Abstract
The polymorphic fungus Candida albicans (C. albicans) can live as an aggressive pathogen and cause many diseases in hosts, for which no effective vaccine exists. The secreted aspartyl proteinase 2 (Sap2) plays a protective role in systemically infected BALB/c mice. Protective cellular immune responses can be preferentially induced when antigens are displayed on small particles. Therefore, the emphasis is placed on developing new phage vaccine to inhibit C. albicans infection. In this study, the ability of the hybrid phage displaying the epitope SLAQVKYTSASSI and recombinant protein of Sap2 (rSap2) for inducing immune protective responses against C. albicans infection was evaluated by lymphoproliferative assay, to gather cytokine and antibody measurements in BALB/c mice. Our results showed that, strong cellular and humoral immune responses were induced in a mouse model immunized with hybrid phage or rSap2. Furthermore, the protection against lethal challenge with C. albicans was observed in mice vaccinated hybrid phage without adjuvant. These findings demonstrate that the hybrid phage displaying the epitope SLAQVKYTSASSI might be a potential vaccine against C. albicans infections.
Keywords: :
Introduction
C. albicans is an opportunistic pathogen yeast, it is implicated as an agent of diseases in immunocompromised groups, diabetes, and cancer patients.Citation1,Citation2Candida species are now the fourth most common microbe isolated from nosocomial bloodstream infections in the United States hospitals with a mortality rate around 40%.Citation3 In addition, carriers of synthesized peptide vaccine are difficult for synthesis, and have safety and solubility issues. Although 2 Candida vaccines were shown to be safe in Phase 1 trials, neither vaccine has been shown to be efficacious in humans.Citation4,Citation5 Therefore, the emphasis is placed on developing new vaccine to inhibit C. albicans infection.
Both basic and clinical studies confirm that T cell-mediated immune responses to C. albicans infection, particularly those associated with Th1- and Th17- type immune responses are pivotal for protection against this pathogen.Citation6-Citation8 The BALB/c mouse is extensively used as an animal model for studies of immunity to C. albicans infection.Citation9,Citation10 Differences in susceptibility to C. albicans infection were observed in a variety of different tissues, Th1-mediated immunity was thought to confer the primary protection, particularly for candidiasis.Citation11-Citation13 Furthermore, Th17 compartment was shown to play a predominant role in mucosal candidiasis.Citation14-Citation16
Numerous studies described the presence of Secreted aspartic proteins (Saps) during C. albicans infections. Sap2 is produced by C. albicans at the early stage of infection, it is well known to degrade many host proteins.Citation17-Citation20 Sap2 could not only provide essential nitrogen for growth, but also enhance attachment, colonization, and penetration of host tissue by the removal of host barriers.Citation21,Citation22 The antibody against Sap2 was protective in systemic candidiasis and an adjuvant-free virosomal formulation of a recombinant Sap2 vaccine induced protection in an experimental model of rat vaginal candidiasis.Citation23,Citation24 These findings led to its evalution as a vaccine antigen where it was demonstrated to have protective efficacy in preclinical animal model of candidiasis. Additionally, we displayed epitope VKYTS of Sap2 on phage and found that the hybrid phage could induce the specific antibody production in mice and recognize the antibody in C.albicans infected patients.Citation25
To evaluate whether hybrid phage represents a potential novel vaccine candidate without adjuvant against C. albicans, we constructed a plasmid pET28a-Sap2 and examined the humoral and cellular immune responses in BALB/c mice after immunization with hybrid phage displaying epitope SLAQVKYTSASSI and rSap2, and assessed the protective immunity induced by the stimulants. In this paper, we describe that the use of the phage for delivery of C. albicans Sap2 epitope for effective immunization of BALB/c mice, and the hybrid phage displaying SLAQVKYTSASSI has a great potential as a vaccine to induce strong Th1 and Th17 response without adjuvant.
Results
Antibody response against hybrid phage and rSap2
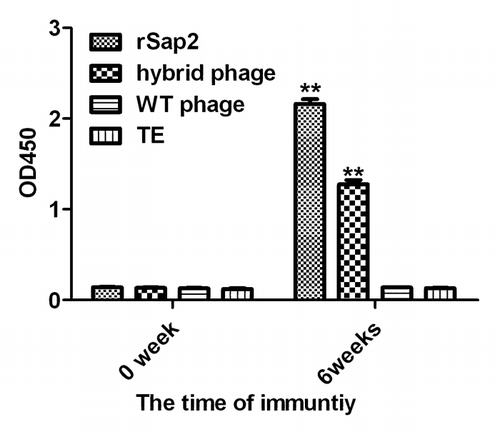
The recombinant phagemid pfd8SHS was transformed into E. coli TG1, and produced hybrid phage displaying epitope SLAQVKYTSASSI. The phage displaying epitope SLAQVKYTSASSI was analyzed by SDS-PAGE () and reacted with anti-sera of rSap2 (). Sequence analysis confirmed that the Sap2 gene was cloned into pET28a successfully. In addition, rSap2 was analyzed by SDS-PAGE, a 43 kDa protein band was visible (), and it could be recognized using the sera from mice immunized with hybrid phage and rSap2 (). The total IgG antibodies against hybrid phage and rSap2 were measured in sera of the immunized mice by ELISA. The results indirectly showed that mice vaccinated with the hybrid phage and rSap2 displayed a significantly high level of total IgG compared with mice vaccinated with TE alone (P < 0.01) (), and demonstrated that the hybrid phage and rSap2 can induce specific antibody production in BALB/c mice.
Figure 1. SDS-PAGE for hybrid phage and WT phage. (A) Lane 1, WT phage, lane 2, Hybrid phage. (B) Western blot assay for hybrid phage with sera. Lane 1, Marker; Lane 2, Sap2 -immunized mice serum; lane 3, non-immunized mice serum.
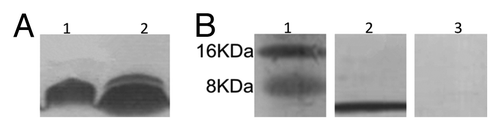
Figure 2. rSap2 recombinant protein and western blot assay. (A) rSap2 protein purified by Ni+ affinity column. Lane1, Marker; Lane2, rSap2. (B) Western blot assay for rSap2 with immunized or non-immunized sera. Lane 1, Marker; Lane 2, Sap2 -immunized mice serum; lane 3, hybrid phage immunized mice sreum; lane 4, WT phage immunized mice sreum; Lane 5, non-immunized mice serum.
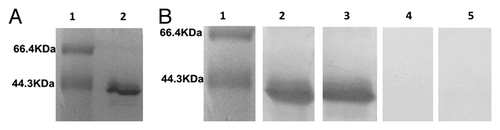
Figure 3. IgG determination by ELISA. Serum IgG responses in mice vaccinated with hybrid, rSap2, WT phage on weeks 0 and 6. Sera were collected on 1 d prior to each immunization, and tested by ELISA using rSap2. The titer was given as the reciprocal of the highest dilution with an OD450 that was 2.5-fold greater than the OD of TE treated mouse sera at the same dilution.
Splenocytes proliferation induced by rSap2 and hybrid phage
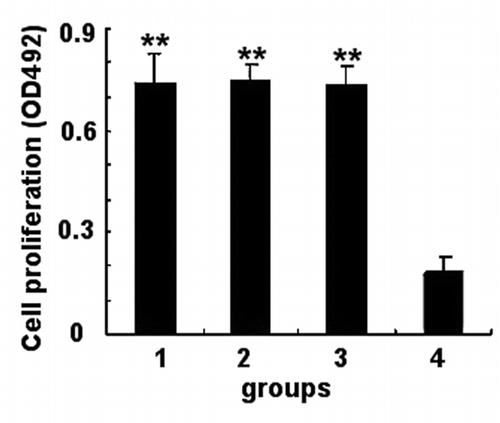
The splenocytes were prepared from the immunized mice to assess the proliferative immune responses. As shown in , compared with those of mice immunized with TE, the splenocytes from all of the mice immunized responded vigorously to rSap2 (P < 0.01). Although no difference was detected among the 3 immunized groups, significant difference compared with TE injected group was found (P < 0.01).
Figure 4. Splenoccytes proliferation measured by MTT. Cells were seeded into a 96-well plate with the density of 5 × 103 cells per well and cultured with stimulus at 37 °C. MTT assay was performed after 72 h. The data shown are means ± SD of 3 independent experiments. Group 1, rSap2 immunized mice; group 2, hybrid phage immunized mice; group 3, WT phage immunized mice; group 4, TE injected mice. **Statistically significant (P < 0.01).
Cytokines levels in splenocytes culture supernatant
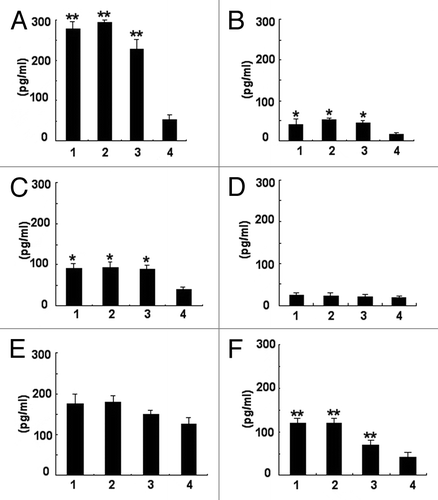
To assess the development of cell-mediated immunity, splenocytes of immunized mice, or TE-injected mice were restimulated in vitro by immunogen for 48 h individually. As shown in , IFN-γ, IL-2, IL-12, and IL-17 production were significantly higher in immunized animals as compared with the control group (P < 0.05). There was no statistically significant difference in the IL-4 and IL-10 production as compared with the control group. Our results showed that both phage displaying epitope and rSap2 could induce Th1- and Th17- type cytokines gene expression.
Figure 5. Quantitative sandwich ELISA assay for cytokines expression in splenocytes culture supernatant. (A) IFN-γ secretion; (B) IL-2 secretion; (C) IL-12 secretion; (D) IL-4 secretion; (E) IL-10 secretion; (F) IL-17 secretion. For (A–F), 1, rSap2 immunized mice; 2, hybrid phage immunized mice; 3, WT phage immunized mice; 4, TE injected mice. The data shown are means ± SD of 3 independent experiments. *Significant (P < 0.05), **Statistically significant (P < 0.01).
Cytokines gene expression in spleens
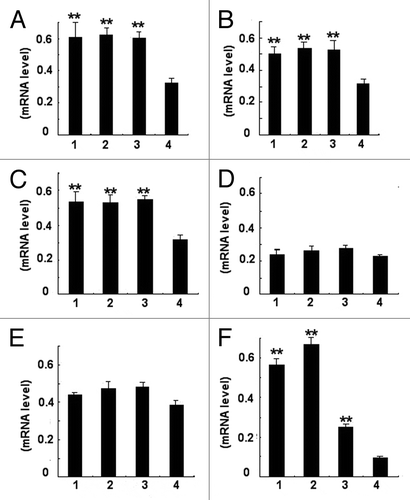
RT-PCR analysis of cytokines was performed on cDNA generated from the total RNA of spleens individually taken from mice. The primers used are shown in , Th1- and Th17- type cytokines, IFN-γ, IL-2, IL-12, and IL-17 were upregulated compared with the control group (P < 0.01) (). However, the level of IL-4 and IL-10 in WT phage, hybrid phage, and rSap2 immunized mice were similar to TE-injected mice.
Table 1. Primer sequences used in polymerase chain reaction to study the gene expression of cytokines
Figure 6. Photodensitometric analysis of PCR products according to RT-PCR assay for cytokines. The histogram showed the ratio of the intensity of cytokines bands to that of β-actin bands. (A) IFN-γ mRNA/β-actin mRNA; (B) IL-2 mRNA/β-actin mRNA; (C) IL- 12mRNA/β-actin mRNA; (D) IL-4 mRNA/β-actin mRNA ; (E) IL-10 mRNA/β-actin mRNA; (F) IL-17 mRNA/β-actin mRNA . For (A–F), 1, rSap2 immunized mice; 2, hybrid phage immunized mice; 3, WT phage immunized mice; 4, TE injected mice. The data shown are means ± SD of 3 independent experiments. **Statistically significant (P < 0.01).
Protection against challenge by intravenous inoculation of C. albicans
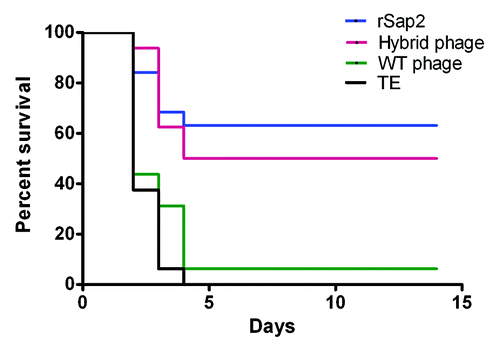
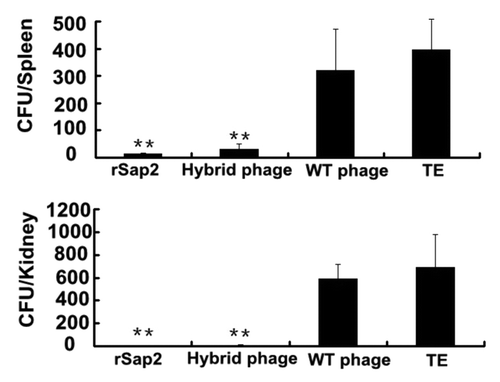
To determine the C. albicans colonization in tissues, 3 mice in each group were challenged by inoculating 106 C. albicans and sacrificed on day 7 post-challenge, and their kidneys and spleens were collected for analysis. In , higher levels of C albicans colonization were detected in the kidneys and spleens of the mice immunized with WT phage or TE, whereas the C. albicans colonization was very low in the mice immunized with rSap2 or hybrid phage. Compared with those of control group, little C. albicans colonization was detected in the kidneys of mice immunized with hybrid phage (P < 0.01). To evaluate the potency of hybrid phage against lethal C. albicans challenge, the immunized mice were challenged with 107 C. albicans on day 10. Starting from day 2 post-challenge, the mice immunized with antigens started dying. The survival rates in the hybrid phage, rSap2, WT phage, and TE groups on 10 d post-challenge were 50%, 62.5%, 8.75%, and 0% respectively ().
Figure 7. Decreased colonization of C. albicans in kidneys and spleens from mice immunized with hybrid phage or rSap2 or wild phage. The numbers of C. albicans CFU recovered from kidneys and spleens of mice treated with three injections administered with a period of 2 wk, and infected with 106C. albicans 2 wk after the third immunization. The results shown indicate the mean value ± standard deviation (SD) of each group. The significance of the results between immunized hybrid mice and each control. (*P < 0.05, **P < 0.01).
Disussion
Altough humoral immune response is critical for protection against candidiasis, several studies showed that cell-mediated immunity also play important roles. Recent studies have focused on the ability of vaccines to polarize the activation of specific T helper cell populations (e.g., Th1, Th2, and Th17) against fungal infections,Citation26 some evidences also showed that Th1 cells conferred resistance to most extracellular microbes, including C. albicans.Citation27-Citation29 Furthermore, emerging data indicated that the newly defined Th17 cells played the predominant role in mucosal candidiasis.Citation30 The protective immunity against C. albicans was described in murine studies. We analyzed T cell immunity by measuring cytokine responses to rSap2 and the phage displaying SLAQVKYTSASSI and found that both were immunogenic.
Sap can modify the epithelial cytokines expression in a vitro model of vaginal candidiasis,Citation31 and Sap2 knockout mice reduces damage to the tissue and has a significantly reduced potential to the cytokines expression.Citation32 On the other side, rSap2 can induce both humoral immune response and cellular immune response, which may play an important role in the anti-Candida protection.Citation24 In this study, rSap2 was demonstrated to induce pro-inflammatory cytokines such as IFN-γ and IL-2 which produce stimulation of Th1 cells and CD8 T cell, and IL-17 which plays a role in C. albicans infection. Moreover, a slight increase in Th2 cytokines was observed following Sap2. Therefore, we hypothesized that suppression/immunoregulation by Th1 cytokines might exist. Vilanova et al. reported that protection against systemic candidiasis in mice immunized with Sap2 was antibody mediated and antibodies also were reported to mediate anti-Candida protection in animals immunized with almost all reported experimental vaccine.Citation4,Citation23,Citation33 Therefore, the protection mediated by Sap2 might refer to antibody, Th1- and Th17-type cytokines.
T cell epitope is located at the C-terminal region of Sap2, which (e.g., SLAQVKYTSASSI) might stimulate the proliferation of splenocyte and then induced the production of cytokines.Citation34 In C. albicans infection, IgG identified epitope in amino acid position 386–390 (VKYTS) is associated with antibody production and survival.Citation35 In previously study, a hybrid phage displaying VKYTS was produced, which was found to have the ability to induce and recognize anti-Sap antibody and could be used to detect C. albicans infection. In contrast to epitope VKYTS, the epitope SLAQVKYTSASSI could both induce cellular and humoral immunity to make it more adaptable for vaccine research.
Previously we reported that hybrid phage could induce Th1 immune response which was related to protection.Citation36 To further evaluate the possible immunomodulatory actions of hybrid phage, cytokines production and cell proliferation by murine splenocytes were examined in the presence of these stimuli. In this study, our data showed that hybrid phage displaying SLAQVKYTSASSI was same as rSap2 which could cause a significantly improvement in stimulation of immune responses. Hybrid phage and rSap2 stimulated splenocytes proliferation, induced Th1 and Th17 response pattern, and enhanced secretion of IL-2, IL-12, IFN-γ, and IL-17. Moreover, serum antibody of mice immunized with hybrid phage could recognize rSap2. Therefore, these findings suggested that hybrid phage had the potential in vaccine production, which probably played a major role in humoral immune response and cell-mediated immunity response.
Phage displaying SLAQVKYTSASSI contains T-cell and B-cell epitopes, which does not need adjuvant to enhance the role, and the hybrid phage delivery induced balanced Th1 and Th2 responses with a potent Th1 response. Similar patterns were observed in our previous studies with the phage displaying epitope LKVIRK.Citation37 In addition, we showed for the first time that the hybrid phage induced strong Th17 response. Therefore, phage seems to be a suitable carrier of an epitope. rSap2 is a good vaccine candidate, which mediates protection by specific antibody and cytokines, however it needs immune adjuvant. The specific immune response, the ability to recruit helper T-cells and the unnecessity for external adjuvant suggest phage may be an inexpensive and particularly useful platform for epitope vaccine design. We also note that, although the mechanistic basis for phage responses is currently unknown, no adverse effects to phage vaccination have been observed in many preclinical studies performed in animal models.Citation38
Our results showed that the hybrid phage immunization had the same protection as recombinant protein immunization. The adjuvant-free phage decorated epitope induced specific antibody response and cell response, as well as provided protection to mice against very high doses of C.albicans challenge. After first challenge, results showed that hybrid phage immunized group and rSap2 immunized group had significant reduction in the number of C. albicans at day 7. After the second survival challenge, mice were inoculated with 107 C. albicans, and it was obvious that immunization with hybrid phage or rSap2 could induce immune response and confer protection against lethal challenge in a mouse model, more important, the characteristic of hybrid phage vaccination was that it could directly be applied in the animals without adjuvant. Therefore, phage-displaying epitope could avoid the problems of adjuvant of recombinant proteins.
Finally, such phage displaying epitope vaccine could be targeted to antigen-presenting dendritic cells by displaying a epitope on the casipd of phage, further stimulating the immunity without adjuvant. In this paper, the hybrid phage displaying the epitope SLAQVKYTSASSI could induce efficient humoral and cell-mediated immune responses, increase survival rate after candidiasis challenge, it may be a potential vaccine against C. albicans infections. With the recent data, we could currently develop the phage vaccine platforms, not only to defend against C.albicans infection, but also to generate efficacious vaccines against other infectious agents.
Materials and Methods
Strains, phage, and animals
Phagemid pfd8SHS which was propagated in Escherichia coli (E. coli) strain TG1 and wild-type phage (WT phage) used in this work were stored in our laboratory. Female BALB/c mice, between 6 and 8 wk of age, were purchased from the Specific-Pathogen-Free Animal Facility of Jilin University, China.
Ethics statement
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of China Association for Laboratory Animal Science. The Ethics Committee of Northeast Normal University approved all animal care and protocols. All sacrifices were performed under pentobarbitone anesthesia, and every effort was made to minimize animal suffering.
Construction of recombinant phagemid pfd8SHS
The construction of recombinant phagemid pfd8SHS and the production of hybrid phage were performed as described previously.Citation25 Briefly, 2 synthesized complementary oligonucleotides encoding the epitope SLAQVKYTSASSI were ligated into SacII-BstBI digested phagemid pfd8. E. coli TG1 transformed by the recombinant phagemid with correct insertion was grown in LB liquid medium supplemented with ampicillin. Hybrid phage was secreted into the culture with the help of WT phage. Then, the culture supernatants containing phage particles was pooled and purified in polyethylene glycol-6000 (final concentration 5% polyethylene glycol-6000, 0.5 M NaCl) by 2 consecutive precipitations for 12 h, and then the phage pellet was resuspended in 2 ml TE (1.0 mM EDTA, 0.01 M Tris–HCl, pH 8.0). The amount of phage produced was estimated by spectrophotometry, where an OD of 1.0 at 270 nm was assumed to correspond to a concentration of 1/3.84 μg/μl.
Construction of plasmids for production of Sap2
The genomic DNA was prepared by mechanical disruption with glass beads. C. albicans ATCC10231 were grown at 28 °C for 18 h and the microorganisms were collected. After 2 washes with 0.9% NaCl, the cells were disrupted with glass beads. Nested PCR was used to amplify Sap2 gene. The PCR product was digested with EcoRI/XhoIand cloned back into the vector pET-28a. The pET28a–Sap2 recombinant plasmid was transformed into E. coli BL21. Expression of recombinant Sap2 (rSap2) was induced by 1 mmol/l IPTG, the protein was extracted in denaturing conditions according to the protocol and its purification was performed by immobilized-metal affinity chromatography with Ni-NTA agarose beads following the manufacturer’s instructions (GE).
Immunization of mice
All experiments were done in compliance with international legal and institutional guidelines. Mice were divided into 4 groups, with 27 mice per group. The mice were immunized on weeks 0, 2, 4, 6 with (1) 25 µg rSap2 coupled with Freund’s complete adjuvant (FCA), (2) 25 µg hybrid phage particles in 200 µl TE, (3) 25 µg wild-type phage particles in 200 µl TE, and (4) 200 µl TE as negative control. One week after the fourth injection, antibody responsed against rSap2 protein were assessed in the sera of vaccinated and control mice by western Blot and ELISA.
Cell proliferation
Cell proliferation was assessed using MTT assay. One week after the fourth immunization, the animals in each group were euthanized, spleens were removed, and a single-cell suspension was prepared by passage through a 100-gauge wire mesh sieve. The cells were cultured in triplicate wells of flat-bottomed 96-well plates at a concentration of 2.5 × 104 cells/ml, and incubated with medium alone or in medium containing a pool of rSap2 or hybrid phage (25 µg/ml). Twenty µl MTT (5mg/ml pH 7.4) was added after 72 h and incubated for 4 h. After removal of MTT, the formazan crystals was solubilized in DMSO (150 μl/well) and measured using a Microplate Reader (Thermo, USA) at 492 nm.
Measurement of cytokines in culture supernatants
Levels of cytokine in culture supernatants were measured by ELISA according to the manufacturer’s instruction (Wuhan Boster Biological Technology, LTD.). Splenocytes of immunized or control mice were prepared as described above. An amount of 5 × 106 splenocytes/ml was cultured in a final volume of 200 µl in 96-well flat-bottom plates in the presence of the relevant stimulants. Culture supernatants were collected after 48 h incubation to estimate cytokines production. The quantity of each cytokine produced in culture supernatant was obtained to determine a standard curve.
RNA extraction, cDNA synthesis, and RT-PCR
One week after the last immunization, spleens were aseptically dissected, sectioned into equal halves and transferred to 1.5 ml polypropylene tubes and frozen on ice. Trizol reagent was used for the extractions of RNA as the manufacturer’s instructions (Invitrogen). Reverse transcription reactions for first strand cDNA synthesis was also performed according to the manufacturer's protocol (Promega). The amplification reaction and primers used were described previously according to Liu (IL-2, IL-4, IFN-γ), Wang (IL-10, IL-12), and Wang (IL-17) ().Citation39-Citation41 The PCR products were then separated by electrophoresis in 1% agarose gels containing 1% ethidium bromide. Densitometric analysis of the bands was performed using the Gel Imager Program. The equivalence in intensity between each cytokine and β-actin indicates the relative concentration of mRNA.
Immunization and challenge
Mice were immunized 3 times. Two weeks after the final immunization, 3 mice of each group were inoculated with 106 C. albicans, 7 d after the infection; each of the challenged mice was sacrificed to count the number of C. albicans that migrated to kidney and spleen. Eight mice of each group were inoculated with 107 C. albicans, and repeat this sample about 2 times. The number of mice that died in 10 d post-infection was counted.
Statistical analysis
The Student t test was used to determine statistical significance between cytokine production and splenocytes proliferation of each immunized group and control group. The criterion for statistical significance was P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81028010 and 81373231).
References
- Vonk AG, Netea MG, van der Meer JWM, Kullberg BJ. Host defence against disseminated Candida albicans infection and implications for antifungal immunotherapy. Expert Opin Biol Ther 2006; 6:891 - 903; http://dx.doi.org/10.1517/14712598.6.9.891; PMID: 16918256
- Kullberg BJ, Oude Lashof AM. Epidemiology of opportunistic invasive mycoses. Eur J Med Res 2002; 7:183 - 91; PMID: 12069910
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309 - 17; http://dx.doi.org/10.1086/421946; PMID: 15306996
- Cassone A, Casadevall A. Recent progress in vaccines against fungal diseases. Curr Opin Microbiol 2012; 15:427 - 33; http://dx.doi.org/10.1016/j.mib.2012.04.004; PMID: 22564747
- Edwards JE Jr.. Fungal cell wall vaccines: an update. J Med Microbiol 2012; 61:895 - 903; http://dx.doi.org/10.1099/jmm.0.041665-0; PMID: 22267544
- Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol 2009; 2:403 - 11; http://dx.doi.org/10.1038/mi.2009.100; PMID: 19587639
- Fidel PL Jr., Ginsburg KA, Cutright JL, Wolf NA, Leaman D, Dunlap K, Sobel JD. Vaginal-associated immunity in women with recurrent vulvovaginal candidiasis: evidence for vaginal Th1-type responses following intravaginal challenge with Candida antigen. J Infect Dis 1997; 176:728 - 39; http://dx.doi.org/10.1086/514097; PMID: 9291322
- La Sala A, Urbani F, Torosantucci A, Cassone A, Ausiello CM. Mannoproteins from Candida albicans elicit a Th-type-1 cytokine profile in human Candida specific long-term T cell cultures. J Biol Regul Homeost Agents 1996; 10:8 - 12; PMID: 9049775
- Cutler JE, Deepe GS Jr., Klein BS. Advances in combating fungal diseases: vaccines on the threshold. Nat Rev Microbiol 2007; 5:13 - 28; http://dx.doi.org/10.1038/nrmicro1537; PMID: 17160002
- Casadevall A, Pirofski LA. Exploiting the redundancy in the immune system: vaccines can mediate protection by eliciting ‘unnatural’ immunity. J Exp Med 2003; 197:1401 - 4; http://dx.doi.org/10.1084/jem.20030637; PMID: 12782708
- Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med 1992; 176:19 - 25; http://dx.doi.org/10.1084/jem.176.1.19; PMID: 1535368
- Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf SF, Bistoni F. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J Immunol 1994; 153:5167 - 75; PMID: 7963574
- Taylor BN, Saavedra M, Fidel PL Jr.. Local Th1/Th2 cytokine production during experimental vaginal candidiasis: potential importance of transforming growth factor-β. Med Mycol 2000; 38:419 - 31; PMID: 11204879
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 2004; 190:624 - 31; http://dx.doi.org/10.1086/422329; PMID: 15243941
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 2009; 206:299 - 311; http://dx.doi.org/10.1084/jem.20081463; PMID: 19204111
- Pirofski LA, Casadevall A. Rethinking T cell immunity in oropharyngeal candidiasis. J Exp Med 2009; 206:269 - 73; http://dx.doi.org/10.1084/jem.20090093; PMID: 19204107
- Colina A-R, Aumont F, Deslauriers N, Belhumeur P, de Repentigny L. Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Infect Immun 1996; 64:4514 - 9; PMID: 8890200
- Goldman RC, Frost DJ, Capobianco JO, Kadam S, Rasmussen RR, Abad-Zapatero C. Antifungal drug targets: Candida secreted aspartyl protease and fungal wall beta-glucan synthesis. Infect Agents Dis 1995; 4:228 - 47; PMID: 8665087
- Rüchel R. Cleavage of immunoglobulins by pathogenic yeasts of the genus Candida. Microbiol Sci 1986; 3:316 - 9; PMID: 3153567
- de Repentigny L, Aumont F, Bernard K, Belhumeur P. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect Immun 2000; 68:3172 - 9; http://dx.doi.org/10.1128/IAI.68.6.3172-3179.2000; PMID: 10816460
- Soll DR. High-frequency switching in Candida albicans. Clin Microbiol Rev 1992; 5:183 - 203; PMID: 1576587
- Schweizer A, Rupp S, Taylor BN, Röllinghoff M, Schröppel K. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol Microbiol 2000; 38:435 - 45; http://dx.doi.org/10.1046/j.1365-2958.2000.02132.x; PMID: 11069668
- Vilanova M, Teixeira L, Caramalho I, Torrado E, Marques A, Madureira P, Ribeiro A, Ferreira P, Gama M, Demengeot J. Protection against systemic candidiasis in mice immunized with secreted aspartic proteinase 2. Immunology 2004; 111:334 - 42; http://dx.doi.org/10.1111/j.1365-2567.2004.01819.x; PMID: 15009435
- De Bernardis F, Amacker M, Arancia S, Sandini S, Gremion C, Zurbriggen R, Moser C, Cassone A. A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine 2012; 30:4490 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.04.069; PMID: 22561143
- Yang Q, Su QP, Wang GY, Wen DZ, Zhang YH, Bao HZ, Wang L. Production of hybrid phage displaying secreted aspartyl proteinase epitope of Candida albicans and its application for the diagnosis of disseminated candidiasis. Mycoses 2007; 50:165 - 71; http://dx.doi.org/10.1111/j.1439-0507.2006.01349.x; PMID: 17472610
- Romani L, Howard DH. Mechanisms of resistance to fungal infections. Curr Opin Immunol 1995; 7:517 - 23; http://dx.doi.org/10.1016/0952-7915(95)80097-2; PMID: 7495516
- Baker DG. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev 1998; 11:231 - 66; PMID: 9564563
- Puccetti P, Romani L, Bistoni F. A TH1-TH2-like switch in candidiasis: new perspectives for therapy. Trends Microbiol 1995; 3:237 - 40; http://dx.doi.org/10.1016/S0966-842X(00)88931-3; PMID: 7648032
- Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, d’Enfert C, Vecchiarelli A. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS One 2011; 6:e22770; http://dx.doi.org/10.1371/journal.pone.0022770; PMID: 21818387
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008; 28:454 - 67; http://dx.doi.org/10.1016/j.immuni.2008.03.004; PMID: 18400188
- Schaller M, Korting HC, Schäfer W, Bastert J, Chen W, Hube B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol 1999; 34:169 - 80; http://dx.doi.org/10.1046/j.1365-2958.1999.01590.x; PMID: 10540295
- Schaller M, Korting HC, Borelli C, Hamm G, Hube B. Candida albicans-secreted aspartic proteinases modify the epithelial cytokine response in an in vitro model of vaginal candidiasis. Infect Immun 2005; 73:2758 - 65; http://dx.doi.org/10.1128/IAI.73.5.2758-2765.2005; PMID: 15845479
- Heuer H, Binh CT, Jechalke S, Kopmann C, Zimmerling U, Krögerrecklenfort E, Ledger T, González B, Top E, Smalla K. IncP-1ε Plasmids are Important Vectors of Antibiotic Resistance Genes in Agricultural Systems: Diversification Driven by Class 1 Integron Gene Cassettes. Front Microbiol 2012; 3:2; http://dx.doi.org/10.3389/fmicb.2012.00002; PMID: 22279444
- Tongchusak S, Brusic V, Chaiyaroj SC. Promiscuous T cell epitope prediction of Candida albicans secretory aspartyl protienase family of proteins. Infect Genet Evol 2008; 8:467 - 73; http://dx.doi.org/10.1016/j.meegid.2007.09.006; PMID: 17974505
- Quanping S, Yanyan H, Yicun W, Zhigang J, Yuling G, Li W. The use of hybrid phage displaying antigen epitope and recombinant protein in the diagnosis of systemic Candida albicans infection in rabbits and cancer patients. Diagn Microbiol Infect Dis 2010; 68:382 - 9; http://dx.doi.org/10.1016/j.diagmicrobio.2010.07.009; PMID: 20884151
- Su QP, Wen DZ, Yang Q, Zhang YH, Liu C, Wang L. Comparison of phage pVIII and KLH as vector in inducing the production of cytokines in C57BL/6J mice. Vaccine 2007; 25:970 - 5; http://dx.doi.org/10.1016/j.vaccine.2006.08.045; PMID: 17055124
- Yang Q, Wang L, Lu DN, Gao RJ, Song JN, Hua PY, Yuan DW. Prophylactic vaccination with phage-displayed epitope of C. albicans elicits protective immune responses against systemic candidiasis in C57BL/6 mice. Vaccine 2005; 23:4088 - 96; http://dx.doi.org/10.1016/j.vaccine.2004.07.005; PMID: 15963364
- Prisco A, De Berardinis P. Filamentous bacteriophage fd as an antigen delivery system in vaccination. Int J Mol Sci 2012; 13:5179 - 94; http://dx.doi.org/10.3390/ijms13045179; PMID: 22606037
- Liu F, Ng TB, Fung MC. Pineal indoles stimulate the gene expression of immunomodulating cytokines. J Neural Transm 2001; 108:397 - 405; http://dx.doi.org/10.1007/s007020170061; PMID: 11475007
- Wang Y, Li SP, Moser SA, Bost KL, Domer JE. Cytokine involvement in immunomodulatory activity affected by Candida albicans mannan. Infect Immun 1998; 66:1384 - 91; PMID: 9529057
- Wang Y, Feng D, Liu G, Luo Q, Xu Y, Lin S, Fei J, Xu L. γ-aminobutyric acid transporter 1 negatively regulates T cell-mediated immune responses and ameliorates autoimmune inflammation in the CNS. J Immunol 2008; 181:8226 - 36; PMID: 19050239