in LTFU. Three of the 29 individuals had no ELISPOT responses at any visit, 20 had sporadic low magnitude responses to one or more of the vaccine-matched peptide pools at one or more visits, and 6 individuals had consistent responses to one or more of the vaccine-matched peptide pools over multiple time points. Of the 29 individuals with ELISPOT data from multiple time points, 23 (79%) had responses to either or both of the Env A or Env B peptide pools, 15 (52%) to Gag peptide pool, 7 (24%) to Pol and 4 (13%) to Nef. The average SFC/m PBMC were 103, 99, 130, 76, 336, and 794 respectively for Env A, Env B, Gag, Pol pool 1 and pool 2 and Nef peptides for all 29 individuals (). The six individuals who repeatedly showed positive responses over several time points were all in the DNA prime-Ad5 boost groups (3 each in the low (1010) and high dose (1011) Ad5 group, respectively, with the highest magnitude responses to Nef ().
Figure 2. ELISPOT Responses of 29 V001 Individuals with 4 or more LTFU visits. (A) Overall median SFC/m PBMC with interquartile ranges (positive responses only) (B) Data from 6 individuals illustrative of responses over time. Each set of symbols (e.g., circles or triangles) represents an individual, the scatterplots show the responses over 2–6 time points spanning the first visit at 1 y post last vaccine to 5 y post last vaccine. The geometric mean is shown by the bar (-).
Social harm
Social harm was reported by 14 volunteers. Misconceptions expressed by parents, partners, neighbors, and clinic staff from non-study sites, etc. included: (1) prevalent HIV infection was the reason for participation in the HIV vaccine trial; (2) volunteers were injected with either live HIV, animal vaccine, harmful substances, or microbes: (3) positive HIV test results due to vaccine-induced antibodies indicate prevalent HIV-infection.
Problems in vaccinated arm
Four events (mild itching, mild scar, occasional numbness, and transient pain) after receipt of MVA.HIVA (n = 2) and DNA prime-Ad5 boost (n = 2) were reported.
Discussion
The long-term follow up (LTFU) study was a prospective, observational study to monitor both vaccine and placebo recipients from previous HIV vaccine trials for any late health effects and the persistence of vaccine-induced immune responses. Placebo recipients were followed up as successfully as vaccine recipients. No significant or potentially vaccine-related medical problems have been detected. No autoimmune or potentially immune mediated disease and no malignancy have been reported in enrolled volunteers. These observations are consistent with previously published data on long-term safety of HIV candidate vaccines.Citation5 To our knowledge, our paper is the only one reporting on long-term safety of HIV vaccines in healthy African adults.
The most common clinical events reported were mild or moderate infectious diseases, and the proportion of volunteers with respective symptoms or conditions did not differ between vaccine and placebo recipients. This observation is consistent with data published on background morbidity, as assessed by unsolicited adverse events in clinical trial participants.Citation6
Three of the IAVI sponsored trials testing an MVA-based HIV vaccine began prior to, and one within a few weeks after the publication that, rarely, pericarditis/myocarditis may occur a few weeks after vaccination against smallpox.Citation7 No such event occurred in our HIV vaccine trials, at the peak time observed for recipients of the replication-competent DryVax®.Citation1,Citation2,Citation8 Therefore we did not look for late occurrences following our highly attenuated MVA. Surveillance in subsequent IAVI trials and a meta-analysis have shown no such events following MVA to date.Citation9-Citation11
No background data on congenital anomalies are available from the countries where the LTFU study was conducted. The types of anomalies observed are also known to occur in children born to women in industrialized countries and to women who have not participated in HIV vaccine trials. The numbers are too small to draw any conclusion on a teratogenic potential of the vaccines tested.
Six participants acquired HIV infection after the end of the respective vaccine trial. The proportions of vaccine and placebo recipients are similar. The number is too small to draw any conclusion as to whether or not receipt of an HIV candidate vaccine affects the susceptibility for HIV-acquisition. False positive HIV test results by HIV rapid test kits in volunteers with no (previously detected) vaccine-induced antibodies were spurious. Persistence of vaccine-induced sero-reactivity was demonstrated in a few VRC DNA prime-Ad5 boost recipients. Overall, the 4th generation HIV ELSA test kit was much more sensitive to detect vaccine-induced antibodies than HIV Rapid kits.Citation12 Therefore, use of rapid tests for standard HIV testing seems appropriate for use in non-research settings and likely eliminates issues associated with VISR in uninfected vaccines who have received the previously studied HIV vaccines.
Isolated incidents of social harm were reported and handled appropriately and efficiently by the volunteers themselves and/or study team members. None of the volunteers who did have vaccine-induced sero-reactivity (VISR) reported social harm. Nevertheless, concepts of HIV vaccine clinical research, such as, the potential of VISR, lack of protection against HIV-infection despite vaccine-induced antibodies and the need for continuous safe sexual practices were re-emphasized and reviewed with study participants, their partners, staff at study sites and at non-study clinics performing HIV tests, as appropriate. Community education is provided continuously by CRC staff members and/or members of the community advisory board (CAB) to address misconceptions about participation in clinical research in general, and in HIV vaccine clinical trials in particular, to alleviate potential social harm.
With an increasing number of trials of prophylactic HIV vaccines enrolling healthy, HIV-uninfected volunteers, the number of individuals who have VISR, but are not HIV-infected, is increasing. VISR varies depending on the HIV antigens in the vaccine construct, the immunogenicity of the vaccine, and the HIV test kits used. VISR may pose problems with discrimination or stigmatization in health care institutions, during ante-natal care, in blood banks or for organ donation. It is crucial to educate volunteers prior to and during their participation in HIV vaccine trials and ensure comprehension of the concept of VISR, assist them and offer long-term post trial follow-up with accurate HIV testing that can differentiate between VISR and natural HIV infection. Health care providers need to be made aware of this problem and trained appropriately.Citation13,Citation14 Collection and publication of VISR data may help alleviate the problem.
The benefit of participating in the LTFU study included general HIV counseling, HIV risk reduction counseling, regular assessments of health status, medical check-ups including children born to female participants and referrals for treatment and care as needed.
Conclusions
The summary analysis of clinical events reported during long-term follow-up is unremarkable. No safety signal has been identified. These data contribute to the long-term safety profile of HIV vaccines tested in healthy, HIV-seronegative African adults. For HIV vaccines studied to date, there was only a small potential for VISR, but current and future vaccines may induce stronger antibody responses that potentially pose difficulties for the individual volunteers, as they may be falsely identified as infected with HIV.
Materials and Methods
Six African CRCs that had conducted IAVI-sponsored HIV vaccine trials (6 double-blind, randomized, placebo-controlled trials and one open label roll-over trial) enrolling a total of 345 volunteers participated in the long-term follow-up LTFU study. Research staff contacted both, prior vaccine and placebo recipients and offered enrolment into the LTFU study. Two hundred 80 seven (287) volunteers provided written informed consent. Clinic visits were scheduled 6-moly. No investigational product was given during LTFU.
A standardized health questionnaire was administered asking for any health problems since the final visit in the vaccine trial, hospitalizations, new chronic diseases, or diseases lasting >4 wk, medications prescribed by a physician and taken for more than 4 wk, problems in the vaccinated arm(s), pregnancies and outcomes, and social harm. In case of (a history of) any complaints or any signs or symptoms, a symptom-directed physical exam was performed. Medical care was provided and/or referrals arranged, as appropriate. Current or anamnestic clinical events were recorded and graded for severity as per “DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events,” Version 6 October 2004, and categorized into System Organ class (SOC) and Preferred Term (PT) using the Medical Dictionary for Regulatory Activities (MedDRA) coding software.Citation15
Blood was drawn at each scheduled visit for HIV testing to detect incident HIV infection or to monitor persistence of vaccine-induced antibodies in HIV-uninfected vaccine recipients. Site-specific HIV testing algorithms used commercially available HIV test kits, including HIV Rapid tests (eg; UnigoldTM HIV [Trinity Biotech]; DetermineTM HIV-1/2 ([Abbot]; Capillus HIV-1/HIV-2 [Trinity Biotech]), 4th generation ELISA (e.g., Vironostika HIV Uniform II Plus O, BioMérieux), p24 Antigen ELISA and RNA HIV PCR (e.g., Roche Amplicor V1.5). Pre- and post HIV test counseling was performed. Individuals found to have acquired HIV infection were referred for care and treatment and followed in prospective IAVI studies looking at clinical, laboratory, immunological and viral markers of disease progression in recently HIV infected volunteers. Volunteers with VISR at the final LTFU study visit will be followed until HIV test results are negative.
Persistence of cellular immune responses was evaluated in selected volunteers who had a positive immune response by INF-γ ELISPOT at the final study visit after receipt of VRC Ad5 alone or VRC DNA prime-Ad5 boost.
IFN-γ ELISPOT assay
Peripheral blood mononuclear cells (PBMC) were isolated using density gradient separation from heparinized whole blood, frozen in a mixture of fetal bovine serum (Sigma-Aldrich) and DMSO (90:10 ratio), using a Kryo 560–16 rate controlled freezer (Planer) and stored in vapor phase liquid nitrogen.Citation16 PBMC were thawed, overnight rested and counted using a Vi-Cell XR counter (Beckman Coulter) for ELISPOT analyses using 6 HIV-1 15mer peptide pools, overlapping by 11 at 1.5 mg/mL per peptide, matching to subtype A; one pool each representing Gag, Pol/Int, RT, Nef and 2 pools representing the Env sequence were used as stimuli.Citation17 A peptide pool consisting of 9–10-mer peptides representing cytomegalovirus, Epstein Barr, and influenza virus (CEF), was used at 1.5 μg/mL, PHA at 10 μg/mL, and a mock stimulus (DMSO/medium), as previously described.Citation11
Spot forming cells (SFC) were counted using an automated AID ELISPOT reader (Autoimmun Diagnostika). The number of SFC/106 PBMC had to satisfy the following criteria: A positive ELISPOT was defined as: (1) an average background (mock)-subtracted count per peptide greater than 38 spot forming units (SFU) per 106 PBMCs with the coefficient of variation no greater than 70%; (2) mean count greater than 4x mean background (mock); and (3) mean background (mock) below 50 SFU/106 PBMCs. Assays with mean background >55 SFC/106 PBMC were considered failures. For any subject, if pre-vaccination ELISPOT responses had a value greater than 38 SFC/106, all subsequent responses to that peptide pool in that individual were considered cross-reactive and were not included in the frequency calculations.
No routine safety laboratory tests were performed.
Use of contraceptives was not required for this study and not recorded. Women who became pregnant and gave birth during or after the HIV vaccine trial or during LTFU were asked questions about the health status and medical history of their children and were invited to bring them for a physical exam at all visits. If medical care was needed, referrals were arranged, as appropriate.
The primary objective of this study was to describe clinical events reported by volunteers who received either vaccine or placebo in previous HIV vaccine studies, the proportion of volunteers who acquire HIV infection after the end of the HIV vaccine trial and the proportion of volunteers with persistent humoral or cellular immune responses.
| Abbreviations: | ||
| CLE | = | clinical event(s) |
| CRC(s) | = | clinical research center(s) |
| LTFU | = | long term follow-up |
| MedDRA | = | Medical Dictionary for Regulatory Activities |
| PBMCs | = | peripheral blood mononuclear cells |
| PT | = | preferred term |
| SOC | = | System Organ Class |
| VISR | = | vaccine-induced sero-reactivity |
Additional material
Download Zip (202.9 KB)Disclosure of Potential Conflicts of Interest
IAVI is a non-profit organization; neither IAVI nor IAVI-affiliated co-authors report any competing interests that may interfere with the objective assessment of this manuscript or with the ability to adhere to this journal’s policies on sharing data and materials. The affiliations of L.D. and C.S. do not alter the authors’ ability to adhere to this journal’s policies on sharing data and materials.
Funding
This study was funded by the International AIDS Vaccine Initiative and its donors, including the generous support of the American people through the United States Agency for International Development (USAID; USAID Cooperative Agreement Number GPO-A-00-06-00006-00). The contents of this manuscript are the responsibility of IAVI and do not necessarily reflect the views of USAID or the US government. The following organizations and institutions played a direct role in study design, data collection and analysis, decision to publish, and preparation of this manuscript: International AIDS Vaccine Initiative (IAVI), the Clinical Research Centers (KAVI, UVRI, PSF, Medunsa, DTHC, ZHERP), and the EMMES Corporation. The EMMES Corporation is a Contract Research Organization (CRO), to which IAVI subcontracted data coordination, management, and analysis for this study.
Acknowledgments
IAVI gratefully acknowledges the time and commitment from volunteers, study teams and all staff at the collaborating centers as well as the generous support of its donors to make this work possible. We thank the IAVI Clinical Program Managers Helen Thomson, James Sherwood, and Andrea van Lieven and the study monitors (Apolo Balyegisawa, Kundai Chinyenze, Mabela Matsoso) for their on-site support at the African Clinical Research Centers. We thank all staff from IAVI regulatory affairs, manufacturing, quality control, project teams, medical affairs, and laboratories.
Ethical Statement
The study protocol was approved by all responsible Ethics Committees and the study was conducted according to Declaration of Helsinki, ICH-GCP, and all applicable local regulations. All subjects provided written informed consent.
References
- Peters BS, Jaoko W, Vardas E, Panayotakopoulos G, Fast P, Schmidt C, Gilmour J, Bogoshi M, Omosa-Manyonyi G, Dally L, et al. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine 2007; 25:2120 - 7; http://dx.doi.org/10.1016/j.vaccine.2006.11.016; PMID: 17250931
- Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, Nanvubya A, Bashir F, Bhatt K, Ogutu H, Wakasiaka S, et al. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine 2008; 26:2788 - 95; http://dx.doi.org/10.1016/j.vaccine.2008.02.071; PMID: 18440674
- Vardas E, Kaleebu P, Bekker LG, Hoosen A, Chomba E, Johnson PR, Anklesaria P, Birungi J, Barin B, Boaz M, et al. A phase 2 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 vaccine based on adeno-associated virus. AIDS Res Hum Retroviruses 2010; 26:933 - 42; http://dx.doi.org/10.1089/aid.2009.0242; PMID: 20666584
- Jaoko W, Karita E, Kayitenkore K, Omosa-Manyonyi G, Allen S, Than S, Adams EM, Graham BS, Koup RA, Bailer RT, et al. Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One 2010; 5:e12873; http://dx.doi.org/10.1371/journal.pone.0012873; PMID: 20877623
- Gilbert PB, Chiu Y-L, Allen M, Lawrence DN, Chapdu C, Israel H, Holman D, Keefer MC, Wolff M, Frey SE, NIAID HIV Vaccine Trials Network. Long-term safety analysis of preventive HIV-1 vaccines evaluated in AIDS vaccine evaluation group NIAID-sponsored Phase I and II clinical trials. Vaccine 2003; 21:2933 - 47; http://dx.doi.org/10.1016/S0264-410X(03)00158-0; PMID: 12798637
- Schmidt C, Smith C, Barin B, Bakhtyari A, Bart PA, Bekker LG, Chomba E, Clumeck N, Ho D, Hoosen A, et al. Background morbidity in HIV vaccine trial participants from various geographic regions as assessed by unsolicited adverse events. Hum Vaccin Immunother 2012; 8:630 - 8; http://dx.doi.org/10.4161/hv.19454; PMID: 22634443
- Centers for Disease Control and Prevention (CDC). Cardiac adverse events following smallpox vaccination--United States, 2003. MMWR Morb Mortal Wkly Rep 2003; 52:248 - 50; PMID: 12680519
- Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program--United States, 2003. MMWR Morb Mortal Wkly Rep 2003; 52:492 - 6; PMID: 12809110
- Vasan S, Schlesinger SJ, Chen Z, Hurley A, Lombardo A, Than S, Adesanya P, Bunce C, Boaz M, Boyle R, et al. Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B’/C candidate vaccine. PLoS One 2010; 5:e8816; http://dx.doi.org/10.1371/journal.pone.0008816; PMID: 20111599
- Ramanathan VD, Kumar M, Mahalingam J, Sathyamoorthy P, Narayanan PR, Solomon S, Panicali D, Chakrabarty S, Cox J, Sayeed E, et al. A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS Res Hum Retroviruses 2009; 25:1107 - 16; http://dx.doi.org/10.1089/aid.2009.0096; PMID: 19943789
- Elizaga ML, Vasan S, Marovich MA, Sato AH, Lawrence DN, Chaitman BR, Frey SE, Keefer MC, MVA Cardiac Safety Working Group. Prospective surveillance for cardiac adverse events in healthy adults receiving modified vaccinia Ankara vaccines: a systematic review. PLoS One 2013; 8:e54407; http://dx.doi.org/10.1371/journal.pone.0054407; PMID: 23349878
- Poster # 164668: Karita E, Kayitesi K, Bayingama R, Sebahungu F, Bizimana J, Grabowski K, et al. Use of HIV rapid test for assessment of persistence of vaccine-induced antibodies among HIV vaccine recipients. AIDS Vaccine 2009, Paris, October 2009.
- Van Braeckel E, Koutsoukos M, Bourguignon P, Clement F, McNally L, Leroux-Roels G. Vaccine-induced HIV seropositivity: a problem on the rise. J Clin Virol 2011; 50:334 - 7; http://dx.doi.org/10.1016/j.jcv.2011.01.003; PMID: 21300566
- Cooper CJ, Metch B, Dragavon J, Coombs RW, Baden LR, NIAID HIV Vaccine Trials Network (HVTN) Vaccine-Induced Seropositivity (VISP) Task Force. Vaccine-induced HIV seropositivity/reactivity in noninfected HIV vaccine recipients. JAMA 2010; 304:275 - 83; http://dx.doi.org/10.1001/jama.2010.926; PMID: 20639561
- DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. . www.rsc-tech.com
- Boaz MJ, Hayes P, Tarragona T, Seamons L, Cooper A, Birungi J, Kitandwe P, Semaganda A, Kaleebu P, Stevens G, et al. Concordant proficiency in measurement of T-cell immunity in human immunodeficiency virus vaccine clinical trials by peripheral blood mononuclear cell and enzyme-linked immunospot assays in laboratories from three continents. Clin Vaccine Immunol 2009; 16:147 - 55; http://dx.doi.org/10.1128/CVI.00326-08; PMID: 19091991
- Keefer MC, Gilmour J, Hayes P, Gill D, Kopycinski J, Cheeseman H, Cashin-Cox M, Naarding M, Clark L, Fernandez N, et al. A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PLoS One 2012; 7:e41936; http://dx.doi.org/10.1371/journal.pone.0041936; PMID: 22870265
