Abstract
ISCOMATRIX™ adjuvant is an integrated adjuvant system due to its ability to both facilitate antigen delivery and immunomodulate the innate and adaptive immune responses to vaccination. ISCOMATRIX™ adjuvant strongly induces both humoral and cell-mediated immunity in formulation with a range of antigens in pre-clinical and clinical evaluations. In this study, we describe the adaptive and innate immune responses associated with ISCOMATRIX™ adjuvant in the context of a previously described HIV-1 vaccine, DP6-001. The DP6-001 vaccine consists of a unique pentavalent HIV-1 Env DNA prime-protein boost regimen. This study demonstrates the potent induction of vaccine-specific antibodies in a mouse model, as well as broadly neutralizing antibodies in immunized rabbits. In addition, we identify a potentially critical role for DNA priming in the induction of the vaccine-specific immune response as well as the serum cytokine profiles associated with ISCOMATRIX™ adjuvant. Most interestingly, DNA prime immunizations made ISCOMATRIX™ adjuvant less dependent on the central innate immune adaptor MyD88, revealing a previously unknown mechanism that may expand our knowledge on the use of adjuvants.
Introduction
Effective prophylactic vaccination against HIV-1 requires induction of a diverse and specific immune response, consisting of protective antibodies, as well as effector CD4+ and CD8+ T cells. In approaching the complex problem of HIV-1 vaccination, the choice of an adjuvant capable of stimulating both humoral and cellular immune responses is a critical component of a protective vaccine.
It is well established that the innate immune response plays an important role in the initiation and regulation of the adaptive immune response.Citation1 Innate immune responses are triggered by recognition of pathogen associated molecular patterns (PAMPS) or of danger associated molecular patterns (DAMPS) by germ-line coded pathogen recognition receptors (PRRs). Engagement of PRR on dendritic cells (DCs) induces their activation and ability to internalize and present pathogen-derived peptides associated with MHC class I and/or II molecules. Antigen (Ag) presentation, the expression of co-stimulatory molecules and secretion of cytokines are all required for optimal priming of T cells and subsequent induction of CTL and antibody (Ab) immune responses.Citation2 Adjuvants mimic the action of PAMPs or DAMPs, and are critical to induce adaptive immune responses against poorly immunogenic Ag, such as recombinant high pure Ags. ISCOMATRIX™ adjuvant possesses the ability to enhance Ab and CTL responses in vivo against the Ags it is formulated with.
ISCOMATRIX™ adjuvant is comprised of cholesterol, phospholipid and ISCOPREP™ saponin, which is purified from the Quillaja saponaria tree. Under defined conditions these components form cage-like structures typically 40 to 50 nm in size.Citation3-Citation5 Like ISCOMATRIX™ adjuvant, the more classical saponin adjuvant QS-21, used in our previous preclinical and clinical evaluations of the DP6-001 vaccine, is a single fraction purified from Quillaja saponaria. This purified saponin fraction QS-21 has despite its potency, long been restricted for use clinically due to local and systemic adverse effects.Citation6,Citation7 However, the formulation of the proprietary ISCOPREP™ saponin along with cholesterol and phospholipid matrix appear to ameliorate these adverse effects without compromising immunogenicity.Citation8,Citation9
The activities of ISCOMATRIX™ adjuvant described above result in transient activation of innate immune cells at the site-of-injection and draining lymph node as well as Ag presentation in association with MHC class I and II molecules leading to induction of CD4+ and CD8+ T cell responses. Observations in pre-clinical and clinical studies have noted that when combined with Ag, ISCOMATRIX™ adjuvant elicits a Th1/Th2 cytokine response as well as robust and persistent Ab responses.Citation9-Citation11 ISCOMATRIX™ adjuvant has been tested in several clinical trials and shown to be well tolerated.Citation8 ISCOMATRIX™ adjuvant is stable for several years when stored at 2–8 °C and can be formulated with virtually any Ag by simple mixing to produce and ISCOMATRIX™ vaccine.Citation12
Although there are no TLR ligands in ISCOMATRIX™ adjuvant, early studies have shown that the immune response to Ag formulated with ISCOMATRIX™ adjuvant were severely compromised in MyD88 deficient mice. It is possible that an endogenous danger signal released upon ISCOMATRIX™ vaccine administration might account for these observations and this hypothesis is currently being investigated.Citation11 The properties of ISCOMATRIX™ adjuvant, together with the ability to manufacture reproducibly at large scale, support its use in therapeutic and prophylactic human vaccines for both intra- and extracellular pathogens.
The current study focuses on the adjuvant effect of ISCOMATRIX™ adjuvant in the unique context of a previously described polyvalent HIV-1 gp120 DNA prime-protein boost construct, DP6-001. We have demonstrated the immunogenicity of DP6-001 in human volunteers, with the induction of envelope (Env)-specific antibody responses with cross-subtype neutralizing ability, as well as induction of vaccine-specific T cell responses.Citation13,Citation14 The protein boost component of DP6-001 was formulated with the saponin adjuvant QS-21 in preclinical and clinical evaluations. As a saponin-based complex with reduced reactogenicity but uncompromised potency, ISCOMATRIX™ adjuvant is a promising alternative to QS-21. In addition, protective immunity against HIV-1 infection requires the action of both the humoral and cellular arms of the adaptive immune response, and so a broadly acting integrated adjuvant system like ISCOMATRIX™ adjuvant is an obvious choice for inclusion in future Env DNA prime-protein boost vaccine formulations.
In the current study, we analyzed the efficacy of a the DP6-001 gp120 DNA prime-protein boost formulated with ISCOMATRIX™ adjuvant in generating a robust antibody response in both Balb/c and C57Bl/6 mice, and New Zealand white (NZW) rabbits, in comparison to DP6-001 formulated with aluminum hydroxide gel adjuvant, Al(OH)3. Serum cytokines following each protein-adjuvant immunization were quantified in mice, demonstrating potent induction of Th1 and Th2 cytokines by ISCOMATRIX™ vaccines. In addition, immunization of MyD88 deficient mice with the protein based ISCOMATRIX™ vaccine, with or without DNA prime, was studied. Our data demonstrate the DP6-001 ISCOMATRIX™ vaccine elicits cross-neutralizing anti-Env antibodies and suggests that DNA priming may overcome the dependence of ISCOMATRIX™ adjuvant on intact MyD88 signaling.
Results
Serum cytokine and antibody responses in Balb/c mice immunized with the DP6-001 HIV-1 vaccine formulation or gp120 protein alone vaccines
The aim of this work was to design an HIV vaccine and immunization protocol able to induce neutralizing Ab (NAb) responses and Th1/Th2 cytokine responses, both parameters potentially important for protective immunity against infection by HIV-1. For this purpose, we used a DNA prime-protein boost regime with gp120 coding DNA for five different HIV subtypes (DP6-001) or the corresponding protein vaccines alone. Al(OH)3 and ISCOMATRIX™ adjuvant were evaluated as candidate adjuvants, due to their clinical relevance or ability to induce Th1/Th2 cellular responses, respectively.
The first study was conducted in Balb/c mice as indicated in . Mice received three DNA immunizations, followed by 2 DP6-001 protein boosts formulated with ISCOMATRIX™ adjuvant or Al(OH)3. Control animals received an empty vector DNA prime followed by two protein boosts formulated with ISCOMATRIX™ adjuvant. Ab titers and cytokine concentrations were determined in sera at the time points indicated.
Figure 1. Study design and immunization schedule. Mice were primed 3 times with a pentavalent DNA vaccine in weeks 0, 2, and 4, followed by 2 pentavalent gp120 protein boosts in weeks 8 and 12. Either the DNA or protein pentavalent vaccine consisted of five HIV-1 Env gp120 immunogens from clades A (92UG037.8), B (92US715.6 and Bal), C (96ZM651), and E (93TH976.17). As a control, mice were immunized with empty pSW3891 vector primes followed by DP6-001 protein and adjuvant boosts. Additional controls included mice immunized with two full dose DP6-001 protein vaccine immunizations and adjuvant, in the absence of DNA priming. Al(OH)3 and ISCOMATRIX™ adjuvant were tested individually as part of the protein boost. Follow-up experiments included 5 immunizations with DP6-001 protein ISCOMATRIX™ vaccine according to the established prime-boost schedule, with the first three immunizations at a 1/5 dose, and the final two immunizations at a full protein dose. A constant dose of ISCOMATRIX™ adjuvant was used for all immunizations. Time points of immunizations, and sample collections for different assays are indicated.
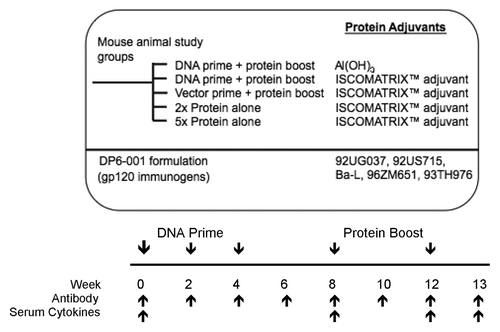
All immunized and naïve animal samples showed very low cytokine concentrations prior to immunization and upon termination at week 13 (). The Al(OH)3 vaccine formulation induced marginal elevated levels of IL-2 and IL-6 and no significant levels of IFNγ. Similarly, priming with empty vector and boosting with proteins did not induce significant levels of cytokine responses. By contrast, the vaccine including DNA prime and protein boost formulated with ISCOMATRIX™ adjuvant induced a more a Th1/Th2 balanced serum cytokine profile following each protein boost.
Figure 2. Kinetics of Th1 and Th2 serum cytokine responses, and gp120-specific IgG responses, in wild type Balb/c mice. Wild type Balb/c mice (n = 5/experimental group) were primed with DP6-001 coding DNA (D) and boosted with DP6-001 protein formulated with Al(OH)3 (D+P-Al(OH)3) or ISCOMATRIX™ adjuvant (P-IMX). Controls received an empty vector DNA prime followed by boost with DP6-001 formulated with ISCOMATRIX™ adjuvant (V+P-IMX). Naïve mice received saline injections. Sera were collected pre-immunization in week 0, and 6 h following the first and second protein-adjuvant boosts in weeks 8 and 12, respectively. Sera were also collected at termination 7 d after the final protein boost in week 13. Levels of serum cytokines in individual mice were measured by CBA. Sera were evaluated for (A) IFNγ, (B) IL-2, or (C) IL-6. Statistical significance was determined by a two-way ANOVA and the Tukey post test (*P < 0.05, **P < 0.01, ***P < 0.001). (D) Total gp120-specific IgG was measured by ELISA in individual sera from immunized Balb/c mice 7 d after final protein boost, on week 13. After DP6-001 DNA priming, protein boosts were formulated with Al(OH)3 (green or D+P-Al(OH)3), or ISCOMATRIX™ adjuvant (purple or D+P-IMX). Control mice were primed with empty vector followed by boosts with DP6-001 protein formulated with ISCOMATRIX™ adjuvant (brown or V+P-IMX). Naïve mice received only saline injections. Data are expressed as mean +/− SEM (n = 5) and statistical significance was determined by the Student t test (*P < 0.05, **P < 0.01, ***P < 0.001). (IMX = ISCOMATRIX™ adjuvant).
Immunization with the DP6-001 ISCOMATRIX™ vaccine induced significantly elevated serum levels of Th1 serum cytokines IFNγ () and IL-2 (), as well as the Th2-associated cytokine IL-6 (). Notably, all three cytokines levels were higher following the first vs. the second protein boost. Other cytokines included in the kit, including IL-4, IL-10, IL-12p70, IL-17, and TNFα, were low to undetectable, or had such a high degree of variability as to be meaningful (data not shown).
We have previously reported robust antibody responses against DP6-001 vaccines in small animals, non-human primates, and clinical settings.Citation13,Citation15 In the current study, high levels of gp120-specific IgG Ab responses were elicited by DP6-001 vaccine in Balb/c mice. shows the antibody titers one week after the final protein boost. Results indicate that both the Al(OH)3 and ISCOMATRIX™ vaccines induced comparable Ab responses. In addition, mice primed with the empty vector and boosted with the DP6-001 ISCOMATRIX™ vaccine showed comparable gp120-specific Ab titers, indicating the strong immunogenicity of the DP6-001 ISCOMATRIX™ vaccine even in the absence of a coding DNA prime.
Serum cytokine and antibody responses in C57Bl/6 mice immunized with the DP6-001 HIV-1 vaccine formulation or gp120 protein alone vaccines
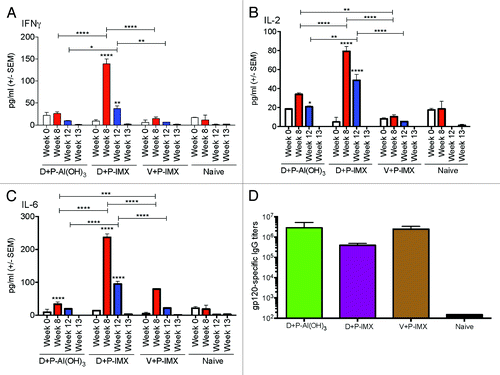
To further confirm that the above cytokine and antibody response were not unique to Balb/c mice, C57Bl/6 mice were immunized with the protocol described in . As we observed in Balb/c mice, the highest serum cytokine levels in C57Bl/6 mice were measured at 6 h following the first protein boost, with reduced levels following the second protein boost with the exception of IL-2 induced by DNA + DP6-001 ISCOMATRIX™ vaccine protocol (). The time point 6 h post-immunization was chosen based our previous adjuvant work in wild type mice as the best time point to approximate peak levels of cytokines with varying kinetics, and supported by recent studies of the kinetics of inflammatory cytokine induction after ISCOMATRIX™ adjuvant administration.Citation11,Citation16 At termination, serum cytokine levels were back to background levels. Animals immunized with DP6-001 formulated with Al(OH)3 or ISCOMATRIX™ adjuvant demonstrated comparable levels of IFNγ () and IL-2 () following the first protein boost. IFNγ levels dropped in both groups to background levels by the second protein boost, however IL-2 was induced after the second boost with DP6-001 ISCOMATRIX™ vaccine. Serum IL-6 was more prominently induced following each protein boost in animals immunized with DP6-001 ISCOMATRIX™ vaccine as compared with the low response observed in mice receiving DP6-001 Al(OH)3 vaccine (). As expected, serum cytokine responses in mice primed with the empty vector were low or at background levels compared with animals primed with the gp120 coding DNA. Serum IL-6 was moderately elevated in vector-primed animals only after the second protein boost before declining to background levels ().
Figure 3. Kinetics of Th1 and Th2 serum cytokine responses, and gp120-specific IgG responses, in wild type C57Bl/6 mice. Wild type C57Bl/6 mice (n = 5/experimental group) were primed with DP6-001 coding DNA (D) and boosted with DP6-001 protein in Al(OH)3 (D+P-Al(OH)3) or ISCOMATRIX™ adjuvant (D+P-IMX). Controls received an empty vector DNA prime followed by boost with DP6-001 protein formulated with ISCOMATRIX™ adjuvant (V+P-IMX). Naïve mice received saline injections. Sera were collected pre-immunization in week 0, and 6 h following the first and second protein-adjuvant boosts in weeks 8 and 12, respectively. Sera were also collected at termination 7 d after the final protein boost in week 13. Levels of serum cytokines in individual mice were measured by CBA. Sera were evaluated for (A) IFNγ, (B) IL-2, and (C) IL-6. Statistical significance was determined by a two-way ANOVA and a Tukey post test (*P < 0.05, **P < 0.01, ***P < 0.001). (D) Total Env-specific IgG was measured by ELISA in sera collected from immunized C57Bl/6 mice (n = 5) 7 d after final protein boost, in week 13. After DP6-001 DNA priming, protein boosts were formulated with Al(OH)3 (green), or ISCOMATRIX™ adjuvant (purple). Control mice were immunized with empty vector prime followed by boosts with DP6-001 protein formulated with ISCOMATRIX™ adjuvant (brown). Naïve mice (black) received “mock” saline injections in lieu of immunization. Env-specific endpoint IgG titer was determined by ELISA using individual serum samples collected in week 13 from immunized wild type mice. Data are expressed as mean +/− SEM and statistical significance was determined by the Student t test (*P < 0.05, **P < 0.01, ***P < 0.001). (IMX = ISCOMATRIX™ adjuvant).
Notably, Th1 cytokine responses measured by CBA differed in Balb/c and C57Bl/6 mice immunized with Al(OH)3. IFNγ responses were greater in C57Bl/6 mice immunized with the DP6-001 Al(OH)3 vaccine as compared with Balb/c mice receiving the same vaccine. This may be attributable to the bias of each mouse strain. Balb/c mice are known to have a more Th2-biased profile, while C57Bl/6 mice are known to have a Th1-biased profile. The Al(OH)3 adjuvant, as a known potent inducer of Th2 responses, may have a more blunted Th1 response in the Th2-biased Balb/c mouse strain. Again, the levels of IL-4, IL-10, IL-12p70, IL-17, and TNFα, were low to undetectable, or had such a high degree of variability as to be meaningful (data not shown).
Similarly to the data from Balb/c mice, there was no significant difference in gp120-specific Ab responses across groups immunized with Al(OH)3 or ISCOMATRIX™ vaccines, or primed with empty vector prior to protein-vaccine boosts (), likely due to the relatively high dose of protein vaccines used in a mouse model.
Induction of neutralizing gp120-specific Abs by ISCOMATRIX™ vaccines in in NZW rabbits
The amount of blood obtained from mice is not enough to determine the titers of NAbs against a standard panel of HIV-1 viruses. For this reason, NZW rabbits were primed with DP6-001 coding DNA prime and boosted with DP6-001 protein formulated in either Al(OH)3 or ISCOMATRIX™ adjuvant. Control rabbits received an empty vector DNA prime followed by boosting with DP6-001 ISCOMATRIX™ vaccine. The titer of gp120-specific IgG was determined in sera collected 7 d following the final protein boost. Rabbits immunized with DP6-001-Al(OH)3 or DP6-001 ISCOMATRIX™ vaccine demonstrated comparable gp120-specific IgG titers across all time points. Priming with DP6-001 DNA induced significant Ab responses by week 6, which was further boosted by DP6-001 protein vaccines (). Priming with empty vector followed by DP6-001 ISCOMATRIX™ vaccine boost induced gp120-specific Abs only after the protein boost. A second DP6-001 ISCOMATRIX™ vaccine boost further amplified the Ab response, which reached similar levels to the other vaccinated groups at week 20 ().
Figure 4. HIV-1 Env-specific IgG titers in rabbits immunized with DP6-001 ISCOMATRIX™ vaccine. New Zealand white rabbits (n = 5/experimental group) were primed with DP6-001 DNA and boosted with DP6-001 protein formulated with Al(OH)3 (D+P-Al[OH]3) or ISCOMATRIX™ adjuvant (D+P-IMX). As a control, rabbits were immunized with empty vector DNA prime followed by boost with the DP6-001 protein formulated with ISCOMATRIX™ adjuvant (V+P-IMX). Env-specific IgG responses were determined at different time points in pooled samples (A) and endpoint titers were determined on week 20 in individual samples (B) by ELISA. (IMX = ISCOMATRIX™ adjuvant)
![Figure 4. HIV-1 Env-specific IgG titers in rabbits immunized with DP6-001 ISCOMATRIX™ vaccine. New Zealand white rabbits (n = 5/experimental group) were primed with DP6-001 DNA and boosted with DP6-001 protein formulated with Al(OH)3 (D+P-Al[OH]3) or ISCOMATRIX™ adjuvant (D+P-IMX). As a control, rabbits were immunized with empty vector DNA prime followed by boost with the DP6-001 protein formulated with ISCOMATRIX™ adjuvant (V+P-IMX). Env-specific IgG responses were determined at different time points in pooled samples (A) and endpoint titers were determined on week 20 in individual samples (B) by ELISA. (IMX = ISCOMATRIX™ adjuvant)](/cms/asset/58e2cb32-b3c4-4962-afc6-4166a6046479/khvi_a_10927907_f0004.gif)
Previous formulations of the polyvalent DP6-001 vaccine in small animal and clinical models were capable of eliciting broadly NAb responses. Immunized rabbit sera were assessed for NAb activity against known sensitive viral isolates SF162 and ss1196 (). No significant difference in neutralizing titers against either sensitive viral isolate was observed between vaccinated groups. Rabbits receiving DP6-001 formulated with either Al(OH)3 or ISCOMATRIX™ adjuvant, as well as those receiving the empty vector prime and DP6-001 ISCOMATRIX™ vaccine, were able to neutralize SF162 () and ss1196 () with similar strength.
Figure 5. ISCOMATRIX™ adjuvant improves breadth of neutralizing antibody response in rabbits immunized with DP6-001. Rabbits were primed with DP6–001 DNA and boosted with DP6–001 protein formulated with either Al(OH)3 (D+P-Al(OH)3) or ISCOMATRIX™ adjuvant (D+P-IMX). Control rabbits were immunized with empty vector DNA prime followed by boosts with DP6-001 protein formulated with ISCOMATRIX™ adjuvant (V+P-IMX). Neutralizing antibody titers were determined against 2 Clade B viral isolates sensitive to neutralization SF162 (A) and ss1196 (B) or against a previously described NIH Tier 2 panel of 12 viruses (C). Percentage of positive neutralizing antibody events is defined as the percentage of Tier 2 viral isolates that were neutralized by vaccine-induced sera antibodies. (IMX = ISCOMATRIX™ adjuvant)
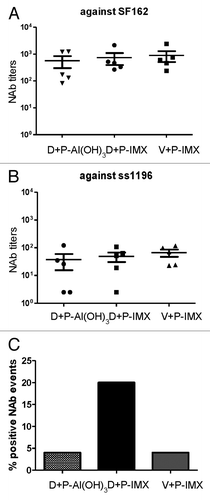
Notable differences emerged upon further dissecting the quality of vaccine-induced NAb responses. Immunized sera were examined for neutralizing ability against the previously described NIH Tier 2 clade B standardized panel (), as these are more resistant to neutralization.Citation17-Citation19 Sera from rabbits immunized with either DP6-001-Al(OH)3 vaccine or empty vector prime and DP6-001 ISCOMATRIX™ vaccine boost failed to elicit greater than 5% neutralization against the less sensitive Tier 2 panel. Rabbits immunized with DP6-001 DNA prime and DP6-001 ISCOMATRIX™ vaccine boost raised a much broader NAb neutralizing response, with an average neutralization of 20% observed against Tier 2 viral isolates. These results identify the importance of both DP6-001-encoding DNA prime components as well as the vaccine adjuvant in inducing an antigen-specific, broadly neutralizing Ab response against HIV-1.
Priming with DNA vaccine overcome the requirement of MyD88 signaling in response to DP6-001 ISCOMATRIX™ vaccines
The induction of Ab responses specific for Ags formulated with ISCOMATRIX™ adjuvant has previously been shown to require signaling through MyD88.Citation11 This dependence was shown in immunization protocols with ISCOMATRIX™ vaccines comprised of proteins, although it was not tested in the context of heterologous prime-boost regimes, including DNA-based vaccines. Here we investigated whether the Ab responses observed with DP6-001 DNA prime and DP6-001 ISCOMATRIX™ vaccine boost were also dependent on MyD88 signaling, a key cytosolic adaptor molecule that functions in regulating the activation of pro-inflammatory genes in IL-1 and TLR signaling pathways.Citation20 For this purpose, we analyzed the Ab response in MyD88 deficient mice using the ISCOMATRIX™ vaccines as described in studies with wild type mice. As the mechanism of action of the Al(OH)3 adjuvant also remains the subject of much study and is itself a complex issue, we focused our efforts here on the role of MyD88 signaling specifically for the ISCOMATRIX™ adjuvant in the context of our DP6-001 vaccine regimen.
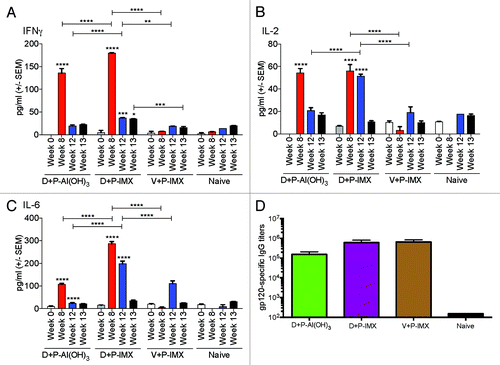
Here we demonstrate similar MyD88 dependency of results in response to DP6-001 ISCOMATRIX™ vaccine alone (). Interestingly, gp120-specific Ab responses were comparable between wild type and MyD88-deficient mice when the vaccination regimen included priming with DNA vaccine, and Ab titers started to rise immediately after the first protein boost in both types of mice (), staying at the same level throughout the experiment (). There was no significant difference in the endpoint gp120-specific Ab responses between wild type and MyD88-deficient mice immunized with the DP6-001 ISCOMATRIX™ vaccine (). In mice that received only two DP6-001 protein ISCOMATRIX™ vaccines in the absence of DNA priming, a significant difference was observed in the endpoint gp120-specific Ab responses ().
Figure 6. DNA priming improves Env-specific IgG response to DP6-001 ISCOMATRIX™ vaccine and overcomes dependency on MyD88. Total Env-specific IgG was measured by ELISA in sera from immunized C57Bl/6 mice 7 d after final protein boost, on week 13. (A and B) DP6-001 DNA-primed wild type (solid) and MyD88 deficient (checkered) mice were immunized with and the DP6-001 ISCOMATRIX™ vaccine boost (D+P-IMX). (C and D) In the absence of DNA priming, wild type and MyD88 deficient mice were immunized twice with the full-dose DP6-001 protein ISCOMATRIX™ vaccine (2P-IMX). (E and F) Also in the absence of DNA priming, wild type and MyD88 deficient mice were immunized five times with the DP6-001 ISCOMATRIX™ vaccine (5P-IMX), receiving 3 immunizations with 1/5 dose DP6-001 protein, followed by 2 full-dose DP6-001 protein immunizations. A constant dose of 1.5 ISCO™ Units of ISCOMATRIX™ adjuvant were used for all immunizations. Naïve mice received only saline injections. Env-specific IgG response titers were in pooled (A, C, E) or individual (B, D, F) sera by ELISA. Time point for data on (B, D, F) corresponds to 7 d after final protein boost. Data are shown as mean +/− SEM and statistical significance was determined by Student’s t test (*P < 0.05, **P < 0.01, ***P < 0.001). (IMX = ISCOMATRIX™ adjuvant)
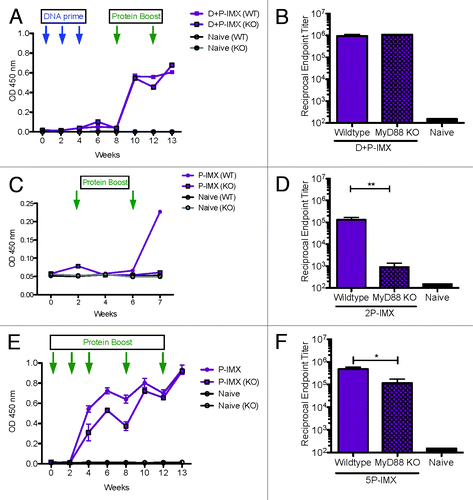
We further investigated the possibility that the above difference between DNA prime-protein boost immunization and two immunizations with a protein-only vaccine in MyD88-deficient mice was due to the different number of immunizations. For this purpose, additional wild type and MyD88-deficient mice were immunized five times with DP6-001 ISCOMATRIX™ vaccine as per the schedule in . A substantial gp120-specific Ab response was detected from the second vaccination, and titers continued to increase with additional boosts (). However, in MyD88-deficient mice, the rise of the Ab response was lower compared with wild type mice (), and a significant different in the endpoint gp120-specific Ab titer was observed between wild type and MyD88-deficient mice immunized with five DP6-001 protein ISCOMATRIX™ vaccines ().
Overall, these results suggest that DNA vaccination primed the immune system so that MyD88 is no longer required for the protein ISCOMATRIX™ vaccines to induce robust Ab responses and demonstrates that MyD88 plays a larger role in immunizations with protein-based ISCOMATRIX™ vaccines.
Serum cytokine responses in MyD88 deficient mice immunized with the DP6-001 HIV-1 vaccine formulated with ISCOMATRIX™ adjuvant
A comparison of the serum cytokine concentrations between wild type and MyD88 KO mice is shown in . Analyses were performed 6 h after the first protein boost, the time point at which wild type mice produced the highest cytokine response (, ).
Figure 7. Th1 and Th2 serum cytokine responses in C57Bl/6 and MyD88 deficient mice boosted with the DP6-001 ISCOMATRIX™ vaccine. Wild type C57Bl/6 mice were immunized with the DP6-001 ISCOMATRIX™ vaccine (D+P-IMX) (purple), or with an empty vector DNA prime followed by the DP6-001 ISCOMATRIX™ vaccine (V+P-IMX) (brown). Naïve mice received saline injections (white). Sera were collected pre-immunization, 6 h following each protein-adjuvant boost, and at termination 7 d after the final protein boost. Samples were evaluated for (A) IFNγ, (B) IL-2, (C) TNF, (D) IL-6, or (E) IL-10. Data show mean + SEM (n = 5). Statistical significance was determined by a one-way ANOVA and a Tukey post test (*P < 0.05, **P < 0.01, ***P < 0.001). (IMX = ISCOMATRIX™ adjuvant)
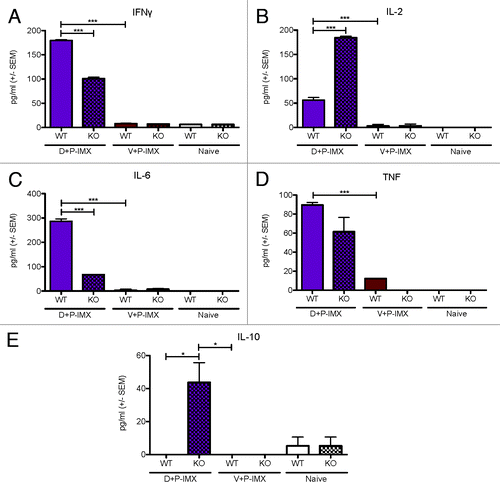
Serum cytokines induced in mice primed with vector and boosted with DP6-001 ISCOMATRIX™ vaccine were comparable to background with the exception of a low but significant level of TNFα. As previously shown, in wild type C57Bl/6 mice the DP6-001 ISCOMATRIX™ vaccine induces significant levels of IFNγ (), IL-2 (), IL-6 (), and TNFα (), as compared with animals primed with the empty vector and boosted with DP6-001 ISCOMATRIX™ vaccine or naïve controls. Notably, in MyD88-deficient mice immunized with DP6-001 ISCOMATRIX™ vaccine, serum IFNγ () and IL-6 () were significantly reduced as compared with wild type.
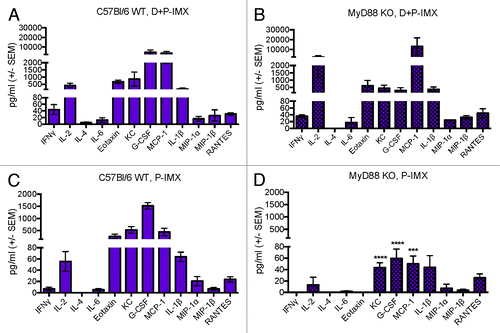
Interestingly, serum IL-2 and IL-10 were significantly elevated in MyD88-deficient mice immunized with DP6-001 ISCOMATRIX™ vaccine compared with wild type mice (). Furthermore, a more comprehensive profile of serum cytokines and chemokines was quantified by a multiplex Luminex array in mice immunized with DP6-001 ISCOMATRIX™ vaccine in the presence or absence of DNA priming. The analysis was performed 6 h after the first protein boost. As we have previously noted, serum cytokine levels were elevated following the first protein immunization. Cytokine levels in wild type mice receiving a DP6-001 DNA prime followed by the DP6-001 ISCOMATRIX™ vaccine were notably higher than in mice receiving only two DP6-001 ISCOMATRIX™ vaccine immunizations (). Most importantly, a similar cytokine response profile was observed between wild type () and MyD88-deficient mice () primed with DP6-001 DNA vaccine before boosted with the DP6-001 ISCOMATRIX™ vaccine. In contrast, serum KC, G-CSF, and MCP-1 were significantly reduced in MyD88-deficient mice immunized with the DP6-001 ISCOMATRIX™ vaccine in the absence of DNA priming () as compared with wild type mice (). Moderate reductions in serum IL-2, Eotaxin, and IL-1β were observed in MyD88 deficient mice only in the absence of DNA prime () compared with wild type animals (). These results further confirmed that MyD88 does play a critical role in the serum cytokine profiles induced by the DP6-001 ISCOMATRIX™ vaccine in the absence of any DNA priming and, more importantly, that DNA priming overcomes such dependency as shown with gp120-specific antibody responses.
Figure 8. Serum cytokine levels after first protein boost in wild type or MyD88 deficient mice immunized with DP6-001 ISCOMATRIX™ vaccine in the presence or absence of DNA priming. Mice C57Bl/6 wild type (left panels, WT) or MyD88 deficient (right panels, KO) were primed with DP6-001 DNA and boosted twice with DP6-001 protein ISCOMATRIX™ (D+P-IMX) vaccine (A and B) or only received two immunizations with the DP6-001 protein ISCOMATRIX™ (P-IMX) vaccine (C and D). Cytokines were quantified in the serum of individual mice 6 h after the last boost with a custom 12-plex Luminex panel. Data represent mean + SEM (n = 5) and statistical significance was determined with a one-way ANOVA and Tukey post test (*P < 0.05, **P < 0.01, ***P < 0.001). (IMX = ISCOMATRIX™ adjuvant).
Discussion
Protective immunity against complex pathogens such as HIV-1 requires a vaccine that is capable of inducing a diverse and potent immune response consisting of both antibody and cell-mediated immunity. The importance of polyfunctional CD4+ helper T cells, CD8+ T cells, and broad neutralizing antibodies have been clearly defined in the effective prophylaxis against HIV-1 challenge. In several clinical trials, including the widely publicized Thailand RV144 trial and our lab’s own trial of the DP6-001 vaccine, a DNA or viral vector prime followed by a protein boost has been employed as an effective strategy to strongly activate both arms of the immune response and confer some level of protective immunity.Citation13,Citation14,Citation19,Citation21,Citation22 The choice of adjuvant to be paired with the protein boost component critically impacts the immunogenicity and efficacy of a vaccine.
In recent years, ISCOMATRIX™ adjuvant has emerged as an ideal candidate for inclusion in future formulations of HIV-1 vaccines, due to its immunomodulatory functions and ability to deliver Ag to DCs for induction of CD4 and CD8 T cell responses, which are crucial components of effective prophylaxis against HIV-1 infection. In addition, ISCOMATRIX™ vaccines (Ag formulated with ISCOMATRIX™ adjuvant) induce potent humoral and cell-mediated immunity, and are well tolerated and safe as demonstrated in a number of Phase I trials of prophylactic and therapeutic vaccines.Citation8
The current study is the first report of ISCOMATRIX™ adjuvant in the context of a heterologous DNA prime-protein boost vaccine strategy. The primary objectives of this study were to confirm the immunogenicity of ISCOMATRIX™ adjuvant formulated with the previously described DP6-001 in multiple animal models, and also to identify the impact of DNA priming on MyD88, a key intracellular molecule important in regulating the immunogenicity of protein based ISCOMATRIX™ vaccines. We demonstrated, in both mouse and rabbit models, that an Env DNA prime-protein boost vaccine formulated with ISCOMATRIX™ adjuvant was able to generate strong Env-specific IgG responses comparable to levels associated with DP6-001-Al(OH)3 immunization. In rabbits, vaccine-induced antibodies generated with DP6-001 DNA prime then boosted with DP6-001-Al(OH)3 or DP6-001 ISCOMATRIX™ vaccines were similarly able to neutralize sensitive HIV-1 viral isolates SF162 and ss1196, while the DP6-001 ISCOMATRIX™ vaccine generated superior neutralizing antibodies against a Tier 2, clade B panel of pseudotyped HIV-1 viruses compared with DP6-001 Al(OH)3 or DP6-001 ISCOMATRIX™ vaccines alone. These data highlight not only the potent immunogenicity of the ISCOMATRIX™ vaccine, but also the substantial impact of a multiclade DNA prime encoding Env antigen on the breadth of vaccine-induced humoral immunity.
We also evaluated the ability of the DP6-001 ISCOMATRIX™ vaccine to generate serum cytokines in comparison to Al(OH)3 vaccine or an empty vector DNA prime. In the current study we observed that serum cytokine responses were robust at 6 h following the first protein boost. In a recent study by Wilson et al., a serum cytokine profile was developed for ISCOMATRIX™ adjuvant 6 h after subcutaneous administration, with the Th1 and Th2 cytokines, as well as the chemokines G-CSF, KC, and MIP-1α, standing out over background levels.Citation11 Wilson et al. correlated this rapid but transient induction of a unique serum cytokine signature with activation of DCs and NKs in the draining lymph node, suggesting that the generation of a potent and unique systemic cytokine response, which may in part facilitate the innate and adaptive immune response to immunization with ISCOMATRIX™ vaccines.Citation11 In the current study, we demonstrated that the DP6-001 Al(OH)3 vaccine and, to a greater extent, the DP6-001 ISCOMATRIX™ vaccine, were able to induce strong non-antigen specific Th1 and Th2 serum cytokines 6 h following the first protein boost following DNA priming. The induction of these inflammatory cytokines was generally greater following the first protein boost after DNA priming, as compared with after subsequent protein boosts. While we have observed this trend in previously published studies examining multiple adjuvants in wild type mice, this reproducible phenomenon warrants further studyCitation16
In contrast, wild type mice primed with empty vector DNA followed by DP6-001 ISCOMATRIX™ vaccine boosts generated low to background levels of serum cytokines by CBA. Similarly, serum cytokines and chemokines analyzed by multiplex array following two DP6-001 ISCOMATRIX™ vaccine immunizations in the absence of DNA priming were reduced across the board in comparison to DNA-primed mice. These results indicate the critical impact of gp120 DNA priming in the generation of an ISCOMATRIX™ adjuvant-associated potent and diverse inflammatory cytokine profile. These data are particularly significant for the gp120 protein antigen, which when delivered by itself has demonstrated poor immunogenicity.Citation23 This likely explains why, formulated with ISCOMATRIX™, the gp120 protein antigen induces a low cytokine response in the absence of DNA priming, while the adjuvant itself performs differently with other protein antigens.Citation24
In this study, we also examined the dependence of the DP6-001 ISCOMATRIX™ vaccine on the broadly utilized innate immune signaling adaptor, MyD88. In a recent study utilizing a recombinant protein tumor antigen vaccine formulated with ISCOMATRIX™ adjuvant, MyD88 was found to be essential for adaptive immune responses such as CD8+ T cell responses, NK cell function, and vaccine-specific antibody responses, while other TLR deficiencies did not compromise adaptive immunity. However, MyD88 appeared to be dispensable for activation of dendritic cells and cross-presentation to CD8+ T cells.Citation11 It was suggested that ISCOMATRIX™ adjuvant produces an indirect effect on innate signaling pathways via the generation of a pro-inflammatory cytokine and chemokine environment, and there is a coexistence of both MyD88-dependent and -independent mechanisms of action for the ISCOMATRIX™ adjuvant.Citation11 Our current study of the role of MyD88 in ISCOMATRIX™ vaccines focused on its impact on adaptive immunity and serum cytokine profiles, as well as its changing function in the context of a DNA prime-protein boost vaccine strategy.
Env-specific IgG endpoint titers associated with DP6-001-Al(OH)3 or DP6-001 ISCOMATRIX™ vaccine were minimally impacted in MyD88-deficient mice. There was some reduction in serum Th1 and Th2 responses measured by CBA associated with DP6-001 ISCOMATRIX™ vaccine in MyD88-deficient mice. In an expanded panel of serum cytokines, mice immunized with the DP6-001 ISCOMATRIX™ vaccine demonstrated a reduced serum cytokine response in comparison to DNA prime-protein DP6-001 ISCOMATRIX™ vaccine boost. Follow-up studies that used two DP6-001 ISCOMATRIX™ vaccine immunizations, however, indicated a dependence of vaccine-induced humoral responses on MyD88 in the absence of DNA priming. In addition, by multiplex array, MyD88 deficiency had a more significant impact on serum cytokine and chemokine profiles in the DP6-001 ISCOMATRIX™ vaccine immunized mice than was observed with DNA prime-DP6-001 ISCOMATRIX™ vaccine boost. These data highlight the significant impact of a DNA priming component on the innate and adaptive immune responses.
Vaccines including a DNA prime component generate improved humoral immunity and enhanced pro-inflammatory response. We observed increased dependence on intact MyD88 signaling for adaptive and innate responses in the absence of any DNA priming with the DP6-001 ISCOMATRIX™ vaccine. Our data are not inconsistent with reports by Wilson et al., which indicate a role for MyD88 signaling with a recombinant protein vaccine formulated with ISCOMATRIX™ adjuvant.Citation11 Rather, our results go further to suggest that DNA priming steps may result in differential regulation and activation of innate immune responses, which shape adaptive immunity.
While an evident but poorly understood role for MyD88 has been identified in the function of ISCOMATRIX™ adjuvant, the exact mechanism of this integrated adjuvant system remains unclear. Studies of other particulate adjuvants have implicated inflammasome pathways such as NLRP3, either directly or indirectly, in their mechanism of action.Citation25 In addition, recent work by Duewell et al. introduced the secretion of mature IL-1β by DCs in vitro in response to stimulation with ISCOMATRIX™ adjuvant in a caspase-1-dependent manner, indicating stimulation of any number of caspase-1-dependent inflammasome pathways.Citation10 The further elucidation of the signaling pathways involved in ISCOMATRIX™ adjuvant-induced adaptive immunity, with a particular focus on the unique impact of DNA priming on these mechanisms, should be a key component of future studies.
The use of animal models for preclinical evaluation of vaccines for use in humans has long been the subject of debate, with concerns that preclinical data may not be suitably predictive of clinic results, prompting the investigation of alternatives such as humanized mouse models.Citation26 In our own experience, preclinical evalulation of the immunogenicity and toxicology of the DP6-001 vaccine and the saponin adjuvant in QS-21 in a rabbit model did not reveal the local or systemic reactogenicity that was later observed in human subjects.Citation14,Citation27 Furthermore, this concern may be most evident for the inbred strains of mice used to model immunological responses, as recent studies comparing models of acute inflammatory stresses in murine and human models demonstrated disparate immunological responses at a genetic level to endotoxemia, injury, and inflammation.Citation28 At the same time, the kinetics of inflammatory cytokine responses to endotoxic stimuli are not entirely dissimilar.Citation29 Still, the preclinical work presented here e has demonstrated the superiority of a novel adjuvant such as ISCOMATRIX™ to classical adjuvants, as well as the substantial impact of a DNA priming component on the immunogenicity and inflammatory cytokine profiles induced by vaccination. Defining a role for innate immune signaling pathways in the efficacy of the ISCOMATRIX™ adjuvant in the context of our vaccine lays the groundwork for future clinical applications of our DNA prime-protein boost HIV-1 vaccine.
In summary, ISCOMATRIX™ adjuvant is a promising candidate for inclusion in future formulations of HIV-1 Env vaccines. In the context of a DNA prime-protein boost strategy, the ISCOMATRIX™ adjuvant was associated with improved neutralizing antibody responses, as well as a potent pro-inflammatory cytokine and chemokine environment that may contribute to the NK cell and DC activation, cross-presentation, and potent CD8+ T cell response characteristic of ISCOMATRIX™ adjuvant. The data presented in the current study not only contributes to our selection of an adjuvant for future optimized HIV-1 Env DNA prime-protein boosts, but also expands our understanding of both the impact of a DNA prime component and also the underlying mechanisms of ISCOMATRIX™ adjuvant in the context of a novel vaccine strategy.
Materials and Methods
HIV-1 gp120 DNA vaccine
The DNA component of the DP6-001 vaccine was composed of equal parts of five plasmids, vector pSW3891, encoding codon-optimized gp120 genes from primary HIV-1 isolates: A (92UG037.8), B (92US715.6), Ba-L, Czm (96ZM651), and E (93TH976.17). The pSW3891 plasmid vector has been previously described.Citation15 DNA vaccine plasmids were grown up in E. coli, and prepared using a Plasmid Giga Kit (Qiagen). DNA plasmid expression was confirmed by transient expression in 293T cells and western blot.
HIV-1 gp120 protein vaccine and adjuvant formulation
The protein vaccine component of DP6-001 was composed of equal parts of five gp120 proteins homologous to DNA vaccine components. The gp120 proteins were produced in CHO cell lines as previously reported.Citation13 The final vaccine antigens consisted of 7 μg of each gp120 protein (35 μg dose) for mice and 80 μg of each gp120 protein (400 μg dose) for rabbits. Antigens were dissolved in Dulbecco’s phosphate buffered saline (DPBS) (Gibco, Invitrogen) and formulated with the candidate adjuvants. For protein immunizations, mice received a total amount of 175 μg Al(OH)3 gel (Sigma Aldrich Corp.) in a 5:1 adjuvant:antigen ratio previously employed by our lab, or 1.5 ISCO™ Units of ISCOMATRIX™ adjuvant (CSL Biotherapies Inc.) formulated with 35 μg total gp120 protein, per the recommendation of the manufacturer. Rabbits immunized with 400 μg protein received a total adjuvant dose of either 2 mg of Al(OH)3 gel (Sigma) or 50 μg ISCOMATRIX™ adjuvant. 1.5 ISCO™ Units is equivalent to 1.5 µg of ISCOPREP™ saponin and to allow easier comparison with other adjuvants the µg measurement is used in this article.
Mouse immunizations
Mixed gender Balb/c and C57Bl/6 (6–8 wk) mice were obtained from Taconic Farms. MyD88-deficient mice on a C57Bl/6 background were generated as previously described.Citation30 Mice were maintained in Department of Animal Medicine animal facility at University of Massachusetts Medical School. Experimental protocols have been approved by IACUC. Mice were immunized with 120 μg gp120 DNA plasmid in each quadriceps at weeks 0, 2, and 4, i.m (n = 5 per experiment group). On weeks 8 and 12, mice were boosted with 35 μg total of five gp120 proteins formulated in Al(OH)3 or ISCOMATRIX™ adjuvant. Blood samples were taken on Day 0 (pre-bleed) and on weeks 2, 4, 6, 8, 10, and 12 for antibody analysis. In addition, 6 h after immunizations at weeks 0, 4, 8, and 12 blood samples were collected for serum cytokine analysis. At termination of the study at week 13, both blood and spleens were collected 7 d after final protein boost. Mouse studies were repeated twice.
Two separate follow-up studies focused on protein immunizations in the absence of DNA priming. In the first study, C57Bl/6 wild type or MyD88-deficient mice were immunized via i.m. with the same DP6-001 gp120 proteins formulated with 1.5 ISCO™ Units of ISCOMATRIX™ adjuvant at weeks 0 and 4, in the absence of DNA priming. In a second study, C57Bl/6 wild type or MyD88-deficient mice were immunized five times with DP6-001 protein formulated with ISCOMATRIX™ adjuvant. In order to mimic the prime-boost regimen employed previously, the first three immunizations administered at weeks 0, 2, and 4, consisted of a fifth of the protein content delivered in the boost immunizations at a total of 7 μg gp120 protein. These immunizations were formulated with 1.5 ISCO™ Units of ISCOMATRIX™ adjuvant. Full-dose immunizations of 35 μg total gp120 protein formulated with 1.5 ISCO™ Units of ISCOMATRIX™ adjuvant were administered at weeks 8 and 12. Sera were collected 6 h after each immunization and at termination on week 13.
Rabbit immunizations
A limited immunization study was done in rabbits to test the neutralizing antibody responses induced by DNA prime-protein boost DP6-001 formulations. NZW rabbits (6–8 wk) were purchased from Millbrook Farm. Rabbits were housed in the Department of Animal Medicine animal facility at University of Massachusetts Medical School, and all experimental protocols were approved by IACUC. Rabbits (n = 5/experimental group) received 400 μg gp120 DNA plasmid in each lumbar muscle at weeks 0, 2, and 4, and were then immunized with 400 μg gp120 protein formulated with Al(OH)3 or ISCOMATRIX™ adjuvant on weeks 8 and 12. All inoculations were performed via i.m. injection. Sera were collected biweekly throughout the immunization schedule until week 16.
Enzyme linked immunosorbant assay
Titers of gp120-specific antibody responses were assessed by ELISA, as previously described.Citation31 Briefly, 96-well EIA/RIA microtiter plates (Costar) were coated with 5 μg/well ConA or 1 μg/mL gp120 protein mix (ABL) diluted in DPBS (Gibco) for 1 h. Wells were blocked with 200 μL/well of blocking buffer (4% whey and 5% powdered milk (weight:volume) in dilution buffer), overnight at 4 °C. Duplicates of 100 μL of 1:3 serially diluted mouse serum (starting dilution of 1:300) were incubated on coated plates, for 1 h. Biotinylated anti-mouse IgG (Vector Laboratories) was used as secondary antibody at 1.5 μg/mL, 100 μL/well, and followed by 100 μL of HRP-conjugated streptavidin (Vector Laboratories) at 0.5 μg/mL. Both reagents were incubated for 1 h. Antibody-antigen reactions were detected by adding 100 μL of fresh 3,3′,5,5′-Tetramethylbenzidine substrate (Sigma-Aldrich) to each well and the reaction was stopped with 25 μL/well of 2 N H2SO4. Absorbance was read as the OD at 450 nm (OD450), using a Multiskan FC (Thermo Fischer Scientific). Titers were determined as the highest dilution at which the OD450 were ≥ 2 × OD450 of negative control wells. Between each step, plates were washed with PBS and 0.1% Triton X-10 five times using AquaMax2000 automatic plate washer (Molecular Devices). gp120-specific IgG antibody titers in rabbits were determined by ELISA as described above, with the following modifications. Rabbit sera were initially diluted at 1:1000, and the secondary antibody used was biotinylated anti-rabbit IgG (Vector) at 1.5 μg/mL.
For temporal analyses, mouse sera dilutions of 1:250 or 1:500 were prepared for each time point, and added to wells in duplicate at 100 μL/well. Plates were processed as described above.
HIV-1 neutralization assay
Neutralization assays on rabbit sera were performed as previously described.Citation31 Neutralizing Ab levels were quantified in immunized mouse sera, as measured against a panel of pseudotyped HIV-1 viruses expressing primary Env Ags. Sensitive pseudotyped viruses included SF162 and ss1196, while separately there were 12 viruses in a larger panel of Tier 2 viruses, as previously described.Citation17-Citation19 HIV-1 pseudovirus was produced in HEK293T cells by co-transfection with pSG3Δenv backbone (NIH AIDS Research References and Reagent Program) and a plasmid encoding gp160. Pseudovirus was titered out using TZM-bl cell line. Rabbit sera were incubated for 1 h at 37 °C with 200 TCID50 (tissue culture infectious dose) of pseudovirus, defined as 200 times the amount of pseudovirus required to infect 50% of the tissue culture. This mixture was added to 1x105 TZM-bl cells in 20 μg/mL DEAE Dextran and incubated for 48 h at 37 °C. Plates were developed with luciferase reagent (Promega). Neutralization activity was expressed as the percent of luciferase activity reduction in serum samples relative to pre-immune sera.
Cytometric Bead Array (CBA)
Cytokines were quantified in serum samples using a Th1/Th2/Th17 CBA kit from BD Biosciences following the manufacturer’s instructions. The cytokines analyzed included IFNγ, IL-2, IL-4, IL-6, IL-10, IL-12p70, IL-17, and TNFα. Samples were read using an LSRII FACS machine (BD Biosciences), and the data was analyzed using FCAP Array Software v3.0 (BD Bioscience).
Luminex assay for cytokine and chemokine analyses
Cytokines and chemokines were quantified in serum samples using a custom Bio-Plex cytokine assay (Bio-Rad) following the manufacturer’s instructions. The cytokines and chemokines analyzed included: IFNγ, IL-1β, IL-2, IL-4, IL-6, Eotaxin, G-CSF, KC, MIP-1α, MIP-1β, MCP-1, and RANTES. Samples were read on a Bio-Plex 200 system with Bio-Plex Manager software (Bio-Rad).
Statistical analysis
Data analysis was performed using Graphpad Prism software and data are presented as the mean of individual samples +/− standard error of the mean (SEM). Statistical analyses were performed using either the Student t test, a one-way ANOVA followed by the Tukey post test, or a two-way ANOVA followed by the Tukey post test. A P value ≤ 0.05 was regarded as significant.
| Abbreviations: | ||
| ANOVA | = | analysis of variance |
| Ag | = | antigen |
| Ab | = | antibody |
| CBA | = | cytometric bead array |
| CTL | = | cytotoxic t lymphocytes |
| DAMPS | = | danger-associated molecular patterns |
| DCs | = | dendritic cells |
| DEAE | = | diethylaminoethanol |
| DPBS | = | Dulbecco’s phosphate buffered saline |
| Env | = | envelope |
| ELISA | = | enzyme-linked immunosorbant assay |
| G-CSF | = | granulocyte colony stimulating factor |
| HRP | = | horseradish peroxidase |
| IFNγ | = | interferon gamma |
| IL | = | interleukin |
| KC | = | keratinocyte chemoattractant |
| MHC | = | major histocompatibility complex |
| MCP | = | monocyte chemoattractant protein |
| MIP | = | macrophage inflammatory protein |
| Nab | = | neutralizing antibody |
| NZW | = | New Zealand white |
| OD | = | optical density |
| PAMPS | = | pathogen-associated molecular patterns |
| PRRs | = | pathogen recognition receptors |
| RANTES | = | regulated on activation normal T cell expressed and secreted |
| SEM | = | standard error of the mean |
| TLR | = | toll-like receptor |
| TNF | = | tumor necrosis factor |
| TCID | = | tissue culture infective dose |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported in part by NIH grants 5 U19 AI082676 and 5 P01 AI082274.
Authors would like to thank Dr Jill M Serrano for critical reading and editing of the manuscript.
References
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010; 327:291 - 5; http://dx.doi.org/10.1126/science.1183021; PMID: 20075244
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140:805 - 20; http://dx.doi.org/10.1016/j.cell.2010.01.022; PMID: 20303872
- Drane DCG, Gittleson C, Boyle J, Maraskovsky E. ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines. Expert Rev Vaccines 2007; 6:761 - 72; http://dx.doi.org/10.1586/14760584.6.5.761; PMID: 17931156
- Sjölander A, Cox JC. Uptake and adjuvant activity of orally delivered saponin and ISCOM vaccines. Adv Drug Deliv Rev 1998; 34:321 - 38; http://dx.doi.org/10.1016/S0169-409X(98)00046-5; PMID: 10837684
- Sjölander A, Drane D, Maraskovsky E, Scheerlinck JP, Suhrbier A, Tennent J, Pearse M. Immune responses to ISCOM formulations in animal and primate models. Vaccine 2001; 19:2661 - 5; http://dx.doi.org/10.1016/S0264-410X(00)00497-7; PMID: 11257406
- Evans TG, McElrath MJ, Matthews T, Montefiori D, Weinhold K, Wolff M, Keefer MC, Kallas EG, Corey L, Gorse GJ, et al, NIAID AIDS Vaccine Evaluation Group. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine 2001; 19:2080 - 91; http://dx.doi.org/10.1016/S0264-410X(00)00415-1; PMID: 11228380
- Waite DC, Jacobson EW, Ennis FA, Edelman R, White B, Kammer R, Anderson C, Kensil CR. Three double-blind, randomized trials evaluating the safety and tolerance of different formulations of the saponin adjuvant QS-21. Vaccine 2001; 19:3957 - 67; http://dx.doi.org/10.1016/S0264-410X(01)00142-6; PMID: 11427271
- McKenzie A, Watt M, Gittleson C. ISCOMATRIX() vaccines: Safety in human clinical studies. Hum Vaccin 2010; 6:237 - 46; http://dx.doi.org/10.4161/hv.6.3.10754; PMID: 20595811
- Morelli AB, Becher D, Koernig S, Silva A, Drane D, Maraskovsky E. ISCOMATRIX: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J Med Microbiol 2012; 61:935 - 43; http://dx.doi.org/10.1099/jmm.0.040857-0; PMID: 22442293
- Duewell P, Kisser U, Heckelsmiller K, Hoves S, Stoitzner P, Koernig S, Morelli AB, Clausen BE, Dauer M, Eigler A, et al. ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J Immunol 2011; 187:55 - 63; http://dx.doi.org/10.4049/jimmunol.1004114; PMID: 21613613
- Wilson NS, Yang B, Morelli AB, Koernig S, Yang A, Loeser S, Airey D, Provan L, Hass P, Braley H, et al. ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway. Immunol Cell Biol 2012; 90:540 - 52; http://dx.doi.org/10.1038/icb.2011.71; PMID: 21894173
- Pearse MJ, Drane D. ISCOMATRIX adjuvant: a potent inducer of humoral and cellular immune responses. Vaccine 2004; 22:2391 - 5; http://dx.doi.org/10.1016/j.vaccine.2003.12.031; PMID: 15193400
- Bansal A, Jackson B, West K, Wang S, Lu S, Kennedy JS, Goepfert PA. Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration. J Virol 2008; 82:6458 - 69; http://dx.doi.org/10.1128/JVI.00068-08; PMID: 18448544
- Kennedy JS, Co M, Green S, Longtine K, Longtine J, O’Neill MA, Adams JP, Rothman AL, Yu Q, Johnson-Leva R, et al. The safety and tolerability of an HIV-1 DNA prime-protein boost vaccine (DP6-001) in healthy adult volunteers. Vaccine 2008; 26:4420 - 4; http://dx.doi.org/10.1016/j.vaccine.2008.05.090; PMID: 18588934
- Pal R, Wang S, Kalyanaraman VS, Nair BC, Whitney S, Keen T, Hocker L, Hudacik L, Rose N, Cristillo A, et al. Polyvalent DNA prime and envelope protein boost HIV-1 vaccine elicits humoral and cellular responses and controls plasma viremia in rhesus macaques following rectal challenge with an R5 SHIV isolate. J Med Primatol 2005; 34:226 - 36; http://dx.doi.org/10.1111/j.1600-0684.2005.00120.x; PMID: 16128917
- Buglione-Corbett R, Pouliot K, Marty-Roix R, West K, Wang S, Lien E, Lu S. Serum cytokine profiles associated with specific adjuvants used in a DNA prime-protein boost vaccination strategy. PLoS One 2013; 8:e74820; http://dx.doi.org/10.1371/journal.pone.0074820; PMID: 24019983
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 2005; 79:10108 - 25; http://dx.doi.org/10.1128/JVI.79.16.10108-10125.2005; PMID: 16051804
- Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, et al. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol 2006; 80:11776 - 90; http://dx.doi.org/10.1128/JVI.01730-06; PMID: 16971434
- Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, Shen S, Green S, Rothman AL, Ennis FA, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine 2008; 26:3947 - 57; http://dx.doi.org/10.1016/j.vaccine.2007.12.060; PMID: 18724414
- Thompson MR, Kaminski JJ, Kurt-Jones EA, Fitzgerald KA. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011; 3:920 - 40; http://dx.doi.org/10.3390/v3060920; PMID: 21994762
- de Souza MS, Ratto-Kim S, Chuenarom W, Schuetz A, Chantakulkij S, Nuntapinit B, Valencia-Micolta A, Thelian D, Nitayaphan S, Pitisuttithum P, et al, Ministry of Public Health–Thai AIDS Vaccine Evaluation Group Collaborators. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol 2012; 188:5166 - 76; http://dx.doi.org/10.4049/jimmunol.1102756; PMID: 22529301
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al, MOPH-TAVEG Investigators. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209 - 20; http://dx.doi.org/10.1056/NEJMoa0908492; PMID: 19843557
- Wang S, Arthos J, Lawrence JM, Van Ryk D, Mboudjeka I, Shen S, Chou TH, Montefiori DC, Lu S. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J Virol 2005; 79:7933 - 7; http://dx.doi.org/10.1128/JVI.79.12.7933-7937.2005; PMID: 15919951
- Morelli AB, Becher D, Koernig S, Silva A, Drane D, Maraskovsky E. ISCOMATRIX: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J Med Microbiol 2012; 61:935 - 43; http://dx.doi.org/10.1099/jmm.0.040857-0; PMID: 22442293
- Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Pétrilli V, Tschopp J, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A 2009; 106:870 - 5; http://dx.doi.org/10.1073/pnas.0804897106; PMID: 19139407
- Hayday AC, Peakman M. The habitual, diverse and surmountable obstacles to human immunology research. Nat Immunol 2008; 9:575 - 80; http://dx.doi.org/10.1038/ni0608-575; PMID: 18490903
- Pal R, Yu Q, Wang S, Kalyanaraman VS, Nair BC, Hudacik L, Whitney S, Keen T, Hung CL, Hocker L, et al. Definitive toxicology and biodistribution study of a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 (HIV-1) vaccine in rabbits. Vaccine 2006; 24:1225 - 34; http://dx.doi.org/10.1016/j.vaccine.2005.07.112; PMID: 16219399
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al, Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013; 110:3507 - 12; http://dx.doi.org/10.1073/pnas.1222878110; PMID: 23401516
- Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D, Inflammation and the Host Response to Injury Investigators. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol 2005; 12:60 - 7; PMID: 15642986
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 1998; 9:143 - 50; http://dx.doi.org/10.1016/S1074-7613(00)80596-8; PMID: 9697844
- Vaine M, Wang S, Hackett A, Arthos J, Lu S. Antibody responses elicited through homologous or heterologous prime-boost DNA and protein vaccinations differ in functional activity and avidity. Vaccine 2010; 28:2999 - 3007; http://dx.doi.org/10.1016/j.vaccine.2010.02.006; PMID: 20170767
