Abstract
This randomized open-label trial was designed to provide preliminary immunogenicity and safety data to support development of the pediatric 13-valent pneumococcal conjugate vaccine (PCV13) for adults. The aims were to: identify an age-appropriate PCV13 formulation, i.e., with (n = 309) or without (n = 304) aluminum phosphate (AlPO4); compare the selected PCV13 formulation (n = 309) with 23-valent pneumococcal polysaccharide vaccine (PPSV23; n = 301); and, together with an extension study, assess sequential use of pneumococcal vaccines at 1-year intervals in adults aged ≥65 years (n = 105) not pre-vaccinated with PPSV23. Immune responses were measured by ELISA and opsonophagocytic activity assays 1 month postvaccination. Immunoglobulin G responses elicited by PCV13 with AlPO4 and PCV13 without AlPO4 were similar for the majority, and noninferior for all PCV13 serotypes. PCV13 with AlPO4 was generally more reactogenic, with reactions mainly mild or moderate. Thus, PCV13 with AlPO4 (hereafter PCV13) became the selected formulation. Immune responses to PCV13 were noninferior for all but one serotype and for most PCV13 serotypes superior to PPSV23. Vaccine sequence assessments showed that for PCV13/PPSV23, the initial PCV13 dose generally enhanced responses to a subsequent PPSV23 dose, compared with PPSV23 alone. For PCV13/PCV13, a second dose did not enhance the first dose response when given after 1 year. For PCV13/PPSV23/PCV13, priming with PCV13 (vaccination 1) did not protect against lower responses induced by PPSV23 to subsequent PCV13 (vaccination 3). In conclusion, the pediatric PCV13 formulation with AlPO4 is well tolerated and immunogenic in adults, is generally more immunogenic than PPSV23, and subsequent vaccination with PPSV23 is possible if required.
Introduction
Diseases caused by Streptococcus pneumoniae remain a significant public health problem, and elderly adults are recognized to be at increased risk.Citation1 A 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax®, Merck and Co Inc.) has been licensed for the elderly population in many countries for nearly 30 y, but despite widespread uptake the morbidity and mortality rate due to pneumococcal disease in adults remains high.Citation1,Citation2 PPSV23 shows efficacy against invasive pneumococcal disease (IPD), but reports on efficacy against pneumonia are inconsistent.Citation2 Importantly, the duration of protection of PPSV23, with its T-cell independent immune response, is limited to 3–5 y, but revaccination has not generally been recommended except in defined high-risk populations.Citation2-Citation4
The 13-valent pneumococcal conjugate vaccine (PCV13; Prevenar 13®, Pfizer Inc) is composed of purified capsular saccharides of S. pneumoniae conjugated to an immunogenic protein carrier (cross-reactive material 197 [CRM197], a nontoxic form of diphtheria toxoid). Conjugation to a protein carrier converts the free-polysaccharide T-cell–independent immune response into a T-cell–dependent immune response. Protein carrier-specific T cells provide the signals needed for maturation of the B-cell response and generation of B-cell memory.Citation5-Citation7 PCV13 has thus the potential for eliciting a memory response on subsequent natural exposure, and permitting revaccination, if required, thus extending the period of protection against vaccine-type pneumococcal disease. In children pneumococcal conjugate vaccines (PCVs) are efficacious against IPD and all-cause pneumonia, and may also offer advantages in adults.Citation8
This trial was conducted as a basis for the adult PCV13 global clinical development program to provide data to support further development of the pediatric vaccine PCV13 for use in adults. The aim of the trial, together with an extension trial, was to: assess the immunogenicity and safety of PCV13 with or without aluminum phosphate (AlPO4) in order to identify an age appropriate formulation, to compare the selected PCV13 formulation with the currently licensed PPSV23, and to assess the sequential use of PCV13 and PPSV23 pneumococcal vaccines in previously unvaccinated older adults.
Results
Baseline characteristics
For the parent study 930 participants were screened, 915 participants were randomized, and 914 were vaccinated. Details of the study flow are included in . For the extension study, 105 participants were enrolled and vaccinated. Baseline demographic characteristics are presented in . There was a higher percentage of female participants in each vaccine group in the parent study, and in the extension study. The mean age of participants at baseline was 70.7 y overall.
Figure 1. CONSORT diagram showing disposition of study participants. Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
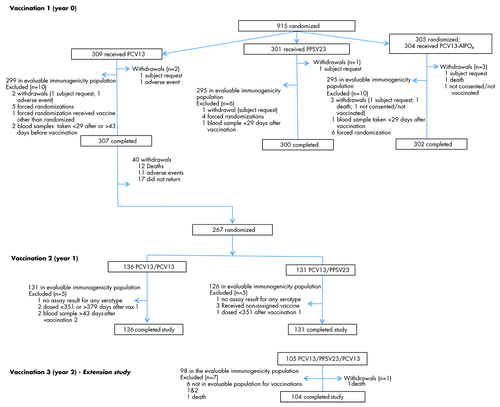
Table 1. Participant demographics of the evaluable immunogenicity population
Immunogenicity
PCV13 without AlPO4 (PCV13−AlPO4) compared with PCV13 with AlPO4 (PCV13)
Immunoglobulin (Ig)G geometric mean concentrations (GMCs) after PCV13−AlPO4 (i.e., PCV13 without AlPO4) were noninferior to those after PCV13 (with AlPO4) for all PCV13 serotypes. For all serotypes, IgG GMCs were generally similar across vaccine groups, with the exception of serotypes 7F and 14, where IgG responses were higher in the PCV13−AlPO4 group ().
Table 2. Pneumococcal IgG GMCs 1 mo after PCV13 without or with AlPO4 (evaluable immunogenicity population)
Based on the IgG immune responses and manufacturing considerations discussed later, PCV13 with AlPO4 was chosen as the selected formulation for further development and will now be referred to as PCV13.
PCV13 compared with PPSV23
PCV13 opsonophagocytic activity (OPA) geometric mean titers (GMTs) were noninferior to PPSV23 GMTs for 11 of 12 common serotypes; the exception was serotype 7F with a lower limit of the 95% confidence interval (CI) of 0.46, just below the pre-specified level of 0.5 (). PCV13 OPA GMTs were statistically significantly higher compared with PPSV23 GMTs for 10 of 12 common serotypes; PCV13 OPA GMTs were statistically significantly lower for serotype 7F. For serotype 6A, contained only in PCV13, the OPA GMT was statistically significantly higher after PCV13 than after PPSV23 ().
Table 3. Pneumococcal immune responses 1 mo after PCV13 and PPSV23 (evaluable immunogenicity population)
IgG GMCs showed a similar pattern of response as those measured by OPA. IgG GMCs in the PCV13 group were significantly higher compared with those of the PPSV23 group for 10 of 12 common serotypes and serotype 6A and noninferior for serotypes 7F and 14 ().
PCV13/PPSV23 compared with PPSV23 alone
OPA GMTs after PCV13 followed one year later by PPSV23 (PCV13/PPSV23) were noninferior to PPSV23 alone for all 12 common serotypes and were statistically significantly higher for 7 of 12 common serotypes; the exceptions were serotypes 4, 7F, 14, 18C, and 19A, which showed similar responses between the vaccine groups (). For serotype 6A, the OPA GMT was statistically significantly higher after PCV13/PPSV23 than after PPSV23.
Table 4. Immune responses 1 mo after vaccine sequence PCV13/PPSV23 given at a 1-y interval compared with after a single dose of PPSV23 (evaluable immunogenicity population)
IgG GMCs showed a similar pattern of responses to those measured by OPA. IgG GMCs after PCV13/PPSV23 were noninferior to PPSV23 for all serotypes and statistically significantly higher for 7 of 12 common serotypes and serotype 6A; the exceptions were serotypes 4, 5, 7F, 14, and 18C, which showed similar IgG responses across the vaccine groups (data not shown).
PCV13/PCV13 compared with PCV13 (dose 2 vs. dose 1)
OPA GMTs after PCV13 followed one year later by a second dose of PCV13 (PCV13/PCV13) were noninferior to PCV13 for 8 of 13 serotypes; serotypes 4, 5, 9V, 14, and 18C did not meet the noninferiority criterion. OPA GMTs after PCV13/PCV13 were statistically significantly lower for 8 of 13 serotypes (serotypes 1, 3, 4, 5, 9V, 14, 18C, and 19A) than after PCV13. The OPA GMT for serotype 23F was significantly higher after PCV13/PCV13 than after PCV13 ().
Table 5. Immune responses 1 mo after dose 1 and dose 2 of PCV13 (evaluable immunogenicity population)
IgG GMCs after PCV13/PCV13 were noninferior to those after PCV13 for 11 of 13 serotypes; serotypes 5 and 18C did not meet the noninferiority criterion. IgG GMCs after PCV13/PCV13 were statistically significantly lower for 8 of 13 serotypes (serotypes 1, 3, 4, 5, 7F, 9V, 18C, and 19A) than after PCV13. IgG GMCs for serotype 6A, 19F, and 23F were significantly higher after PCV13/PCV13 than after PCV13 (data not shown).
PCV13/PPSV23/PCV13 compared with PCV13 (vaccination 3 vs. vaccination 1)
For the vaccine sequence PCV13/PPSV23/PCV13, OPA GMTs after vaccination 3 were noninferior to vaccination 1 for 3 of 13 serotypes. OPA GMTs were statistically significantly lower after vaccination 3 than after vaccination 1 for 10 of 13 serotypes (1, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, and 19F), and were similar for the other 3 serotypes ().
Table 6. Immune responses after each vaccination in the vaccine sequence PCV13/PPSV23/PCV13
PCV13/PPSV23/PCV13 compared with PCV13/PPSV23 (vaccination 3 vs. vaccination 2)
For the vaccine sequence PCV13 followed 1 y later by PPSV23 and then 1 y later by PCV13 (PCV13/PPSV23/PCV13), OPA GMTs after vaccination 3 were noninferior to OPA GMTs after vaccination 1 for 9 of 13 serotypes. OPA GMTs were statistically significantly lower for 8 of 13 serotypes (1, 3, 5, 7F, 9V, 14, 19A, and 19F) and were significantly higher for 3 of 13 serotypes (6A, 6B, 23F) after vaccination 3 vs. after vaccination 2 ().
Safety after vaccination
Compliance with e-diary completion in this elderly population was high, with 74.3–77.5% of participants completing all 14 d after each vaccination, and >91.4% completing each day of the 14 daily assessments.
Safety after PCV13 (with AlPO4), PCV13−AlPO4, and PPSV23
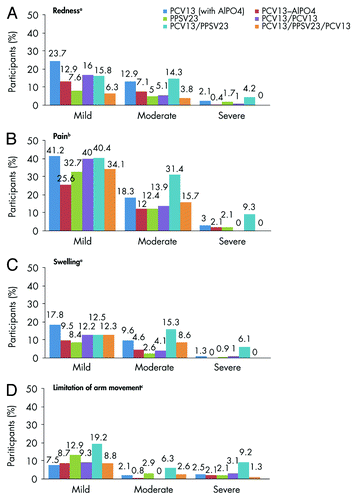
Most local reactions within 14 d after any vaccination were mild in severity. The mean durations of local reactions did not exceed 3.5 d. Mild and moderate redness, and mild and moderate swelling were significantly more frequent after PCV13 (with AlPO4) than after PPSV23 (P < 0.01) (). Mild pain was significantly different across the 3 vaccine groups with higher frequency in PCV13 and PPSV23 ().
Figure 2. Local reactions reported within 14 d after vaccination. aSeverity of redness and swelling: absent (no or minimal redness/swelling present, 0 to <2.5 cm; 0 to <5 caliper units), mild (2.5–5.0 cm; ≥5 to ≤10 caliper units), moderate (5.1–10.0 cm; >10 to ≤20 caliper units), or severe (>10.0 cm; >20 caliper units). bSeverity of pain at the injection(s) site: mild (awareness of sign/symptom but easily tolerated), moderate (enough discomfort to interfere with usual activity), or severe (inability to do usual activity).cSeverity of limitation of arm movement: absent (no limitation), mild (some limitation), moderate (able to move arm above shoulder-level but not above head), or severe (unable to move arm above shoulder). Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Systemic events within 14 d after any vaccination were similar with the exception of rash, which was more frequent after PCV13 than after PCV13−AlPO4 or after PPSV23 (P < 0.003 for overall comparison across the 3 vaccine groups) ().
Table 7. Systemic events reported within 14 d after vaccination
Adverse events (AEs) within 29–43 d after vaccination were similar across the PCV13, PCV13−AlPO4, and PPSV23 groups (23.1%, 20.1%, and 26.8%, respectively). Of these, 0.6%, 1.3%, and 0.3%, respectively, were serious AEs (SAEs) and not related to the study vaccines. A total of 13 deaths occurred after vaccination 1: one death due to myocardial infarction occurred within 29 d after vaccination with PCV13−AlPO4, and 12 deaths occurred during the following 11-mo interval before vaccination 2, mostly due to cardiovascular disease or cancer. No deaths were related to the study vaccines.
Safety after vaccination 2: PCV13/PCV13 vs. PCV13/PPSV23
There was a lower percentage of local reactions after PCV13 compared with PPSV23 when given as vaccination 2 of the vaccine sequence PCV13/PCV13 and PCV13/PPSV23 given at 1-y intervals (). Systemic events were generally similar across groups ().
The incidences of any AEs after vaccination 2 were 20.1% and 28.1%, in the PCV13/PCV13 and PCV13/PPSV23 groups, respectively. Of these, 4 participants experienced SAEs, including 3 participants after PCV13/PCV13 (1 with outcome death) and 1 participant after PCV13/PPSV23. None of the SAEs was related to study vaccine.
Safety profile after PCV13/PPSV23/PCV13
Generally, fewer local reactions and systemic events were reported after vaccination 3 compared with after vaccinations 1 and 2 of the sequence PCV13/PPSV23/PCV13, when given at 1-y intervals ( and ); similarly for AEs (data not shown). Two SAEs, neither of which was related to the study vaccine, including one cardio-respiratory arrest with an outcome of death, were reported within 29 d after vaccination 3.
Discussion
The parent study and its extension were designed to provide data to support further development of the pediatric vaccine PCV13 for use in adults. First, an age appropriate formulation was defined, followed by comparison with the licensed PPSV23, and finally the potential of re-administering pneumococcal vaccines at a 1-y interval was assessed.
PCV13 containing AlPO4, the same formulation as marketed for pediatric vaccination, was shown in adults to be noninferior to PCV13 without AlPO4 for all serotypes, with generally similar IgG immune responses across groups. Of interest, although there is evidence that AlPO4 contained in conjugate vaccines enhances the immune response in infants,Citation9,Citation10 it did not result in an enhanced antibody response in adults when compared with PCV13 without AlPO4. Although PCV13 containing AlPO4 was the more reactogenic of the 2 formulations, reactions were mainly mild or moderate. Formulations with AlPO4 and polysorbate 80 (which was added later) combine to protect against vaccine product aggregation and undergo less protein and antigenicity loss than formulations without these constituents (data on file). Based on the comparable immunogenicity, the favorable safety profile, and better product stability, PCV13 containing AlPO4 was chosen as the formulation of choice for the adult indication. All additional analyses in this study included the chosen formulation, i.e., now referred to as PCV13.
PCV13 was then compared with the currently licensed PPSV23. PCV13 OPA immune responses were shown to be noninferior to PPSV23 for all PCV13 serotypes, except for serotype 7F; the majority of serotypes were significantly higher than PPSV23 (exceptions were serotypes 7F and 14). A similar pattern of response was observed with IgG responses. The generally higher functional antibody immune responses observed after PCV13 compared with PPSV23 for the majority of PCV13 serotypes were later confirmed by the US pivotal trial in subjects aged 50–64 y not previously vaccinated with PPSV23.Citation11 Although a specific level of OPA antibody has not been shown to correlate with protection against pneumococcal disease in adults, OPA antibody responses are believed to provide the best functional correlate of vaccine-induced protection in humans.Citation12,Citation13
The vaccine sequence PCV13/PPSV23, where the vaccines were given at a 1-y interval, was compared with PPSV23 given alone. OPA responses after PCV13/PPSV23 were statistically significantly higher for 7 of 12 serotypes compared with the responses observed after a single dose of PPSV23, and were noninferior for the remainder. These findings are consistent with studies in the US where vaccines were given at 1-yCitation14 and 3.5- to 4-yCitation15 intervals, which also showed that the initial dose of PCV13 generally enhanced the response to a subsequent dose of PPSV23. This indicates that PCV13 establishes an immunological memory state that increases the anti-pneumococcal responses to subsequent administration of PPSV23.
Of interest, a second PCV13 dose given 1 y after the first PCV13 dose (PCV13/PCV13) did not enhance the immune response observed after the first PCV13 dose. This is in contrast to the increased immune response observed in children after the booster dose administered at 12 to 15 mo of age following a 2- or 3-dose primary vaccination series during infancy.Citation8 In adults, no booster effects were observed after a second dose of PCV7 when vaccine doses were given at a 6-mo or 1-y interval.Citation16-Citation18 In the present study, OPA GMTs were significantly lower for the majority of serotypes (8 of 13) after PCV13/PCV13 compared with after dose 1 of PCV13 and were significantly higher for serotype 23F only. This finding was later confirmed by another study of PCV13 in adults aged 60–64 y, with significantly lower OPA GMTs for 7 of 13 serotypes and statistically significantly higher responses only for serotype 23FCitation14 It was speculated that the lack of enhancement of the immune response may be due to high circulating antibody titers following initial PCV13 vaccination interfering with response to subsequent vaccination when the interval between doses is short. This perspective was supported by a separate study which assessed a longer interval of 3.5–4.0 y between PCV13 administrations.Citation15 In this study, responses to a second PCV13 dose for the majority of serotypes were generally at least comparable to initial PCV13 responses and statistically significantly greater for many of the serotypes. These latter observations showed that it is possible to revaccinate PCV13 recipients with PCV13 at an appropriate interval, thereby maintaining or increasing antibody levels, which may optimize protection over time. In the same study, revaccination with PPSV23, in which PPSV23 was administered twice with a 3.5- to 4.0-y interval, resulted in an overall lower immune response.Citation15
As PPSV23 is generally associated with reduced responses to subsequent PPSV23 or PCV vaccination,Citation15,Citation19 we extended the study to determine if prior PCV13 might protect against lower responses induced by PPSV23 to subsequent PCV13 vaccination. This extension study showed that the reduced immune responses after PPSV23 were not overcome by priming with PCV13 when administered as the vaccine sequence PCV13/PPSV23/PCV13 at 1-y intervals.
For safety evaluations in both studies, reactogenicity was collected using an e-diary, which prompted assessment of vaccine-associated symptoms and completion of a checklist within a fixed time window, providing an accurate representation of each participant’s experience for 14 d after vaccination. Compliance with e-diary completion in this elderly population was high.
Overall, PCV13 was well tolerated and safe, and was comparable to PPSV23—a licensed vaccine with an acceptable safety profile.Citation13 Revaccination with a second dose of PCV13 at a 1-y interval did not increase reactogenicity. In contrast, administration of PPSV23 after PCV13 showed increased reactogenicity for local reactions and some systemic events compared with after 2 doses of PCV13. These findings were later confirmed by other studies using the final manufacturing formulation.Citation14
A limitation of this study was that PCV13 did not contain 0.02% polysorbate 80, which was later added to PCV13 to improve the robustness of the manufacturing process. Nevertheless, as noted above, immune responses were consistent with those from other adult studies using the final formulation.Citation14,Citation15,Citation20,Citation21 Other limitations included the short 1-y interval between vaccinations, and the lack of a comparative arm assessing PPSV23 followed by PPSV23. These limitations have been subsequently addressed in another PCV13 clinical development study.Citation15
Based on data from this study and others, PCV13 has since been licensed in the US, Europe, and other countries for adults aged ≥50 y.Citation21-Citation23 The US Advisory Committee on Immunization Practices recently recommended that certain immunocompromised populations be vaccinated first with PCV13 followed by a dose of PPSV23.Citation24
Overall, PCV13 in adults was well tolerated and immunogenic. With its T-cell–dependent immune response and memory effect on subsequent natural exposure or revaccination, PCV13 has the potential to protect against vaccine-type pneumococcal disease over an extended time period.
Methods
Trial design
The parent trial was a randomized, open-label, multicenter trial in adults aged ≥65 y who were not previously vaccinated with PPSV23, conducted at 19 sites in South Africa (ClinicalTrials.gov identification number NCT00269672) between September 30, 2005, and December 19, 2006. The extension trial was an open-label, single-arm trial (ClinicalTrials.gov identification number NCT00500357) conducted at 17 of the 19 sites between November 5, 2007, and January 14, 2008. Both trials were conducted in accordance with the ethical principles originating in the Declaration of Helsinki and were each approved by 2 ethics committees: Pharma-Ethics at Lyttelton Manor and the Committee for Pharmaceutical Trials at University of Stellenbosch. The study was conducted in compliance with the local legal requirements in South Africa.
Participants
Healthy men and women in South Africa aged ≥65 y were eligible for enrolment. Participants were ineligible if they: had a known hypersensitivity to any vaccine or vaccine component; had a history of S. pneumoniae infection within the previous 5 y; were previously vaccinated with PPSV23 or any vaccine within past 30 d, or a diphtheria-containing vaccine within 6 mo prior to study vaccine; had known or suspected immunodeficiency or suppression or serious chronic illness with pulmonary, renal, or cardiac failure; had evidence of severe cognitive impairment (mini-mental state examination score ≤21); or were residents in a nursing home or other long-term care facility. Participants on anticoagulants were eligible unless current use contraindicated intramuscular injection.
Randomization
In the parent trial, participants were randomized in a 1:1:1 ratio to receive PCV13 formulated with AlPO4 (hereafter PCV13), PCV13 formulated without AlPO4 (hereafter PCV13−AlPO4), or PPSV23. After 1 y, participants who received the selected PCV13 formulation were randomly assigned (1:1 ratio) to receive either PCV13 or PPSV23 (). Participants who received the vaccine sequence PCV13/PPSV23 in the parent study were invited to participate in an open-label extension trial, and received a further dose of PCV13 (PCV13/PPSV23/PCV13). All vaccines were given at 1-y intervals (). Participants who received PCV13−AlPO4 received PPSV23 after 1 y (data not shown).
The randomization schedule was determined through an interactive voice response telephone system for vaccination 1 and by vaccination envelopes for vaccination 2.
Vaccines and administration
The formulation choice was between PCV13 with AlPO4 (lot number 7-5064-002B7-5064-002B) and without AlPO4. PCV13 (lot number 7-5088-002A) contains pneumococcal serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F (containing 2.2 µg of each saccharide, except for 4.4 µg of serotype 6B), individually conjugated to CRM197, 0.85% sodium chloride, and 0.125 mg aluminum as AlPO4 per 0.5-ml dose. PCV13−AlPO4 was identical to PCV13 except that PCV13−AlPO4 contains no AlPO4. The vaccine was prefilled into single-dose syringes without preservatives.
PPSV23 (lot number 0815P) consists of a purified capsular polysaccharide from 12 of the serotypes included in PCV13 (all except 6A), as well as 11 additional serotypes (2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, and 33F). The vaccine is formulated to contain 25 µg of each of the 23 purified polysaccharide serotypes per 0.5-mL dose of vaccine in isotonic saline solution containing 0.25% phenol as preservative.
Vaccines were administered intramuscularly into the deltoid muscle of the arm.
Immunogenicity assessment
PCV13 serotype-specific immune responses were measured in a central laboratory by ELISA measuring IgG binding antibodiesCitation25 or by standardized microcolony OPA assays measuring functional antibody titers.Citation26-Citation28 OPA titers were defined as the interpolated reciprocal serum dilution that resulted in complement-mediated killing of 50% of the assay bacteria. The lowest titer that can be determined in the assay (limit of detection [LOD]), regardless of serotype, is 1:8. However, to quantify functional antibodies with appropriate precision and accuracy, the lower limit of quantitation (LLOQ) was determined for each serotype-specific OPA assay during assay validation. Titers below the LLOQ were set to a value of 1:4 (half of the LOD). IgG concentrations and OPA titers were measured in blood samples obtained immediately before and approximately 1 mo (29–43 d) after each vaccination. Prevaccination antibody levels are not presented in this manuscript (Pfizer data on file).
Safety assessments
Participants recorded local reactions (redness, swelling, pain, and limitation of arm movement) and systemic events (oral temperature to measure fever, fatigue, headache, chills, rash, vomiting, decreased appetite, and new and aggravated generalized muscle and joint pain) daily for 14 d after vaccination in an e-diary. Other AEs were collected by the investigator at each visit (29–43 d after each vaccination) based on clinical evaluation as well as in response to nonspecific questions. AEs were categorized according to the Medical Dictionary for Regulatory Activities (MedDRA).
Sample size estimation
A sample size of 278 evaluable participants per group was to provide 90% overall power to demonstrate noninferiority between PCV13 with AlPO4 and PCV13 without AlPO4 using a 2-fold criterion for the geometric mean IgG concentration ratio (i.e., lower limit of the 95% CI > 0.5). Additionally, a sample of 278 participants per vaccine group was to provide 80% power to demonstrate noninferiority of the immune responses elicited by PCV13 compared with PPSV23 based on the pneumococcal OPA titers ≥ 1:128 observed during a previous trialCitation16 using the noninferiority criterion −10%. Based on an assumed dropout rate of ≤7%, approximately 900 participants were enrolled to achieve 278 evaluable participants per group. Target enrolment for the extension trial was estimated at 150 participants and included PCV13/PPSV23 recipients from the parent trial.
The evaluable immunogenicity population included all eligible participants, with no other major protocol violations, who received the assigned vaccines and had ≥1 valid and determinate assay result for the comparison of interest. The all-available immunogenicity population included all participants who had ≥1 valid and determinate assay result (data not shown). The safety population included any participant who received ≥1 dose of study vaccine.
Study objectives
The parent trial was conducted to:
1) demonstrate that PCV13−AlPO4 is as immunogenic as PCV13 with AlPO4 as determined by serotype-specific IgG antibody concentrations; OPA assays were not performed.
2) demonstrate that the selected formulation of PCV13 is as immunogenic as PPSV23, as determined by serotype-specific OPA antibody titers and IgG concentrations.
3) compare the responses elicited by PPSV23 when given 1 y after PCV13 with a single dose of PPSV23 (hereafter PCV13/PPSV23 vs. PPSV23).
4) compare the responses of a second dose of PCV13 relative to the first dose of PCV13 (hereafter PCV13/PCV13 vs. PCV13) as determined by serotype-specific OPA antibody titers.
The extension study compared OPA responses after vaccination 3 with those after vaccination 1, and vaccination 2 of the vaccine sequence PCV13/PPSV23/PCV13 given at a 1-y interval.
The safety and tolerability of PCV13 (with and without AlPO4) and PPSV23 were assessed.
Statistical analyses
For the 13 serotypes contained in PCV13, serotype-specific IgG concentrations/OPA titers were logarithmically transformed for analysis. IgG GMCs and OPA GMTs were computed for each serotype with corresponding 95% CIs constructed by back transformation of the CIs for the mean of the logarithmically transformed assay results, computed using the Student t distribution. To assess differences between vaccines, 2-sided 95% CIs for the ratios of GMCs or GMTs were constructed by back transformation of the CIs for the mean difference of the logarithmically transformed assay results computed using the Student t distribution. For each serotype comparison, the noninferiority criterion was met if the lower limit of the 2-sided 95% CI for the ratios of the GMCs or GMTs was >0.5 (2-fold criterion). Differences between groups were considered statistically significantly lower when the upper limit of the 2-sided 95% CI of the GMC or GMT ratio was <1 or significantly higher when the lower limit of the 2-sided 95% CI of the GMC or GMT ratio was >1.
Rates of local reactions, systemic events, and AEs between vaccine groups were compared using Fisher exact test.
The statistical software system used for generation of the tables and figures was SAS® Release 9.1 or higher.
| Abbreviations: | ||
| AE | = | adverse event |
| AlPO4 | = | aluminum phosphate |
| CI | = | confidence interval |
| CRM197 | = | cross-reactive material 197 |
| GMC | = | geometric mean concentration |
| GMT | = | geometric mean titer |
| Ig | = | immunoglobulin |
| IPD | = | invasive pneumococcal disease |
| LLOQ | = | lower limit of quantitation |
| LOD | = | limit of detection |
| OPA | = | opsonophagocytic activity |
| PCV13 | = | 13-valent PCV |
| PPSV23 | = | 23-valent pneumococcal polysaccharide vaccine |
| PCV | = | pneumococcal conjugate vaccine |
| SAE | = | serious AE |
Disclosure of Potential Conflicts of Interest
C.J., D.J., K.U.J., D.A.S., E.A.E., W.C.G., B.S.-T. are employees of Pfizer Inc and may hold stock options. P.d.V. and K.M. received financial compensation from Wyeth to conduct these studies. Wyeth was acquired by Pfizer Inc in October 2009.
Acknowledgments
This trial was sponsored by Wyeth, which was acquired by Pfizer Inc in October 2009. Medical writing support was provided by Nancy Price, PhD and editorial support was provided by Vicki Schwartz, PhD at Excerpta Medica, and was funded by Pfizer Inc. Support with data analysis was provided by James Trammel and programming staff at PharmaNet/i3. We wish to thank all study coordinators involved in the trials as well as all research participants who were recruited.
References
- World Health Organization. 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008; 83:373 - 84; PMID: 18927997
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013; 1:CD000422; PMID: 23440780
- Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012; 30:6802 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.09.019; PMID: 23000122
- Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010; 59:1102 - 6; PMID: 20814406
- Ada G. Vaccines and vaccination. N Engl J Med 2001; 345:1042 - 53; http://dx.doi.org/10.1056/NEJMra011223; PMID: 11586958
- Clutterbuck EA, Oh S, Hamaluba M, Westcar S, Beverley PC, Pollard AJ. Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine. Clin Vaccine Immunol 2008; 15:182 - 93; http://dx.doi.org/10.1128/CVI.00336-07; PMID: 18032593
- Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis 2012; 205:1408 - 16; http://dx.doi.org/10.1093/infdis/jis212; PMID: 22457293
- Klugman KP, Black S, Dagan R, Malley R, Whitney CG. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. China: Saunders; 2012. p. 504-41.
- Lockhart SP, Hackell JG, Fritzell B. Pneumococcal conjugate vaccines: emerging clinical information and its implications. Expert Rev Vaccines 2006; 5:553 - 64; http://dx.doi.org/10.1586/14760584.5.4.553; PMID: 16989635
- Lockhart S, Watson W, Fletcher P, Leeper A, Edwards S, McCaughey M, Dunning A, Sikkema D, Siber G. Aluminum Phosphate is an Active Adjuvant for CRM197 Pneumococcal Conjugate Vaccine (PnC) in Infants. Eighth Annual Conference on Vaccine Research. Baltimore, Maryland, USA; May 8-11, 2005. [Abstract P4] Available at: http://www.nfid.org/professional-education/archives/acvr/acvr05.pdf. Accessed on January 6, 2014.
- Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013; 31:3577 - 84; http://dx.doi.org/10.1016/j.vaccine.2013.04.085; PMID: 23688526
- Lee LH, Frasch CE, Falk LA, Klein DL, Deal CD. Correlates of immunity for pneumococcal conjugate vaccines. Vaccine 2003; 21:2190 - 6; http://dx.doi.org/10.1016/S0264-410X(03)00025-2; PMID: 12706710
- Jackson LA. Pneumococcal polysaccharide vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. China: Saunders; 2012. p. 542-72.
- Greenberg RN, Gurtman A, Frenck R, Strout CB, Jansen KU, Trammel J, Scott DA, Emini EA, Gruber W, Schmoele-Thoma B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal-vaccine naïve adults 60–64 years of age. Vaccine 2013; Forthcoming
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013; 31:3594 - 602; http://dx.doi.org/10.1016/j.vaccine.2013.04.084; PMID: 23688525
- de Roux A, Schmöle-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008; 46:1015 - 23; http://dx.doi.org/10.1086/529142; PMID: 18444818
- Lazarus R, Clutterbuck E, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin Infect Dis 2011; 52:736 - 42; http://dx.doi.org/10.1093/cid/cir003; PMID: 21367726
- Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50-80 years. Clin Infect Dis 2009; 49:1318 - 25; http://dx.doi.org/10.1086/606046; PMID: 19814624
- Törling J, Hedlund J, Konradsen HB, Örtqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 2003; 22:96 - 103; http://dx.doi.org/10.1016/S0264-410X(03)00521-8; PMID: 14604576
- Schwarz TF, Flamaing J, Rümke HC, Penzes J, Juergens C, Wenz A, Jayawardene D, Giardina P, Emini EA, Gruber WC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥65 years. Vaccine 2011; 29:5195 - 202; http://dx.doi.org/10.1016/j.vaccine.2011.05.031; PMID: 21619909
- Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis 2012; 55:259 - 64; http://dx.doi.org/10.1093/cid/cis359; PMID: 22495545
- Prevnar 13: United States Prescribing Information. Available from: http://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm201669.pdf. Accessed on March 6, 2013.
- European Medicines Agency. Prevenar 13: Summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001104/WC500057247.pdf. Accessed on March 6, 2014
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816 - 9; PMID: 23051612
- Quataert S, Martin D, Anderson P, Giebink GS, Henrichsen J, Leinonen M, Granoff DM, Russell H, Siber G, Faden H, et al. A multi-laboratory evaluation of an enzyme-linked immunoassay quantitating human antibodies to Streptococcus pneumoniae polysaccharides. Immunol Invest 2001; 30:191 - 207; http://dx.doi.org/10.1081/IMM-100105064; PMID: 11570640
- Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997; 4:415 - 22; PMID: 9220157
- Hu BT, Yu X, Jones TR, Kirch C, Harris S, Hildreth SW, Madore DV, Quataert SA. Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae.. Clin Diagn Lab Immunol 2005; 12:287 - 95; PMID: 15699424
- Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, Jansen KU. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine 2011; 29:7207 - 11; http://dx.doi.org/10.1016/j.vaccine.2011.06.056; PMID: 21689707
