Abstract
Bladder cancer is a common urologic malignancy with rising incidence in the elderly population. In most cases, bladder cancer is non-muscle-invasive at diagnosis and shows dramatically high recurrence rates, although current treatments often reduce the risk of disease progression. Immunotherapy using intravesical instillation of Bacillus Calmette-Guérin (BCG) remains the most effective therapy for patients with high risk tumors. However, BCG-therapy has important limitations including substantial adverse events and frequent treatment failure. Thus, it appears crucial to either improve or replace current therapy using new immunotherapeutic strategies. Here, we discuss the clinical trials that assessed therapeutic vaccination of bladder cancer patients using tumor associated antigens and we also argue for novel approaches arising from murine models. Vaccination routes to induce appropriate T-cell homing in the tumor site as well as the use of local immunostimulation to enhance recruitment of vaccine-induced T cells are discussed to highlight what we believe is a promising therapeutic vaccination strategy for patients with non-muscle-invasive bladder cancer.
Introduction
Bladder cancer is the fourth most common cancer in men both in Europe and USA.Citation1,Citation2 With a median age at diagnosis of 73 y, the incidence rates in patients over 65 y are high and rising, suggesting that the burden of this cancer will increase in countries where the population is aging.Citation3 Bladder cancer is three times more common in men than in women. Tobacco smoking is considered as the most important factor associated with bladder cancer but other causes include environmental/occupational exposure to aromatic amines, high arsenic levels in drinking water, or pelvic radiotherapy.Citation4
More than 90% of bladder cancers are transitional cell carcinomas, while the remaining are adenocarcinoma and squamous cell carcinoma. Approximately 70–80% of bladder tumors are superficial at initial diagnosis and confined to the mucosa (urothelium and/or lamina propria) therefore being termed as non-muscle-invasive bladder cancer (NMIBC). NMIBC consists of three stages: non-muscle-invasive papillary carcinoma (Ta), carcinoma in situ (CIS), and tumor invading the lamina propria/chorion (T1).Citation3Depending on tumor stage and grade characteristics the recurrence rate of these tumors can be 30 to 80%, with 1 to 45% (high risk patients) that will progress to muscle-invasive bladder cancer (stages T2 to T4) over a 5-y period.Citation5 Treatment/diagnostic of NMIBC is performed by transurethral resection of the bladder (TURB) possibly followed by intravesical chemotherapy or immunotherapy to avoid recurrence and/or progression. For patients with low or intermediate risk tumors, the European Association of Urology (EAU) guidelinesCitation5 recommend an immediate postoperative instillation of chemotherapeutic agent. Patients with high risk disease should receive a six weekly intravesical immunotherapy with Bacillus Calmette-Guérin (BCG), potentially completed by a yearly maintenance therapy. In some situations, this recommendation also applies to intermediate risk tumors. Muscle-invasive stages usually require radical cystectomy possibly preceded by neoadjuvant chemotherapy.
The Need to Improve Bladder Cancer Immunotherapy
The anti-tumor action of BCG is not fully understood, but instillation of BCG results in the induction of an inflammatory response. After the standard six weekly BCG instillations, different cell populations are recruited and found in the urine, mostly neutrophils (around 75% of cells in the urine), monocytes/macrophages, and T cells.Citation6 Importantly, both CD4 and CD8 T cells are essential for BCG-mediated antitumor activityCitation7 and it is largely accepted that a predominant Th1 immune response is associated with effective BCG therapy, while Th2 response is rather associated with BCG failure.Citation8-Citation13
But on the other side of the coin, the inflammatory response is also responsible for local and systemic adverse events. Indeed, the vast majority of patients undergoing BCG therapy experience mild side-effects, mostly cystitis-like symptoms with dysuria, urinary frequency or urgency, hematuria, as well as flu-like symptoms.Citation14 Less frequent but potentially severe complications can also occur in some patients and include bladder contracture, allergic reactions, high grade fever, and even life-threatening BCG sepsis (<0.4%).Citation14 Of note, BCG therapy is contraindicated in patients who are immunosuppressed—e.g., with untreated HIV infection—, have history of BCG sepsis, gross hematuria, traumatic catheterization, or active urinary tract infection.Citation15
Another significant limitation of BCG treatment is the lack of response in a substantial number of patients. Indeed, 20 to 50% of patients (depending on the definition, e.g., recurrence in the following 6 mo) fail to respond to BCG and show high risk of progression to muscle-invasive disease and death. Moreover about one-third of responders will recur in the following years.Citation16 The causes of BCG failure have been poorly investigated. A recent study showed that patients with recurrence after repeated BCG courses may have urothelial carcinoma in the upper urinary tract or in the prostatic urethra that had remained undetected by the urologist.Citation17 These tumors are not treated by intravesical BCG and can thus lead to disease recurrence. It was also recently reported that patients lacking response to purified protein derivative (PPD), i.e., patients with no sustained preexisting immunity to BCG (due to previous parenteral BCG vaccine or mycobacterial infection), showed a lower recurrence-free survival.Citation18 Furthermore, we are currently investigating whether BCG failure may also be due to an immunosuppressive tumor environment that may differ among patients and that may lead to inefficient immune responses to intravesical BCG therapy.
In conclusion, although BCG is still the most successful therapy for NMIBC to date, high incidence of adverse events and relatively high rate of treatment failures point up the crucial need to improve bladder cancer treatment strategies. Thus, further research is warranted to either improve (e.g., with combinatorial strategies) or replace BCG-therapy with less toxicity and/or better efficacy. Along this line, we are currently testing the efficacy and safety of other bacteria in murine bladder cancer models.
Therapeutic Vaccination: Clinical Trials in Bladder Cancer Patients
Vaccination is being investigated as a novel immunotherapeutic strategy to treat bladder cancer. This aims at inducing adaptive immunity as an alternative to the non-specific BCG-induced immunostimulation. One first question is the type of response that has to be induced. As shown in other tumor types,Citation19 the presence of intratumoral CD8 T cells is associated with better disease-free and overall survival rates in patients with muscle-invasive bladder cancer.Citation20 These important findings evoke a protective mechanism that may rely on potential tumor-specific T cells to confer a survival benefit. Hence, it appears important to design effective vaccine strategies that elicit CD8 T-cell responses.
What antigens?
Designing such a vaccine requires relevant target antigen(s) to be identified. Characteristics of ideal candidates include (1) high specificity of expression in cancer cells (no expression in healthy tissue) (2) high expression level and high frequency of cancer cells expressing the antigen, and (3) ability to elicit cellular immune responses in patients. In that respect, cancer-testis antigens (CTA) have been established as promising target candidates in vaccine development for patients with bladder cancer.Citation21 CTAs are a family of tumor-associated antigens (TAA) with both potent immunogenicity and restricted expression in various types of cancers and only in testicular germ cells in healthy individuals. In a screening of nine CTAs (NY-ESO-1, LAGE-1, MAGE-A1, MAGE-A3, MAGE-A4, MAGE-A10, CT7, CT10, and GAGE) in tumors samples from patients with bladder cancer, it has been reported that at least one CTA was expressed in tumors from most patients and about half of these tumors expressed ≥3 CTAs.Citation21
Clinical trials
To date, only few pilot clinical trials have been conducted to evaluate vaccine candidates for bladder cancer. These trials were conducted on a limited number of study patients and only in the context of advanced invasive bladder cancers.Citation22-Citation24 All tested vaccines were well tolerated with no significant adverse events in patients.
In a first study, a dendritic cell (DC)-based vaccine was assessed in four HLA-A24+ patients with advanced metastatic MAGE-A3+ bladder cancers. Following subcutaneous (SC) injections of autologous DCs pulsed with a HLA-A24-restricted MAGE-A3 peptide, it was reported that 3 of 4 patients showed reduction in the size of lymph node and/or liver metastases.Citation22
In another trial, immunogenicity of a protein-based vaccine was tested in six patients who underwent a cystectomy or nephroureterectomy.Citation23 Six weekly intradermal injections of NY-ESO-1 recombinant protein adjuvanted with BCG or GM-CSF (depending on the dose) induced vaccine-specific CD4 T cells in all patients, whereas only 1/6 patients had a detectable CD8 T-cell response. Antibody responses were detected in 5/6 patients.
More recently, Obara et al. identified two HLA-A24-restricted immunogenic peptides from two proteins, MPHOSPH1 (M phase phosphoprotein-1) and DEPDC1 (DEP domain containing-1 protein), involved in bladder carcinogenesis.Citation24 Six patients with advanced metastatic bladder cancers received SC injections of these peptides (with Freund’s adjuvant). Vaccine-specific CD8 T-cell responses were detected in 4 of 6 patients. Of note, the two “non-responding” patients had lower overall survival as compared with responders, yet the small number of enrolled patients does not allow to draw conclusions about the clinical benefits. Interestingly, MPHOSPH1 and DEPDC1 are expressed in both muscle-invasive and non-muscle-invasive bladder cancer cells, indicating that they are also attractive candidates for vaccination of NMIBC patients.Citation24
Therefore, although promising results were reported, additional trials in larger cohorts of patients are required to further correlate immune responses with clinical outcomes in bladder cancer patients. Of note, an ongoing Phase II trial is evaluating safety and efficacy of MAGE-A3 recombinant protein vaccination in patients following cystectomy (NCT01435356 in ClinicalTrials.gov).
Immunization Routes to Target the Bladder
In above-mentioned clinical trials, vaccine-induced immune responses were evaluated in peripheral blood. An effective vaccine needs certainly to elicit anti-tumor T-cell responses but what is the point in it if these effector cells never reach the tumor site?
The journey of vaccine-induced T cells
Trafficking of T cells toward various peripheral tissues is a multistage process that relies on tissue-specific signals allowing T cells to extravasate from the blood circulation and to enter and be retained in a particular tissue. The final destination depends on their “homing” program, i.e., a set of cell-surface receptors expressed on T-cells. Of note, a global switch occurs in the homing program of naïve T cells after their priming in a lymph node.Citation25 Therefore, vaccination can only be effective when the set of chemokine receptors and integrin molecules expressed on vaccine-induced effector T cells match the chemokines and cell-associated tissue-specific ligands expressed in the targeted tumor environment. In this respect, recent murine studies clearly demonstrated that the vaccination route can critically influence the localization—and thus efficacy—of vaccine-induced antitumor responses.Citation26 Sandoval et al. showed that a cancer vaccine delivered by the intranasal (IN) mucosal route was able to inhibit the growth of head and neck or lung cancers, while the same vaccine delivered by the intramuscular route was poorly effective.Citation27 Moreover, the authors demonstrated that IN immunization preferentially upregulated a particular set of integrins (CD49a) on CD8 T cells allowing their retention in the mucosal head and neck tumors. However superior efficacy of mucosal immunization may not always hold true for inducing regression of mucosal tumors as we recently reported in an orthotopic genital tumor model.Citation28 In this context, SC vaccination was more efficient to regress tumors as compared with IN or intravaginal (IVAG) mucosal routes.
Lessons from bladder cancer vaccination in mice
Since the immunization route seems to directly affect the mucosal homing program on induced CD8 T cells—and possibly the efficacy of vaccine to control mucosal tumors—, it is then of great interest to also compare different vaccination routes in the context of bladder cancer. We established an orthotopic murine bladder-tumor model expressing E7, an HPV tumor-antigen (no murine bladder cancer model expressing a tumor-antigen relevant in human is available). We vaccinated the mice with a previously developed E7-polypetide vaccineCitation28,Citation29 and evaluated which immunization routes efficiently induced E7-specific CD8 T cells infiltration in the bladder, as well as effective anti-tumor activity as assessed by subsequent tumor regression.Citation30
When comparing the SC, IVAG, and IN routes, we found that both SC and IVAG immunization can induce high number of vaccine-specific CD8 T cells in the bladder and—most importantly—bladder tumor regression. Surprisingly, while we and others found that IN immunization is effective at eliciting immune responses in the genital mucosa,Citation28,Citation31 this vaccination route, however, was inadequate to induce vaccine-specific T-cells in the bladder.Citation30 Our preliminary data suggests that different homing molecules are involved and that vaccine-specific CD8 T cells homing to the bladder may require a unique pattern of integrin/selectin to match the receptors/ligands expressed in the bladder.
Interestingly, the same SC vaccine was able to induce 90% regression of tumors located in the genital mucosaCitation28 vs. 50% for bladder tumors. This was associated with higher specific-CD8/Treg ratio in the genital mucosa, suggesting a more immunosuppressive environment in the bladder, which is in line with transcriptional studies showing a broadly tolerogenic transcriptional program following bacterial infection of the urinary tract.Citation32 Based on our observations, it seems to be more difficult to induce tumor regression in the bladder than in the genital mucosa. Thus, one needs to elaborate strategies to boost T-cell infiltration in the bladder following vaccination.
The Use of Local Immunostimulants
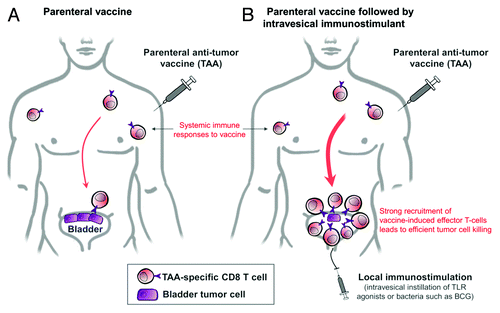
As single therapeutic approaches may turn to lack clinical efficacy, the use of combinatorial strategies has become an appealing alternative. In this respect, we recently described a novel approach in which vaccination was followed by local immunostimulation at the mucosal tumor site.Citation33 In mice, IVAG instillation of TLR agonists—CpG and poly-(I:C)—was found to dramatically increase number and frequency of both total and vaccine-specific CD8 T cells in the genital mucosa. Vaccine-induced systemic responses were not altered. In our murine model of genital cancer, vaccination combined with local CpG administration led to remarkably better regression of large established genital tumors as compared with vaccination alone.
The bladder also represents an ideal target for local therapies owing to its anatomical properties. Thus, we are now testing that same strategy in our bladder tumor model. Ongoing experiments indicate that intravesical immunostimulation with CpG after vaccination can similarly increase recruitment/retention of vaccine-specific CD8 T cells in the bladder. Besides, we showed that local immunostimulation can also be performed with bacteria.Citation34 Therefore, a strategy combining TAA vaccination with intravesical BCG immunostimulation may be of particular interest in patients with bladder cancer (). We are currently conducting a clinical trial aiming to assess how vaccination of NMIBC patients with the adjuvanted recombinant MAGE-A3 protein (5 doses every 3 wk) combined with subsequent standard intravesical BCG-therapy (weekly doses for 6 wk) may enhance innate and vaccine-specific T-cell responses both systemically and locally in the bladder (NCT01498172 in ClinicalTrials.gov).
Conclusion
Overall it seems to us that TAA vaccination using the SC route combined with local immunostimulation is a promising strategy for treatment of bladder cancer. New strategies have to be tested in large cohorts of NMIBC patients to shrink disease recurrence rates. Of note, bladder cancer is the most expensive malignancy to treat per-patient,Citation35 on account of a lifelong follow-up with multiple surgical procedures and high recurrence rates. Hence, it appears crucial to improve current standard therapies and worthwhile to increase funding for basic and clinical research in this field.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Swiss Cancer League (KFS 2808-08-2011 to D.N.H. and KLS 2744-02-2011 to L.D.) and the Swiss National Science Foundation (FNS 31003A-135109 to D.N.H. and FNS 32003B-146638/1 to L.D.).
References
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010; 46:765 - 81; http://dx.doi.org/10.1016/j.ejca.2009.12.014; PMID: 20116997
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10 - 29; http://dx.doi.org/10.3322/caac.20138; PMID: 22237781
- Nielsen ME, Smith AB, Meyer AM, Kuo TM, Tyree S, Kim WY, Milowsky MI, Pruthi RS, Millikan RC. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer 2014; 120:86 - 95; http://dx.doi.org/10.1002/cncr.28397; PMID: 24122346
- Kiriluk KJ, Prasad SM, Patel AR, Steinberg GD, Smith ND. Bladder cancer risk from occupational and environmental exposures. Urol Oncol 2012; 30:199 - 211; http://dx.doi.org/10.1016/j.urolonc.2011.10.010; PMID: 22385990
- Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J, Rouprêt M, European Association of Urology (EAU). EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 2011; 59:997 - 1008; http://dx.doi.org/10.1016/j.eururo.2011.03.017; PMID: 21458150
- De Boer EC, De Jong WH, Van Der Meijden AP, Steerenberg PA, Witjes JA, Vegt PD, Debruyne FM, Ruitenberg EJ. Presence of activated lymphocytes in the urine of patients with superficial bladder cancer after intravesical immunotherapy with bacillus Calmette-Guérin. Cancer Immunol Immunother 1991; 33:411 - 6; http://dx.doi.org/10.1007/BF01741603; PMID: 1878894
- Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol 1993; 150:1018 - 23; PMID: 8102183
- Riemensberger J, Böhle A, Brandau S. IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol 2002; 127:20 - 6; http://dx.doi.org/10.1046/j.1365-2249.2002.01734.x; PMID: 11882028
- Saint F, Patard JJ, Maille P, Soyeux P, Hoznek A, Salomon L, Abbou CC, Chopin DK. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette-Guerin treatment for superficial bladder cancer. J Urol 2002; 167:364 - 7; http://dx.doi.org/10.1016/S0022-5347(05)65469-9; PMID: 11743357
- de Reijke TM, de Boer EC, Kurth KH, Schamhart DH. Urinary cytokines during intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer: processing, stability and prognostic value. J Urol 1996; 155:477 - 82; http://dx.doi.org/10.1016/S0022-5347(01)66424-3; PMID: 8558640
- Nadler R, Luo Y, Zhao W, Ritchey JK, Austin JC, Cohen MB, O’Donnell MA, Ratliff TL. Interleukin 10 induced augmentation of delayed-type hypersensitivity (DTH) enhances Mycobacterium bovis bacillus Calmette-Guérin (BCG) mediated antitumour activity. Clin Exp Immunol 2003; 131:206 - 16; PMID: 12562379
- Luo Y, Chen X, O’Donnell MA. Role of Th1 and Th2 cytokines in BCG-induced IFN-gamma production: cytokine promotion and simulation of BCG effect. Cytokine 2003; 21:17 - 26; http://dx.doi.org/10.1016/S1043-4666(02)00490-8; PMID: 12668155
- Bockholt NA, Knudson MJ, Henning JR, Maymí JL, Weady P, Smith GJ 3rd, Eisenbraun MD, Fraser JD, O’Donnell MA, Luo Y. Anti-interleukin-10R1 monoclonal antibody enhances bacillus Calmette-Guérin induced T-helper type 1 immune responses and antitumor immunity in a mouse orthotopic model of bladder cancer. J Urol 2012; 187:2228 - 35; http://dx.doi.org/10.1016/j.juro.2012.01.030; PMID: 22503050
- Koya MP, Simon MA, Soloway MS. Complications of intravesical therapy for urothelial cancer of the bladder. J Urol 2006; 175:2004 - 10; http://dx.doi.org/10.1016/S0022-5347(06)00264-3; PMID: 16697786
- Kresowik TP, Griffith TS. Bacillus Calmette-Guerin immunotherapy for urothelial carcinoma of the bladder. Immunotherapy 2009; 1:281 - 8; http://dx.doi.org/10.2217/1750743X.1.2.281; PMID: 20046960
- Sylvester RJ. Bacillus Calmette-Guérin treatment of non-muscle invasive bladder cancer. Int J Urol 2011; 18:113 - 20; http://dx.doi.org/10.1111/j.1442-2042.2010.02678.x; PMID: 21091799
- Giannarini G, Birkhäuser FD, Recker F, Thalmann GN, Studer UE. Bacillus Calmette-Guérin Failure in Patients with Non-Muscle-invasive Urothelial Carcinoma of the Bladder May Be Due to the Urologist’s Failure to Detect Urothelial Carcinoma of the Upper Urinary Tract and Urethra. Eur Urol 2013; Forthcoming http://dx.doi.org/10.1016/j.eururo.2013.09.049; PMID: 24144432
- Biot C, Rentsch CA, Gsponer JR, Birkhäuser FD, Jusforgues-Saklani H, Lemaître F, Auriau C, Bachmann A, Bousso P, Demangel C, et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med 2012; 4:37ra72; http://dx.doi.org/10.1126/scitranslmed.3003586; PMID: 22674550
- Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy?. Lancet Oncol 2012; 13:e32 - 42; http://dx.doi.org/10.1016/S1470-2045(11)70155-3; PMID: 22225723
- Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A 2007; 104:3967 - 72; http://dx.doi.org/10.1073/pnas.0611618104; PMID: 17360461
- Sharma P, Shen Y, Wen S, Bajorin DF, Reuter VE, Old LJ, Jungbluth AA. Cancer-testis antigens: expression and correlation with survival in human urothelial carcinoma. Clin Cancer Res 2006; 12:5442 - 7; http://dx.doi.org/10.1158/1078-0432.CCR-06-0527; PMID: 17000678
- Nishiyama T, Tachibana M, Horiguchi Y, Nakamura K, Ikeda Y, Takesako K, Murai M. Immunotherapy of bladder cancer using autologous dendritic cells pulsed with human lymphocyte antigen-A24-specific MAGE-3 peptide. Clin Cancer Res 2001; 7:23 - 31; PMID: 11205913
- Sharma P, Bajorin DF, Jungbluth AA, Herr H, Old LJ, Gnjatic S. Immune responses detected in urothelial carcinoma patients after vaccination with NY-ESO-1 protein plus BCG and GM-CSF. J Immunother 2008; 31:849 - 57; http://dx.doi.org/10.1097/CJI.0b013e3181891574; PMID: 18833002
- Obara W, Ohsawa R, Kanehira M, Takata R, Tsunoda T, Yoshida K, Takeda K, Katagiri T, Nakamura Y, Fujioka T. Cancer peptide vaccine therapy developed from oncoantigens identified through genome-wide expression profile analysis for bladder cancer. Jpn J Clin Oncol 2012; 42:591 - 600; http://dx.doi.org/10.1093/jjco/hys069; PMID: 22636067
- Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy 2011; 3:1223 - 33; http://dx.doi.org/10.2217/imt.11.100; PMID: 21995573
- Nardelli-Haefliger D, Dudda JC, Romero P. Vaccination route matters for mucosal tumors. Sci Transl Med 2013; 5:fs4; http://dx.doi.org/10.1126/scitranslmed.3005638; PMID: 23408051
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, et al. Mucosal imprinting of vaccine-induced CD8⁺ T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med 2013; 5:72ra20; http://dx.doi.org/10.1126/scitranslmed.3004888; PMID: 23408053
- Decrausaz L, Domingos-Pereira S, Duc M, Bobst M, Romero P, Schiller JT, Jichlinski P, Nardelli-Haefliger D. Parenteral is more efficient than mucosal immunization to induce regression of human papillomavirus-associated genital tumors. Int J Cancer 2011; 129:762 - 72; http://dx.doi.org/10.1002/ijc.25973; PMID: 21384340
- Decrausaz L, Revaz V, Bobst M, Corthésy B, Romero P, Nardelli-Haefliger D. Induction of human papillomavirus oncogene-specific CD8 T-cell effector responses in the genital mucosa of vaccinated mice. Int J Cancer 2010; 126:2469 - 78; PMID: 19816937
- Domingos-Pereira S, Derré L, Warpelin-Decrausaz L, Haefliger JA, Romero P, Jichlinski P, Nardelli-Haefliger D. Intravaginal and Subcutaneous Immunization Induced, Vaccine Specific CD8 T Cells and Tumor Regression in the Bladder. J Urol 2013; Forthcoming http://dx.doi.org/10.1016/j.juro.2013.08.009; PMID: 23954582
- Naz RK. Female genital tract immunity: distinct immunological challenges for vaccine development. J Reprod Immunol 2012; 93:1 - 8; http://dx.doi.org/10.1016/j.jri.2011.09.005; PMID: 22154945
- Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity 2013; 38:349 - 59; http://dx.doi.org/10.1016/j.immuni.2012.10.019; PMID: 23415912
- Domingos-Pereira S, Decrausaz L, Derré L, Bobst M, Romero P, Schiller JT, Jichlinski P, Nardelli-Haefliger D. Intravaginal TLR agonists increase local vaccine-specific CD8 T cells and human papillomavirus-associated genital-tumor regression in mice. Mucosal Immunol 2013; 6:393 - 404; http://dx.doi.org/10.1038/mi.2012.83; PMID: 22968420
- Decrausaz L, Pythoud C, Domingos-Pereira S, Derré L, Jichlinski P, Nardelli-Haefliger D. Intravaginal live attenuated Salmonella increase local antitumor vaccine-specific CD8(+) T cells. Oncoimmunology 2013; 2:e22944; http://dx.doi.org/10.4161/onci.22944; PMID: 23483225
- Messing EM. Why should we increase public awareness of bladder cancer, and how can we do it?. Nat Clin Pract Urol 2008; 5:117; http://dx.doi.org/10.1038/ncpuro1061; PMID: 18322472