Abstract
Generation and maintenance of high quantity and quality memory CD8+ T cells determine the level of protection from viral, bacterial, and parasitic re-infections, and hence constitutes a primary goal for T cell epitope-based human vaccines and immunotherapeutics. Phenotypically and functionally characterizing memory CD8+ T cells that provide protection against herpes simplex virus type 1 and type 2 (HSV-1 and HSV-2) infections, which cause blinding ocular herpes, genital herpes, and oro-facial herpes, is critical for better vaccine design. We have recently categorized 2 new major sub-populations of memory symptomatic and asymptomatic CD8+ T cells based on their phenotype, protective vs. pathogenic function, and anatomical locations. In this report we are discussing a new direction in developing T cell-based human herpes vaccines and immunotherapeutics based on the emerging new concept of “symptomatic and asymptomatic memory CD8+ T cells.”
Introduction
Following repeated infections, various populations of memory CD8+ T cells develop and might be separated into major sub-populations that differ in their phenotype, effector functions, proliferative capacity, anatomical locations, and long-term fates.Citation1-Citation4 However, the continuous evolution of the CD8+ T cell population over time following an infection often makes it difficult to track the developmental lineage of memory CD8+ T cells. Several research groups are presently studying the mechanisms of establishment, persistence, and regulation of local memory CD8+ T cells (i.e., tissue resident memory CD8+ T cells [TRM] and effector memory CD8+ T cells [TEM]). Local TRM and TEM cells reside within infected muco-cutaneous tissues and are relatively important, compared with central memory CD8+ T cells (TCM), in providing immediate protection against subsequent re-infection and reactivation.Citation5 However, until our recent work on HSV-1 and HSV-2-specific memory CD8+ T cells,Citation6 the segregation of memory CD8+ T cells into asymptomatic (ASYMP) memory CD8+ T cell sub-population (i.e., those memory CD8+ T cells that actually lead to protection against herpes) and symptomatic (SYMP) memory CD8+ T cell sub-populations (i.e., those memory CD8+ T cells that, in contrast, lead to immunopathology and perhaps even lead to the exacerbation of herpetic disease) has not been reported.
Ocular HSV-1 infection is the leading cause of viral induced corneal blindness in the United States.Citation6-Citation16 Genital HSV-2 infection is among the most common sexually transmitted viral infections.Citation8,Citation9,Citation17-Citation19 The prevalence of HSV seropositive adults, 15 y and older, is estimated to be at least 61 million within the United States, and over half billion worldwide.Citation20-Citation22 After the initial viral exposure, HSV-1 and HSV-2 replicate in mucosal epithelial cells causing primary clinical inflammatory manifestations characterized by painful mucocutaneous vaginal blisters, corneal disease, and oro-facial herpes (cold sore).Citation23-Citation29 Newly infected seronegative pregnant women can vertically transmit the virus to their newborns, causing encephalitis and death.Citation19,Citation30,Citation31 Once the primary infection is cleared the HSV establishes life-long latency in sensory neurons of dorsal and trigeminal ganglia.Citation32,Citation33 Several factors, including stress, elevated body temperature, UV exposure, and hormonal changes trigger HSV-1 and HSV-2 reactivation from latently infected sensory neurons. The reactivated virus travels back and re-infects the primary site of infection resulting in recurrent painful inflammatory lesions.Citation32 The infection process and the therapeutics currently used against HSV-1 and HSV-2 replication and fusion have been recently reviewed in.Citation34 Infected individuals must rely on sustained or intermittent antiviral drugs (Acyclovir and derivatives), sexual behavior education and barrier methods to limit the spread of HSV-1 and HSV-2.Citation35,Citation36 The effectiveness of antiviral therapy is sometimes limited by the development of antiviral resistance. Controlling the spread of herpes through vaccine or immunotherapeutic approaches still remains a challenge.Citation18,Citation22,Citation37-Citation39 It is generally known that: (1) Natural immunity does not efficiently protect against new or recurrent herpes infection or disease,Citation38,Citation40-Citation45 suggesting that an effective vaccine will have to induce a more vigorous immune response than sub-optimal natural immunity. (2) The immune system causes the majority of mucocutaneous herpetic lesions.Citation46 (3) Recurrent herpetic corneal disease is mediated by T cellsCitation47-Citation52 and in mice CD4+ and CD8+ effector T cells can either protect against or potentiate herpetic corneal disease.Citation53-Citation56 (4) Inducing local HSV-specific CD8+ T cell immunity at the sites of infection (i.e., the ocular, oral, and vaginal muco-cutaneous tissues, and sensory ganglia) or in the draining lymph node is necessary to prevent virus transmission/reactivation and/or limit the severity of ocular herpes.Citation16,Citation57-Citation60
This review: (1) describes our novel concept of “development of symptomatic/asymptomatic CD8+ T cell sub-populations”; (2) describes the spatial and temporal position of the new symptomatic and asymptomatic memory CD8+ T cell sub-populations within the widely known TEM, TRM, and TCM CD8+ T cell populations; (3) sheds the light on the mechanisms of induction, establishment, persistence, and regulation of symptomatic and asymptomatic memory CD8+ T cells in ocular, oral, and genital herpes infection models; and (4) discusses how the new “symptomatic/asymptomatic CD8+ T cell sub-populations” concept provides new immunological insights and a new direction for the design of efficient and safe T cell-based human vaccines and immunotherapeutics.
Heterogeneity of Symptomatic and Asymptomatic Memory CD8+ T Cells
After the clearance of pathogens, the vast majority (often as much as 90 to 95%) of effector CD8+ T cells die, leaving behind only 5 to 10% of effector CD8+ T cells to differentiate into a heterogeneous pool of memory CD8+ T cells.Citation61-Citation65 The level of heterogeneity of memory CD8+ T cells is an important aspect that has come to light over the past few years. Tracking the developmental lineage of CD8+ T cells between effector and memory stages is complicated by the continuously evolving memory CD8+ T cell sub-populations that develop long after the infections are cleared.Citation66-Citation73 These diverse populations of memory CD8+ T cells can at times be categorized into sub-populations that differ in their phenotype, effector functions, proliferative capacity, anatomical locations, and long-term fates.Citation61,Citation74
Phenotypic markers
Several phenotypic markers are used to separate diverse sub-populations of memory CD8+ T cells include: CD62L, IL-7R (CD127), CD69, CD11a, CCR5, CCR7, and CD103+ (known as α4β7 integrin) (anatomical localization), IL-2/IFN-γ/TNF-α, perforin, granzymes A/B/C/K, programmed death-1 (PD-1) (effector functions/dysfunction), Bcl-2, CD122, CD28, CD57, CD27, KLRG1, CXCR3, and CD43 (survival and/or proliferative capacity).Citation75-Citation77 Various factors affect the expression of these markers, including: (1) the type and duration of infection; (2) inflammatory cytokines; (3) antigen specificity; (4) naïve T cell precursor frequency; and (5) anatomic location within the host’s body.Citation9 Thus, CD8+ T cells may exhibit a range of differentiated phenotypes spanning from IL7RlowKLRG1high short-lived effector cells (SLECs) to IL7RhighKLRG1low memory precursor effector cells (MPECs) that are capable of generating long-lived memory CD8+ T cells,Citation71,Citation78-Citation80 TCM, TEM, and TRM cells.Citation81,Citation82
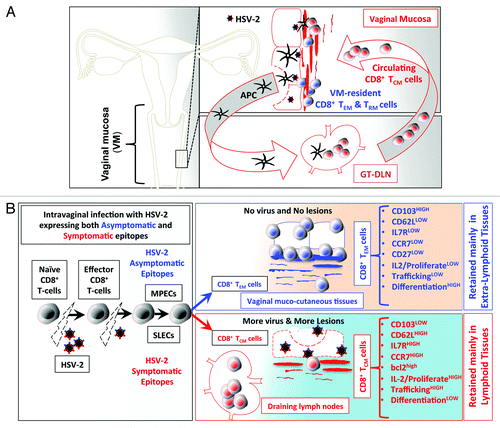
In addition to the 5 factors above, we recently found that symptomatic and asymptomatic epitope stimulations are among the major factors that affect the development of memory CD8+ T cell sub-populations and hence, they define the fate of antigen specific symptomatic and asymptomatic memory CD8+ T cells post-infection. For instance, following intravaginal infection with HSV-1 or HSV-2, asymptomatic epitopes appear to preferentially induce effector memory CD8+ TEM cells (). These asymptomatic CD8+ TEM cells are destined to survive and become long-lived CD8+ T cells that reside within the vaginal mucosal tissues, which are the sites of recurent infections and disease (). In contrast, symptomatic epitopes appear to preferentially induce central memory symptomatic CD8+ T cells that are not destined to survive within the mucosal tissues, but rather reside in the lymphoid organs ().
Figure 1. (A) Following intravaginal infection with HSV-2; stimulation with pathogenic symptomatic and protective asymptomatic human epitopes, expressed by the virus, greatly contributes to development of HSV-specific memory CD8+ T cell subsets, with either VM-resident effector memory (TEM) and tissue-resident memory (TRM) or lymphoid resident central memory CD8+ T cells (TCM). (See text for details). We hypothesize that virus derived symptomatic epitopes boost primarily CD8+ TCM, that reside primarily in the secondary lymphoid tissues, such as GT-DLN, which, once re-activated, re-circulate through the bloodstream into the vaginal mucosa (VM). In contrast, virus derived asymptomatic epitopes boost primarily VM-resident CD8+ TEM/TRM that are retained in the mucosal site of viral entry. (B). Anatomic-distribution, phenotypic, and functional characteristics of HSV-specific symptomatic central memory CD8+ T cells (TCM) vs. asymptomatic effector memory (TEM) CD8+ T-cells induced following intravaginal infection with HSV-2. Intra-vaginal (VAG) infection with HSV-2 bearing both symptomatic or asymptomatic epitopes prime naïve T cells into effector T cells to clear the virus infection while simultaneously generating memory CD8+ T cells to protect against subsequent encounter with the virus following re-infection or reactivation from latently infected sensory ganglia. We hypothesize that after the clearance of the virus, there is an optimum equilibrium between SLECs (IL7RlowKLRG1high) and MPECs (IL7RhighKLRG1low) cells derived from an early effector cells (EECs, IL7RlowKLRG1low), which then transit to 2 main categories of memory CD8+ T cells: TCM CD8+ T cells specific to symptomatic epitopes and TEM CD8+ T cells specific to asymptomatic epitopes. TEM CD8+ T-cells are mainly retained in the vaginal mucosa (see text for detail).
Functional assays
Functional assays, such as cytokine production, are combined with IL7R (CD127) and KLRG1 phenotyping to assist discriminate between MPECs and SLECs subsets. For instance, the IL-6 receptors is used to help distinguish memory precursor cell subsets, as the IL-6RαhighIL7Rhigh pre-memory CD8+ T cells survive after priming and contribute to the majority of functional memory CD8+ T cells after the contraction phase.Citation83 Compared with mono-functional CD8+ T cells that only produce one cytokine; poly-functional CD8+ T cells produce IFN-γ, TNF-α, and IL-2 and preferentially survive and develop into MPECs. While MPECs can give rise to long-lived TCM, some SLECs can give rise to a terminally differentiated population of TEM cells.Citation71,Citation84,Citation85 We recently showed that induction of polyfunctional CD8+ T cells is more protective against ocular and genital herpes than mono-functional CD8+ T cells.Citation6,Citation17 These poly-functional CD8+ T cells appeared to be a differentiated population of TEM cells. It remains to be determined whether symptomatic and asymptomatic epitope stimulation can lead to vaginal mucosa (VM)-resident CD8+ T cells differentiation into MPECs or SLECs.
Lectins and integrins
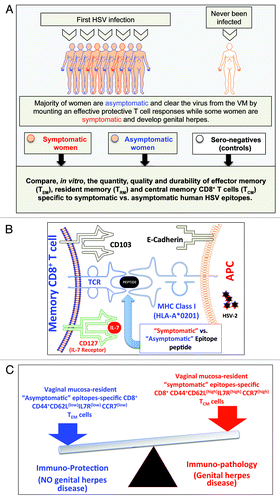
Lectins and integrins also contribute to heterogeneity of memory CD8+ T cells in the mucocutanoues tissues.Citation17,Citation86 High levels of α4β7 integrin (CD103) are expressed on mucosal tissue resident memory CD8+ T cells. CD103 binds to epithelial cadherin (E-Cadherin) expressed by epithelial cells. We found that compared with CD8+ T cells from HSV-seropositive symptomatic individuals, CD8+ T cells from HSV-seropositive asymptomatic individuals express high levels of CD103 (CD103high) (). Thus, CD103 and E-Cadherin interaction is crucial for retention of asymptomatic memory CD8+ TEM cells in the epithelium. The rapid upregulation of CD103 on asymptomatic CD8+ TRM/TEM cells is mediated by TGF-β, which plays a critical role during TRM/TEM differentiation and rapid control of infection in barrier tissues, such as skin and mucosa.Citation87,Citation88 We are currently investigating the underlying mechanisms of retention of asymptomatic memory CD8+ TRM/TEM cells within the ocular and vaginal mucosal tissues as well as the possible regulation by TGF-β of CD103 expression on ocular and vaginal tissue-resident HSV-specific memory CD8+ TRM/TEM cells.
Cytokines
The progression of the anti-viral CD8+ T cell response is controlled by cytokine milieu.Citation89-Citation94 The decision of an effector CD8+ T cell to develop into SLECs or MPECs appears to be regulated by the amount of inflammatory cytokines (i.e., IL-6, IL-8, and IL-12) present during T cell priming.Citation71 Memory T cell survival depends on IL-15, however, this alone is not sufficient for long-term maintenance of these cells.Citation69,Citation71,Citation95 The reliance of CD8+ T cell expansion on inflammatory cytokines (IL-12, IFN-α/β, and IFN-γ) or on IL-2, depends on the tissues in which they reside.Citation95-Citation99 In fact, a handful of inflammatory cytokines have been found to influence CD8+ T cell transition from the effector state to the memory state.Citation9 TGF-β also affects differentiation of memory T cells within the mucosa.Citation100 The mechanisms by which cytokine milieu controls the heterogeneity of symptomatic and asymptomatic memory CD8+ T cell subsets remain to be fully elucidated.
Intrinsic (transcription) factors
Many survival and transcriptional factors are involved in the regulation of effector state to memory state transition.Citation71,Citation101 These include Bcl2, Eomes, Tcf1, Blimp1, Id2, Id3, and T-bet.Citation102 T-bet is a key lineage-determining transcription factor that controls the fate SLECs/MPECs during infection.Citation68,Citation71,Citation103 Depending on the amount of inflammation, a gradient of T-bet is created in which high T-bet promotes SLECs and low T-bet promotes MPECs.Citation71,Citation104 The control of intrinsic (transcription) factors in the heterogeneity of symptomatic and asymptomatic memory CD8+ T cells also remain to be elucidated.
Anatomical locations
Anatomical locations play a crucial role in the distribution of memory CD8+ T cell subsets in different organs. Mucosal surfaces, that constitute an impressive and powerful first-line of defense system that is frequently exposed to an array of exogenous antigens and pathogens, contain mainly CD8+ TRM/TEM cells.Citation6,Citation9 The mucosal immune system is largely separate and distinct from the systemic immune system. Several infectious diseases with the highest rates of morbidity and mortality begin primarily as local infections at one of the mucosal barrier sites. CD8+ TRM/TEM cells from muco-cutaneous tissues, such as the skin or mucosal lining of the respiratory tract, gut, and genital tract, defend against pathogen invasion. As illustrated in and , with respect to symptomatic/asymptomatic memory CD8+ T cells, most asymptomatic CD8+ T cells appear to reside in non-lymphoid mucosal tissues. Additionally, they have a decreased ability to traffic to lymphoid tissues and appear more terminally differentiated due to a lower proliferation capacity in response to Ag or homeostatic cytokines (IL-15 and IL-7).Citation95,Citation105-Citation107 In contrast, most symptomatic CD8+ T cells that are traditionally defined by the presence of CD62L and/or CCR7 lymph-node-directing molecules and low expression of chemokine receptors, such as CCR5, appear to be associated with homing to inflammatory sites.Citation108
Figure 2. A proposed model of phenotypic and functional characteristics of HSV-specific CD8+ T cells in HSV-seropositive symptomatic vs. asymptomatic individuals: (A) Majority of women are asymptomatic and clear the virus from the VM by mounting a protective T cell responses while some women are symptomatic and develop genital herpes. Significant differences in the quantity, the quality and the durability of effector memory (TEM), tissue-resident memory (TRM) and central memory(TCM) CD8+ T cells specific to symptomatic vs. asymptomatic human HSV-2 epitopes are detected in symptomatic and asymptomatic women. (B) Potential interactions between an APC, presenting an asymptomatic vs. symptomatic epitope, (right) and a memory CD8+ T cell (left) occur in HSV-2 infected vaginal mucosa, and appear to be mediated by TCR/MHC, CD103/E-Cadherin, and CD127-IL7 pathways. (C) The balance between the asymptomatic vs. symptomatic epitopes will determine herpes protection vs. immunopathology.
Establishing HSV-Specific Asymptomatic Memory CD8+ T Cell Responses at the Mucosal Tissues
Based on T cell circulation, tissues are characterized into 3 classes: permissive tissues, effector permissive tissues and restrictive tissues.Citation9
Permissive tissues
Permissive tissues are readily accessible by both effector and CD8+ TEM cells, even in the absence of local inflammation or antigens. These constitute the spleen, lung, liver, kidney, and adipose tissue.Citation109-Citation111
Effector permissive tissues
Effector permissive tissues are attainable by effector CD8+ T cells, but not by asymptomatic memory CD8+ TEM and TCM cells. These tissues are generally colonized by CD8+ T cells early during the effector phase and consist of the gut, brain, and peritoneal cavity. Though the presence of antigens or inflammatory factors may enhance recruitment, migration of effector CD8+ T cells to these tissues does not require direct infection. These tissues also become inaccessible to circulating symptomatic memory CD8+ T cells after resolution of infection.Citation72,Citation112-Citation114
Restrictive tissues
Restrictive tissues are inaccessible by either effector or memory CD8+ T cells at steady-state of infection and are only accessible to effector CD8+ T cells when there is a local inflammation. These tissues include the skin epidermis, vaginal epithelial layer, salivary glands, lung airways, cornea, and the sensory ganglia—all of which lack tissue tropic chemokines or adhesion molecules. Thus, systemic and local immunization at distal sites may often result in a diminished level of access for effector T cells into restrictive tissues.Citation72,Citation112-Citation114
Many infections, including HSV-1 and HSV-2, occur at the ocular, oral and vaginal mucosal tissues.Citation17,Citation18,Citation115,Citation116 At the steady-state, the mucosal epithelial layer and the sub-mucosa are protected by innate leukocytes and lymphocytes. The restrictive mucosal tissues are inaccessible to effector and memory CD8+ T cells at a steady-state but can be reached by effector CD8+ T cells when there is a local inflammation induced, for example, by primary or recurrent herpes infection.Citation115-Citation117 Thus, the female genital tract is an immunologically restrictive tissue that normally prevents entry of activated T cells in the absence of inflammation or infection.Citation115 Recruitment of HSV-specific CD8+ T cells to the vaginal mucosal (VM) tissues is therefore thought to be restricted because effector and memory CD8+ T cells does not circulate through the genital tract.Citation115-Citation117 However, while the VM tissue is considered mainly an effector site for CD8+ T cells, recent data demonstrates that the VM tissue may also be an inductive site for CD8+ T cells (Wang Y., AAI 2013, Abstract #3186). CD8+ T cells persist in the VM tissue at the dermal–epidermal junction (DEJ)—the portal of release of viral particles that reactivate from latently infected neurons of sensory ganglia—for prolonged periods of time after herpes lesions are cleared.Citation118,Citation119 However, the mechanisms by which vaginal DEJ-resident memory CD8+ T cells are generated and maintained remain to be fully elucidated. Recently, it has been reported that CD8αα+ T cells resident in DEJ tissue play an important role in immune surveillance and in initial containment of HSV-2 reactivation in human VM tissue.Citation119 Generation of long-term VM-resident memory CD8+ T cell immunity against sexually transmitted pathogens depends on the formation of long-lasting asymptomatic memory TEM and TRM CD8+ cells.Citation68,Citation71,Citation95,Citation120,Citation121 Compared with other mucosal tissues, such as the gut, induction of VM-resident CD8+ T cell immunity has received much less attention. In general, parenteral immunization induces systemic but not mucosal immune responses, while mucosal immunization induces both systemic and mucosal immune responses.Citation9,Citation17,Citation122-Citation124 We have recently described, for the first time, a Lipo/rAdv5 prime/boost mucosal vaccine delivered intravaginally (IVAG) that induced robust and long-lived HSV-specific asymptomatic CD8+ T cell responses which protect against genital herpes infection and disease.Citation17,Citation18 The induced long-lasting memory CD8+ T cell responses persisted in both the VM and GT-DLN for up to 8 mo post immunization. To avoid exacerbation of vaginal mucosal disease it is imperative to avoid inducing pathogenic symptomatic CD8+ T cells. Our lab is curently exploring the determining factors that regulate the induction, persistence, and retention of asymptomatic CD8+ TEM cells witin the ocular and genital mucosal tissues, the sites of primary and recurent HSV-1 and HSV-2 infections, respectively (). Additionally, the development of asymptomatic CD8+ T cells whithin the ocular and genital mucosal tissues most likely displays a more profound complexity and is more uniquely regulated in comparison to the development on other mucosal surfaces.Citation115,Citation125
In the following paragraphs we will discuss our novel concept of “symptomatic/asymptomatic CD8+ T cell development” within the widely known TEM, TRM, and TCM CD8+ cell populations. It will also shed some light on the mechanisms of induction, establishment, persistence, and regulation of “asymptomatic” memory CD8+ T cells at the ocular and genital mucocutaneous tissues and the new direction for the design of efficient and safe “asymptomatic” T cell-based human vaccines and immunotherapeutics.
Contribution of Memory Precursor Effector Cells (MPECs) and Short-Lived Effector Cells (SLECs) in the Development of Symptomatic vs. Asymptomatic Memory CD8+ T Cells
T cell responses to a primary viral infection are optimally designed to generate large numbers of effector CD8+ T cells that clear the virus infection while simultaneously generating memory CD8+ T cells that protect against subsequent encounter with the virus following re-infection or reactivation. During a primary T cell response, the effector T cell pool contains 2 cellular subsets: short-lived effector cells (SLECs), a majority of which undergo apoptosis, and the memory precursor effector cells (MPECs) that differentiate into memory cells.Citation126-Citation129 To ensure the balance between these equally essential processes, CD8+ T cells integrate multiple signals and undergo an exponential increase in the number of T cells that is associated with a dynamic CD8+ T cell differentiation process.Citation130 Such regulation results in the equilibrium between SLECs (IL7RlowKLRG1high) and MPECs (IL7RhighKLRG1low) that are derived from early effector cells (EECs, IL7RlowKLRG1low).Citation130 What properties distinguish terminally differentiated CD8+ T cell sub-populations and how this process influences HSV-specific memory CD8+ T cell developments in the ocular, oral, and genital mucosal tissues are important questions that remain to be answered. Interestingly, recent results from our laboratory suggest that CD8+ T cells specific to herpes asymptomatic epitopes tend to have mainly a SLECs phenotype while CD8+ T cells specific to herpes symptomatic epitopes tend to have mainly a MPECs phenotype (Khan et al., unpublished data).
In general, after several rounds of cell division, the proliferative potential of effector CD8+ T cells declines and they become highly sensitive to apoptosis and death.Citation95,Citation131 In contrast, memory CD8+ T cells are multi-potent and, thus, are less terminally differentiated (they can remain resting memory CD8+ T cells or re-differentiate into effector CD8+ T cells at any time), can self-renew, have a high proliferative potential and increased longevity.Citation71,Citation95,Citation131-Citation135 Recent genomic and proteomic studies revealed many differences between gene expression profiles of memory and effector CD8+ T cell.Citation136,Citation137 During early infection, virus-specific effector cells that are generated from SLECs, (KLRG1highIL7Rlow) rarely persist into “memory” time points as they often die from apoptosis once the acute infection is cleared. In contrast, memory CD8+ T cells generated from MPECs (KLRG1lowIL7Rhigh) often develop into a mixture of TEM, TRM, and TCM cells.Citation71,Citation138-Citation140 We hypothesize that the delicate balance of MPEC vs. SLEC and the TCM vs. TRM/TEM cell lineages decisions is strongly influenced by the repertoire of TCR signals that are generated by symptomatic and asymptomatic epitopes displayed by a viral pathogen. Specifically, TCR recognition of asymptomatic epitopes will drive development of CD8+ T cells with preferentially SLEC phenotype, while recognition of symptomatic epitopes will drive preferentially MPEC phenotype. The challenge we now face is to determine the molecular mechanisms that lead symptomatic and asymptomatic epitopes-specific effector CD8+ T cell development to ultimately choose the MPEC vs. SLEC fate following an infection.Citation141 A better knowledge of MPECs and SLECs regulation and its association with symptomatic and asymptomatic T cells responses should help develop a safe and effective herpes vaccine.Citation126-Citation129
Following CD8+ T cell responses to a primary viral infection, the majority of SLECs undergo apoptosis, while the MPECs differentiate into memory cells.Citation126-Citation129 The equilibrium between MPEC vs. SLEC and the TCM vs. TEM cell lineage are integrated by multiple external and internal signals associated with an exponential increase in the number of cells and a dynamic CD8+ T cell differentiation process.Citation130 This review highlights how symptomatic and asymptomatic immune responses contribute in shaping the MPEC vs. SLEC and the TCM vs. TEM cell fate. The symptomatic and asymptomatic mechanisms that lead to terminally differentiated CD8+ T cells and how this process influences memory CD8+ T cell developments in the ocular and genital mucosal tissues are important questions that remain to be answered. We hypothesize that the delicate balance of MPEC vs. SLEC and the TCM vs. TEM cell lineages decisions is likely influenced by the repertoire of TCR signals that are generated by “symptomatic” and “asymptomatic” epitopes displayed by a viral pathogen.
Anatomic-Distribution, Phenotypic, and Functional Characteristics of Various Subsets of Symptomatic and Asymptomatic Memory CD8+ T Cells
Although memory CD8+ T cell sub-populations are widely heterogeneous in terms of their phenotype, function, and anatomic distribution, they can be divided into 3 major subtypes: (1) the effector memory CD8+ T cells (TEM); (2) the central memory CD8+ T cells (TCM); and (3) the tissue-resident memory CD8+ T cells (TRM).Citation142 These 3 major populations of memory T cells play cooperative and complementary roles in protecting the host from re-infection.Citation143,Citation144 As illustrated in , these 3 populations differ in their anatomic-distribution, phenotype, and function.
Anatomic distribution
TCM cells circulate in blood and reside mainly in lymphoid tissues. TEM and TRM cells are retained in tissues within the portal entry sites of potential invading pathogens, such as the ocular, oral and vaginal muco-cutaneous tissues. They are rapidly mobilized (within hours of re-infections). While CD8+ TEM cells are also present in blood and splenic red pulp, they are the dominant T cell sub-population in extra-lymphoid tissues.Citation88,Citation145 TEM are sequestered in epithelia and underlying stroma,Citation88 and protect these tissues.Citation144,Citation146 CD8+ TEM cells have altered T cell trafficking patterns due to: (1) downregulation of T cell homing molecules (CD62L, CCR7)Citation147,Citation148; and (2) constitutive upregulation of non-lymphoid homing lectins, integrins, and chemokine receptors. Unique skin-resident HSV-specific CD8+ TEM/TRM cell subset have recently been reported to persist up to 8 mo after clearance of skin infection.Citation17,Citation86 They express high level of αE integrin (CD103). CD103 binds to epithelial cadherin (E-Cadherin) in epithelial cells. CD103-E-Cadherin binding is critical for the retention of CD8+ TEM/TRM cells within the epithelium. The role of TEM/TRM cells in protection against ocular, oral, and genital herpes, as often seen in asymptomatic individuals, remains to be determined.
Phenotype
CD8+ TCM cells are CD8+CD103lowCD62LhighCCR7high. CD8+ TEM cells are CD8+CD103lowCD62LlowCCR7low. CD8+ TRM cells are CD8+CD103highCD62LlowCCR7low. Markers other than CD103, CD62L, and CCR7 that are used to identify the CD8+ TRM cell sub-population include a high expression of CD11a, CD49a, and CD69.Citation142,Citation149-Citation151
Function
CD8+ TCM cells have high proliferation potential and high capacity to secrete IL-2 upon re-stimulation.Citation88,Citation108,Citation152 CD8+ TCM cells must undergo differentiation for effector functions (e.g., cytotoxic and cytokine production).Citation88,Citation153,Citation154 In contrast, CD8+ TEM/TRM cells that reside in extra-lymphoid tissues are already differentiated and poised for immediate effector function.Citation143 Tissue resident CD8+ TRM cells are potent effectors that provide rapid long-term protection against tissue re-infection, express constitutively high levels of granzyme B, and eliminate infected target cells with secreted perforin.Citation82,Citation155-Citation158 After clearance of epithelial HSV-1 and HSV-2 infections, CD8+ TRM cells resident in the skin contain lower perforin and granzyme transcripts as compared with the effector phase.Citation159-Citation161 However, HSV-specific CD8+ T cells in the sensory ganglia express high level of granzymes and perforin due to TCR engagement by latently infected neurons, which are critical for suppressing reactivation of HSV-1 and HSV-2.Citation82,Citation158-Citation160 Tissue resident CD8+ TRM cells produce high levels of effector IFN-γ, TNF-α, IL-22, and IL-17 cytokines and chemokines such as MIP-1, upon viral antigen re-encounter.Citation162 Tissue resident CD8+ TRM cells efficiently and immediately interfere with virus replication in peripheral non-lymphoid tissues, such as the skin. Tissue resident CD8+ TRM cells express cytotoxic granules to provide rapid cytotoxic response against viral infections.Citation88,Citation163 We have recently showed that vaginal mucosa-resident HSV-specific memory CD8+ T cells induced by our intravaginal Lipo/rAdv prime-boost vaccine is correlated with protection against genital herpes infection and disease (R2 = 0.7836; P < 0.0001).Citation17 However, the relative contribution of TEM/TRM vs. TCM in long-term protective memory against genital herpes remains to be elucidated.
Development of Central Memory (TCM), Effector Memory (TEM), and Tissue-Resident Memory (TRM) CD8+ T Cells in Symptomatic vs. Asymptomatic Settings
Understanding the molecular mechanisms by which memory CD8+ T cells are established and maintained within the tissues will allow us to develop new vaccine and immunotherapeutic approaches to induce antigen-specific activation vs. tolerance depending on patient's clinical needs. Memory CD8+ T cells can survive long-term in the absence of antigens (over 2 y in mice and over 50 y in humans).Citation68,Citation71,Citation95,Citation120,Citation121 As mentionned above, there are several subsets of memory T cells, including central memory (TCM), effector memory (TEM), and tissue-resident memory (TRM) cells (based on CD62L, IL7R, CCR7, CD11a, and CD103 expression).Citation95 TCM cells are mainly CD8+CD103lowCD62LhighCCR7high. TEM cells are mainly CD8+CD103lowCD62LlowCCR7low. Another sub-population, has been recently described as permanently residing in peripheral tissues, is called tissue-resident memory CD8+ T cells (TRM cells)Citation142,Citation149-Citation151 and is CD8+CD103highCD62LlowCCR7low. TRM cells are also CD11ahigh, CD49ahigh, and CD69high. Although central memory CD8+ T cells (TCM) appear to provide some protection against systemic infection, TEM and TRM cells have special features that make them well suited to respond quickly and effectively when infection is localized to peripheral compartments, such as the vaginal, the oral, and the ocular mucosal surfaces. As mentioned above, CD8+ TCM vs. CD8+ TEM/TRM cell lineage decision is influenced by the nature and strength of TCR signaling and IL-2 in addition to IL-15 and other exogenous and endogenous factors.Citation164,Citation165 HSV-specifc TRM cells are preferentially retained in close proximity to the epidermis and peripheral nerves in vaginal muco-cutaneous tissues, following HSV-1 and HSV-2 infections, whereas clusters of neuronal TRM cells are retained in areas of previous infections for at least several weeks.Citation82,Citation151,Citation155-Citation159,Citation161,Citation166 Our lab is actively engaged in determining the relative contribution of HSV symptomatic vs. asymptomatic epitopes in the induction of CD8+ TCM, TEM, and TRM cell sub-populations and their homing in lymphoid vs. the muco-cutaneous tissues. Additionally, our lab is investigating the role of symptomatic vs. asymptomatic CD8+ T cell sub-populations in the protection against herpes at the ocular (HSV-1), oral (HSV-1) vaginal (HSV-1/HSV-2) muco-cutaneous sites of infection. The project involves in vitro studies in symptomatic vs. asymptomatic humans as well as in vivo studies using our novel susceptible “humanized” HLA transgenic mouse, guinea pig, and rabbit models of ocular, oral, and genital herpes. Determining how CD8+ TCM, TEM and TRM cell sub-population develop and protect against infections and diseases that might tightly depend on the models by which symptomatic vs. asymptomatic epitope-specific memory CD8+ T cells develop.
Models for Memory CD8+ T Cells Development Within Symptomatic and Asymptomatic Memory CD8+ T Cells Concept
After an acute infection, the memory CD8+ T cell population evolves progressively over time into sub-populations that are enriched with cells with higher proliferative capacity, greater longevity, and with slight alterations during latent, chronic, and persistent phases of infections.Citation149,Citation167-Citation169 How effector CD8+ T cell differentiation is balanced to permit the formation of effector cell properties in the MPECs and yet still prevents the MPECs from acquiring a terminal SLECs state is still controversial. More so, it is also unclear whether naïve (N) and effector (E) CD8+ T cells specific to symptomatic vs. asymptomatic epitopes follow a different path of development into memory (M) cells (i.e., designated in this review as symptomatic vs. asymptomatic N > > > E > > > M transitions). Nevertheless, based on the studies from many murine models of persistent infections, 3 major models have been proposed to explain naïve to effector to memory CD8+ T cell transitions:
Model 1: De-differentiation model
De-differentiation model implies that after antigenic activation most naïve CD8+ T cells reach a terminally differentiated effector stage, where the cells become fully functional effector CD8+ T cells that have cytotoxic activity and produce cytokines. However, some effector CD8+ T cells are capable of de-differentiating into memory CD8+ T cells that gain longevity and a high proliferative potential (). During an effector to memory transition, MPECs gradually acquire proliferative and survival capacity and produce IL-2, Bcl-2, and CD62L.Citation170-Citation176 The underlying mechanisms behind this functional maturation remain to be fully elucidated. However, the exposure to IL-2 during infection, the presence of CD4+ T helper cells, and the maintenance of lower level of expression of T-bet appear to be important factors for establishing a memory CD8+ T cell population with a higher proliferative capacity.Citation95,Citation105,Citation137,Citation165,Citation177-Citation180 The de-differentiation model would elegantly fit into the ocular, oral, and genital herpes asymptomatic infections. In theory, after activation by both symptomatic and asymptomatic epitopes, most CD8+ T cells would reach a terminally differentiated effector stage but only asymptomatic epitopes-specific CD8+ T cells would be able to de-differentiate into memory CD8+ TEM/TRM cells, as illustrated in . We are currently trying to test the de-differentiation model in ocular, oral, and genital herpes asymptomatic infections and to understand the contribution of symptomatic and asymptomatic stimulations in the phenotypic and functional maturation of CD8+ T cells during the naïve to effector to memory transitions.
Figure 3. Models for generating diverse differentiated states of effector and memory CD8+ T cells following stimulation by symptomatic vs. asymptomatic epitopes. (A) First working model of de-differentiation for generating CD8+ T cell diversity proposes that soon after symptomatic or asymptomatic activation daughter naïve CD8+ cells become fully differentiated and functional by producing cytokines and exhibiting cytotoxic activity. The majority of effector CD8+ T cell then die. While a minority of asymptomatic epitope specific CD8+ T cells will de-differentiate into MPEC and long-lived memory CD8+ T cells. (B) Second working linear model for generating CD8+ T cell diversity proposes that degree of effector cell differentiation is regulated by the duration of exposure of daughter cells to symptomatic and asymptomatic epitopes following repetitive reactivation of HSV-2 from latency. The majority of terminally differentiated effector CD8+ T cell will die following cumulative encounter with the virus. While a minority of asymptomatic epitope specific CD8+ T cells which did not reach the end stage of differentiation will develop into MPEC and long-lived memory CD8+ T cells. The memory CD8+ T cell phenotypes vary according to the differentiation state of the effector cells from which they descended; curved arrows with bold, thin, or dashed lines indicate a high, medium, or low degree of proliferative potential and longevity. (C) Third working divergent model for generating CD8+ T cell diversity is similar to B, except that the strength of symptomatic vs. asymptomatic signal (instead of the cumulative symptomatic vs. asymptomatic stimulation) will determine the degree of effector cell differentiation and formation MPEC and long-lived memory CD8+ T cells.
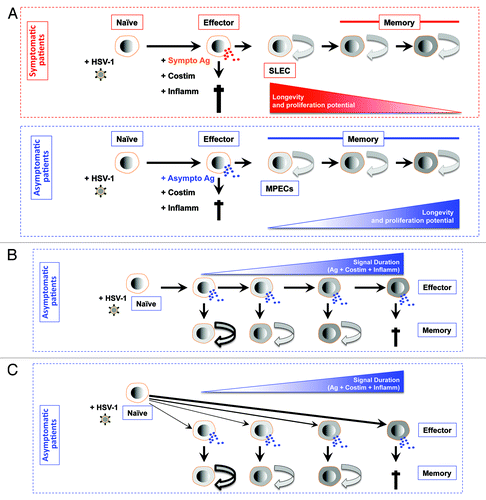
Model 2: Decreasing potential or linear model
Decreasing potential or linear model implies that after antigenic stimulation, naïve CD8+ T cells differentiate into a stepwise manner and, in doing so, progressively acquire a more terminally differentiated phenotype. Thus, CD8+ T cells that become MPECs do not progress as far as the SLECs (). In this model, the progression of differentiation may be controlled by successive antigenic stimulations of CD8+ T cells.Citation95,Citation181,Citation182 This model would also fit ocular, oral, and genital herpes symptomatic and asymptomatic concept. In theory, during the latency/reactivation cycles, spontaneous intermittent reactivation of the virus from latency leads to successive antigenic stimulation of CD8+ T cells with symptomatic and asymptomatic epitopes expressed by the virus proteins. Thus, the differentiation state of an effector CD8+ T cell would reflect the number of times in which CD8+ T cells encountered either symptomatic or asymptomatic epitopes during infection/reactivation. This linear model would provide a likely mechanism for generating a spectrum of symptomatic or asymptomatic effector and memory CD8+ T cells.Citation95,Citation108,Citation181,Citation183,Citation184 In the case of HSV latent infection, the successive reactivation of the virus and re-stimulation of CD8+ T cells with symptomatic vs. asymptomatic epitopes will contribute to dictating phenotypical and functional maturation of CD8+ T cells throughout the naïve to effector to memory transition, as illustrated in . The successive stimulation of CD8+ T cells with asymptomatic epitopes would lead to the formation of more TEM/TRM cells and more CD8+ T cell memory to effector transitions. In contrast, a successive stimulation of CD8+ T cells with symptomatic epitopes will lead to TCM and less memory to effector transitions.
Model 3: Divergent model
Divergent model for generating CD8+ T cell diversity proposes that soon after antigenic activation, daughter CD8+ T cells are instructed to generate either SLECs or MPECs subsets, as illustrated in . Although the divergent model is asymmetric and produces only 2 cell types, it also incorporates a range of “in-between” differentiated states that depend on the overall nature and strength of symptomatic vs. asymptomatic epitopes stimulation encountered by a T cell at or near the time of priming.Citation95,Citation185-Citation188 Following a first encounter with an antigen, dividing CD8+ T cells exhibit unequal partitioning of symptomatic and asymptomatic epitopes that mediate signaling, cell fate specification, and an asymetric cell division and lead to the differentiation toward either an effector or memory T cell lineage.Citation189 This model would also suit the scenario where a primary ocular, oral, or genital herpes infection would lead to various strengths of CD8+ T cell stimulations depending on whether HSV symptomatic or asymptomatic epitopes were encountered. The differentiation state of an effector CD8+ T cell would then denote the strength of symptomatic or asymptomatic T cell epitope signal during the priming of naïve CD8+ T cells, as illustrated in . Specifically, a strong stimulation of naïve CD8+ T cells with asymptomatic epitopes would lead to the formation of more TEM/TRM and more CD8+ T cell memory to effector transitions. In contrast, a robust stimulation of CD8+ T cells with symptomatic epitopes would lead to TCM and less memory to effector transitions.
The Role Of Cytokines In Symptomatic Vs. Asymptomatic Memory CD8+ T Cell Development
As mentioned above, a handful of inflammatory cytokines influence CD8+ T cell transition from effector to memory state.Citation190 IL-12, IFN-α/β, and IFN-γ inflammatory cytokines strongly enhance the expansion of effector CD8+ T cells, their cytotoxic activity, and the production of antiviral cytokines.Citation95-Citation98,Citation103,Citation131,Citation191-Citation194 Optimal effect of IL-12, IFN-α/β, and IFN-γ inflammatory cytokines appear to occur particularly when effector CD8+ T cells are activated by weak stimuli or following a cross-presentation of antigens. However, these inflammatory cytokines may also act as a double-edged sword: (1) on one hand, they stimulate effector CD8+ T cell expansion and function; (2) on the other hand, they drive the terminal maturation of effector CD8+ T cells, hence, limitimg the potential transition of effector to memory state. Among the IL-12, IFN-α/β, and IFN-γ inflammatory cytokines, the role of IFN-γ in the transition of effector to memory still remains controversial. A recent work has shown that IFN-γ promotes effector CD8+ T cell contraction and downregulation of IL7R.Citation95,Citation193 Yet, another recent report showed that IFN-γ receptor (IFN-γR) is needed for normal memory CD8+ T cell formation.Citation95 In IL12−/− mice, a higher frequency of IL7Rhigh MPECs and memory CD8+ T cells form following infection,Citation95,Citation97,Citation103,Citation194 suggesting that IL-12 plays a critical role in the effector CD8+ T cell fate decisions. Our recent findings showed that stimulation of CD8+ T cells with HSV-1 asymptomatic epitopes preferentially induced poly-functional T cells that produce IL-12, TNF-α, and IFN-γ inflammatory cytokines.Citation6 In contrast, stimulation of CD8+ T cells with HSV-1 symptomatic epitopes preferentially induced mono-functional T cells.Citation6 Moreover, we found that HSV-1 asymptomatic epitopes preferentially induced CD8+ TEM cells (Khan et al., unpublished data). In contrast, HSV-1 symptomatic epitopes favor induction of CD8+ TCM cells (Khan et al., unpublished data). We, therefore, hypothesize that the delicate balance of TCM vs. TEM cell lineages decision is strongly influenced by the profile of cytokines generated following stimulation with symptomatic vs. asymptomatic epitopes. Specifically, the recognition of asymptomatic epitopes will drive the development of tissue-resident memory CD8+ T cells with preferentially a TEM/TRM phenotype, while the recognition of symptomatic epitopes will drive preferentially a TCM phenotype.
Homeostatic cytokines IL-7 and IL-15 are also critical for the long-term survival and turnover of memory CD8+ T cells.Citation85,Citation95,Citation195 Prolonged deprivation of IL-7 and IL-15 homeostatic cytokines has considerable consequences on the formation and maintenance of memory CD8+ T cells.Citation95,Citation121,Citation177,Citation196-Citation199 Formation of KLRG1highIL-7Rlow SLECs depends on production of IL-15, but this alone is not sufficient to maintain these cells for a long-term period.Citation69,Citation71,Citation95 Once T cells enter peripheral tissues, various molecules from tissue microenvironment may further influence their effector heterogeneity. For example, TGF-β have various tissue-specific effects on the differentiation of T cells in the intestinal mucosa,Citation100 but its effect on ocular, oral, and vaginal mucosa-resident CD8+ T cells remains to be determined. In general, a rapid upregulation of CD103, an integrin that is expressed by T cells and required for tissue retention, is mediated by TGF-β1.Citation87
Given the importance of tissue-resident memory CD8+ TEM/TRM cells in providing local protection against subsequent HSV re-infection and reactivation, the factors that regulate memory CD8+ T cell retention within the ocular, oral, and vaginal mucosa where protection is required is not yet known. To date, virtually no results are available on the mechanisms involved in the retention of memory CD8+ T cells within the ocular, oral, and vaginal mucosa. We have recently demonstratedCitation17,Citation18 that: (1) targeting the vaginal mucosa (VM) with a lipopeptide/recombinant adenovirus (Lipo/rAdv) heterologous prime/boost vaccine, containing a mouse CD8+ T cell epitope, induced a robust and durable VM-resident CD8+ T cells that protects against genital herpes infections and disease; and (2) VM-resident IFN-γ-producing CD8+ T cell responses correlated positively with protection (R2 = 0.7836; P < 0.0001). Moreover, we found a higher expression of CD103 on asymptomatic CD8+ T cells vs. symptomatic CD8+ T cells, responding to HSV-2 infection (unpublished data). These results suggest that priming conditions dominated by asymptomatic viral epitopes stimulation may increase CD103 expression on memory CD8+ T cells and, thus, might promote long-lasting retention of memory CD8+ T cells within the VM ( and ). Even so, the mechanisms that regulate the retention of HSV-specific memory CD8+ T cells in the ocular and mucosal tissues; and particularly (1) the role of CD103 in the retention of ocular and vaginal mucosa-resident HSV-specific CD8+ T cells; (2) whether the expression of CD103 on ocular, oral, and vaginal mucosa-resident HSV-specific memory CD8+ T cells are affected with TGF-β; and (3) whether stimulation with symptomatic vs. asymptomatic epitopes would affect the expression of CD103, and the production of inflammatory and homeostatic cytokines by memory CD8+ T cells and hence affect memory CD8+ T cells development and retention within the peripheral tissues, such as the ocular, oral, and vaginal mucosal tissues remain largely unexplored.
The Role of Cell Intrinsic (Transcription) and Epigenetic Factors in the Development of Symptomatic Vs. Asymptomatic Memory CD8+ T Cells
As mentioned above, modulation of many cell-intrinsic factors, in part achieved through epigenetic modifications of DNA and histones, are involved in the regulation of effector to memory transition. Transcription factors that regulate effector T cell differentiation, such as T-bet, controls SLEC vs. MPEC fate decisions through its level of expression.Citation69,Citation71,Citation103,Citation194 Higher expression of T-bet favors the formation of KLRG1highIL7Rlow SLECs while lower expression of T-bet promotes KLRG1lowIL7Rhigh MPECs.Citation69,Citation71,Citation95 Similar to T-bet, the transcriptional regulator ID2 (inhibitor of differentiation 2) plays a critical role in effector to memory CD8+ T cell transition.Citation95,Citation200 ID2-deficient CD8+ T cells have a more IL7RhighCD27high “MPEC-like” phenotype.Citation95,Citation200 Another transcription factor, Blimp-1 (prdm1) is expressed at high levels by KLRG1highIL7Rlow SLECs.Citation180 CD8+ T cells in Blimp1-deficient mice have an activated and highly proliferative phenotype, suggesting that Blimp1 expression is anti-proliferative.Citation95,Citation201,Citation202 Moreover, the potential antagonists to Blimp-1, Bcl-6, and its homolog, Bcl-6b appear to promote the development of memory CD8+ T cells and thus, increasing their proliferation.Citation95,Citation203-Citation205 Furthermore, recent evidence indicates that the apparent reduction in the proliferative potential of KLRG1high CD8+ T cells is also regulated by an increase in the expression of p27kip, a cell cycle inhibitorCitation95 and a decrease in the expression of Bmi-1, a transcriptional repressor, both promoting T cell proliferation.Citation95,Citation206 Undoubtedly, as more genes are discovered and examined in epigenitic studies, the list of intrinsic factors involved in this process will continue to grow in the next few years. Increasing the expression of Bcl-2 and Bcl-XL or blocking their actions in effector CD8+ T cells does not greatly affect the normal rate of effector cell death following infection.Citation72,Citation85,Citation95,Citation195,Citation196,Citation199 Among the molecules that have been found to promote and prevent effector CD8+ T cell contraction are the pro-apoptotic Bim and the serine protease inhibitor 2A (Spi2A), respectively.Citation95,Citation207-Citation211 Inflammatory cytokines can also modulate the expression of key transcription factors that regulate effector T cell differentiation, such as T-bet and eomesodermin (eomes).Citation95,Citation212 IL-12 augments T-bet expression and diminishes eomes expression in activated CD8+ T cells.Citation69,Citation71,Citation103,Citation194 Our current understanding of epigenetic mechanisms that regulate the off-on-off expression of CD8+ T cell effector molecules at the naïve-effector-memory stages of differentiation, and how modifications to the genome/epigenitic factors in coming years, will likely serve as novel targets to regulate naïve-effector-memory CD8+ T cell transistions.Citation213-Citation216
The Novel “Symptomatic/Asymptomatic” Concept: Considerations for Herpes Vaccines and Immunotherapeutics
The memory CD8+ T cells, designated as protective or asymptomatic memory CD8+ T cells, can induce lifelong protection from re-infections with a wide variety of viral, bacterial, and parasitic pathogens. Conversely, the memory CD8+ T cells, designated as pathogenic or symptomatic CD8+ T cells, can cause immunopathology when not properly regulated.Citation15,Citation217-Citation221 The in vivo immunogenicity and protective efficacy of human CD8+ T cell epitopes found to be antigenic in vitro is crucial in vaccines design.Citation222-Citation225 Up to 50% of HSV-1 and HSV-2 CD8+ epitopes recently reported as recognized by human CD8+ T cells in vitro, may not be protective as expected.Citation118,Citation226-Citation232 Moreover, some of the HSV-1/HSV-2 human T cell epitopes may actually be pathogenic and contribute to exacerbation of disease.Citation218 A human CD8+ T cell epitope derived from HSV-1 gK caused a significant increase in virus replication in the eyes of HLA-A*0201 transgenic mice associated with increased acute corneal scarring.Citation217-Citation221 The gK CD8+ T cell epitope also exacerbated ocular disease in HLA-A*0201 transgenic rabbits (BenMohamed, unpublished data). This suggests that gK CD8+ T cell epitope might be clinically harmful and as such is considered as a symptomatic epitope and thus must be excluded from future asymptomatic vaccine. The mechanisms by which symptomatic CD8+ T cell epitopes exacerbate ocular, oro-facial, and genital herpetic diseases remain to be fully elucidated (see our recent reviewCitation233). Regardless of the nature of that mechanism, for symptomatic epitopes are associated with recurrent herpetic disease, it is logical to exclude them from future herpes vaccines and immunotherapeutics, since they may actually exacerbate, rather than protect from, recurrent herpetic disease.Citation218,Citation234-Citation238 Thus following a primary mucosal infection with a pathogen, such as HSV-1 or HSV-2, virus-specific asymptomatic epitopes presented by infected antigen presenting cells (APCs) would induce activation, clonal expansion, and differentiation of naive asymptomatic epitope-specific CD8+ TEM/TRM cells into a pool of “protective” effector cytotoxic T lymphocytes. This is necessary for the clearance of the virus. In contrast, virus specific symptomatic epitopes presented by infected APCs to naive symptomatic epitope-specific CD8+ TCM cells would instead induce a “pathogenic” T cell population and may exacerbate the viral disease.Citation9,Citation38,Citation122,Citation123,Citation239-Citation242 Therefore, the central hypothesis of our novel “symptomatic vs. asymptomatic T cells” concept is that, following intravaginal infection by a pathogen, symptomatic and asymptomatic activation phenotype would recall memory CD8+ T cells, with either a central or effector memory phenotype.
In the most recent clinical herpes vaccine trials, therapeutic vaccination with recombinant herpes proteins that presumably contained both asymptomatic and symptomatic T cell epitopes, only led to moderate and transient protection.Citation6,Citation122,Citation239 Based on our “symptomatic vs. asymptomatic T cells” concept, a human vaccine containing asymptomatic (protective) CD8+ T cell epitopes, but not symptomatic (pathogenic) CD8+ T cell epitopes, would foster better protective and long-lasting CD8+ TEM/TRM cells against HSV re-infections and reactivations. The immune mechanisms by which HSV-specific asymptomatic CD8+ T cells control herpes infection and disease, and by which HSV-specific symptomatic CD8+ T cells may exacerbate herpes infection and disease, remain to be fully elucidated. Following an asymptomatic vaccination, protective asymptomatic HSV-specific CD8+ TEM/TRM cells would be profoundly localized to sites of primary and recurrent infections such as, the ocular, oral, and vaginal muco-cutaneous surfaces, the skin, the cervix, and the eye, along with sites of latent infection, in the sensory ganglia, such as the trigeminal ganglia and the dorsal sacral ganglia. Although HSV-1/2 infections co-exist with brisk T-cell responses, pathogenic symptomatic HSV-specific CD8+ TCM cells would be associated with worsened recurrent infection.Citation6,Citation122,Citation239 Hence, induction of protective asymptomatic T cells, and suppression of pathogenic symptomatic T cells, are essential futures for a safe and efficient herpes vaccinations. Therefore, identifying HSV-1 and HSV-2 asymptomatic and symptomatic T cell epitopes from all the 84+ herpes proteins is critical for a rational design of asymtomatic and effective ocular, oral, and genital herpes vaccines.
Evidence from B6 mice suggests that CD8+ T cells, specific to the immunodominant HSV-1 and HSV-2 glycoprotein B (gB) H2b-restricted epitope (gB498–505), protect against ocular and genital herpes infection and diseases. However, the possible role of human CD8+ T cells, specific to HLA-restricted gB epitopes, in protective immunity seen in HSV-1 and/or HSV-2 seropositive asymptomatic healthy individuals (who have never had clinical herpes) remains to be determined. In our recent study,Citation6 6 out of 10 potential HLA-A*02:01-restricted CD8+ T cell epitopes from the HSV-1 gB amino acid sequence exhibited high binding affinity to HLA-A*02:01 molecules. In 10 sequentially studied HLA-A*02:01 positive and HSV-1 seropositive asymptomatic individuals, the most frequent, robust, and polyfunctional CD8+ T-cell responses, as assessed by a combination of tetramer, IFN-γ-ELISpot, CFSE proliferation, CD107a/b cytotoxic degranulation and multiplex cytokine assays, were directed mainly against epitopes gB342–350 and gB561–569. In contrast, in 10 HLA-A*02:01 positive, HSV-1 seropositive symptomatic individuals (with a history of numerous episodes of recurrent clinical herpes disease) resulted in frequent, but less robust, CD8+ T-cell responses directed mainly against non-overlapping epitopes (gB183–191 and gB441–449). Asymptomatic individuals had a significantly higher proportion of HSV-gB-specific CD8+ T cells expressing CD107a/b degranulation marker and produced more effector cytokines IL-2, IFN-γ, and TNF-α compared with symptomatic individuals. Furthermore, immunization of a novel herpes susceptible HLA-A*02:01 transgenic mouse model with asymptomatic epitopes, but not with symptomatic epitopes, promoted strong CD8+ T cell-dependent protective immunity against ocular herpes infection and disease. This study is first to directly confirm the new “symptomatic/asymptomatic CD8+ T cell development” concept. As per our new “symptomatic/asymptomatic” concept, inflammatory corneal lesions are not a direct consequence of the damage caused by the virus or by auto-reactive or bystander T cells, but rather a result of an immunopathological T cell reaction caused by symptomatic HSV-1 epitopes. In contrast, T cell responses specific to asymptomatic HSV-1 epitopes would result in immuno-protection. Regardless of the mechanisms involved, if symptomatic individuals tend to generate CD8+ T cells that recognize symptomatic epitopes, it would therefore be rational to exclude such symptomatic epitopes from future herpes vaccines, based on the grounds that they may exacerbate rather than cure recurrent herpetic disease.
Concluding Remarks
Tracking the developmental lineage of CD8+ T cells between effector and memory stages is complicated by the continuously evolving memory CD8+ T cell sub-populations that develop long after infections are cleared. With respect to symptomatic and asymptomatic memory CD8+ T cell sub-populations, most asymptomatic CD8+ T cells appeared to be of the CD8+ TEM/TRM cell phenotype, reside in non-lymphoid mucosal tissues, have a reduced ability to traffic to lymphoid tissues and appear more terminally differentiated due to a lower proliferation capacity in response to antigen or homeostatic IL-15 and IL-7 cytokines.Citation95,Citation105-Citation107 In contrast, symptomatic epitope stimulations likely induce memory CD8+ TCM cells.
During early infection, virus-specific effector cells that are generated from SLECs, (KLRG1highIL7Rlow) rarely persist into “memory” time points and often die from apoptosis once the acute infection is cleared. In contrast, memory CD8+ T cells generated from MPECs (KLRG1lowIL7Rhigh) often develop into a mixture of TEM/TRM and TCM cells.Citation71,Citation137,Citation139,Citation140 It is likely that the delicate balance of MPEC vs. SLEC and the TEM/TRM vs. TCM cell lineages decision is strongly influenced by the repertoire of TCR signaling that is generated following symptomatic vs. asymptomatic epitopes stimulation. Specifically, recognition of asymptomatic epitopes will mainly drive the development of tissue-resident memory CD8+ T cells with preferentially an SLEC and TEM/TRM cell phenotype, while recognition of symptomatic epitopes will mainly drive preferentially a MPEC and TCM cell phenotype.
The above remark is supported by our recent findings showing that stimulation of CD8+ T cells with asymptomatic epitopes preferentially induce polyfunctional T cells that produce IL-12, TNF-α, and IFN-γ inflammatory cytokines. In contrast, stimulation of CD8+ T cells with symptomatic epitopes preferentially induce monofunctional T cells. Moreover, we found that asymptomatic epitopes favorably induce TEM cells. In contrast, symptomatic epitopes favorably induce TCM cells. It is likely that the delicate balance of TCM vs. TEM cell lineages decision is strongly influenced by the profile of cytokines generated following stimulation with symptomatic and asymptomatic epitopes. Specifically, recognition of asymptomatic epitopes will mainly drive development of tissue-resident memory CD8+ T cells with preferentially a TEM/TRM phenotype, while recognition of symptomatic epitopes will mainly drive preferentially a TCM phenotype.
While developing an effective herpes vaccine is scientifically feasible, virologists, and immunologists still remain puzzled by the relationship between the effector/memory T cell sub-populations and the infection/disease process.Citation6 The key for an ultimate development of an effective herpes vaccine can be drawn from studies of T cells from HSV-seropositive asymptomatic individuals who manage to “naturally” and continuously control recurrent herpetic disease to clinically undetectable levels.Citation6 One ongoing research goal in our laboratory is to explore the underlying mechanisms of “symptomatic vs. asymptomatic T cells” concept with respect to (1) CD103 expression promoting the long-term persistence of memory CD8+ TEM/TRM cells at the ocular, oral, and genital muco-cutaneous site of HSV-1 and HSV-2 infectionsCitation118,Citation119; (2) CD103 regulation of the retention of a mucosal tissues-resident asymptomatic CD8+ T cells; and (3) TGF-β regulation of CD103 expression on mucosal tissues-resident memory CD8+ T cells that promote their adhesion to the extracellular matrix.Citation243
The immune mechanism(s) by which HSV-specific asymptomatic CD8+ T cells control herpes disease and HSV-specific symptomatic CD8+ T cells remain to be fully elucidated in humans. Following an asymptomatic vaccination, protective asymptomatic HSV-specific CD8+ TEM/TRM cells would localize to sites of primary and recurrent infections such as, the ocular, oral, and vaginal mucosal surfaces, the skin, the cervix, and the eye, as well as to sites of latent infection in the sensory ganglia. Although HSV-1/2 infections co-exist with brisk T-cell responses, pathogenic symptomatic HSV-specific CD8+ TCM cells would be associated with worsened recurrent infection.Citation6,Citation122,Citation239 Hence, positive selection of protective asymptomatic T cells and negative selection of pathogenic symptomatic T cells through a molecular-based approach is essential for future herpes vaccines. Identifying viral asymptomatic and symptomatic T cell epitopes from all the 84+ herpes proteins is a good starting point and critical for a rational design of an effective ocular and genital herpes vaccine.
We recently reported, for the first time, that a Lipo/rAdv5 prime/boost mucosal vaccine delivered intravaginally, induced a robust and long-lasting HSV-specific asymptomatic CD8+ T cells that protect against genital herpes infection and disease.Citation17,Citation18 The induced long-lasting memory CD8+ T cell responses persisted in both the VM and GT-DLN for up to 8 mo post immunization. To avoid exacerbation of vaginal mucosal disease, it is imperative to avoid inducing symptomatic CD8+ T cells. We also found that immunization of a novel herpes susceptible HLA-A*02:01 transgenic mouse model with asymptomatic epitopes, but not with symptomatic epitopes, induced strong CD8+ T cell-dependent protective immunity against ocular herpes infection and disease. This study is first to directly confirm our new “symptomatic/asymptomatic CD8+ T cell development” concept. As per this cencept, inflammatory corneal lesions are not a direct consequence of the damage caused by the virus or by auto-reactive or bystander T cells, but rather a result of the balance between immunopathological T cell responses specific to symptomatic HSV-1 epitopes and immuno-protective T cell responses specific to asymptomatic HSV-1 epitopes.
The new “symptomatic/asymptomatic CD8+ T cell” concept provides new immunological insights and a new direction for the design of an efficient and safe T cell-based human vaccines and immunotherapeutics, not only for herpes but for other infectious diseases. Regardless of the mechanisms involved, if symptomatic individuals tend to generate CD8+ T cells that recognize symptomatic epitopes, it would be rational to exclude such symptomatic epitopes from any future vaccines on the grounds that they may exacerbate, rather than cure, recurrent infectious disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by Public Health Service research grants NIH-EY14900 and NIH-EY019896 to LBM, by The Discovery Eye Foundation, by The Henry L. Guenther Foundation, and by an unrestricted Research to Prevent Blindness Challenge grant.
References
- Belkaid Y, Bouladoux N, Hand TW. Effector and memory T cell responses to commensal bacteria. Trends Immunol 2013; 34:299 - 306; http://dx.doi.org/10.1016/j.it.2013.03.003; PMID: 23643444
- Masopust D, Vezys V, Wherry EJ, Ahmed R. A brief history of CD8 T cells. Eur J Immunol 2007; 37:Suppl 1 S103 - 10; http://dx.doi.org/10.1002/eji.200737584; PMID: 17972353
- Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8+ T-cell memory. Nat Rev Immunol 2005; 5:101 - 11; http://dx.doi.org/10.1038/nri1550; PMID: 15662368
- Masopust D, Ahmed R. Reflections on CD8 T-cell activation and memory. Immunol Res 2004; 29:151 - 60; http://dx.doi.org/10.1385/IR:29:1-3:151; PMID: 15181278
- Gebhardt T, Mackay LK. Local immunity by tissue-resident CD8(+) memory T cells. Front Immunol 2012; 3:340; http://dx.doi.org/10.3389/fimmu.2012.00340; PMID: 23162555
- Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, Diaz OR, Gottimukkala C, Kalantari M, Villacres MC, et al. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol 2013; 191:5124 - 38; http://dx.doi.org/10.4049/jimmunol.1301415; PMID: 24101547
- Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, Diaz OR, Gottimukkala C, Kalantari M, Villacres MC, et al. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol 2013; 191:5124 - 38; http://dx.doi.org/10.4049/jimmunol.1301415; PMID: 24101547
- Chentoufi AA, BenMohamed L, Van De Perre P, Ashkar AA. Immunity to ocular and genital herpes simplex viruses infections. Clin Dev Immunol 2012; 2012:732546; PMID: 23326289
- Chentoufi AA, Benmohamed L. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin Dev Immunol 2012; 2012:149135; PMID: 23320014
- Chentoufi AA, Dasgupta G, Nesburn AB, Bettahi I, Binder NR, Choudhury ZS, Chamberlain WD, Wechsler SL, BenMohamed L. Nasolacrimal duct closure modulates ocular mucosal and systemic CD4(+) T-cell responses induced following topical ocular or intranasal immunization. Clin Vaccine Immunol 2010; 17:342 - 53; http://dx.doi.org/10.1128/CVI.00347-09; PMID: 20089796
- Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, Jester JV, Nesburn AB, Wechsler SL, BenMohamed L. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol 2010; 184:2561 - 71; http://dx.doi.org/10.4049/jimmunol.0902322; PMID: 20124097
- Nesburn AB, Bettahi I, Dasgupta G, Chentoufi AA, Zhang X, You S, Morishige N, Wahlert AJ, Brown DJ, Jester JV, et al. Functional Foxp3+ CD4+ CD25(Bright+) “natural” regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J Virol 2007; 81:7647 - 61; http://dx.doi.org/10.1128/JVI.00294-07; PMID: 17475646
- Bettahi I, Nesburn AB, Yoon S, Zhang X, Mohebbi A, Sue V, Vanderberg A, Wechsler SL, BenMohamed L. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest Ophthalmol Vis Sci 2007; 48:4643 - 53; http://dx.doi.org/10.1167/iovs.07-0356; PMID: 17898288
- Nesburn AB, Bettahi I, Zhang X, Zhu X, Chamberlain W, Afifi RE, Wechsler SL, BenMohamed L. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul Surf 2006; 4:178 - 87; http://dx.doi.org/10.1016/S1542-0124(12)70164-7; PMID: 17146573
- Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J Virol 2005; 79:15289 - 301; http://dx.doi.org/10.1128/JVI.79.24.15289-15301.2005; PMID: 16306600
- Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine 2005; 23:873 - 83; http://dx.doi.org/10.1016/j.vaccine.2004.08.019; PMID: 15603887
- Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, Benmohamed L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol 2012; 189:4496 - 509; http://dx.doi.org/10.4049/jimmunol.1201121; PMID: 23018456
- Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2009; 2:129 - 43; http://dx.doi.org/10.1038/mi.2008.81; PMID: 19129756
- Bettahi I, Zhang X, Afifi RE, BenMohamed L. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol 2006; 19:220 - 36; http://dx.doi.org/10.1089/vim.2006.19.220; PMID: 16817765
- Kalantari-Dehaghi M, Chun S, Chentoufi AA, Pablo J, Liang L, Dasgupta G, Molina DM, Jasinskas A, Nakajima-Sasaki R, Felgner J, et al. Discovery of potential diagnostic and vaccine antigens in herpes simplex virus 1 and 2 by proteome-wide antibody profiling. J Virol 2012; 86:4328 - 39; http://dx.doi.org/10.1128/JVI.05194-11; PMID: 22318154
- Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Badakhshan T, Kim SM, Nesburn AB, Wechsler SL, Benmohamed L. Future of an “Asymptomatic” T-cell Epitope-Based Therapeutic Herpes Simplex Vaccine. Future Virol 2012; 7:371 - 8; http://dx.doi.org/10.2217/fvl.12.22; PMID: 22701511
- Dasgupta G, Chentoufi AA, Kalantari M, Falatoonzadeh P, Chun S, Lim CH, Felgner PL, Davies DH, BenMohamed L. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol 2012; 86:4358 - 69; http://dx.doi.org/10.1128/JVI.07107-11; PMID: 22318137
- Peterslund NA. Herpesvirus infection: an overview of the clinical manifestations. Scand J Infect Dis Suppl 1991; 80:15 - 20; PMID: 1666443
- Rozenberg F, Deback C, Agut H. Herpes simplex encephalitis : from virus to therapy. Infect Disord Drug Targets 2011; 11:235 - 50; http://dx.doi.org/10.2174/187152611795768088; PMID: 21488834
- Nikkels AF, Pièrard GE. Treatment of mucocutaneous presentations of herpes simplex virus infections. Am J Clin Dermatol 2002; 3:475 - 87; http://dx.doi.org/10.2165/00128071-200203070-00004; PMID: 12180895
- Inoue T, Inoue Y, Nakamura T, Yoshida A, Inoue Y, Tano Y, Shimomura Y, Fujisawa Y, Aono A, Hayashi K. The effect of immunization with herpes simplex virus glycoprotein D fused with interleukin-2 against murine herpetic keratitis. Jpn J Ophthalmol 2002; 46:370 - 6; http://dx.doi.org/10.1016/S0021-5155(02)00501-4; PMID: 12225814
- Honda M, Niimura M. [Alphaherpesvininae--dermatology]. Nihon Rinsho 2000; 58:895 - 900; PMID: 10774212
- Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea 1999; 18:127 - 43; http://dx.doi.org/10.1097/00003226-199903000-00001; PMID: 10090358
- O’Brien JJ, Campoli-Richards DM. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1989; 37:233 - 309; PMID: 2653790
- Whitley RJ. Herpes simplex virus infections of women and their offspring: implications for a developed society. Proc Natl Acad Sci U S A 1994; 91:2441 - 7; http://dx.doi.org/10.1073/pnas.91.7.2441; PMID: 8146137
- Westhoff GL, Little SE, Caughey AB. Herpes simplex virus and pregnancy: a review of the management of antenatal and peripartum herpes infections. Obstet Gynecol Surv 2011; 66:629 - 38; http://dx.doi.org/10.1097/OGX.0b013e31823983ec; PMID: 22112524
- Ohashi M, Bertke AS, Patel A, Krause PR. Spread of herpes simplex virus to the spinal cord is independent of spread to dorsal root ganglia. J Virol 2011; 85:3030 - 2; http://dx.doi.org/10.1128/JVI.02426-10; PMID: 21159869
- Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med 1987; 316:1444 - 9; http://dx.doi.org/10.1056/NEJM198706043162304; PMID: 3033506
- Antoine TE, Park PJ, Shukla D. Glycoprotein targeted therapeutics: a new era of anti-herpes simplex virus-1 therapeutics. Rev Med Virol 2013; 23:194 - 208; http://dx.doi.org/10.1002/rmv.1740; PMID: 23440920
- Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, Douglas JM Jr., Paavonen J, Morrow RA, Beutner KR, et al, Valacyclovir HSV Transmission Study Group. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004; 350:11 - 20; http://dx.doi.org/10.1056/NEJMoa035144; PMID: 14702423
- Conant MA, Spicer DW, Smith CD. Herpes simplex virus transmission: condom studies. Sex Transm Dis 1984; 11:94 - 5; http://dx.doi.org/10.1097/00007435-198404000-00009; PMID: 6087482
- Dasgupta G, Nesburn AB, Wechsler SL, BenMohamed L. Developing an asymptomatic mucosal herpes vaccine: the present and the future. Future Microbiol 2010; 5:1 - 4; http://dx.doi.org/10.2217/fmb.09.101; PMID: 20020824
- Zhang X, Castelli FA, Zhu X, Wu M, Maillère B, BenMohamed L. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol 2008; 15:1436 - 49; http://dx.doi.org/10.1128/CVI.00123-08; PMID: 18667634
- Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, BenMohamed L. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin Dev Immunol 2012; In press
- BenMohamed L, Bertrand G, McNamara CD, Gras-Masse H, Hammer J, Wechsler SL, Nesburn AB. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J Virol 2003; 77:9463 - 73; http://dx.doi.org/10.1128/JVI.77.17.9463-9473.2003; PMID: 12915561
- Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillère B, BenMohamed L. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol 2008; 82:11792 - 802; http://dx.doi.org/10.1128/JVI.00692-08; PMID: 18799581
- Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol 2008; 180:426 - 37; PMID: 18097044
- Koelle DM. Vaccines for herpes simplex virus infections. Curr Opin Investig Drugs 2006; 7:136 - 41; PMID: 16499283
- Bernstein D. Glycoprotein D adjuvant herpes simplex virus vaccine. Expert Rev Vaccines 2005; 4:615 - 27; http://dx.doi.org/10.1586/14760584.4.5.615; PMID: 16221064
- Hoshino Y, Dalai SK, Wang K, Pesnicak L, Lau TY, Knipe DM, Cohen JI, Straus SE. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J Virol 2005; 79:410 - 8; http://dx.doi.org/10.1128/JVI.79.1.410-418.2005; PMID: 15596834
- Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today 1997; 18:443 - 9; http://dx.doi.org/10.1016/S0167-5699(97)01114-6; PMID: 9293161
- Doymaz MZ, Rouse BT. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+ T lymphocytes. Invest Ophthalmol Vis Sci 1992; 33:2165 - 73; PMID: 1351475
- Jayaraman S, Heiligenhaus A, Rodriguez A, Soukiasian S, Dorf ME, Foster CS. Exacerbation of murine herpes simplex virus-mediated stromal keratitis by Th2 type T cells. J Immunol 1993; 151:5777 - 89; PMID: 8228262
- Boorstein SM, Elner SG, Meyer RF, Sugar A, Strieter RM, Kunkel SL, Elner VM. Interleukin-10 inhibition of HLA-DR expression in human herpes stromal keratitis. Ophthalmology 1994; 101:1529 - 35; http://dx.doi.org/10.1016/S0161-6420(94)31149-3; PMID: 7916449
- Deshpande S, Zheng M, Lee S, Banerjee K, Gangappa S, Kumaraguru U, Rouse BT. Bystander activation involving T lymphocytes in herpetic stromal keratitis. J Immunol 2001; 167:2902 - 10; PMID: 11509638
- Bouley DM, Kanangat S, Wire W, Rouse BT. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol 1995; 155:3964 - 71; PMID: 7561104
- Babu JS, Thomas J, Kanangat S, Morrison LA, Knipe DM, Rouse BT. Viral replication is required for induction of ocular immunopathology by herpes simplex virus. J Virol 1996; 70:101 - 7; PMID: 8523513
- Banerjee K, Biswas PS, Rouse BT. Elucidating the protective and pathologic T cell species in the virus-induced corneal immunoinflammatory condition herpetic stromal keratitis. J Leukoc Biol 2005; 77:24 - 32; PMID: 15496448
- Chen H, Hendricks RL. B7 costimulatory requirements of T cells at an inflammatory site. J Immunol 1998; 160:5045 - 52; PMID: 9590254
- Stuart PM, Summers B, Morris JE, Morrison LA, Leib DA. CD8(+) T cells control corneal disease following ocular infection with herpes simplex virus type 1. J Gen Virol 2004; 85:2055 - 63; http://dx.doi.org/10.1099/vir.0.80049-0; PMID: 15218191
- Banerjee K, Deshpande S, Zheng M, Kumaraguru U, Schoenberger SP, Rouse BT. Herpetic stromal keratitis in the absence of viral antigen recognition. Cell Immunol 2002; 219:108 - 18; http://dx.doi.org/10.1016/S0008-8749(02)00601-9; PMID: 12576029
- Nesburn AB, Burke RL, Ghiasi H, Slanina S, Bahri S, Wechsler SL. Vaccine therapy for ocular herpes simplex virus (HSV) infection: periocular vaccination reduces spontaneous ocular HSV type 1 shedding in latently infected rabbits. J Virol 1994; 68:5084 - 92; PMID: 8035508
- Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology 1998; 252:200 - 9; http://dx.doi.org/10.1006/viro.1998.9454; PMID: 9875329
- Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthalmol Vis Sci 1998; 39:1163 - 70; PMID: 9620075
- Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J Virol 1998; 72:7715 - 21; PMID: 9733807
- Huang Y, Park Y, Wang-Zhu Y, Larange A, Arens R, Bernardo I, Olivares-Villagómez D, Herndler-Brandstetter D, Abraham N, Grubeck-Loebenstein B, et al. Mucosal memory CD8⁺ T cells are selected in the periphery by an MHC class I molecule. Nat Immunol 2011; 12:1086 - 95; http://dx.doi.org/10.1038/ni.2106; PMID: 21964609
- Arens R, Loewendorf A, Redeker A, Sierro S, Boon L, Klenerman P, Benedict CA, Schoenberger SP. Differential B7-CD28 costimulatory requirements for stable and inflationary mouse cytomegalovirus-specific memory CD8 T cell populations. J Immunol 2011; 186:3874 - 81; http://dx.doi.org/10.4049/jimmunol.1003231; PMID: 21357256
- Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev 2010; 235:190 - 205; PMID: 20536564
- Toka FN, Gieryńska M, Suvas S, Schoenberger SP, Rouse BT. Rescue of memory CD8+ T cell reactivity in peptide/TLR9 ligand immunization by codelivery of cytokines or CD40 ligation. Virology 2005; 331:151 - 8; http://dx.doi.org/10.1016/j.virol.2004.10.022; PMID: 15582662
- Toka FN, Gieryńska M, Suvas S, Schoenberger SP, Rouse BT. Rescue of memory CD8+ T cell reactivity in peptide/TLR9 ligand immunization by codelivery of cytokines or CD40 ligation. Virology 2005; 331:151 - 8; http://dx.doi.org/10.1016/j.virol.2004.10.022; PMID: 15582662
- Bajénoff M, Egen JG, Qi H, Huang AY, Castellino F, Germain RN. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends Immunol 2007; 28:346 - 52; http://dx.doi.org/10.1016/j.it.2007.06.005; PMID: 17625969
- Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med 2007; 204:1625 - 36; http://dx.doi.org/10.1084/jem.20070322; PMID: 17606632
- Joshi NS, Cui W, Dominguez CX, Chen JH, Hand TW, Kaech SM. Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells. J Immunol 2011; 187:4068 - 76; http://dx.doi.org/10.4049/jimmunol.1002145; PMID: 21930973
- Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A 2010; 107:16601 - 6; http://dx.doi.org/10.1073/pnas.1003457107; PMID: 20823247
- Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine 2009; 27:2177 - 87; http://dx.doi.org/10.1016/j.vaccine.2009.01.088; PMID: 19201385
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 2007; 27:281 - 95; http://dx.doi.org/10.1016/j.immuni.2007.07.010; PMID: 17723218
- Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A 2007; 104:11730 - 5; http://dx.doi.org/10.1073/pnas.0705007104; PMID: 17609371
- de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med 2005; 202:1433 - 42; http://dx.doi.org/10.1084/jem.20051365; PMID: 16301748
- Wolkers MC, Gerlach C, Arens R, Janssen EM, Fitzgerald P, Schumacher TN, Medema JP, Green DR, Schoenberger SP. Nab2 regulates secondary CD8+ T-cell responses through control of TRAIL expression. Blood 2012; 119:798 - 804; http://dx.doi.org/10.1182/blood-2011-08-373910; PMID: 22128144
- Tani-ichi S, Shimba A, Wagatsuma K, Miyachi H, Kitano S, Imai K, Hara T, Ikuta K. Interleukin-7 receptor controls development and maturation of late stages of thymocyte subpopulations. Proc Natl Acad Sci U S A 2013; 110:612 - 7; http://dx.doi.org/10.1073/pnas.1219242110; PMID: 23267098
- Crawley AM, Faucher S, Angel JB. Soluble IL-7R alpha (sCD127) inhibits IL-7 activity and is increased in HIV infection. J Immunol 2010; 184:4679 - 87; http://dx.doi.org/10.4049/jimmunol.0903758; PMID: 20304824
- Parish IA, Rao S, Smyth GK, Juelich T, Denyer GS, Davey GM, Strasser A, Heath WR. The molecular signature of CD8+ T cells undergoing deletional tolerance. Blood 2009; 113:4575 - 85; http://dx.doi.org/10.1182/blood-2008-10-185223; PMID: 19204323
- Kim EH, Suresh M. Role of PI3K/Akt signaling in memory CD8 T cell differentiation. Front Immunol 2013; 4:20; http://dx.doi.org/10.3389/fimmu.2013.00020; PMID: 23378844
- Amsen D, Backer RA, Helbig C. Decisions on the road to memory. Adv Exp Med Biol 2013; 785:107 - 20; http://dx.doi.org/10.1007/978-1-4614-6217-0_12; PMID: 23456843
- Wiesel M, Crouse J, Bedenikovic G, Sutherland A, Joller N, Oxenius A. Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo. Eur J Immunol 2012; 42:320 - 9; http://dx.doi.org/10.1002/eji.201142091; PMID: 22102057
- Shin H, Iwasaki A. Tissue-resident memory T cells. Immunol Rev 2013; 255:165 - 81; http://dx.doi.org/10.1111/imr.12087; PMID: 23947354
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 2013; 31:137 - 61; http://dx.doi.org/10.1146/annurev-immunol-032712-095954; PMID: 23215646
- Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high prememory CD8+ T cells. J Immunol 2007; 178:778 - 87; PMID: 17202339
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med 2008; 205:625 - 40; http://dx.doi.org/10.1084/jem.20071641; PMID: 18316415
- Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15Ralpha. Proc Natl Acad Sci U S A 2006; 103:9166 - 71; http://dx.doi.org/10.1073/pnas.0600240103; PMID: 16757567
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012; 483:227 - 31; http://dx.doi.org/10.1038/nature10851; PMID: 22388819
- Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J Immunol 2007; 179:3504 - 14; PMID: 17785784
- Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol 2012; 188:5811 - 7; http://dx.doi.org/10.4049/jimmunol.1102695; PMID: 22675215
- Keppler SJ, Rosenits K, Koegl T, Vucikuja S, Aichele P. Signal 3 cytokines as modulators of primary immune responses during infections: the interplay of type I IFN and IL-12 in CD8 T cell responses. PLoS One 2012; 7:e40865; http://dx.doi.org/10.1371/journal.pone.0040865; PMID: 22815848
- Morimoto J, Sato K, Nakayama Y, Kimura C, Kajino K, Matsui Y, Miyazaki T, Uede T. Osteopontin modulates the generation of memory CD8+ T cells during influenza virus infection. J Immunol 2011; 187:5671 - 83; http://dx.doi.org/10.4049/jimmunol.1101825; PMID: 22021613
- Keppler SJ, Aichele P. Signal 3 requirement for memory CD8+ T-cell activation is determined by the infectious pathogen. Eur J Immunol 2011; 41:3176 - 86; http://dx.doi.org/10.1002/eji.201141537; PMID: 21830209
- Valkenburg SA, Day EB, Swan NG, Croom HA, Carbone FR, Doherty PC, Turner SJ, Kedzierska K. Fixing an irrelevant TCR alpha chain reveals the importance of TCR beta diversity for optimal TCR alpha beta pairing and function of virus-specific CD8+ T cells. Eur J Immunol 2010; 40:2470 - 81; http://dx.doi.org/10.1002/eji.201040473; PMID: 20690181
- Cox MA, Zajac AJ. Shaping successful and unsuccessful CD8 T cell responses following infection. J Biomed Biotechnol 2010; 2010:159152; http://dx.doi.org/10.1155/2010/159152; PMID: 20379363
- Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: Role of direct IL-12 signals for cell fate decision of CD8(+) T cells. Eur J Immunol 2009; 39:1774 - 83; http://dx.doi.org/10.1002/eji.200839093; PMID: 19548244
- Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol 2008; 180:1309 - 15; PMID: 18209024
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med 2005; 202:637 - 50; http://dx.doi.org/10.1084/jem.20050821; PMID: 16129706
- Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol 2007; 179:2074 - 81; PMID: 17675465
- Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol 2006; 177:1746 - 54; PMID: 16849484
- D’Souza WN, Schluns KS, Masopust D, Lefrançois L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J Immunol 2002; 168:5566 - 72; PMID: 12023352
- El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-beta-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med 2005; 201:1647 - 57; http://dx.doi.org/10.1084/jem.20041044; PMID: 15897278
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009; 460:103 - 7; http://dx.doi.org/10.1038/nature08097; PMID: 19494812
- Masson F, Minnich M, Olshansky M, Bilic I, Mount AM, Kallies A, Speed TP, Busslinger M, Nutt SL, Belz GT. Id2-mediated inhibition of E2A represses memory CD8+ T cell differentiation. J Immunol 2013; 190:4585 - 94; http://dx.doi.org/10.4049/jimmunol.1300099; PMID: 23536629
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 2005; 6:1236 - 44; http://dx.doi.org/10.1038/ni1268; PMID: 16273099
- Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med 2007; 204:1405 - 16; http://dx.doi.org/10.1084/jem.20062363; PMID: 17535971
- Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 2003; 4:225 - 34; http://dx.doi.org/10.1038/ni889; PMID: 12563257
- Plunkett FJ, Franzese O, Belaramani LL, Fletcher JM, Gilmour KC, Sharifi R, Khan N, Hislop AD, Cara A, Salmon M, et al. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech Ageing Dev 2005; 126:855 - 65; http://dx.doi.org/10.1016/j.mad.2005.03.006; PMID: 15992610
- Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol 2005; 17:480 - 5; http://dx.doi.org/10.1016/j.coi.2005.07.019; PMID: 16098721
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745 - 63; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104702; PMID: 15032595
- Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol 2008; 180:3122 - 31; PMID: 18292535
- Kolls JK, Habetz S, Shean MK, Vazquez C, Brown JA, Lei D, Schwarzenberger P, Ye P, Nelson S, Summer WR, et al. IFN-gamma and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J Immunol 1999; 162:2890 - 4; PMID: 10072538
- Reddehase MJ, Mutter W, Münch K, Bühring HJ, Koszinowski UH. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol 1987; 61:3102 - 8; PMID: 3041033
- Denton AE, Russ BE, Doherty PC, Rao S, Turner SJ. Differentiation-dependent functional and epigenetic landscapes for cytokine genes in virus-specific CD8+ T cells. Proc Natl Acad Sci U S A 2011; 108:15306 - 11; http://dx.doi.org/10.1073/pnas.1112520108; PMID: 21876173
- Araki Y, Wang Z, Zang C, Wood WH 3rd, Schones D, Cui K, Roh TY, Lhotsky B, Wersto RP, Peng W, et al. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity 2009; 30:912 - 25; http://dx.doi.org/10.1016/j.immuni.2009.05.006; PMID: 19523850
- Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med 2006; 203:919 - 32; http://dx.doi.org/10.1084/jem.20052237; PMID: 16567385
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012; 491:463 - 7; http://dx.doi.org/10.1038/nature11522; PMID: 23075848
- Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol 2010; 10:699 - 711; http://dx.doi.org/10.1038/nri2836; PMID: 20829886
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature 2009; 462:510 - 3; http://dx.doi.org/10.1038/nature08511; PMID: 19898495
- Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 2007; 204:595 - 603; http://dx.doi.org/10.1084/jem.20061792; PMID: 17325200
- Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 2013; 497:494 - 7; http://dx.doi.org/10.1038/nature12110; PMID: 23657257
- Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J Immunol 2010; 185:5315 - 25; http://dx.doi.org/10.4049/jimmunol.1001948; PMID: 20921525
- Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol 2008; 180:5309 - 19; PMID: 18390712
- Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Badakhshan T, Kim SM, Nesburn AB, Wechsler SL, Benmohamed L. Future of an “Asymptomatic” T-cell Epitope-Based Therapeutic Herpes Simplex Vaccine. Future Virol 2012; 7:371 - 8; http://dx.doi.org/10.2217/fvl.12.22; PMID: 22701511
- Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, Benmohamed L. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin Dev Immunol 2012; 2012:187585; PMID: 22548113
- Chentoufi AA, Dervillez X, Rubbo PA, Kuo T, Zhang X, Nagot N, Tuaillon E, Van De Perre P, Nesburn AB, Benmohamed L. Current trends in negative immuno-synergy between two sexually transmitted infectious viruses: HIV-1 and HSV-1/2. Curr Trends Immunol 2012; 13:51 - 68; PMID: 23355766
- Kumamoto Y, Iwasaki A. Unique features of antiviral immune system of the vaginal mucosa. Curr Opin Immunol 2012; 24:411 - 6; http://dx.doi.org/10.1016/j.coi.2012.05.006; PMID: 22673876
- Tejera MM, Kim EH, Sullivan JA, Plisch EH, Suresh M. FoxO1 controls effector-to-memory transition and maintenance of functional CD8 T cell memory. J Immunol 2013; 191:187 - 99; http://dx.doi.org/10.4049/jimmunol.1300331; PMID: 23733882
- Sullivan JA, Kim EH, Plisch EH, Suresh M. FOXO3 regulates the CD8 T cell response to a chronic viral infection. J Virol 2012; 86:9025 - 34; http://dx.doi.org/10.1128/JVI.00942-12; PMID: 22675000
- Sullivan JA, Kim EH, Plisch EH, Peng SL, Suresh M. FOXO3 regulates CD8 T cell memory by T cell-intrinsic mechanisms. PLoS Pathog 2012; 8:e1002533; http://dx.doi.org/10.1371/journal.ppat.1002533; PMID: 22359505
- Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol 2012; 188:4305 - 14; http://dx.doi.org/10.4049/jimmunol.1103568; PMID: 22467649
- Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham QM, Zickovich JM, Lefrançois L. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol 2011; 187:4967 - 78; http://dx.doi.org/10.4049/jimmunol.1102335; PMID: 21987662
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 2006; 211:81 - 92; http://dx.doi.org/10.1111/j.0105-2896.2006.00382.x; PMID: 16824119
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27:670 - 84; http://dx.doi.org/10.1016/j.immuni.2007.09.006; PMID: 17950003
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 2007; 27:393 - 405; http://dx.doi.org/10.1016/j.immuni.2007.08.007; PMID: 17892848
- Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med 1994; 179:1127 - 35; http://dx.doi.org/10.1084/jem.179.4.1127; PMID: 8145034
- Sprent J, Tough DF. Lymphocyte life-span and memory. Science 1994; 265:1395 - 400; http://dx.doi.org/10.1126/science.8073282; PMID: 8073282
- Peixoto A, Evaristo C, Munitic I, Monteiro M, Charbit A, Rocha B, Veiga-Fernandes H. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J Exp Med 2007; 204:1193 - 205; http://dx.doi.org/10.1084/jem.20062349; PMID: 17485515
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 2002; 111:837 - 51; http://dx.doi.org/10.1016/S0092-8674(02)01139-X; PMID: 12526810
- Kaech SM, Ahmed R. Immunology. CD8 T cells remember with a little help. Science 2003; 300:263 - 5; http://dx.doi.org/10.1126/science.1084511; PMID: 12690179
- Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol 2007; 179:53 - 63; PMID: 17579021
- Sarkar S, Teichgräber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol 2007; 179:6704 - 14; PMID: 17982060
- Amoah S, Holbrook BC, Yammani RD, Alexander-Miller MA. High viral burden restricts short-lived effector cell number at late times postinfection through increased natural regulatory T cell expansion. J Immunol 2013; 190:5020 - 9; http://dx.doi.org/10.4049/jimmunol.1200971; PMID: 23589620
- Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 2011; 477:216 - 9; http://dx.doi.org/10.1038/nature10339; PMID: 21841802
- Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity 2009; 31:859 - 71; http://dx.doi.org/10.1016/j.immuni.2009.11.007; PMID: 20064446
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001; 291:2413 - 7; http://dx.doi.org/10.1126/science.1058867; PMID: 11264538
- Sheridan BS, Lefrançois L. Regional and mucosal memory T cells. Nat Immunol 2011; 12:485 - 91; http://dx.doi.org/10.1038/ni.2029; PMID: 21739671
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 1996; 272:60 - 6; http://dx.doi.org/10.1126/science.272.5258.60; PMID: 8600538
- Rott LS, Briskin MJ, Andrew DP, Berg EL, Butcher EC. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with beta 7 integrins and memory differentiation. J Immunol 1996; 156:3727 - 36; PMID: 8621908
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med 2000; 343:1020 - 34; http://dx.doi.org/10.1056/NEJM200010053431407; PMID: 11018170
- Mackay LK, Wakim L, van Vliet CJ, Jones CM, Mueller SN, Bannard O, Fearon DT, Heath WR, Carbone FR. Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. J Immunol 2012; 188:2173 - 8; http://dx.doi.org/10.4049/jimmunol.1102719; PMID: 22271651
- Mackay LK, Wakim L, van Vliet CJ, Jones CM, Mueller SN, Bannard O, Fearon DT, Heath WR, Carbone FR. Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. J Immunol 2012; 188:2173 - 8; http://dx.doi.org/10.4049/jimmunol.1102719; PMID: 22271651
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 2012; 109:7037 - 42; http://dx.doi.org/10.1073/pnas.1202288109; PMID: 22509047
- Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 2001; 410:106 - 11; http://dx.doi.org/10.1038/35065118; PMID: 11242051
- Jiang X, Chentoufi AA, Hsiang C, Carpenter D, Osorio N, Benmohamed L, et al. The herpes simplex virus type 1 latency associated transcript (LAT) can protect neuronal derived C1300 and Neuro2A cells from Granzyme B induced apoptosis and CD8 T-cell killing. J Virol 2010; 85:2325 - 2; http://dx.doi.org/10.1128/JVI.01791-10
- Harari A, Enders FB, Cellerai C, Bart PA, Pantaleo G. Distinct profiles of cytotoxic granules in memory CD8 T cells correlate with function, differentiation stage, and antigen exposure. J Virol 2009; 83:2862 - 71; http://dx.doi.org/10.1128/JVI.02528-08; PMID: 19176626
- Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 2013; 39:687 - 96; http://dx.doi.org/10.1016/j.immuni.2013.08.019; PMID: 24076049
- Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 2013; ; Forthcoming http://dx.doi.org/10.1189/jlb.0313180; PMID: 24006506
- Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 2013; Forthcoming http://dx.doi.org/10.1038/mi.2013.67; PMID: 24064670
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 2013; 14:1285 - 93; http://dx.doi.org/10.1038/ni.2745; PMID: 24162775
- Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, Bevan MJ. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol 2012; 189:3462 - 71; http://dx.doi.org/10.4049/jimmunol.1201305; PMID: 22922816
- Himmelein S, St Leger AJ, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae 2011; 2:5; http://dx.doi.org/10.1186/2042-4280-2-5; PMID: 21429183
- Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A 2010; 107:17872 - 9; http://dx.doi.org/10.1073/pnas.1010201107; PMID: 20923878
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 2009; 15:293 - 9; http://dx.doi.org/10.1038/nm.1935; PMID: 19219024
- Piet B, de Bree GJ, Smids-Dierdorp BS, van der Loos CM, Remmerswaal EB, von der Thüsen JH, van Haarst JM, Eerenberg JP, ten Brinke A, van der Bij W, et al. CD8⁺ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest 2011; 121:2254 - 63; http://dx.doi.org/10.1172/JCI44675; PMID: 21537083
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682 - 7; http://dx.doi.org/10.1038/nature04444; PMID: 16382236
- Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol 2000; 164:3950 - 4; PMID: 10754284
- Hofmann M, Oschowitzer A, Kurzhals SR, Krüger CC, Pircher H. Thymus-resident memory CD8+ T cells mediate local immunity. Eur J Immunol 2013; 43:2295 - 304; http://dx.doi.org/10.1002/eji.201343519; PMID: 23715993
- de Niet A, de Bruijne J, Plat-Sinnige MJ, Takkenberg RB, van Lier RA, Reesink HW, van Leeuwen EM. Upregulation of CXCR3 expression on CD8+ T cells due to the pervasive influence of chronic hepatitis B and C virus infection. Hum Immunol 2013; 74:899 - 906; http://dx.doi.org/10.1016/j.humimm.2013.04.017; PMID: 23643635
- Evans AG, Moser JM, Krug LT, Pozharskaya V, Mora AL, Speck SH. A gammaherpesvirus-secreted activator of Vbeta4+ CD8+ T cells regulates chronic infection and immunopathology. J Exp Med 2008; 205:669 - 84; http://dx.doi.org/10.1084/jem.20071135; PMID: 18332178
- Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol 2006; 177:450 - 8; PMID: 16785542
- Forget MA, Huon Y, Reuben A, Grange C, Liberman M, Martin J, Mes-Masson AM, Arbour N, Lapointe R. Stimulation of Wnt/ß-catenin pathway in human CD8+ T lymphocytes from blood and lung tumors leads to a shared young/memory phenotype. PLoS One 2012; 7:e41074; http://dx.doi.org/10.1371/journal.pone.0041074; PMID: 22859966
- Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol 2010; 184:35 - 44; http://dx.doi.org/10.4049/jimmunol.0803355; PMID: 19949092
- Miles DJ, van der Sande M, Jeffries D, Kaye S, Ojuola O, Sanneh M, Cox M, Palmero MS, Touray ES, Waight P, et al. Maintenance of large subpopulations of differentiated CD8 T-cells two years after cytomegalovirus infection in Gambian infants. PLoS One 2008; 3:e2905; http://dx.doi.org/10.1371/journal.pone.0002905; PMID: 18682836
- Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med 2008; 14:542 - 50; http://dx.doi.org/10.1038/nm1744; PMID: 18425131
- Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, Shen H, Kuwano H, Yoshikai Y. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol 2006; 176:507 - 15; PMID: 16365444
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med 2005; 11:1299 - 305; http://dx.doi.org/10.1038/nm1326; PMID: 16288282
- Tewari K, Sacha J, Gao X, Suresh M. Effect of chronic viral infection on epitope selection, cytokine production, and surface phenotype of CD8 T cells and the role of IFN-gamma receptor in immune regulation. J Immunol 2004; 172:1491 - 500; PMID: 14734726
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 2003; 4:1191 - 8; http://dx.doi.org/10.1038/ni1009; PMID: 14625547
- Bachmann MF, Wolint P, Schwarz K, Jäger P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol 2005; 175:4686 - 96; PMID: 16177116
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 2006; 441:890 - 3; http://dx.doi.org/10.1038/nature04790; PMID: 16778891
- Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med 2007; 204:2015 - 21; http://dx.doi.org/10.1084/jem.20070841; PMID: 17698591
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science 1996; 272:54 - 60; http://dx.doi.org/10.1126/science.272.5258.54; PMID: 8600537
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol 2002; 2:251 - 62; http://dx.doi.org/10.1038/nri778; PMID: 12001996
- Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrançois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol 2005; 6:793 - 9; http://dx.doi.org/10.1038/ni1227; PMID: 16025119
- Gattinoni L, Powell DJ Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 2006; 6:383 - 93; http://dx.doi.org/10.1038/nri1842; PMID: 16622476
- Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol 2004; 5:809 - 17; http://dx.doi.org/10.1038/ni1098; PMID: 15247915
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol 2002; 3:619 - 26; PMID: 12055624
- Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol 2002; 2:982 - 7; http://dx.doi.org/10.1038/nri959; PMID: 12461571
- Lanzavecchia A. Lack of fair play in the T cell response. Nat Immunol 2002; 3:9 - 10; http://dx.doi.org/10.1038/ni0102-9; PMID: 11753400
- Butler NS, Nolz JC, Harty JT. Immunologic considerations for generating memory CD8 T cells through vaccination. Cell Microbiol 2011; 13:925 - 33; http://dx.doi.org/10.1111/j.1462-5822.2011.01594.x; PMID: 21501363
- Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 1995; 2:271 - 9; http://dx.doi.org/10.1016/1074-7613(95)90051-9; PMID: 7697544
- Mescher MF, Agarwal P, Casey KA, Hammerbeck CD, Xiao Z, Curtsinger JM. Molecular basis for checkpoints in the CD8 T cell response: tolerance versus activation. Semin Immunol 2007; 19:153 - 61; http://dx.doi.org/10.1016/j.smim.2007.02.007; PMID: 17382557
- Hernández J, Aung S, Marquardt K, Sherman LA. Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J Exp Med 2002; 196:323 - 33; http://dx.doi.org/10.1084/jem.20011612; PMID: 12163561
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 2000; 290:1354 - 8; http://dx.doi.org/10.1126/science.290.5495.1354; PMID: 11082062
- Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol 2006; 177:7515 - 9; PMID: 17114419
- Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol 2007; 19:320 - 6; http://dx.doi.org/10.1016/j.coi.2007.04.015; PMID: 17433869
- Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol 2000; 1:426 - 32; http://dx.doi.org/10.1038/80868; PMID: 11062503
- Wherry EJ, Becker TC, Boone D, Kaja MK, Ma A, Ahmed R. Homeostatic proliferation but not the generation of virus specific memory CD8 T cells is impaired in the absence of IL-15 or IL-15Ralpha. Adv Exp Med Biol 2002; 512:165 - 75; http://dx.doi.org/10.1007/978-1-4615-0757-4_22; PMID: 12405201
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 2002; 195:1541 - 8; http://dx.doi.org/10.1084/jem.20020369; PMID: 12070282
- Osborne LC, Dhanji S, Snow JW, Priatel JJ, Ma MC, Miners MJ, Teh HS, Goldsmith MA, Abraham N. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R alpha mutant mice. J Exp Med 2007; 204:619 - 31; http://dx.doi.org/10.1084/jem.20061871; PMID: 17325202
- Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol 2006; 7:1317 - 25; http://dx.doi.org/10.1038/ni1403; PMID: 17086188
- Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, Hodgkin PD, Nutt SL. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol 2006; 7:466 - 74; http://dx.doi.org/10.1038/ni1321; PMID: 16565720
- Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, Calame K. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol 2006; 7:457 - 65; http://dx.doi.org/10.1038/ni1320; PMID: 16565721
- Manders PM, Hunter PJ, Telaranta AI, Carr JM, Marshall JL, Carrasco M, Murakami Y, Palmowski MJ, Cerundolo V, Kaech SM, et al. BCL6b mediates the enhanced magnitude of the secondary response of memory CD8+ T lymphocytes. Proc Natl Acad Sci U S A 2005; 102:7418 - 25; http://dx.doi.org/10.1073/pnas.0501585102; PMID: 15833813
- Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol 2004; 173:883 - 91; PMID: 15240675
- Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol 2002; 3:558 - 63; http://dx.doi.org/10.1038/ni802; PMID: 12021781
- Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc Natl Acad Sci U S A 2007; 104:13414 - 9; http://dx.doi.org/10.1073/pnas.0706040104; PMID: 17686974
- Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity 2008; 28:218 - 30; http://dx.doi.org/10.1016/j.immuni.2007.12.014; PMID: 18275832
- Grayson JM, Weant AE, Holbrook BC, Hildeman D. Role of Bim in regulating CD8+ T-cell responses during chronic viral infection. J Virol 2006; 80:8627 - 38; http://dx.doi.org/10.1128/JVI.00855-06; PMID: 16912311
- Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol 2006; 36:1694 - 706; http://dx.doi.org/10.1002/eji.200635897; PMID: 16761315
- Phillips T, Opferman JT, Shah R, Liu N, Froelich CJ, Ashton-Rickardt PG. A role for the granzyme B inhibitor serine protease inhibitor 6 in CD8+ memory cell homeostasis. J Immunol 2004; 173:3801 - 9; PMID: 15356127
- Liu N, Phillips T, Zhang M, Wang Y, Opferman JT, Shah R, Ashton-Rickardt PG. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nat Immunol 2004; 5:919 - 26; http://dx.doi.org/10.1038/ni1107; PMID: 15311278
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 2003; 302:1041 - 3; http://dx.doi.org/10.1126/science.1090148; PMID: 14605368
- Youngblood B, Noto A, Porichis F, Akondy RS, Ndhlovu ZM, Austin JW, Bordi R, Procopio FA, Miura T, Allen TM, et al. Cutting edge: Prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol 2013; 191:540 - 4; http://dx.doi.org/10.4049/jimmunol.1203161; PMID: 23772031
- Youngblood B, Hale JS, Ahmed R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology 2013; 139:277 - 84; http://dx.doi.org/10.1111/imm.12074; PMID: 23347146
- Scharer CD, Barwick BG, Youngblood BA, Ahmed R, Boss JM. Global DNA methylation remodeling accompanies CD8 T cell effector function. J Immunol 2013; 191:3419 - 29; http://dx.doi.org/10.4049/jimmunol.1301395; PMID: 23956425
- Youngblood B, Wherry EJ, Ahmed R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr Opin HIV AIDS 2012; 7:50 - 7; http://dx.doi.org/10.1097/COH.0b013e32834ddcf2; PMID: 22134341
- Allen SJ, Mott KR, Ljubimov AV, Ghiasi H. Exacerbation of corneal scarring in HSV-1 gK-immunized mice correlates with elevation of CD8+CD25+ T cells in corneas of ocularly infected mice. Virology 2010; 399:11 - 22; http://dx.doi.org/10.1016/j.virol.2009.12.011; PMID: 20079918
- Mott KR, Chentoufi AA, Carpenter D, BenMohamed L, Wechsler SL, Ghiasi H. The role of a glycoprotein K (gK) CD8+ T-cell epitope of herpes simplex virus on virus replication and pathogenicity. Invest Ophthalmol Vis Sci 2009; 50:2903 - 12; http://dx.doi.org/10.1167/iovs.08-2957; PMID: 19168902
- Mott KR, Perng GC, Osorio Y, Kousoulas KG, Ghiasi H. A recombinant herpes simplex virus type 1 expressing two additional copies of gK is more pathogenic than wild-type virus in two different strains of mice. J Virol 2007; 81:12962 - 72; http://dx.doi.org/10.1128/JVI.01442-07; PMID: 17898051
- Mott KR, Osorio Y, Maguen E, Nesburn AB, Wittek AE, Cai S, Chattopadhyay S, Ghiasi H. Role of anti-glycoproteins D (anti-gD) and K (anti-gK) IgGs in pathology of herpes stromal keratitis in humans. Invest Ophthalmol Vis Sci 2007; 48:2185 - 93; http://dx.doi.org/10.1167/iovs.06-1276; PMID: 17460278
- Osorio Y, Mott KR, Jabbar AM, Moreno A, Foster TP, Kousoulas KG, Ghiasi H. Epitope mapping of HSV-1 glycoprotein K (gK) reveals a T cell epitope located within the signal domain of gK. Virus Res 2007; 128:71 - 80; http://dx.doi.org/10.1016/j.virusres.2007.04.007; PMID: 17499382
- Remakus S, Rubio D, Ma X, Sette A, Sigal LJ. Memory CD8+ T cells specific for a single immunodominant or subdominant determinant induced by peptide-dendritic cell immunization protect from an acute lethal viral disease. J Virol 2012; 86:9748 - 59; http://dx.doi.org/10.1128/JVI.00981-12; PMID: 22740418
- Moutaftsi M, Salek-Ardakani S, Croft M, Peters B, Sidney J, Grey H, Sette A. Correlates of protection efficacy induced by vaccinia virus-specific CD8+ T-cell epitopes in the murine intranasal challenge model. Eur J Immunol 2009; 39:717 - 22; http://dx.doi.org/10.1002/eji.200838815; PMID: 19224639
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol 2011; 12:509 - 17; http://dx.doi.org/10.1038/ni.2039; PMID: 21739679
- Bevan MJ. Understand memory, design better vaccines. Nat Immunol 2011; 12:463 - 5; http://dx.doi.org/10.1038/ni.2041; PMID: 21587308
- Laing KJ, Magaret AS, Mueller DE, Zhao L, Johnston C, De Rosa SC, Koelle DM, Wald A, Corey L. Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes. J Clin Immunol 2010; 30:703 - 22; http://dx.doi.org/10.1007/s10875-010-9441-2; PMID: 20635156
- Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, Elliott M, Grabstein K, Posavad C, Corey L. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol 2006; 80:5509 - 15; http://dx.doi.org/10.1128/JVI.02659-05; PMID: 16699031
- Jing L, Chong TM, McClurkan CL, Huang J, Story BT, Koelle DM. Diversity in the acute CD8 T cell response to vaccinia virus in humans. J Immunol 2005; 175:7550 - 9; PMID: 16301664
- Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, Meseda CA, Snow DC, Wald A, Corey L. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci U S A 2003; 100:12899 - 904; http://dx.doi.org/10.1073/pnas.2131705100; PMID: 14566059
- Koelle DM, Liu Z, McClurkan CM, Topp MS, Riddell SR, Pamer EG, Johnson AS, Wald A, Corey L. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest 2002; 110:537 - 48; PMID: 12189248
- Koelle DM, Chen HB, McClurkan CM, Petersdorf EW. Herpes simplex virus type 2-specific CD8 cytotoxic T lymphocyte cross-reactivity against prevalent HLA class I alleles. Blood 2002; 99:3844 - 7; http://dx.doi.org/10.1182/blood.V99.10.3844; PMID: 11986245
- Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol 2001; 166:4049 - 58; PMID: 11238653
- Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New Concepts in Herpes Simplex Virus Vaccine Development: Notes and Challenges from the Battlefield. Expert Rev Vaccines 2009; 8:1023 - 35; http://dx.doi.org/10.1586/erv.09.60; PMID: 19627185
- Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, et al. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J Virol 2011; 85:9127 - 38; http://dx.doi.org/10.1128/JVI.00587-11; PMID: 21715478
- Allen SJ, Hamrah P, Gate D, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, et al. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. J Virol 2011; 85:4184 - 97; http://dx.doi.org/10.1128/JVI.02290-10; PMID: 21307196
- Frank GM, Lepisto AJ, Freeman ML, Sheridan BS, Cherpes TL, Hendricks RL. Early CD4(+) T cell help prevents partial CD8(+) T cell exhaustion and promotes maintenance of Herpes Simplex Virus 1 latency. J Immunol 2010; 184:277 - 86; http://dx.doi.org/10.4049/jimmunol.0902373; PMID: 19949087
- Allen SJ, Mott KR, Zandian M, Ghiasi H. Immunization with different viral antigens alters the pattern of T cell exhaustion and latency in herpes simplex virus type 1-infected mice. J Virol 2010; 84:12315 - 24; http://dx.doi.org/10.1128/JVI.01600-10; PMID: 20861248
- Mott KR, Bresee CJ, Allen SJ, BenMohamed L, Wechsler SL, Ghiasi H. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J Virol 2009; 83:2246 - 54; http://dx.doi.org/10.1128/JVI.02234-08; PMID: 19091870
- Dasgupta G, Chentoufi AA, Kalantari M, Falatoonzadeh P, Chun S, Lim CH, Felgner PL, Davies DH, BenMohamed L. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol 2012; 86:4358 - 69; http://dx.doi.org/10.1128/JVI.07107-11; PMID: 22318137
- Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines 2009; 8:1023 - 35; http://dx.doi.org/10.1586/erv.09.60; PMID: 19627185
- Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines 2009; 8:1023 - 35; http://dx.doi.org/10.1586/erv.09.60; PMID: 19627185
- Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillère B, BenMohamed L. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol 2008; 82:11792 - 802; http://dx.doi.org/10.1128/JVI.00692-08; PMID: 18799581
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 2013; 14:1294 - 301; http://dx.doi.org/10.1038/ni.2744; PMID: 24162776
