Abstract
Understanding the relevant biological activity of any pharmaceutical formulation destined for human use is crucial. For vaccine-based formulations, activity must reflect the expected immune response, while for non-vaccine therapeutic agents, such as monoclonal antibodies, a lack of immune response to the formulation is desired.
During early formulation development, various biochemical and biophysical characteristics can be monitored in a high-throughput screening (HTS) format. However, it remains impractical and arguably unethical to screen samples in this way for immunological functionality in animal models. Furthermore, data for immunological functionality lag formulation design by months, making it cumbersome to relate back to formulations in real-time. It is also likely that animal testing may not accurately reflect the response in humans.
For a more effective formulation screen, a human whole blood (hWB) approach can be used to assess immunological functionality. The functional activity relates directly to the human immune response to a complete formulation (adjuvant/antigen) and includes adjuvant response, antigen response, adjuvant-modulated antigen response, stability, and potentially safety.
The following commentary discusses the hWB approach as a valuable new tool to de-risk manufacture, formulation design, and clinical progression.
Introduction
Over several decades, the pharmaceutical industry has become heavily reliant on demonstrating functionality in animal models, especially mouse models, as a way to characterize and select vaccine formulations. However, it is becoming increasingly clear that animal testing may not always accurately reflect the response in humans and may not necessarily provide the most meaningful data. The issues surrounding animal use and human immunofunctionality are well-known and have been highlighted in several recent publications.Citation1-Citation8 The difference in immune function is hardly surprising given the vast evolutionary distance between mouse and human, thought to be in the order of 65 million years.Citation1 There are many examples where differences between animals and humans may have profound implications for formulation development. One example is Toll-like receptor (TLR) usage, relevant to the development of new generation adjuvants.Citation9 Currently, the response initiated by a TLR may not be fully known until clinical testing is initiated. Also the immunogenicity of DNA vaccines in mice did not translate to humans.Citation10 Another example was highlighted in a clinical trial for an immunomodulatory anti-CD28 monoclonal antibody (mAb) (TGN1412). During the trial, delivery of the antibody triggered an immediate systemic inflammatory response and a serious adverse event (SAE) referred to as a “cytokine storm.”Citation11 This life-threatening event was unexpected since standard non-clinical safety studies in animal models raised no alarm.
During pharmaceutical development of vaccines, it is crucial to identify optimal conditions for long-term storage and for boosting immunogenicity through, for example, the addition of adjuvants. To this end, a large number of variables must be examined during pre-formulation development to identify optimal pH, buffer ingredients, ionic strength, stabilizer excipients, as well as optimal adjuvant-antigen composition. Multiwell-based assays are readily available to monitor biophysical and biochemical properties of macromolecules as a function of different formulation parameters under normal or stressed conditions.Citation12-Citation14 However, there is a lack of simple and cost efficient in vitro high throughput screening methods to evaluate the biological activity of complete adjuvanted formulations. This is because responses at the organism level require a number of components. The hWB approach aims to emulate this.
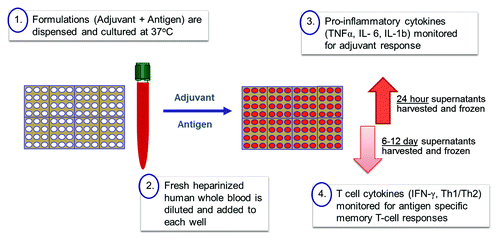
In order to begin addressing some of the issues mentioned above and to bridge preclinical testing in animal models, Sanofi Pasteur has looked into strategies that focus on in vitro testing using human cells.Citation4 Compatible with these initiatives and as a way to further bridge formulation development to vaccine functionality and to human clinical studies, we now describe a new way to evaluate the immune response to adjuvanted formulations using a fresh human whole blood (hWB) approach (). The approach may be particularly useful in an early clinical phase when a formal in vitro potency assay may not be available.
Monitoring an Adjuvant Response
Innate immunity is an immediate response to a stimulus or “danger signal” typically triggered through ancient receptors inherited at birth, which sense conserved pathogen-associated molecular patterns (PAMPs) found on a variety of bacteria, fungi, and other pathogens.Citation15 One of the best characterized of these innate signaling pathways is that for the bacterial endotoxin, lipopolysaccharide (LPS), which triggers a pro-inflammatory response through Toll-like receptor 4 (TLR4). Derivatives of LPS that mimic innate immunity are now key components of a new generation of vaccine adjuvants that use a TLR4 agonist (TLR4A).Citation16
Studies were initially conducted using TLR4A combined with an aluminum salt adjuvant (ASA), hereafter referred to as TLR4-Adjuvant. Several of these adjuvant formulations stimulated a pro-inflammatory cytokine (TNFα) response in fresh human whole blood (hWB). The response was initiated after as little as 6 h of incubation and more optimally between 18–24 h. To further characterize the adjuvant response, individual components of the adjuvant were used in a follow-up study to stimulate hWB over a broad range of concentrations. The data indicated a consistent trend in response across 3 different subjects (). Although there was a lack of response to the ASA alone (not shown), the response to TLR4A was significantly augmented in the presence of ASA. Indeed, there were responses to the TLR4-Adjuvant at concentrations of the TLR4A alone that would otherwise have failed to induce a detectable response. The combination of TLR4A with ASA is therefore crucial to the adjuvant effect observed.
Figure 2. Innate immune response to vaccine formulation. TNFα is released from human whole blood of three different subjects after 24h stimulation with LPS, TLR4-adjuvant, TLR4A, and ASA (ASA alone was consistently negative and is not shown).
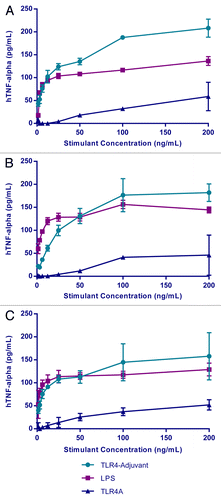
While there is expected variability between subjects, there may be ways to normalize the data against a standard reference such as LPS or other TLR4As. Evaluating adjuvant responses in this way is inexpensive, relatively quick, and technically easy to perform. Depending on the technology used it may be possible to generate functional data comfortably within a single working day.
Monitoring an Antigen Specific Memory Response
Typically, the memory T cell population is rapidly activated and proliferates upon re-encounter with a specific antigen, with immediate cytokine-secreting cells being described as effector memory, and with proliferating T cells being described as central memory.Citation17,Citation18 A methodology developed by scientists at the London School of Hygiene and Tropical Medicine and based on earlier studies in Leishmania has been in use for approximately two decades as a way to monitor antigen specific, vaccine-induced memory after bacillus Calmette-Guérin (BCG) vaccination. The response is monitored by stimulating hWB with antigens from M. tuberculosis and detection of T cell cytokines such as IFNγ after 6–7 d of culture by ELISA,Citation19,Citation20,Citation21,Citation22 or by multiplex bead array assays.Citation23
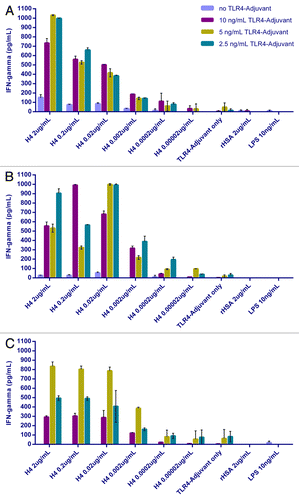
To investigate antigen specific responses to complete vaccine formulations, an advanced TB vaccine candidate referred to as H4Citation24 was formulated with the TLR4-Adjuvant and tested using fresh hWB recovered from 3 healthy BCG vaccinated volunteers ().
The data demonstrated that an antigen-specific IFNγ memory response can be monitored in a simple 6 d assay when blood is incubated with the complete formulation. In all cases illustrated, an antigen and adjuvant dose response was detected. The optimum concentration of H4 was relatively broad (2.0 μg and 0.02 μg) and was dependent on the concentration of TLR4-Adjuvant. Similarly, the optimum dose of adjuvant varied between 2.5 ng and 10 ng. While responses were variable between subjects, the expectation is that loss of potency, between different formulations, would be apparent in any subject tested. The ability to demonstrate profound immunomodulation in terms of quantitative (measurable) and qualitative (cytokine profile) responses by adjuvant on the T cell response through this in vitro approach is of particular interest.
Adjuvant Immunomodulation
The use of hWB may provide a new way to monitor ‘complete’ vaccine formulations in terms of both the antigen and the adjuvant response. The ability to demonstrate and understand modulation in vitro in this manner is relatively novel.Citation25 Since a TLR4-Adjuvant is expected to drive Th1-like immunity, it is thus important to show and quantify this effect for any formulation being developed.
The example chosen here uses a TB vaccine to boost the memory response primed by BCG or prior mycobacterial exposure. However, the approach is likely to be applicable to any other T cell inducing vaccine that aims to boost existing memory.
Formulation Stability
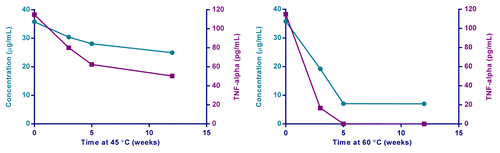
Once the functionality of a formulation can be demonstrated, the ability to detect changes to the stability of the formulation is then possible. To this end, TLR4-Adjuvant formulations under different conditions of accelerated temperature storage were monitored using hWB and chemical integrity by reversed phase high-performance liquid chromatography (RP-HPLC). The fresh blood from a single subject was tested on multiple weeks using different adjuvant preparations in an overnight TNFα assay. The data revealed a loss of functional activity (ability to stimulate TNFα) when formulations were stored at 45 °C and 60 °C for 3, 5, and 12 wk (). Interestingly, the loss of functional activity agreed with changes in the RP-HPLC profile. The ability to link specific chemical or structural changes to an immunofunctionality will be particularly useful. Stability studies could also be extended to antigen-specific responses discussed above.
Formulation Safety
Formulation safety may be an important potential application for the hWB approach. Since the approach identifies both innate signaling and the modulated adaptive response, the use of cells from healthy humans may be a quick way to confirm absence of unwanted or unexpected immunity in any formulation at any time during development. Such testing might also identify contamination with endotoxin or any other toxins that might not be recognized in other standard tests (i.e., Limulus Amebocyte Lysate – LAL and rabbit pyrogenicity). Indeed, a commercial kit using hWB is already being marketed for this purpose (Biotest PyroDetect System).Citation26 Application of the approach may also be helpful to a non-clinical safety assessment. Certainly, innovative approaches to address safety are of major interest to the industry (e.g., BioVacSafe).
Concluding Remarks
The hWB approach enables the determination of the functionality of a complete formulation (adjuvant and antigen) within 6 d. Multiple formulations can be monitored simultaneously and responses compared for several subjects. The approach presents an opportunity for a broad formulation screen and to select optimal, possibly safer, formulations based on a functional response on fresh human cells. The functional response can be used in conjunction with standard biochemical and biophysical tests. The ability to re-stimulate antigen-specific memory B cells may also be possible with this approach. Moreover, the ability to correlate a memory T helper cell response with antibody elicited in vivo would make the approach especially attractive for screening other vaccine targets.Citation27 Innovative technologies that monitor T cell immunity are continuously being developed. These technologies and other assays such as qPCR or proteomics could be readily applied to the hWB approach.Citation28,Citation29
A convincing argument for the advantages of using fresh hWB is already reported and discussed.Citation30,Citation31 Provided that drawn blood is returned to culture within a few hours, there is no need for cellular enrichment or centrifugation. Blood is maintained in the most physiologically relevant condition. In addition, hWB contains components essential to stimulate both innate and memory T cell immunity. Such factors may include neutrophils and eosinophils, as well as platelets and other cells and plasma constituents, that have been shown to play a role in cellular function and cytokine production.Citation32,Citation33 Since hWB is used for immunomonitoring clinical trials,Citation34 there may be a direct link between formulation screening in vitro and actual clinical trial monitoring. Indeed, the approach may be compatible with a ‘trial-in-a-test tube’Citation35, where an in vitro response may be predictive of the clinical response.
The use of point-of-care devices (PCD) to screen whole blood for an array of markers is becoming a reality.Citation36,Citation37,Citation38 The hWB approach is ideally placed to exploit these and other innovative technologies.
Variability of the response between different subjects is expected. Such variability allows for a more realistic understanding of vaccine performance. In terms of formulation screening, multiple formulations can be monitored relative to a reference formulation within a single subject.
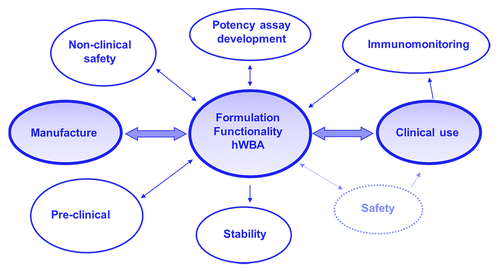
In summary, the hWB approach is a relatively inexpensive, fast HTS methodology that can monitor complete vaccine formulation functionality in terms of the adjuvant response, the antigen response, and the modulation of the antigen response by the adjuvant. In addition, the approach can detect a loss of functionality thus supporting structure-function characterization of vaccine antigens and optimization of antigen-adjuvant interactions. Finally, the application of a formulation to human cells can be a sensitive indicator of unwanted or unexpected immunological response. The approach would considerably de-risk progression of vaccine formulations through early clinical trials ().
| Abbreviations: | ||
| BCG | = | Bacillus Calmette-Guérin |
| SP | = | Sanofi Pasteur |
| TB | = | tuberculosis |
| hWB | = | human whole blood |
| H4 | = | TB antigen |
| IFNγ | = | interferon gamma |
| TNFα | = | tumor necrosis factor alpha |
| HTS | = | high-throughput screening |
| mAB | = | monoclonal antibody |
| TLR | = | toll-like receptor |
| TLR4 | = | toll-like receptor 4 |
| SAE | = | serious adverse event |
| TLR4A | = | TLR4 agonist |
| ASA | = | aluminum salt adjuvant |
| TLR4-adjuvant | = | TLR4A combined with an ASA |
| RP-HPLC | = | reversed phase high-performance liquid chromatography |
| LAL | = | limulus amebocyte lysate |
| PCD | = | point-of-care devices |
| PAMPs | = | pathogen-associated molecular patterns |
| LPS | = | lipopolysaccharide |
Disclosure of Potential Conflicts of Interest
R.H.B., J.H., Y.H., S.A., S.F.A., M.H., and N.R. are employees of Sanofi Pasteur. All other authors have no conflict of interest
References
- Davis MM. A prescription for human immunology. Immunity 2008; 29:835 - 8; http://dx.doi.org/10.1016/j.immuni.2008.12.003; PMID: 19100694
- Germain RN. Vaccines and the future of human immunology. Immunity 2010; 33:441 - 50; http://dx.doi.org/10.1016/j.immuni.2010.09.014; PMID: 21029956
- Of men, not mice. Nat Med 2013; 19:379; http://dx.doi.org/10.1038/nm.3163; PMID: 23558605
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al, Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013; 110:3507 - 12; http://dx.doi.org/10.1073/pnas.1222878110; PMID: 23401516
- Rice J. Animal models: Not close enough. Nature 2012; 484:S9; http://dx.doi.org/10.1038/nature11102; PMID: 22509510
- Cook N, Jodrell DI, Tuveson DA. Predictive in vivo animal models and translation to clinical trials. Drug Discov Today 2012; 17:253 - 60; http://dx.doi.org/10.1016/j.drudis.2012.02.003; PMID: 22493784
- Greek R, Menache A, Rice MJ. Animal models in an age of personalized medicine. Personalized Medicine 2012; 9:47 - 64; http://dx.doi.org/10.2217/pme.11.89
- Mullane K, Williams M. Translational semantics and infrastructure: another search for the emperor’s new clothes?. Drug Discov Today 2012; 17:459 - 68; http://dx.doi.org/10.1016/j.drudis.2012.01.004; PMID: 22269133
- Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev 2011; 239:178 - 96; http://dx.doi.org/10.1111/j.1600-065X.2010.00978.x; PMID: 21198672
- Klinman DM, Klaschik S, Tross D, Shirota H, Steinhagen F. FDA guidance on prophylactic DNA vaccines: analysis and recommendations. Vaccine 2010; 28:2801 - 5; http://dx.doi.org/10.1016/j.vaccine.2009.11.025; PMID: 19941989
- Stebbings R, Findlay L, Edwards C, Eastwood D, Bird C, North D, Mistry Y, Dilger P, Liefooghe E, Cludts I, et al. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol 2007; 179:3325 - 31; PMID: 17709549
- Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2007; 2:2212 - 21; http://dx.doi.org/10.1038/nprot.2007.321; PMID: 17853878
- Ausar SF, Espina M, Brock J, Thyagarayapuran N, Repetto R, Khandke L, Middaugh CR. High-throughput screening of stabilizers for respiratory syncytial virus: identification of stabilizers and their effects on the conformational thermostability of viral particles. Hum Vaccin 2007; 3:94 - 103; http://dx.doi.org/10.4161/hv.3.3.4149; PMID: 17426457
- Kissmann J, Ausar SF, Rudolph A, Braun C, Cape SP, Sievers RE, Federspiel MJ, Joshi SB, Middaugh CR. Stabilization of measles virus for vaccine formulation. Hum Vaccin 2008; 4:350 - 9; http://dx.doi.org/10.4161/hv.4.5.5863; PMID: 18382143
- Janeway CA Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20:197 - 216; http://dx.doi.org/10.1146/annurev.immunol.20.083001.084359; PMID: 11861602
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 2009; 183:6186 - 97; http://dx.doi.org/10.4049/jimmunol.0901474; PMID: 19864596
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745 - 63; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104702; PMID: 15032595
- Todryk SM, Pathan AA, Keating S, Porter DW, Berthoud T, Thompson F, Klenerman P, Hill AVS. The relationship between human effector and memory T cells measured by ex vivo and cultured ELISPOT following recent and distal priming. Immunology 2009; 128:83 - 91; http://dx.doi.org/10.1111/j.1365-2567.2009.03073.x; PMID: 19689738
- Weir RE, Morgan AR, Britton WJ, Butlin CR, Dockrell HM. Development of a whole blood assay to measure T cell responses to leprosy: a new tool for immuno-epidemiological field studies of leprosy immunity. J Immunol Methods 1994; 176:93 - 101; http://dx.doi.org/10.1016/0022-1759(94)90353-0; PMID: 7963598
- Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, Ngwira B, Sichali L, Nazareth B, Blackwell JM, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 2002; 359:1393 - 401; http://dx.doi.org/10.1016/S0140-6736(02)08353-8; PMID: 11978337
- Lalor MK, Ben-Smith A, Gorak-Stolinska P, Weir RE, Floyd S, Blitz R, Mvula H, Newport MJ, Branson K, McGrath N, et al. Population differences in immune responses to Bacille Calmette-Guérin vaccination in infancy. J Infect Dis 2009; 199:795 - 800; http://dx.doi.org/10.1086/597069; PMID: 19434928
- Lee H, Cho SN, Kim HJ, Anh YM, Choi JE, Kim CH, Ock PJ, Oh SH, Kim DR, Floyd S, et al. Evaluation of cell-mediated immune responses to two BCG vaccination regimes in young children in South Korea. Vaccine 2011; 29:6564 - 71; http://dx.doi.org/10.1016/j.vaccine.2011.07.003; PMID: 21763746
- Lalor MK, Floyd S, Gorak-Stolinska P, Ben-Smith A, Weir RE, Smith SG, Newport MJ, Blitz R, Mvula H, Branson K, et al. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J Infect Dis 2011; 204:1075 - 85; http://dx.doi.org/10.1093/infdis/jir515; PMID: 21881123
- Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, Andersen P. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol 2005; 174:6332 - 9; PMID: 15879133
- Gaur RL, Suhosk MM, Banaei N. In vitro immunomodulation of a whole blood IFN-γ release assay enhances T cell responses in subjects with latent tuberculosis infection. PLoS One 2012; 7:e48027; http://dx.doi.org/10.1371/journal.pone.0048027; PMID: 23144722
- Daneshian M, von Aulock S, Hartung T. Assessment of pyrogenic contaminations with validated human whole-blood assay. Nat Protoc 2009; 4:1709 - 21; http://dx.doi.org/10.1038/nprot.2009.159; PMID: 19893507
- Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A 2009; 106:3877 - 82; http://dx.doi.org/10.1073/pnas.0813390106; PMID: 19237568
- Kasprowicz VO, Mitchell JE, Chetty S, Govender P, Huang KH, Fletcher HA, Webster DP, Brown S, Kasmar A, Millington K, et al. A molecular assay for sensitive detection of pathogen-specific T-cells. PLoS One 2011; 6:e20606; http://dx.doi.org/10.1371/journal.pone.0020606; PMID: 21853018
- Chakera A, Bennett SC, Cornall RJ. A whole blood monokine-based reporter assay provides a sensitive and robust measurement of the antigen-specific T cell response. J Transl Med 2011; 9:143; http://dx.doi.org/10.1186/1479-5876-9-143; PMID: 21871084
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011; 11:519 - 31; http://dx.doi.org/10.1038/nri3024; PMID: 21785456
- Damsgaard CT, Lauritzen L, Calder PC, Kjaer TM, Frøkiaer H. Whole-blood culture is a valid low-cost method to measure monocytic cytokines - a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J Immunol Methods 2009; 340:95 - 101; http://dx.doi.org/10.1016/j.jim.2008.10.005; PMID: 19000693
- Chen J, Bruns AH, Donnelly HK, Wunderink RG. Comparative in vitro stimulation with lipopolysaccharide to study TNFalpha gene expression in fresh whole blood, fresh and frozen peripheral blood mononuclear cells. J Immunol Methods 2010; 357:33 - 7; http://dx.doi.org/10.1016/j.jim.2010.03.006; PMID: 20307542
- Silva D, Ponte CGG, Hacker MA, Antas PRZ. A whole blood assay as a simple, broad assessment of cytokines and chemokines to evaluate human immune responses to Mycobacterium tuberculosis antigens. Acta Trop 2013; 127:75 - 81; http://dx.doi.org/10.1016/j.actatropica.2013.04.002; PMID: 23571106
- Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, et al. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods 2004; 291:185 - 95; http://dx.doi.org/10.1016/j.jim.2004.06.010; PMID: 15345316
- Higbee RG, Byers AM, Dhir V, Drake D, Fahlenkamp HG, Gangur J, Kachurin A, Kachurina O, Leistritz D, Ma Y, et al. An immunologic model for rapid vaccine assessment -- a clinical trial in a test tube. Altern Lab Anim 2009; 37:Suppl 1 19 - 27; PMID: 19807200
- Yin YP, Chen XS, Wei WH, Gong KL, Cao WL, Yong G, Feng L, Huang SJ, Wang DM, Han Y, et al. A dual point-of-care test shows good performance in simultaneously detecting nontreponemal and treponemal antibodies in patients with syphilis: a multisite evaluation study in China. Clin Infect Dis 2013; 56:659 - 65; http://dx.doi.org/10.1093/cid/cis928; PMID: 23132172
- Scott T. Point-of-care tetanus immunoassay: an audit of unscheduled tetanus prophylaxis. Int J Orthop Trauma Nurs 2012; 16:97 - 103; http://dx.doi.org/10.1016/j.ijotn.2011.06.005
- Hortensius J, Slingerland RJ, Kleefstra N, Logtenberg SJ, Groenier KH, Houweling ST, Bilo HJ. Self-monitoring of blood glucose: the use of the first or the second drop of blood. Diabetes Care 2011; 34:556 - 60; http://dx.doi.org/10.2337/dc10-1694; PMID: 21289231