?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The incidence of reported meningococcal disease in Italy is among the lowest in Europe. The trend of the disease was increasing up to 2005 and then declined after the gradual introduction of a universal Men C vaccination program in 17/21 Italian regions. Since 2006, in Emilia-Romagna region vaccination against Neisseria meningitidis serogroup C was actively offered free of charge in a single dose to the age groups 12–15 months and 14–15 years, in addition to people with defined epidemiological risk. Our aim was to measure the impact of vaccination on the incidence of meningococcal disease caused by different serogroups among the population of Emilia Romagna Region, Northern Italy (approximately 4.5 million inhabitants) subdivided by age. Using surveillance data, we computed the incidence rates of Neisseria meninigitidis related invasive disease per 100.000 inhabitants for the years 2000 to 2012. In addition, the percentage change in incidence and the mortality rates were calculated. Results indicate a 70.1% decrease in the incidence of meningococcus C-related invasive disease after the introduction of MenC universal vaccination. No case of serogroup C related infection was observed since 2006 in children aged 1–4 years. These findings suggest that the single-dose vaccination strategy against serogroup C N.meningitidis targeted to the age groups 12–15 months and 14–15 years was effective in the Emilia-Romagna population. However, the occurrence of two cases of meningiditis in a 5-month child and in a 9-years child suggests caution and careful consideration in surveillance for the next years.
Introduction
Neisseria meningitidis is a gram-negative diplococcus which normally colonizes the pharynx and upper respiratory tract without causing invasive disease. The point-prevalence carriage rate in Europe and the US has been estimated to range from 10 to 35% in young adults.Citation1 In a recent Italian study, meningococcal carriage prevalence was 2% in 583 university students.Citation2 Most isolates were nontypeable and none belonged to serogroup C. Only approximately 2% of students had received MenC conjugate vaccine. However, MenC vaccine coverage in younger children was 60% and the authors considered that a herd effect of vaccination on carriage could not be excluded.Citation2
Despite advances in antibiotic therapy, improvements in intensive care and the increasing use of vaccines, N.meningitidis continues to be a major cause of invasive disease, including meningitis, leading to increased morbidity, mortality, and neurological sequelae. This is particularly evident among young children and adolescents, both in developing and in developed countries.Citation3 The following six serogroups out of the 13 currently identified for N.meningitidis account for up to 90% of the infections: A, B, C, W135, X, and Y. The geographic distribution and epidemic potential differ according to different serogroups. In Europe, most cases of meningococcal diseases are caused by serogroups B and C and the incidence of laboratory-confirmed disease is 1/100.000 population.Citation4 In sub-Saharan Africa (the so-called Africa meningitis belt) explosive epidemics of meningococcal disease are experienced every 5 to 10 y and are usually caused by serogroup A (http://www.who.int/mediacentre/factsheets/fs141/en/, accessed October 2013).
Between 1999 and 2006, in response to the identification of relatively high endemic rates of serogroup C related disease, the United KingdomCitation5 and many European countries (Belgium, Iceland, Ireland, Netherlands, and Catalonia, Spain),Citation6-Citation8 introduced meningococcal serogroup C conjugate (MCC) vaccines as part of their national immunization programs.
In Germany, Portugal, Switzerland, and France, where the incidence of confirmed and probable cases of meningococcal disease remained low and stable during the 1999–2006 periodCitation9 routine vaccination with meningococcal C conjugate vaccines was not initially implemented but has now been introduced into the routine schedule.Citation10 Following the MCC, conjugated tetravalent vaccines, covering serogroups A, C, W135, and Y, were more recently licensed in USA, Canada, and Europe. However, due to the rather low incidence of meningococcal disease related to capsular groups A, W135, and Y in Europe, recommendations for the use of quadrivalent conjugate vaccine in Europe are still not uniform across the continent.
In Italy, a national monitoring program of invasive meningococcal diseases was set up in 1994. This program was modified in October 2006 to include surveillance of invasive bacterial caused by N. menigitidis, Streptococcus pneumoniae, and Hemophilus influenzae.Citation11 Although Italy has a low endemicity for meningococcal disease, MCC vaccination was recommended under the 2005–2007 National Vaccine Plan and, in 2011 17 out of the 21 regions had introduced universal vaccination.Citation12 Starting from 2003, in Tuscany (a region located in the Central Italy bordering Emilia Romagna with about 3.7 million inhabitants) MCC vaccine was offered to all subjects at risk, and from March 2005 the vaccine was universally offered free of charge to newborns (three doses at 3, 5, and 13 mo of age and in a single dose to unvaccinated children under 6 y of age in a catch-up program). This campaign proved to be successful in preventing meningococcal disease, with no cases observed since 2006 in vaccinated subjects and a herd immunity effect in the unvaccinated age groups.Citation13
Similarly, in Emilia-Romagna, a region located in North-eastern Italy with about 4 million inhabitants, MCC vaccine was offered to people at risk since 2003. Starting from 2006, it has been actively offered free of charge in a single dose to children aged 12–15 mo (together with vaccines against measles, mumps, and rubella) and adolescents aged 14–15 y (in conjunction with anti-diphtheria and tetanus active immunization). No catch-up strategy was implemented.
This paper examines the impact of the universal MCC vaccination campaign in Emilia-Romagna, and provides evidence on age-specific vaccine effectiveness.
Results
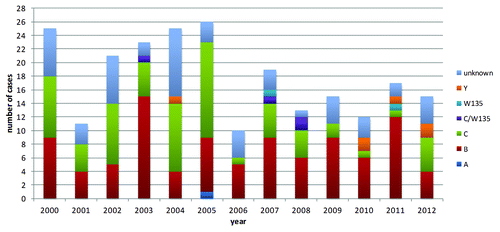
Surveillance data are shown in by infecting N. meningitidis serogroup for the years 2000–2012. From 2001 to 2005, a constant increase in the number of infections was detected, with a peak of 26 cases in 2005 and the main drop occurring soon after the implementation of the vaccine campaign in 2006. This decrement was followed by an increase of notified cases in 2007. This might be partially explained by an increased sensitivity of the surveillance system, that became fully implemented from 2007.
The vaccination coverage increased constantly since 2007 and reached 92.2% at 24 mo of age and 80.4% at 15–16 y of age in 2012 (). The vaccine effectiveness was estimated as 99.0%.
Table 1. Vaccination coverage with MCC vaccine at 24 mo of age and at 15–16 y. Emilia Romagna, 2007–2012
shows the incidence of N. meningiditis infection before and after the implementation of the vaccination campaign, and the percentage change in incidence between the two periods by age group and by infecting serogroup. The average incidence of meningitis caused by meningococcus declined from 0.54/100.000 (years 2000–2005) to 0.33/100.000 (years 2006–2012). The impact of MCC vaccination on the incidence of serogroup C related disease was remarkable, with a significant decline in the temporal trend since 2006 (Poisson regression, P = 0.038). The total number of notified serogroup C cases dropped from 51 in 2000–2005 to 19 in 2006–2012, corresponding to an overall change in incidence from 0.2/100.000 to 0.06/100.000 (–70.1%). This change reached –100% (no case notified) and -83.1% in the two target age groups 1–4 and 15–24 y.
Table 2. Incidence of invasive meningococcal disease in Emilia-Romagna before and after the implementation of the universal vaccination campaign against serogroup C, 2000–2012
Notably, no case of serogroup C infection was observed after the introduction of the MCC vaccination in the age group 1–4 y. However, after 6 y of herd protection in infants <1 y, one case of meningiditis was recorded in 2012 in a 5-mo child without risk factors who recovered completely from the disease.
The incidence of serogroup B infections declined overall by 9.1% (). However, this temporal variation was not significant (P = 0.988). The decline was observed in the age groups 1–4, 15–24, and 25–64 y, while a non-significant increase (from 0.16/100.000 to 0.35/100.000) occurred in the age group 5–14. In the age group <1 y, the incidence slightly increased over time (from 2.429/100.000 to 2.854/100.000).
The overall mortality rate for meningococcal diseases decreased from 0.616 to 0.264 per 1 000 000 across the two periods (). Eight deaths were observed in 2006–2012. Five of these cases, aged 9, 31, 32, 52, and 76 y, were caused by serogroup C and were not vaccinated. The remaining three deceased patients were infected by serogroup B; one (2 y old) was previously vaccinated for serogroup C, and the others (38 and 54 y old, respectively) were unvaccinated. None of them was suffering from specific pathologies or predisposing conditions. During the period 2006–2012, one vaccine failure was reported in a 17 y old boy, who was immunodepressed and developed N.meningitidis infection with mild clinical symptoms one year after vaccination.
Table 3. Mortality rate for the total population, for children aged 0–4 y and by serogroups before and after the implementation of the universal vaccination campaign against Serogroup C. Emilia-Romagna, 2000–2012
Discussion
This study evaluates the impact of a universal MCC vaccination program in the Emilia-Romagna Region, based on a single dose and targeted to children aged 12–15 mo and adolescents aged 14–15 y.
Our findings indicate that the immunization coverage reached very high values: 92.2% at 24 mo and 80.4% at 15–16 y of age in 2012. A high coverage was achieved because MCC vaccination at 12–15 mo was added to the existing universal vaccination schedule for measles, mumps, rubella and at 14–15 y to the vaccination schedule for diphtheria and tetanus and therefore was well accepted.Citation14 In fact, in 2012, coverage at 24 mo for MMR1 was 92.4% and coverage for dT booster in adolescence was 85.8%.
The observed decline of meningitis caused by N. meningiditis and particularly by serogroup C is consistent with the rates recently reported from Tuscany, where a clear drop of meningococcus C meningitis was also observed.Citation13 Specifically, in Tuscany the number of notified cases (all serogroups) in 2011 was 12, corresponding to 18% of cases of bacterial meningitis. Only 1 case of serogroup C invasive disease was reported in the age group 21–30 (not involved in the routine immunization program). This finding is noteworthy, given that the two regions are neighboring but the vaccine strategies adopted differed in the schedule and the target population. Provisional data from the same Region show that 18 overall cases occurred in 2012 (4 due to Men C), and confirm, as for the previous years, that no case occurred in vaccinated subjects and that, since 2010, no case occurred in subjects aged <20 y.
The slight fluctuations in the number of meningococcal C diseases registered both in Emilia Romagna and in Tuscany could be ascribed to the variable activity of respiratory viral infections (especially influenza) in different winter seasons. As a matter of fact, it is well known that viral infections can often pave the way to bacterial invasive diseases. However, the dramatic impact of routine vaccination programmes against Men C in spite of different target groups for the two Regions is highlighted by the immediate reduction to zero cases in immunized subjects, and by the constant incidence or only marginal decrease of meningococcal B invasive disease in the same time interval.
A recent reviewCitation15 classified the available vaccination regimens for meningococcus serogroup C into four strategies and summarized their pros and cons: (A) three doses during the first year of life (for instance Spain, Ireland, and UK – up to 2006), (B) two doses in the first year of life and another dose in the second, (C) one dose during the second year of life (with a catch-up campaign) (for instance, the Netherlands and Belgium), (D) one dose during the second year of life and a booster dose in teenaged.
With strategy (A), 98–100% of vaccinated children developed a protective antibody titer after the second dose.Citation16 Within 12–18 mo of vaccine introduction, a marked decline was observed in the number of cases and deaths caused by serogroup C disease and vaccine effectiveness was estimated as 90% or above in the age groups targeted by the vaccination campaign. However, while in children vaccinated in the catch-up campaign the vaccine effectiveness remained high, for those vaccinated in the routine infant immunization program, the effectiveness of the MCC vaccine fell to low levels after only 1 y.Citation17 Therefore, a new vaccination schedule was introduced in 2006, consisting of two doses administered at 3 and 4 mo of age and an additional dose at 12 mo. In 2013 the routine immunization schedule was further modified in the UK to include a primary dose at 3 mo and 2 booster doses at 13–14 mo and 14 y.Citation18 This strategy has also been endorsed by Spanish health care experts.Citation19
Strategy (B) integrated with a catch-up campaign in children aged 1–6 y proved to generate a long-term protection in Tuscany.Citation13 Additional earlier reports described the effectiveness of this scheduleCitation20,Citation21 although its low cost/benefit ratio lead the Tuscany Region to shift to the vaccination strategy based on a single dose at the second year of life (with continuing catch-up until 6 y) and in adolescents in 2008.
Strategy (C), based on epidemiological and cost/benefit studies, resulted in a consistent decrease in the number of cases of N. meningitidis related meningitis in the Netherlands, from 20 in 2000 to 1 in 2004.Citation6
Strategy (D) has not been adopted in Europe, but it was supported by preliminary evidence and simulations.Citation22 Recent results based on a cross-sectional survey in EnglandCitation23 using bio-banked serum samples supported the need of a booster dose at the age of 12 to deal with the increasing number of childhood population lacking anti-meningococcus specific individual protection. Evidence about the long-term protection of children vaccinated at the age 10 or above suggests that the titer of specific antibodies was higher than in those vaccinated before this age.Citation24 Moreover, data from a Dutch study also suggest that long-term persistence of vaccine-induced antibodies is age-dependent.Citation25
Taken together, these studies indicate that vaccination at teenage may be useful because children at that age may exhibit a stronger immunological response to vaccine than those detected among younger children, but further evidence supporting this hypothesis is warranted. In addition, adolescents seem to play a crucial role as main reservoir of meningococci, thus representing the main spreader of infection to other age groups.Citation26
The Emilia-Romagna region adopted a vaccination regimen that differs from those described in the cited paper.Citation15 Results indicate that a single dose administered to 2 different age groups with no catch-up proved to be effective in children of 1–4 y. It is important to underline that this finding was obtained with a very high vaccination coverage. The occurrence of two cases of mengiditis in a 5-mo child and in a 9 y old boy might be occasional, or might mean that herd protection in childhood is weaker if toddler and adolescent immunization is not followed by a catch up in older children. This aspect deserves further evaluation and a careful consideration in surveillance for the next years.
Another point to emphasize is that replacement of N.meningitidis serogroup B did not apparently occur since the implementation of the vaccine strategy. The number of serogroup B related cases slightly declined since the beginning of the MCC vaccination campaign, on average, in a way compatible with the natural fluctuations over time.
In conclusion, our vaccination strategy proved to be effective, but trends need to be examined over longer periods before drawing definite conclusions. Furthermore, when the two vaccinated cohorts will merge, a decision will need to be made about the administration of a booster dose during adolescence. Data from the international literature suggest that such booster is crucial in order to maintain the herd protection effect in the population.Citation26
The strengths of the current study include the completeness of the surveillance system and the single reference laboratory, which eliminates the bias related to variability in serotyping procedures.
In addition, there is an established electronic recording of vaccinations at Local Health Authority level that allows a reliable calculation of vaccination coverages. Still, although the surveillance system has been in place for the entire period of observation in our region, we cannot exclude a possible underestimation of cases due to lack of notification.
Concerning the larger applicability of the Emilia Romagna strategy, it should be considered that, as other authors have emphasized, decision-making about vaccination strategy has to be targeted to the ‘local epidemiology’ and the ‘changing epidemiology’ of meningococcal disease and it should be based on reliable surveillance data combined with vaccination coverage data. The development of new vaccines might open up new perspectives and help designing improved or new strategies against meningococcal infections.
Recently, the Italian national vaccination plan 2012–2014 has adopted the Emilia Romagna vaccination strategy for MCC and provides for an active and free of charge offer of the vaccination in a single dose to children of 13–15 mo of age and adolescents, in addition to people with selected risk conditions. This plan has currently been acknowledged by all Italian regions, but to date has not been completely and uniformly enforced. Thus the data reported in the present paper are not representative of the entire country.
The MCC vaccination is included among the essential health interventions (LEA, Livelli Essenziali di Assistenza): this implies that all the Regions are required to ensure its uniform provision and the necessary financial resources. Given the relatively low frequency of meningococcal invasive disease, and the absence of randomized trials to support specific schedules, observational studies like the present one provide useful evidence to decision makers in order to allow the best implementation of vaccination campaigns nationwide.
Methods
Data collection
In Emilia-Romagna, invasive diseases caused by bacterial agents (MIB) are monitored through the National Infectious Disease Information System,Citation11 a comprehensive, passive, and compulsory surveillance system. Surveillance records are filled in by the Department of Public Health of the Local Health Authority, which collects all the information from clinical services and diagnostic laboratories. Data reported in this paper are incident cases in the Region for the years 2000–2012. Immunization coverage is calculated on the resident population, although the vaccination campaign is directed to the whole population present in the territory. Coverage is defined as the percentage of vaccinated individuals in the target age groups, over the resident population of the same age. Vaccine effectiveness was estimated by use of the following equation: VE = 1 – [PCV(1 – PPV)]/[(1 – PCV)PPV], where PCV is the proportion of the cases of serogroup C disease in individuals who were vaccinated and PPV is the proportion of the population that was vaccinated (i.e., vaccine coverage).Citation27
Identification of the meningococcal serogroup
Based on clinical diagnosis of meningitis, meningococcal meningitis was defined by the presence of N. meningitidis (either detected by standard culture or by DNA amplification of a pathogen specific genomic target) in a cerebrospinal fluid (CSF) sample.
N. meningitidis strains isolated from blood and CSF specimens from MIB patients admitted to hospitals of Emilia-Romagna are collected and sent to Regional Reference Center for Microbiological Emergencies-CRREM, Unit of Clinical Microbiology, S. Orsola Hospital, Bologna. Identification of suspected colonies is obtained by the API NH test (Biomerieux), and the serogrouping is checked by the “Difco N. meningitidis antiserum” panel (Becton Dickenson and Company, BD). In case of a negative culture assay, the CSF specimens are analyzed by Real Time PCR: DNA was extracted by using an automated nucleic acids extractor (NucliSens EasyMag, Biomerieux) and the meningococcal DNA is detected by using the EuSepScreen Kit (Eurospital S.p.A). In case of a positive amplification for N. meningitides, the serogroup is detected by using the Neisseria meningitidis & Serogroup Real-Time PCR assay (DIA-Men Serogroup-020, Diagenode Diagnostics).
All notified cases of meningiditis were analyzed by the reference laboratory (CRREM) and all strains diagnosed in hospitals were confirmed.
Statistical analysis
The incidence of invasive meningococcal diseases was calculated for the years 2000–2012 as the number of cases per 100 000 population, by using data provided by the regional registry office for the denominators. The percentage change was calculated as follows:
, where x = incidence rate in 2000–2005, y = incidence rate in 2006–2012
Mortality rates per 1 000 000 population were calculated for all patients using surveillance data. The temporal variation of the incidence of meningococcal infection was modeled using Poisson regression. The introduction of the vaccination campaign was included in the model as a time-dependent covariate. Analyses were performed using STATA 10 software (StataCorp, www.stata.com).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Caugant DA, Maiden MC. Meningococcal carriage and disease--population biology and evolution. Vaccine 2009; 27:Suppl 2 B64 - 70; http://dx.doi.org/10.1016/j.vaccine.2009.04.061; PMID: 19464092
- Germinario C, Tafuri S, Napoli C, Montagna MT, Balducci MT, Fortunato F, Martinelli D, Prato R. Young-adult carriers of Neisseria meningitidis in Puglia (Italy): will the pattern of circulating meningococci change following the introduction of meningococcal serogroup C conjugate vaccines?. Hum Vaccin 2010; 6:1025 - 7; http://dx.doi.org/10.4161/hv.6.12.13145; PMID: 21150271
- Dickinson FO, Pérez AE, Cuevas IE. Meningococcal disease serogroup C. Risk Manag Healthc Policy 2012; 5:1 - 15; http://dx.doi.org/10.2147/RMHP.S12711; PMID: 22427737
- 2012 Annual Epidemiological Report, 6th Edition http://www.ecdc.europa.eu/en/publications/Publications/Annual-Epidemiological-Report-2012.pdf.
- Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. [Review] J Med Microbiol 2002; 51:717 - 22; PMID: 12358061
- de Greeff SC, de Melker HE, Spanjaard L, Schouls LM, van Derende A. Protection from routine vaccination at the age of 14 months with meningococcal serogroup C conjugate vaccine in the Netherlands. Pediatr Infect Dis J 2006; 25:79 - 80; http://dx.doi.org/10.1097/01.inf.0000195594.41449.c6; PMID: 16395110
- de Voer RM, Mollema L, Schepp RM, de Greeff SC, van Gageldonk PG, de Melker HE, Sanders EA, Berbers GA, van der Klis FR. Immunity against Neisseria meningitidis serogroup C in the Dutch population before and after introduction of the meningococcal c conjugate vaccine. PLoS One 2010; 5:e12144; http://dx.doi.org/10.1371/journal.pone.0012144; PMID: 20730091
- Martínez AI, Dominguez A, Oviedo M, Minguell S, Jansa JM, Codina G, Vazquez JA, Meningococcal Disease Working Group of Catalonia. Changes in the evolution of meningococcal disease, 2001-2008, Catalonia (Spain). Vaccine 2009; 27:3496 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.01.045; PMID: 19200816
- European Union Invasive Bacterial Infections Surveillance Network (EU-IBIS). Commission of the European Communities Programme on Community action on the prevention of AIDS and certain other communicable diseases. Invasive Neisseria meningitidis in Europe 2006. Available at: http://www.hpa-bioinformatics.org.uk/euibis/documents/2006_meningo.pdf (Accessed October 2013).
- Granoff DM, Harrison LH, Pelton S. Meningococcal vaccines. In: Plotkin SA, Orenstein WA, editors. Vaccines. 6th ed. Amsterdam: Saunders Elsevier; 2011.
- Protocollo per la sorveglianza nazionale delle malattie invasive da meningococco, pneumococco ed emofilo in Italia (12/3/2007). http://www.simi.iss.it/meningite_batterica.htm
- Alfonsi V, D’Ancona F, Giambi C, Nacca G, Rota MC, Regional Coordinators for Infectious Diseases and Vaccinations. Current immunization policies for pneumococcal, meningococcal C, varicella and rotavirus vaccinations in Italy. Health Policy 2011; 103:176 - 83; http://dx.doi.org/10.1016/j.healthpol.2011.10.002; PMID: 22030308
- Bechini A, Levi M, Boccalini S, Tiscione E, Balocchini E, Canessa C, Azzari C, Bonanni P. Impact on disease incidence of a routine universal and catch-up vaccination strategy against Neisseria meningitidis C in Tuscany, Italy. Vaccine 2012; 30:6396 - 401; http://dx.doi.org/10.1016/j.vaccine.2012.08.019; PMID: 22921931
- Coperture vaccinali nell’infanzia e nell’adolescenza – Anno 2012, Regione Emilia-Romagna – Assessorato Politiche per la Salute http://www.saluter.it/documentazione/rapporti/vaccinazioni_infanzia_settembre2013.pdf
- Chiappini E, Venturini E, Bonsignori F, Galli L, de Martino M. Serogroup C Neisseria meningitidis invasive infection: analysis of the possible vaccination strategies for a mass campaign. [Review] Acta Paediatr 2010; 99:1609 - 14; http://dx.doi.org/10.1111/j.1651-2227.2010.01908.x; PMID: 20545931
- Richmond P, Borrow R, Goldblatt D, Findlow J, Martin S, Morris R, Cartwright K, Miller E. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J Infect Dis 2001; 183:160 - 3; http://dx.doi.org/10.1086/317646; PMID: 11078484
- Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 2004; 364:365 - 7; http://dx.doi.org/10.1016/S0140-6736(04)16725-1; PMID: 15276396
- https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book.
- Borrow R, Abad R, Trotter C, van der Klis FR, Vazquez JA. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine 2013; 31:4477 - 86; http://dx.doi.org/10.1016/j.vaccine.2013.07.083; PMID: 23933336
- Hamlin J, Senthilnathan S, Bernstein HH. Update on universal childhood immunizations. Curr Opin Pediatr 2008; 20:483 - 9; http://dx.doi.org/10.1097/MOP.0b013e328306ebd1; PMID: 18622208
- Velentgas P, Bohn RL, Brown JS, Chan KA, Gladowski P, Holick CN, Kramer JM, Nakasato C, Spettell CM, Walker AM, et al. A distributed research network model for post-marketing safety studies: the Meningococcal Vaccine Study. Pharmacoepidemiol Drug Saf 2008; 17:1226 - 34; http://dx.doi.org/10.1002/pds.1675; PMID: 18956428
- De Wals P, Trottier P, Pépin J. Relative efficacy of different immunization schedules for the prevention of serogroup C meningococcal disease: a model-based evaluation. Vaccine 2006; 24:3500 - 4; http://dx.doi.org/10.1016/j.vaccine.2006.02.010; PMID: 16517032
- Ishola DA Jr., Borrow R, Findlow H, Findlow J, Trotter C, Ramsay ME. Prevalence of serum bactericidal antibody to serogroup C Neisseria meningitidis in England a decade after vaccine introduction. Clin Vaccine Immunol 2012; 19:1126 - 30; http://dx.doi.org/10.1128/CVI.05655-11; PMID: 22647271
- Snape MD, Kelly DF, Lewis S, Banner C, Kibwana L, Moore CE, Diggle L, John T, Yu LM, Borrow R, et al. Seroprotection against serogroup C meningococcal disease in adolescents in the United Kingdom: observational study. BMJ 2008; 336:1487 - 91; http://dx.doi.org/10.1136/bmj.39563.545255.AE; PMID: 18535032
- de Voer RM, Mollema L, Schepp RM, de Greeff SC, van Gageldonk PG, de Melker HE, Sanders EA, Berbers GA, van der Klis FR. Immunity against Neisseria meningitidis serogroup C in the Dutch population before and after introduction of the meningococcal c conjugate vaccine. PLoS One 2010; 5:e12144; http://dx.doi.org/10.1371/journal.pone.0012144; PMID: 20730091
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol 2009; 9:213 - 20; http://dx.doi.org/10.1038/nri2494; PMID: 19214194
- Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol 2010; 17:840 - 7; http://dx.doi.org/10.1128/CVI.00529-09; PMID: 20219881