 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Rabies claims about 55 000 human lives and many hundreds of thousands of livestock every year, worldwide. Despite a heavy disease burden, laboratory facilities to diagnose the infection remain scarce in most countries of the developing world where the disease is endemic. Rapid Fluorescent Focus Inhibition Test (RFFIT) and Fluorescent Antibody Virus Neutralization Test (FAVN) are the common tests done in the rabies diagnostic laboratories to detect and quantitate Rabies Virus Neutralizing Antibodies (RVNA). RFFIT is most often employed in confirming seroconversion following prophylactic vaccination, and to aid ante-mortem diagnosis in suspected cases of rabies. Though this remains one of the most sought-after diagnostic services in rabies laboratories, the requirements for expensive anti-rabies fluorochrome antibody conjugate and a fluorescent microscope restrict its performance to only a few reference laboratories. Cost-effective laboratory diagnostic methods employing affordable technology are a need of the hour in the rabies-endemic countries. In this study we have developed a new immunohistochemistry-based neutralization test and extensively evaluated it along with RFFIT. One hundred and 20 human serum samples collected after post-exposure vaccination were subjected to both the tests for determining RVNA titers. The results obtained with the new test correlated significantly with those of RFFIT. Further validation of the inter- and intra- assay precision, lower limit of quantification (LLOQ) and specificity was also performed. The best correlation between the 2 methods, however, was observed only when the RVNA concentrations in the samples were >20 IU/mL. Overall, the immunohistrochemistry-based neutralization test yielded satisfactory results. We suggest that it might serve as a cost-effective alternative to RFFIT in low-resource settings in the developing countries.
Introduction
Rabies is considered as a major neglected zoonotic disease worldwide. Over 55 000 people die of rabies every year.Citation1 This disease still continues to be a major public health problem in most of the developing countries of Asia, Africa, and South America. In India, 20 000 human deaths are reported every year and nearly 5 million people take post exposure prophylaxis.Citation2 A variety of modern cell culture vaccines are available and cost effective intradermal vaccination is now implemented in most states within India. Protection from rabies is due to induction of adequate levels of rabies virus neutralizing antibodies.Citation3 Detection and quantification of rabies antibodies is intended in the first place for checking the immune status in humans following pre- or post-exposure vaccination. It will also assist in the ante-mortem diagnosis of human rabies, particularly the paralytic form. The methods currently recommended by WHO for estimation of RVNA are the Rapid Fluorescent Focus Inhibition Test (RFFIT)Citation4 and Fluorescent Antibody Virus Neutralization test (FAVN).Citation5 These methods, though rapid, require an expensive fluorescence microscope and fluorescently labeled rabies conjugate and hence are done mostly in reference laboratories. There is a need to develop an economical test which can be used in routine microbiology laboratories so that sero-conversion can be monitored in peripheral laboratories.
Recently, the Centers for Disease Control and Prevention (CDC), Atlanta, USA has developed an immunohistochemistry-based rapid diagnostic test for the detection of rabies virus antigen in brain smears.Citation6 The test, called Direct Rapid Immunohistochemistry Test (DRIT) is reported to be as sensitive and specific as the conventional Fluorescent Antibody Test (FAT).Citation7-Citation9 The test procedure is simple and involves binding of rabies nucleoprotein antigen by a cocktail of biotinylated anti-nucleoprotein (N) monoclonal antibodies. Subsequent steps involve addition of streptavidin peroxidase and color development with the chromogenic agent 3-amino-9-ethylcarbazole (AEC). The rabies N antigen if present appears as reddish brown particles of varying size and shape against a blue background produced by hematoxylin. In the present work, we have adapted the DRIT principle for detection of rabies N protein in cell culture and used it to quantify rabies virus neutralizing antibodies. It was evaluated extensively in comparison with RFFIT. Herein we report our findings and suggest that this new test may serve as an alternative to RFFIT and FAVN particularly in the developing countries.
Results
An immunohistochemical staining process was successfully substituted for the fluorescent staining procedure for detection of RVNA, and yielded comparable results to the latter. In the RFFIT method upon completion of the staining procedure, the uninfected cells did not exhibit green fluorescent foci suggestive of rabies nucleoprotein antigen, and the serum dilution exhibiting 50% reduction of fluorescent foci was taken as the endpoint. In comparison, no reddish-brown staining (indicating viral nucleoprotein) was observed in uninfected cells stained by the immunohistochemistry-based neutralization test, and the end points were arrived at by examining the dilutions for a similar reduction in staining intensity to 50% ( and ). The RVNA concentrations estimated were expressed in International Units per milliliter (IU/mL) in comparison to that produced by the Reference Serum. All the confirmed negative samples (n = 30) were found to be negative by both RFFIT and the immunohistochemistry-based neutralization test.
Figure 1. Showing the 50% end point dilution in the Rapid Fluorescent Focus Inhibition Test. Note approximately 50% BHK 21 cells in the microscopic field are infected as evidenced by presence of fluorescent foci representing rabies nucleoprotein (×400).
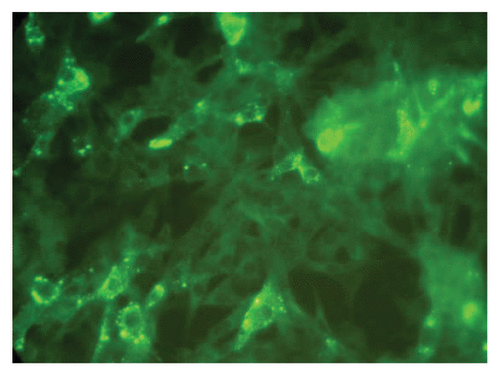
Figure 2. Showing 50% end point dilution in the immunohistochemistry based test. Note the presence of dark brown inclusions representing rabies nucleoprotein in approximately 50% of BHK 21 cells (×400).
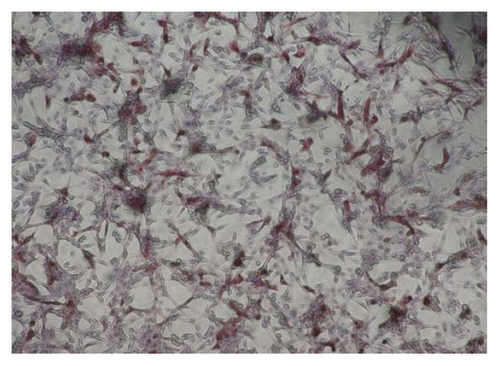
Calculation of RVNA concentration
The RVNA concentration in the serum samples tested by both RFFIT and the immunohistochemistry-based neutralization test was calculated using the following formula:
Out of 120 sera tested by both methods, 110 yielded identical end point dilutions. The end point differed by one order of dilution in the remaining 10 samples. The 30 serum samples taken as negative controls were found to be negative by both RFFIT and immunohistochemistry-based neutralization test (RVNA concentrations were less than the LLOQ). The Geometric Mean Concentrations (GMC) with 95% confidence interval obtained by these 2 tests is given in The GMC of RFFIT was 27.87 (CI: 26.71 to 29.08) and that of the immunohistochemistry-based neutralization test was 28.18 (CI: 27.17 to 29.23). The limits of agreement () between the difference between the 2 tests were 7.260 to 6.919 with a mean difference of –0.171 (CI –0.812 to 0.470). There was a significant correlation between correlation of RFFIT and the immunohistochemistry-based neutralization test (r = 0.669; P < 0.001), indicating a significant relation between RFFIT and the immunohistochemistry-based neutralization test. The scatter plot of RVNA concentration obtained in the 2 tests is given in . However, as can be seen from , the best correlation between RFFIT and the immunohistochemistry-based test was observed only when the samples had RVNA concentrations more than 20 IU/mL ().
Table 1. Results for repeatability/intra-assay precision
Figure 3. Bland Atman plot showing the agreement between the 2 tests. Please see section “results” for explanation.
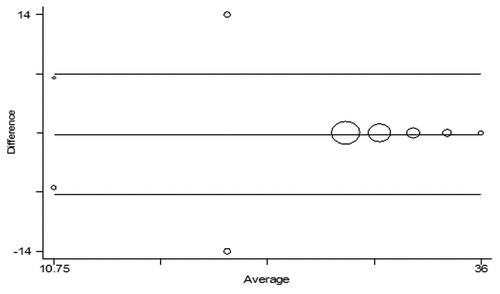
Figure 4. Scatter plot graph of results obtained between the 2 tests. Note the good correlation among samples with high titers (>20 IU/mL). There was no correlation with samples having titer less than 20 IU/mL.
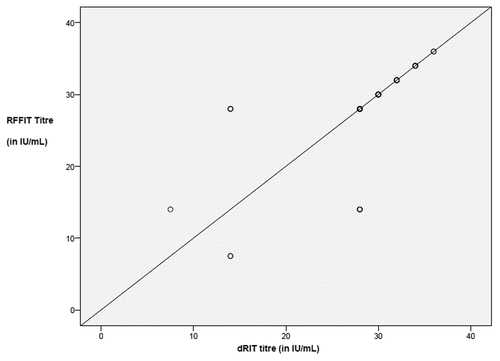
Table 2. Comparison of antibody titers observed by RFFIT and immunohistochemistry test
Results of validation
The new method was validated by assessing 4 parameters, viz., inter- and intra-assay precision, specificity and Lower Limit of Quantitation (LLOQ), against those previously obtained in our laboratory for the RFFIT method. The new method performed very well in these parameters in comparison to RFFIT. and show the results of inter- and intra-assay precision and specificity tests. As can be seen from and , testing of a set of 12 samples by 2 independent individuals on 2 different days yielded results within an acceptable range. In the specificity test employing inactivated rabies virus as a homologous inhibitor and measles virus as the heterologous inhibitor, a marked fall in the RVNA concentration was observed with homologous, compared with the heterologous inhibitor (). The LLOQ was calculated by using increasing dilutions of the Reference Serum (with an assigned unitage of 120 U/mL), and was found to be 0.1 IU/mL which was same as that observed for RFFIT method upon earlier validation of the latter ().
Table 3. Results for intermediate/inter-assay precision
Table 4. Results for the lower limit of quantitation
Table 5. Results of specificity of immunohistochemistry based neutralization test
Discussion
Rabies has the highest fatality rates among the known zoonotic infections. However, the disease is 100% preventable with the help of timely and systematic prophylaxis. RVNA targeted principally against the viral glycoprotein represent the prime correlate of protective immunity against rabies and their induction using potent cell culture vaccines remain the mainstay in rabies prophylaxis. Immune-enhancing effects of anti-nucleoprotein antibodies have also been recognized, but their specific roles in protection are not clear. Verifying sero-conversion assumes importance in post-exposure settings, and is primarily attempted by virus neutralization tests. Though a number of commercial ELISA-based platforms are available for detecting rabies virus-specific antibodies, neutralization tests remain the only methods recommended by the WHO at present. A simple and rapid immunohistochemical staining method suitable for field-level testing was developed by the CDC, Atlanta for detection of rabies virus antigen in infected brains. In the present study, we explored the possibility of application of this method in assaying RVNA in serum specimens. Our results indicate that this technique can be adapted to detect presence of un-neutralized virus following a conventional serum virus neutralization test. The major advantage of the modified test is the requirement of only a light microscope for interpretation of the results, and detection of end point by presence of reddish-brown aggregates instead of fluorescent foci of cells. A 100% correlation could not be observed between the results of the conventional RFFIT staining and the immunohistochemistry-based neutralization test, as the RVNA concentrations of a few samples differed by one order of dilution between the 2. This could be explained by relative subjectivity of the 2 independent readers who interpreted the results. Another observation was that the results obtained from both the methods had the best correlation when the samples had RVNA concentrations >20 IU/mL. Sparing this, a good correlation was found between the RVNA concentrations obtained for the antibody-positive as well as antibody-negative samples when tested by both methods.
A few modifications of the conventional DRIT staining procedure were made in order to adapt it to a microneutralization format for detection of RVNA. Notably, cell fixing prior to staining was done using chilled acetone instead of 10% buffered formalin recommended in DRIT staining, as the former was found to yield a better background upon counterstaining with Gill’s hematoxylin. In addition, the incubation periods with 3% H2O2, biotinylated N-monoclonal antibody cocktail and the streptavidin-peroxidase conjugate were increased to 30 min each, as the usual incubation time of 10 min with these reagents as employed in conventional DRIT procedure yielded poor staining. The duration of incubation with the chromogenic reagent, viz., 3-amino-9-ethylcarbazole (AEC) was retained at 10 min. Further, a 1:5 dilution of Gill’s hematoxylin was found to improve the contrast.
Infected cells stained by the modified procedure were clearly discernible by the presence of reddish-brown inclusions representing N protein aggregates, as against fluorescent foci observed in conventional RFFIT procedure ( and ). In addition, counterstaining with hematoxylin afforded a clear and easier interpretation of the results in the cells stained by the immunohistochemistry-based staining procedure. Non-specific staining, which sometimes is observed with the RFFIT procedure, was not observed in the cells stained by the immunohistochemistry-based neutralization test, in our study. The 50% end points calculated for the samples were nearly identical in both the methods employed, and the RVNA concentrations expressed in IU/mL were also similar. None of the antibody-negative samples showed the presence of neutralizing antibodies, in either method.
Two previous studies have reported the utility of an indirect immunoperoxidase test using polyclonal anti-rabies antisera for the detection of RVNA in serum samples.Citation10,Citation11 In the present study, the sensitivity and specificity of the test were enhanced further with the use of a biotinylated anti-N monoclonal antibody cocktail, which was previously shown to be 100% specific and sensitive when employed in detection of rabies virus N antigen in brain smears in our laboratory.
As the modified staining technique was developed and used for the first time in detecting RVNA, we included several parameters for evaluating it, as previously applied for RFFIT in our laboratory. As can be seen from , , and , the LLOQ, inter- and intra-assay precision, and specificity of the modified staining procedure were comparable to those of RFFIT. Findings from our study suggest that a modified immunohistochemical staining procedure employing a cocktail of biotinylated anti-N monoclonal antibodies can substitute the fluorescent staining procedure for detection of RVNA in serum samples, with the added advantage of interpretation using a light microscope. One of the major constraints for use of RFFIT is the cost involved in procurement of a fluorescence microscope and the rabies FITC conjugate. In our laboratory the approximate cost of RFFIT per one serum sample is US$50 as against US$20 estimated for the immunohistochemistry-based neutralization test. Thus the latter affords a cost saving of more than 50% than incurred in the conventional fluorescent staining procedure for assaying RVNA.
To conclude, the present study evaluated for the first time, the feasibility of adapting the DRIT procedure for detecting un-neutralized virus in serum neutralization tests for detection and quantitation of RVNA. Preliminary results show that it is easier, more economical and well-suited for application in resource-limited settings, for the purpose. The utility of the modified test needs to be evaluated further using larger sets of clinical specimens and at more laboratories receiving serum samples for evaluation of RVNA response.
Materials and Methods
Blood samples
Our laboratory routinely performs RFFIT on serum samples referred from different hospitals in the country. Included in the study were 120 serum samples obtained during the period January 2011-December 2012, from individuals who received post-exposure rabies prophylaxis. Thirty serum samples from individuals with no prior history of animal bites and rabies prophylaxis were included as negative controls. The sera were heat-inactivated at 56 °C for one hr and subsequently tested by the RFFIT and the immunohistochemistry-based neutralization test, in parallel. Ethical clearance was not required for the study, as it was done on samples received for routine laboratory diagnosis.
Rapid Fluorescent Focus Inhibition Test
The RFFIT procedure as recommended by WHO was followed, with a few modifications.Citation11 Serial dilutions of the test sera (1:32 till 1:8096) were made in duplicate in Eagle’s Minimum Essential Medium (EMEM, Sigma-Aldrich) containing 2% Fetal Bovine Serum (European Grade Fetal Bovine Serum, Biological Industries) in a 96-well tissue culture plate (CellStar®, Greiner Bio-One). Serial dilutions of the Reference Serum were also made similarly. The Reference Serum used was an in-house preparation calibrated against the Second International Reference Serum with a potency of 30 IU/mL (National Institute of Biological Standards). One hundred microlitres of Challenge Virus Standard-11 strain of rabies virus (adapted to BHK-21 cells) containing 100 Fluorescent Focus Forming Dose (FFD50) was added to each serum dilution. The plates were incubated at 37 °C in a CO2 incubator (NuAire) for one hour. Subsequently, 100 µL of BHK-21 cell suspension (trypsinized from a confluent monolayer in a 25 cm2 tissue culture flask into 10 mL of growth medium) was added to each well and the plate returned to the 37 °C incubator, after including appropriate virus controls and negative controls. Following 24 hours, the cell monolayers in the wells were fixed using chilled acetone and stained using the Polyclonal DFA Reagent (Catalog No.5199, Light Diagnostics). The stained plate was observed under UV light in an inverted fluorescent microscope (Nikon Eclipse TS100, Nikon Instruments Inc.).
Immunohistochemistry-based neutralization test
The procedures for serum dilution, preparation of serum-virus mixture and incubation were done exactly as for RFFIT. Following the 24-h incubation period, the growth medium in the wells was discarded by careful inversion into a disinfectant, and the cell monolayers were fixed using chilled acetone (35 µL/well) for a period of 10 min. The acetone was then removed from the wells by inverting the plates, and the wells gently rinsed in Phosphate Buffered Saline (PBS) (pH 7.2) once. Endogenous peroxidase activity was quenched by incubating the wells with freshly prepared 3% hydrogen peroxide solution (50 µL/well), for 10 min at room temperature. Following a PBS wash, the monolayers were incubated with a biotinylated anti-N monoclonal antibody cocktail (a generous gift from CDC) (50 µL/well) for 30 min at 37 °C. The wells were washed in 3 changes of PBS, and then incubated with streptavidin-peroxidase conjugate (Merck Biosciences) (50 µL/well) for 30 min at 37 °C. After 3 washes in PBS, the wells were incubated with 50 µL/well of the chromogen solution (prepared using acetate buffer, pH 5.2; 9-amino-ethyl-carbazole, and 3% H2O2 added just prior to addition to the wells; exactly as recommended by CDC for DRIT staining) for 10 min at room temperature in the dark. The wells were washed in 3 changes of distilled water, and then incubated with the counterstain, Gill’s #2 hematoxylin solution (Sigma Aldrich) (diluted 1:5 in distilled water) exactly for 2 min. The wells were washed thrice with distilled water, and observed under a light microscope.
Validation of the immunohistochemistry-based neutralization test
The new test developed was validated for several parameters like the intra- and inter-assay precision, specificity and LLOQ. The test performed very well in these validation tests and results were comparable with those from RFFIT validation performed earlier in our laboratory. The results of evaluation of inter- and intra-assay precision are given in , , and . It can be seen that the results obtained on 12 samples tested on different days and by 2 independent persons were within the acceptable range. In the specificity test wherein inactivated rabies virus was used as a homologous inhibitor and measles as a heterologous inhibitor, there was a marked fall in the RVNA concentration with the homologous inhibitor in comparison to the heterologous inhibitor. The LLOQ of RVNA concentration, which was evaluated using decreasing dilutions of commercial HRIG having an assigned titer of 120 IU correlated with respective dilutions. The LLOQ of the immunohistochemistry-based neutralization test was found to be 0.1 IU/mL, identical to that obtained for RFFIT method when the latter was evaluated previously in our laboratory.
Statistical analysis
The data were expressed using geometric mean and 95% confidence interval. The agreement between RVNA concentrations calculated with RFFIT and with the immunohistochemistry-based neutralization test was established by Bland Altman plot along with limits of agreement. The Pearson correlation coefficient was calculated between RFFIT and immunohistochemistry-based neutralization test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to acknowledge the significant help received from Prof K Thennarasu for the statistical analyses performed in the study.
References
- Knobel DL, Cleaveland S, Coleman PG, Fèvre EM, Meltzer MI, Miranda ME, Shaw A, Zinsstag J, Meslin FX. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ 2005; 83:360 - 8; PMID: 15976877
- Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NS, Ashwath Narayana DH, Abdul Rahman S, Meslin F-, Lobo D, Ravikumar K, Gangaboraiah. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J Infect Dis 2007; 11:29 - 35; http://dx.doi.org/10.1016/j.ijid.2005.10.007; PMID: 16678463
- Nicholson KG. Cell culture vaccines for human use. General considerations. In: Meslin FX, Koprowsky H and Kaplan MM. (eds.). Laboratory techniques in Rabies. 4th ed. Geneva, WHO, 1996, pp.271-9.
- Smith SJ, Yager AP, Baer MG. Rapid Fluorescent Focus Inhibition Test for determining rabies virus neutralizing antibodies. In: Meslin FX, Kaplan MM, Koprowski H (eds.). Laboratory techniques in Rabies. 4th ed. Geneva: WHO, 1996, pp. 181-91.
- Cliquet F, Aubert M, Sagne L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies virus neutralising antibody. J Immunol Methods 1998; 212:79 - 87; http://dx.doi.org/10.1016/S0022-1759(97)00212-3; PMID: 9671155
- Lembo T, Niezgoda M, Velasco-Villa A, Cleaveland S, Ernest E, Rupprecht CE. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg Infect Dis 2006; 12:310 - 3; http://dx.doi.org/10.3201/eid1202.050812; PMID: 16494761
- Dürr S, Naïssengar S, Mindekem R, Diguimbye C, Niezgoda M, Kuzmin I, Rupprecht CE, Zinsstag J. Rabies diagnosis for developing countries. PLoS Negl Trop Dis 2008; 2:e206; http://dx.doi.org/10.1371/journal.pntd.0000206; PMID: 18365035
- Saturday GA, King R, Furhmann L. Validation and operational application of a rapid method for rabies antigen detection. US Army Med Dep J 2009;42-5.
- Madhusudana SN, Subha S, Thankappan U, Ashwin YB. Evaluation of a direct rapid immunohistochemical test (dRIT) for rapid diagnosis of rabies in animals and humans. Virol Sin 2012; 27:299 - 302; http://dx.doi.org/10.1007/s12250-012-3265-6; PMID: 23055005
- Cardoso TC, Silva LH, Albas A, Ferreira HL, Perri SH. Rabies neutralizing antibody detection by indirect immunperoxidase serum neutralization assay performed on chicken embryo related cell line. Mem Inst Oswaldo Cruz 2004; 99:531 - 4; PMID: 15543419
- Ogawa T, Gamoh K, Aoki H, Kobayashi R, Etoh M, Senda M, Hirayama N, Nishimura M, Shiraishi R, Servat A, et al. Validation and standardization of virus neutralizing test using indirect immunoperoxidase technique for the quantification of antibodies to rabies virus. Zoonoses Public Health 2008; 55:323 - 7; http://dx.doi.org/10.1111/j.1863-2378.2008.01128.x; PMID: 18489544
