Abstract
Individuals infected with human immunodeficiency virus (HIV) have excess risk of developing human papillomavirus (HPV)-related disease. A substantial fraction of HPV-associated cancers is caused by HPV serotypes not included in the currently available vaccines. Among healthy women, both Cervarix® (HPV-16/18, GlaxoSmithKline Biologicals, GSK) and Gardasil® (HPV-6/11/16/18, Merck) have demonstrated partial cross-protection against certain oncogenic non-vaccine HPV-types. Currently, there are no available data on vaccine-induced cross-protection in men and little is known about cross-reactive immunity after HPV-vaccination of HIV-infected individuals.
In an investigator-initiated trial, we randomized 91 HIV-positive men and women to receive vaccination with Cervarix® or Gardasil®. The HPV-DNA status of the participants was determined with pcr before and after immunization. Cross-reactive antibody responses against HPV-31, HPV-33, and HPV-45 were evaluated for up to 12 months using a pseudovirion-based neutralization assay (PBNA). Geometric mean antibody titers (GMTs) were compared among vaccine groups and genders at 7 and 12 months.
Both vaccines induced anti-HPV-31, -33, and -45 neutralizing antibodies in participants who were seronegative and HPV-DNA negative for those types at study entry. Geometric mean antibody titers were comparable between vaccine groups. Interestingly, anti-HPV-31 and -33 antibody titers were higher among women compared with men at 7 and 12 months.
In conclusion, both licensed HPV-vaccines induced cross-neutralizing antibodies against frequent oncogenic non-vaccine serotypes HPV-31, HPV-33, and HPV-45 in HIV-infected adults, and women had greater serological responses against HPV-31 and -33 compared with men.
Introduction
Persistent infection with human papillomavirus (HPV) is a major health concern, causing a variety of cancers including cervical, anal, vulvar, vaginal, penile, head/neck, and oropharyngeal cancer.Citation1-Citation4 Non-oncogenic HPV-types can cause benign anogenital and non-genital warts. Currently, there are 2 licensed HPV vaccines: Cervarix® and Gardasil®. Both vaccines protect against infection with HPV-16 and HPV-18, the 2 serotypes that cause the majority of HPV-associated cancers. In addition, Gardasil® protects against HPV-6 and -11, the most common causative agents of genital warts.Citation5-Citation8
Around 30% of all invasive cervical cancers and a substantial fraction of other HPV-associated cancers are caused by oncogenic HPV-types not covered by the available HPV-vaccines.Citation2,Citation9 However, both vaccines have been shown to induce some cross-protection against closely related oncogenic HPV serotypes in healthy young women. In the PATRICIA and Costa Rica Trials, Cervarix® induced partial cross-protection against HPV-31, -33, and -45,Citation9-Citation12 and Gardasil induced partial protection against HPV-31 and -33 in the FUTURE studies.Citation13,Citation14 An association between vaccine-induced clinical cross-protection and humoral immune responses has recently been demonstrated.Citation15 To our knowledge, HPV-vaccine induced cross-neutralizing antibody responses have not been evaluated in men and cross-protective efficacy data have not been published in trials including male participants.Citation16,Citation17
People with HIV infection have an increased risk of developing HPV-associated disease.Citation16-Citation22 HPV-vaccination efficacy data are currently unavailable for HIV-infected individuals. A limited number of studies have demonstrated immunogenicity of Gardasil®Citation23-Citation26 and Cervarix®Citation27 in HIV-infected populations and one study has compared the immunogenicity of the 2 vaccines.Citation28 Recently, it has been demonstrated that Gardasil® induces HPV-31 cross-neutralizing antibodies in HIV-infected children.Citation29 The potential induction of HPV-33 and -45 cross-neutralizing antibodies from Gardasil® and Cervarix® vaccination has never been investigated in HIV-infected individuals.
We previously published results from a comparative trial of Cervarix® and Gardasil® in HIV-infected adults.Citation28 This study presents data on cross-reactive serological neutralizing antibody responses against HPV-31, -33, and HPV-45 for Cervarix® and Gardasil® in an HIV-infected adult population.
Results
Study population
91 HIV-infected individuals were screened for HPV-DNA and vaccinated as previously described.Citation28 Baseline characteristics (sex, race, age, body mass index (BMI), CD4 t-cell count, HIV-RNA level) were comparable between vaccine groups at the time of inclusion. In the Gardasil® group there was a higher proportion of current smokers than in the Cervarix® group (16 vs. 6; P = 0.026). Of particular importance for inter-sex analyses, men and women did not differ significantly in immune status or age.
Baseline HPV-seropositivity and HPV-DNA status
An ED50 of 40, the lower limit of detection in the pseudovirion-based neutralization assay (PBNA), was chosen as cut-off to define sera as negative for antibodies capable of neutralizing a given HPV-type. Baseline anogenital HPV-DNA status was available in 86 participants. Baseline HPV-seropositivity and HPV-DNA status are summarized in . Twenty-six study subjects were DNA and seronegative for HPV-31 at study inclusion, 40 were baseline DNA and seronegative for HPV-33, and 48 were negative DNA and seronegative for HPV-45. Baseline HPV-DNA and seronegative subjects are referred to as “baseline HPV-negative cohorts.”
Table 1. Baseline HPV serostatus and HPV-DNA status at time of inclusion in the study
Immunogenicity
An analysis of antibody titers according to baseline serostatus and anogenital DNA status is shown in . In the baseline HPV-31 negative cohort, both vaccines increased anti-HPV-31 GMTs from baseline to 7 and 12 mo with no significant differences in crude or adjusted GMT ratios between vaccine groups (adjustment for potential confounders: current smoking, baseline CD4+ cell count, current use of HAART (yes or no), BMI, sex, age and baseline HPV-16 and -33 sero and DNA status). Antibody titers peaked at 7 mo at which point they were comparable to the titers derived from natural infection (baseline HPV-31 titers in the HPV-31 seropositive, DNA negative-group). Both vaccines also increased anti-HPV-33 GMTs at 7 and 12 mo in the baseline HPV-33 negative cohort. We found no significant differences in crude or adjusted GMT ratios for anti-HPV-33 between vaccine groups at either 7 or 12 mo. The vaccine-induced anti-HPV-33 titers peaked at 7 mo at which point they were approximately half of those derived from natural infection (baseline HPV-33 titers in the HPV-33 seropositive, DNA negative-group). In the HPV-45 baseline negative cohort, both vaccines increased anti-HPV-45 GMTs at 7 and 12 mo. No significant differences in crude GMT ratios were found between vaccine groups at either 7 or 12 mo. After adjustment for potential confounders (current smoking, baseline CD4+ cell count, current use of HAART, BMI, sex, age and baseline HPV-18 DNA and serostatus), 7-mo GMTs were 1.94 (95% CI: 1.07–3.52) fold higher in the Cervarix® compared with the Gardasil® group. Adjustment did not alter 12-mo GMT ratios. Vaccine-induced anti-HPV-45 titers peaked at 7 mo at which point they were approximately half of those derived from natural infection (baseline HPV-45 titers in the HPV-45 seropositive, DNA negative-group).
Table 2. Geometric mean antibody titers (GMTs) and GMT ratios according to baseline seropositivity and anogenital human papillomavirus (HPV)
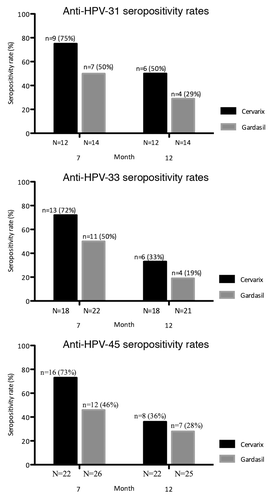
Participants with a baseline ED50 value of 40 who had a subsequent ED50 value >40 at 7 mo were defined as seroconverter for a given HPV type. shows seropositivity rates in baseline HPV-negative cohorts at 7 and 12 mo. Slightly higher seropositivity rates were found in the Cervarix® group compared with the Gardasil® group, but inter-group differences were not statistically significant for any single HPV-type at any time point or for the total number of seropositives for the 3 HPV-types combined (data not shown).
Figure 1. Seropositivity rates for neutralizing anti-HPV-31, -33, and -45 antibodies in baseline HPV-negative cohorts. Seropositivity rates for neutralizing anti-HPV-31, -33, and -45 antibodies measured by Pseudovirion-based neutralization assay at months 7 and 12. (HPV-negative cohorts, seronegative, and DNA-negative at baseline for HPV type analyzed). N, number of evaluable subjects; n, number of seropositive subjects, per vaccine, per timepoint. Percentages indicate seropositivity rates at each timepoint (seropositivity defined as PBNA ED50 > 40). HPV, human papillomavirus; GMTs, geometric mean titers.
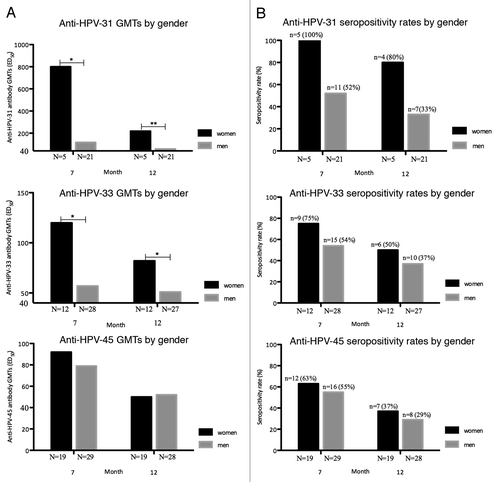
shows GMTs and seropositivity rates stratified by sex in baseline HPV-negative cohorts. shows that women had higher anti-HPV-31 and -33 titers when compared with men at 7 and 12 mo. At 7 and 12 mo, Anti-HPV-31 GMTs were 6.85 (95% CI: 1.41–33.2) and 3.79 (95% CI: 1.59–9.03) fold higher among women compared with men and anti-HPV-33 GMTs were 2.09 (95% CI: 1.11–3.92) and 1.61 (95% CI: 1.00–2.61) fold higher among women compared with men. shows trends toward slightly higher seropositivity rates in women compared with men but this was not statistically significant for any single HPV-type at any time point or for the total number of seropositives for the 3 HPV-types combined (data not shown).
Figure 2. Gender-specific geometric mean titers (GMTs) and seropositivity rates for neutralizing anti-HPV-31, -33, and -45 antibodies in baseline HPV-negative cohorts. (A) Gender-specific geometric mean antibody titer computed using the log10 transformation of titers as measured by Pseudovirion-based neutralization assay at months 7 and 12. (HPV-negative cohorts, seronegative, and DNA-negative at baseline for HPV type analyzed). N, number of evaluable subjects. *P < 0.05, **P < 0.001. HPV, human papillomavirus; GMTs, geometric mean titers. (B) Gender-specific seropositivity rates for neutralizing anti-HPV-31, -33, and -45 antibodies measured by Pseudovirion-based neutralization assay at months 7 and 12. (HPV-negative cohorts, seronegative, and DNA-negative at baseline for HPV type analyzed). N, number of evaluable subjects; n, number of seropositive subjects, per gender, per timepoint. Percentages indicate seropositivity rates at each timepoint (seropositivity defined as PBNA ED50 > 40). HPV, human papillomavirus; GMTs, geometric mean titers.
Anogenital HPV DNA detection at 7 months
Eighty participants had available anogenital HPV DNA detection at both baseline and 7 mo. summarizes incident and persistent HPV-31, -33, and -45 infections. One incident HPV-31 infection occurred in a female participant who did not seroconvert to HPV-31 and one incident HPV-33 infection occurred in a male participant who had seroconverted to vaccination. The remaining 4 incident infections occurred among male participants who were baseline seropositive for incident infection HPV-types.
Table 3. Week 28 human papillomavirus (HPV) DNA at 7 mo, according to baseline results
Discussion
The current analysis was designed to directly compare the induction of cross-reactive neutralizing antibodies of Cervarix® and Gardasil® in HIV-infected adults. Our principal finding was that both HPV-vaccines induced anti-HPV-31, -33, and -45 neutralizing antibodies in participants who were HPV DNA and seronegative for the respective types at study inclusion. The kinetics of the serological responses and seropositivity rates reported in our study corresponded quite well with previous findings by Einstein et al. who compared the induction of anti-HPV-31 and -45 neutralizing antibodies in healthy adult women after vaccination with Gardasil® vs. Cervarix®.Citation25 We did find, however, higher antibody titers in comparison with the Einstein study. We believe the higher titers to be a result of differences in the sensitivity of the assays in the 2 studies. The PBNA used in the present study is based on Gaussia Luciferase whereas the assay used in the Einstein study uses secreted alkaline phosphatase as reporter. A direct comparison between the 2 assays revealed that antibody titers were approximately 10-fold higher using the assay based on Gaussia Luciferase.Citation30 We cannot directly compare the anti-HPV-31 titers in our study to those previously observed in HIV-infected children because Weinberg et al. used a competitive Luminex immunoassay and hence reported antibody concentrations in milli-Merck units.Citation29 In our study, HPV-31 antibody titers reached the same magnitude as those derived from natural infection whereas HPV-33 and -45 titers only reached half that level. Whether higher antibody titers provide improved clinical cross-protection remains unanswered. In study participants who were seropositive at baseline, we also observed marked increases in antibody titers. However, the clinical importance of boosting pre-existing antibody responses derived from natural infection is unknown. Efficacy trials have shown that adult women with serological signs of previous infection with HPV vaccine-types benefit from vaccination.Citation31,Citation32
The major limitation of the present analysis is the low numbers of participants who were both DNA and seronegative for the investigated HPV-types at study entry. The study was not powered to compare serological responses among baseline DNA and seronegative participants, because the primary endpoint was a comparison of 7 mo HPV-16, and -18 titers between vaccine groups regardless of the baseline HPV-DNA and serostatus of study participants.Citation28 Thus, the comparison of cross-reactive neutralizing antibody titers between vaccine groups should be interpreted with caution given to the low numbers of participants in the subgroup analyses. Collectively, our results suggest that partial cross-protection against frequent non-vaccine type HPVs may be attainable in HIV-infected individuals using either of the licensed HPV-vaccines.
To our knowledge, the question of HPV-vaccine induced cross-protection has only been explored and demonstrated in efficacy studies among women.Citation10-Citation14,Citation33,Citation34 In the present study, we found that women had higher neutralizing antibody responses against HPV-31 and -33 when compared with men. This raises the intriguing and clinically very important question if HPV-vaccine induced cross-protection might be partly dependent on sex. Sex differences in humoral immune responses following especially viral vaccinations have been reported in numerous studies and several potential mechanistic explanations have been hypothesized, e.g., gonadal hormone levels and yet undefined antigen-specific interactions with the immune system.Citation35,Citation36 We cannot rule out that that possible priming due to previous HPV exposure, even if not detectable by a highly sensitive PBNA, could explain the higher anti-HPV-31 and -45 titers among women compared with men. Furthermore, the study was not powered to detect sex differences in GMTs among baseline HPV-negative cohorts, and the observed sex differences are based on a very limited number of observations. It seems evident that any future findings regarding possible cross-protection from clinical trial in HIV-infected individuals should be very carefully interpreted and increased attention should be assigned to possible sex differences. The observed trends toward more seroconverters in the Cervarix® group compared with the Gardasil® group remain uncertain but for HPV-31 and -45, these trends are supported by similar previous findings by Einstein et al. in healthy women.Citation25
Swabs were collected for HPV-DNA detection from both the anal canal and the cervix uteri in women whereas male participants only had anal swabs. Hence, the sensitivity of HPV-DNA detection was increased in women compared with men. We do not believe that this has affected serological results and conclusions in the present analysis. We cannot completely rule out that the incident HPV-33 infection in a male participant who seroconverted to HPV-33 was due to lack of sensitivity in the baseline HPV-DNA detection. However, the particular participant had an anal HPV-61 infection that persisted from baseline to 7 mo and we have no reason to think that either his baseline or 7-mo HPV-DNA detection was inadequate. Additionally, he also had an incident HPV-68 infection from baseline to 7 mo which further strengthens the argument that the incident HPV-33 infection was not due to lack of sensitivity in the assay.
HIV-infected individuals have an increased risk of HPV-related disease and vaccination may reduce their risk of HPV-related cancers. The immunological data from our study suggest that both licensed HPV-vaccines induce low titers of neutralizing anti-HPV-31, -33, and -45 antibodies in well-controlled HIV patients with no signs of prevalent or previous infection with these HPV-types. It remains to be seen if cross-neutralizing antibodies afforded by HPV-vaccination can translate into cross-protection against HPV-associated disease in HIV patients. This study strongly supports carefully designed efficacy trials with enhanced focus on any sex-related differences in cross-protection following HPV-vaccination in HIV-infected individuals.
Materials and Methods
Study design
This was a pre-planned subanalysis of a single-site investigator-initiated randomized, double-blind trial that randomized HIV-infected adults to vaccination with either Cervarix® or Gardasil® (Trial Registration ClinicalTrials.gov identifier: NCT01386164). The overall study design has previously been described.Citation28 The study was conducted at the Department of Infectious Diseases, Aarhus University Hospital, Denmark. HIV-seropositive volunteers 18 y or older were stratified according to use of highly active antiretroviral therapy (HAART) and sex at a ratio of 1:1 in blocks of 4 to receive 3 doses of Cervarix® or Gardasil® at days 0, 45, and 180. Participants were seen at months 7 and 12 for immunogenicity and safety follow up. The study protocol was approved by the Danish Medicines Agency, the Regional Ethical Committee and the Danish Data Protection Agency. The study was monitored for regulatory compliance and data quality by the Division of Good Clinical Practice at Aarhus University Hospital
Sampling and assays
Anal and cervical swabs for HPV-DNA testing and blood samples were collected and processed as previously described.Citation28 Serum samples were heat inactivated for 60 min at 56 °C and analyzed for neutralizing anti-HPV-31, -33, and -45 antibodies at the joint Chemical Biology Core Facility of the German Cancer Research Center and the European Molecular Biology Laboratory, Heidelberg, Germany, using a pseudovirion-based neutralization assay (PBNA).
HPV-31,-33, and -45 pseudovirions were prepared as previously described.Citation37-Citation39 ED50-values were calculated as previously described.Citation28,Citation30
Baseline HPV-seronegative subjects were defined as those with ED50 value = 40 for individual HPV-types as measured by Pseudovirion-based neutralization assay (PBNA).
Statistical analysis
The primary exploratory objective of this clinical trial was to compare anti-HPV-16/18 antibody titers induced by Cervarix® and Gardasil® in HIV-infected adults measured by PBNA. Secondary objectives included comparison of serum neutralizing antibody titers against HPV-31/33/45 measured by PBNA at months 7 and 12 among vaccine groups. We calculated crude GMTs on anti-HPV-31/33/45 antibodies. In HPV-negative cohorts, an adjusted estimate was obtained using a multivariate linear regression that included the following variables: current smoking, baseline CD4+ cell count, current use of HAART (yes or no), BMI, sex, and age. Adjustment in the HPV-31 negative cohort additionally included baseline anti-HPV-16/-33 sero- and DNA status, in the HPV-33 negative cohort anti-HPV-16/-31 sero- and DNA status were included in the adjustment and anti-HPV-18 baseline DNA and serostatus were used in the HPV-45 negative cohort. Statistical analyses of GMTs (within-group and between-group comparisons) were performed at months 7 and 12 using χ2 test. We used the Fischer exact test to compare the proportion of seroconverters between the vaccine groups. All statistical analyses were performed using Stata version 12.2 (StataCorp).
| Abbreviations: | ||
| BMI | = | body mass index |
| GMTs | = | geometric mean titers |
| GSK | = | GlaxoSmithKline Biologicals |
| HPV | = | human papillomavirus |
| HIV | = | human immunodeficiency virus |
| PBNA | = | pseudovirion-based neutralization assay |
Disclosure of Potential Conflicts of Interest
This work was supported by Aarhus University, Henrik Henriksen’s Foundation, Aase and Ejnar Danielsen’s Foundation, Jørgen Holm and Wife’s Foundation, Lykfeldt and Wife’s Foundation and The Danish Medical Association
All potential conflicts have been declared to the editors of Human Vaccines and Immunotherapeutics.
Acknowledgments
We thank the participants for their involvement in the trial. We also thank the study nurses, Iben Loftheim and Inge Arbs, for their important job vaccinating the participants and laboratory technician Lene Svinth-Jøhnke for her excellent handling of all the samples.
Trial Registration
ClinicalTrials.gov identifier: NCT01386164.
References
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244 - 65; http://dx.doi.org/10.1136/jcp.55.4.244; PMID: 11919208
- Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24 Suppl 3:S3/1-10.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890 - 907; http://dx.doi.org/10.1016/S0140-6736(07)61416-0; PMID: 17826171
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12 - 9; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F; PMID: 10451482
- Barr E, Tamms G. Quadrivalent human papillomavirus vaccine. Clin Infect Dis 2007; 45:609 - 7; http://dx.doi.org/10.1086/520654; PMID: 17682997
- Keam SJ, Harper DM. Human papillomavirus types 16 and 18 vaccine (recombinant, AS04 adjuvanted, adsorbed) [Cervarix]. [Cervarix] Drugs 2008; 68:359 - 72; http://dx.doi.org/10.2165/00003495-200868030-00007; PMID: 18257611
- Monie A, Hung CF, Roden R, Wu TC. Cervarix: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics 2008; 2:97 - 105; PMID: 19707432
- Siddiqui MA, Perry CM. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil). Drugs 2006; 66:1263 - 71, discussion 1272-3; http://dx.doi.org/10.2165/00003495-200666090-00008; PMID: 16827602
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al, Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048 - 56; http://dx.doi.org/10.1016/S1470-2045(10)70230-8; PMID: 20952254
- Malagón T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Brisson M. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:781 - 9; http://dx.doi.org/10.1016/S1473-3099(12)70187-1; PMID: 22920953
- Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmerón J, Chow SN, Apter D, Kitchener H, et al, HPV PATRICIA Study Group. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:100 - 10; http://dx.doi.org/10.1016/S1470-2045(11)70287-X; PMID: 22075170
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al, HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301 - 14; http://dx.doi.org/10.1016/S0140-6736(09)61248-4; PMID: 19586656
- Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, García P, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis 2009; 199:936 - 44; http://dx.doi.org/10.1086/597309; PMID: 19236277
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis 2009; 199:926 - 35; http://dx.doi.org/10.1086/597307; PMID: 19236279
- Safaeian M, Kemp TJ, Pan DY, Porras C, Rodriguez AC, Schiffman M, Cortes B, Katki H, Wacholder S, Schiller JT, et al. Cross-protective vaccine efficacy of the bivalent HPV vaccine against HPV31 is associated with humoral immune responses: results from the Costa Rica Vaccine Trial. Hum Vaccin Immunother 2013; 9:1399 - 406; http://dx.doi.org/10.4161/hv.24340; PMID: 23571174
- Hagensee ME, Cameron JE, Leigh JE, Clark RA. Human papillomavirus infection and disease in HIV-infected individuals. Am J Med Sci 2004; 328:57 - 63; http://dx.doi.org/10.1097/00000441-200407000-00008; PMID: 15254442
- Gormley RH, Kovarik CL. Dermatologic manifestations of HPV in HIV-infected individuals. Curr HIV/AIDS Rep 2009; 6:130 - 8; http://dx.doi.org/10.1007/s11904-009-0018-8; PMID: 19589298
- De Panfilis G, Melzani G, Mori G, Ghidini A, Graifemberghi S. Relapses after treatment of external genital warts are more frequent in HIV-positive patients than in HIV-negative controls. Sex Transm Dis 2002; 29:121 - 5; http://dx.doi.org/10.1097/00007435-200203000-00001; PMID: 11875372
- Sunesen KG, Nørgaard M, Thorlacius-Ussing O, Laurberg S. Immunosuppressive disorders and risk of anal squamous cell carcinoma: a nationwide cohort study in Denmark, 1978-2005. Int J Cancer 2010; 127:675 - 84; http://dx.doi.org/10.1002/ijc.25080; PMID: 19960431
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370:59 - 67; http://dx.doi.org/10.1016/S0140-6736(07)61050-2; PMID: 17617273
- Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, Holmberg SD, Brooks JT, Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med 2008; 148:728 - 36; http://dx.doi.org/10.7326/0003-4819-148-10-200805200-00005; PMID: 18490686
- Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, Abramowitz L, Costagliola D, Mary-Krause M, FHDH-ANRS CO 4. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS 2008; 22:1203 - 11; http://dx.doi.org/10.1097/QAD.0b013e3283023f78; PMID: 18525266
- Jessica A.. Kahn, Jiahong Xu, Bill G. Kapogiannis, Bret Rudy, René Gonin, Nancy Liu, et al. Immunogenicity and Safety of the Human Papillomavirus-6, -11, -16, -18 Vaccine in HIV-Infected Young Women. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2013;
- Levin MJ, Moscicki AB, Song LY, Fenton T, Meyer WA 3rd, Read JS, Handelsman EL, Nowak B, Sattler CA, Saah A, et al, IMPAACT P1047 Protocol Team. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr 2010; 55:197 - 204; http://dx.doi.org/10.1097/QAI.0b013e3181de8d26; PMID: 20574412
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Lebacq M, van der Most R, et al, HPV-010 Study Group. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum Vaccin 2011; 7:1359 - 73; http://dx.doi.org/10.4161/hv.7.12.18282; PMID: 22048172
- Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, Jay N, Aboulafia D, Cohn DL, Einstein MH, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis 2010; 202:1246 - 53; http://dx.doi.org/10.1086/656320; PMID: 20812850
- Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K, Durand C, Hervé C, Descamps D. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine 2013; 31:5745 - 53; http://dx.doi.org/10.1016/j.vaccine.2013.09.032; PMID: 24091311
- Toft L, Storgaard M, Müller M, Sehr P, Bonde J, Tolstrup M, Ostergaard L, Søgaard OS. Comparison of the Immunogenicity and Reactogenicity of Cervarix and Gardasil Human Papillomavirus Vaccines in HIV-Infected Adults: A Randomized, Double-Blind Clinical Trial. J Infect Dis 2013; http://dx.doi.org/10.1093/infdis/jit657; PMID: 24273179
- Weinberg A, Song LY, Saah A, Brown M, Moscicki AB, Meyer WA 3rd, Bryan J, Levin MJ, IMPAACT/PACTG P1047 Team. Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J Infect Dis 2012; 206:1309 - 18; http://dx.doi.org/10.1093/infdis/jis489; PMID: 22859825
- Sehr P, Rubio I, Seitz H, Putzker K, Ribeiro-Müller L, Pawlita M, Müller M. High-throughput pseudovirion-based neutralization assay for analysis of natural and vaccine-induced antibodies against human papillomaviruses. PLoS One 2013; 8:e75677; http://dx.doi.org/10.1371/journal.pone.0075677; PMID: 24124504
- Castellsagué X, Muñoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br J Cancer 2011; 105:28 - 37; http://dx.doi.org/10.1038/bjc.2011.185; PMID: 21629249
- Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin 2009; 5:696 - 704; http://dx.doi.org/10.4161/hv.5.10.9515; PMID: 19855170
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr., Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576 - 85; http://dx.doi.org/10.1056/NEJMoa1010971; PMID: 22029979
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr., Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401 - 11; http://dx.doi.org/10.1056/NEJMoa0909537; PMID: 21288094
- Green MS, Shohat T, Lerman Y, Cohen D, Slepon R, Duvdevani P, Varsano N, Dagan R, Mendelson E. Sex differences in the humoral antibody response to live measles vaccine in young adults. Int J Epidemiol 1994; 23:1078 - 81; http://dx.doi.org/10.1093/ije/23.5.1078; PMID: 7860159
- Klein SL. Sex differences in prophylaxis and therapeutic treatments for viral diseases. Handb Exp Pharmacol 2012; •••:499 - 522; PMID: 23027464
- Seitz H, Schmitt M, Böhmer G, Kopp-Schneider A, Müller M. Natural variants in the major neutralizing epitope of human papillomavirus minor capsid protein L2. Int J Cancer 2013; 132:E139 - 48; http://dx.doi.org/10.1002/ijc.27831; PMID: 22961598
- Castro F, Dirks WG, Fähnrich S, Hotz-Wagenblatt A, Pawlita M, Schmitt M. High-throughput SNP-based authentication of human cell lines. Int J Cancer 2013; 132:308 - 14; http://dx.doi.org/10.1002/ijc.27675; PMID: 22700458
- Schmitt M, Pawlita M. High-throughput detection and multiplex identification of cell contaminations. Nucleic Acids Res 2009; 37:e119; http://dx.doi.org/10.1093/nar/gkp581; PMID: 19589807
