Abstract
A novel therapeutic strategy is required for autoimmune diseases characterized by the production of autoantibody, because current clinical strategies have limitations. Vaccination against autoimmune diseases is a feasible strategy because vaccines induce immune response memory and the antigen specificity. However, no suitable adjuvant is available to direct the immune response toward tolerance or suppression. In the current study, we evaluated whether kynurenine (Kyn) could serve as a novel suppressive adjuvant to decrease the humoral immune responses against hepatitis A virus (HAV) in the ICR mouse model in vivo and lipopolysaccharide (LPS) in B cells in vitro. The underlying mechanisms of Kyn-mediated suppression of LPS-induced IgM responses were explored. The results showed that Kyn significantly decreased HAV immunogenicity when co-administered with HAV, and that Kyn (100 μM/1000 μM) impaired IgM generation compared with that induced by LPS alone. We also demonstrated that microRNA30b (miR30b) played a critical role in the process of Kyn-mediated suppression of IgM responses induced by LPS, and that Bach2, a transcriptional repressor of B cell terminal differentiation, was a novel target of miR30b. These findings suggest that Kyn can serve as a novel and effective suppressive adjuvant for vaccines.
Introduction
The immune system continuously maintains the balance between tolerance to self-antigen and responsiveness to pathogen. When the tolerance is inhibited, autoimmune diseases usually occur and are characterized by the production of autoantibody that is secreted by plasma cell and that plays a key role in their pathogenesis. Autoimmune diseases have been estimated to affect 4–5% of the population.Citation1 However, there are many side effects of the currently-used therapeutic strategies, such as causing tumors, infection, as well as delayed dysfunction, and especially reduced targeting effectiveness. Thus, a novel strategy is required to improve therapeutic effects. Compared with the therapeutic strategies including immunosuppressive agents, cytokines, and TNF-α antagonists, recovering tolerance or downregulating the immune response to non-harmful antigens is a better ideal way to treat autoimmune diseases through administrating a mixture of special antigen with adjuvant that decreases the strength of the immune response.Citation2 Recent medical advances have included determining the self-antigens for various autoimmune diseases. Thus, it is difficult to find novel tolerogenic/suppressive adjuvants and to understand their biological mechanisms of action for a successful new therapeutic strategy.
Indoleamine-2, 3-dioxygenases (IDO) was recently shown to suppress the immune response, and investigators have gradually begun to focus on tryptophan degradation products, including Kynurenine (Kyn), 3-hydroxykynurenine (3-HK), kynurenic acid (KYNA), and quinolinic acid (QUIN), through the IDO metabolic pathway. Kyn is the first metabolite of tryptophan through tryptophan-2, 3-dioxygenase (TDO) degradation in liver under physiological conditions,Citation3 and through the IDO in the extrahepatic tissues such as kidney, blood, lung, and lymph tissue during infection, inflammation, or oxidative stress,Citation4,Citation5 and is excreted in the urine after conversion into xanthurenic acid in the kidney and liver.Citation6 Attenuation of the immune response is an important factor in the formation of cancer where the level of Kyn is often several times higher than under physiological conditions.Citation7 This suggests that Kyn may be related to regulation of immune response. Kyn can induce apoptosis in natural killer cells through generation of reactive oxygen species,Citation8 inhibit the natural killer cell and T cell proliferation,Citation9 and promote the differentiation of naïve T cells into regulatory T cell (Treg) rather than into Th17 cells by negatively regulating dendritic cell immunogenicity.Citation10 In addition, it has been confirmed that Kyn activates the aryl hydrocarbon receptor (AHR) in a ligand-receptor manner,Citation11 and the activation leads to the generation of Treg, which supports the role of AHR as a central player in T cell differentiation.Citation12 Several studies have demonstrated that cytochrome P450 1A1 (CYP1A1) is upregulated by AHR when activated by ligands such as dioxin and β-naphthoflavoneCitation13 and many other potential endogenous ligands.Citation14,Citation15 The available data on the effect of Kyn on the immune system mainly focuses on the areas of dendritic cells, natural killer cells and T cells. However, the influence of Kyn on B cells has not been reported.
B cells play a key role in the humoral immune response. Upon activation by antigen, mature B cells undergo an immunoglobulin class switch recombination and differentiate into antibody-secreting plasma cells, the process is called the B cell terminal differentiation. In addition to the experimental animal model, B cell terminal differentiation also can be studied successfully in vitro, because B cells are capable of both immunoglobulin class switch recombination and differentiation toward antibody-secreting plasma cells in response to a TD stimulus or a TI-related signal such as lipopolysaccharide (LPS). The process of B cell terminal differentiation is mainly regulated by 2 groups of transcription factors that are mutually antagonistic. The factors include those that maintain the B cell program, including Bach2, Bcl6, and Pax5, and those that facilitate plasma cell differentiation, especially Blimp1. Bach2 is a transcriptional repressor that is expressed throughout B cell differentiation. By contrast, Bach2 is downregulated in antibody-secreting plasma cells. Ochiai et al. found that Bach2 suppressed the B cell terminal differentiation through negatively regulating Blimp1 in B cells.Citation16 While Bach2 is absent, Blimp1 is expressed early in the process, which facilitates the process of B cell terminal differentiation.Citation17 In addition, the expression of Bach2 is positively controlled by Pax5.Citation18 Thus, Bach2 is an important component of the genetic network controlling B cells differentiation into antibody-secreting plasma cells. In addition to the transcriptional factors, post-transcriptional control of gene expression is also a crucial mechanism for the humoral immune response. MicroRNAs are small RNA molecules that drive post-transcriptional negative regulation of gene expression by promoting the degradation or translational block of their target mRNAs. Inhibition of Dicer, a type-III RNase endonuclease that cleaves the primary transcript into mature microRNA with another type-III RNase endonuclease, Drosha, prevents most mature microRNAs generation. In the Dicer−/− mouse model, the mice were unable to produce high-affinity class-switched antibodies, long-lived plasma cells, and memory B cells.Citation19 To date, several microRNAs, such as microRNA155Citation20,Citation21 and microRNA181b,Citation22 have been found to play crucial roles in the regulation of B cell terminal differentiation.
In this research, we evaluated the effectiveness of Kyn as an adjuvant, and its effects on humoral immune responses against Hepatitis A virus (HAV) in the ICR mouse model in vivo and in LPS-stimulated B cells in vitro. We further explored the underlying mechanism about how Kyn suppresses production of IgM induced by LPS. Our data showed that Kyn was a novel adjuvant, and when it was co-administered with HAV or LPS, Kyn significantly downregulated the humoral immune responses compared with that induced by HAV or LPS alone. Moreover, in the LPS-stimulated B cells, a model used to study the B cell terminal differentiation in vitro, we demonstrated that microRNA30b (miR30b) plays an important role in the process of Kyn-mediated suppression of IgM responses. We also found that Bach2 was a novel target of miR30b.
Results
The adjuvant effect of Kyn on humoral immune response
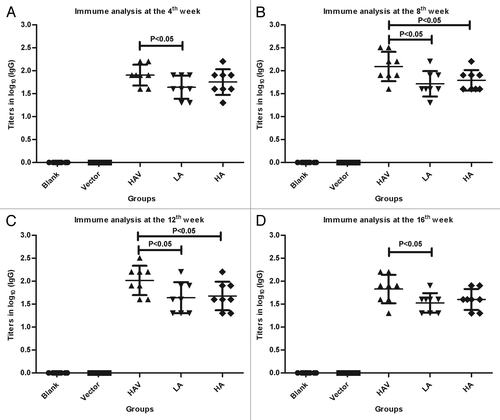
We evaluated the effect of co-delivering HAV and Kyn on the HAV-specific antibody response in the ICR mouse model, which is highly sensitive to HAV and is also used to investigate the HAV vaccine’s safety in Chinese Good Manufacturing Practices. Kyn and the HAV were administered subcutaneously, as described in the experimental procedures listed in . To better show the individual differences against HAV, the results from a single representative experiment are presented. As shown in , there were no significant HAV-specific antibody response differences between the single HAV group and HAV plus high-dose Kyn group at the 4th and 16th week after vaccination. However, HAV co-immunization with low-dose Kyn generated a significantly lower level of HAV-specific IgG titers than HAV alone at the 4th, 8th, 12th, and 16th week (P < 0.05, P < 0.05, P < 0.05, P < 0.05, respectively; ). Similar results also were found in the high-dose Kyn group at the 8th and 12th week (P < 0.05, P < 0.05, respectively; ). The results from 3 independent experiments are presented in Figure S1.
Table 1. Animal group and immunogens
Figure 1. HAV-specific antibody (IgG) titers. Female ICR mice were divided into 5 groups (8/group) and were subcutaneously immunized with Physiological saline, HAV or HAV mixed with the selected dose of Kyn (except for the control group), as described in the experimental procedures listed in . The serum samples were collected and analyzed using ELISA on the 4th (A), 8th (B), 12th (C), and 16th (D) week. The Y value is the log value of the endpoint titers. Blank, blank control group; vector, negative control group; HAV, HAV group; LA, HAV co-administrated with low dose adjuvant group; HA, HAV co-administrated with high dose adjuvant group.
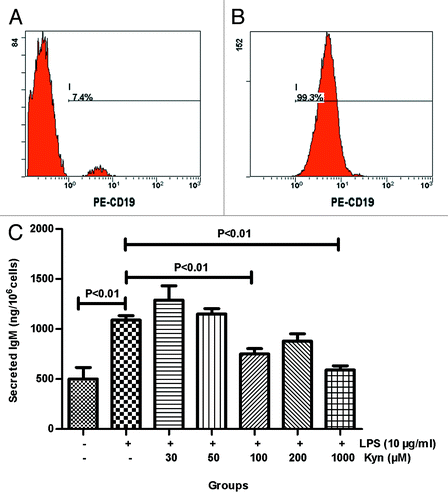
Normal human B cells were separated from the volunteer’s peripheral blood using the Human B Cell Isolation Kit II to evaluate the adjuvant effect of Kyn on the production of LPS-induced IgM in vitro. The purity of isolated B cells was routinely found to be ≥95% CD19+, which satisfied the requirements of the study. The percentage of B cells in the peripheral blood mononuclear cell (PBMC) about was 7.4% before separation (), while the purity of isolated B cells approximately was 99.3% after separation (). The data showed that Kyn (100 μM/1000 μM) significantly decreased the IgM responses when co-administered with 10 μg/mL LPS in normal human B cells (P < 0.01, P < 0.01, respectively; ). No significant differences for secreted IgM were detected between the 10 μg/mL LPS group and the 200 μM Kyn plus 10 μg/mL LPS group (), which suggested that the effect of Kyn on the IgM responses induced by LPS was dependent on concentration in vitro. As we expected, Kyn significantly attenuated the antigenic immunogenicity.
Figure 2. Effect of Kyn on LPS-induced IgM secretion in B cells. FCM was performed to determine B cell purity. (A) Before separation, (B) after separation. B cells were stimulated with the indicated combination of LPS (10 μg/mL) and selected concentrations of Kyn. Supernatants were collected after 72 h and analyzed for IgM using ELISA, as described in the experimental procedures (C). The experiment was repeated 3 times.
Kyn affects miR30b expression level
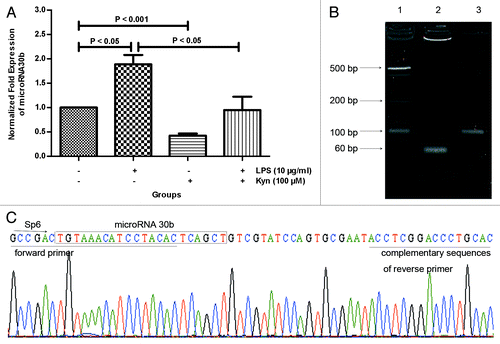
MicroRNAs play important and broad roles in a wide variety of biological processes, including embryogenesis, carcinogenesis, proliferation and apoptosis, differentiation, and signal transduction.Citation23,Citation24,Citation25,Citation26,Citation27,Citation28 Thus, we hypothesize that microRNAs are involved in the process of Kyn-mediated humoral immune response suppression. Let-7e, microRNA9, 23a, 24, 216, and 30b were selected using several web-accessible microRNA database searching programs (http://www.targetscan.org, http://www.microrna.org, and http://www.miRBase.org). In normal human B cells, the level of miR30b was significantly decreased in the 100 μM Kyn group compared with that in control group (P < 0.001; ). By contrast, LPS significantly increased the miR30b level (P < 0.05; ). In addition, Kyn significantly decreased the level of miR30b when co-administered with 10 μg/mL LPS (P < 0.05; ). Data from the other microRNAs are not presented in this manuscript. Subsequently, to evaluate the accuracy of the methods used to detect miR30b, agarose gel electrophoresis was performed to analyze the products of real-time PCR. As we expected, a stripe of approximately 60 bp was detected (). Furthermore, the sequence matched that of miRBase (MIMAT0000420) ().
Figure 3. Effect of Kyn on miR30b expression level. Real-time PCR was used to assay the miR30b expression level (A) in B cells treated with the indicated combination of LPS (10 μg/mL) and Kyn (100 μM) at 8 h. The results represent 3 separate experiments. The products of real-time PCR were evaluated by agarose gel electrophoresis (B); line 1, 20bp ladder; line 2, miR30b; line 3, U6 snRNA; and sequencing (C).
Involvement of miR30b in Kyn-mediated suppression of IgM responses induced by LPS
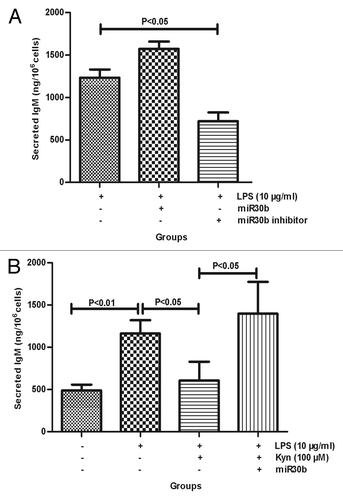
The transfection efficiency of miR30b and the miR30b inhibitor was analyzed using real-time PCR (Fig. S2). ELISA was performed to examine the effect of miR30b on the IgM responses. The data suggested that miR30b inhibitor significantly decreased the secreted IgM when co-administrated with 10 μg/mL LPS (P < 0.05; ). This result is in agreement with the results that the miR30b expression level and the strength of IgM responses are decreased by Kyn. However, although secreted IgM tended to increase in the miR30b + 10 μg/mL LPS group compared with that in the 10 μg/mL LPS group, there was no significant difference in the IgM concentration between these groups. We next wanted to determine the role of miR30b in the process of Kyn-mediated suppression of IgM responses. As shown in , the effect of Kyn-mediated inhibition of IgM responses induced by LPS was reduced by transfecting miR30b into B cells (P < 0.05), which demonstrated that miR30b was an important component in the process of downregulating IgM production induced by Kyn.
Figure 4. Involvement of miR30b in Kyn-mediated suppression of IgM responses induced by LPS in B cells. Supernatants were harvested from the B cells incubated with LPS (10 μg/mL), LPS (10 μg/mL) + miR30b, LPS (10 μg/mL) + miR30b inhibitor, LPS (10 μg/mL) + Kyn (100 μM), LPS (10 μg/mL) + Kyn (100 μM) + miR30b, or without at 72 h. The effect of miR30b on the generation of IgM (A) or on kyn-mediated suppression of IgM responses (B) was assayed using ELISA. The experiment was repeated 3 times.
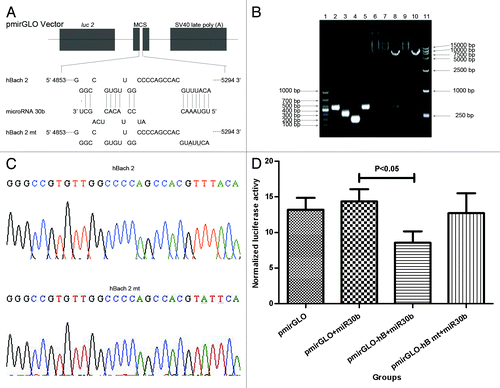
BACH2, a novel microRNA30b target
In the RNA-induced silencing complex, microRNA downregulates the expression of the target gene either by direct degradation or destabilization and eventually suppresses translation of the target through binding to the target sequence in the 3′ UTR via the 2–7 nucleotide seed region.Citation29,Citation30,Citation31 Determining the miR30b target may explain its action in the humoral immune response. The results that Kyn reduces the level of miR30b and the strength of the LPS-induced IgM responses, described above, suggest that repressor of B cell terminal differentiation could be the miR30b target. We hypothesized that Bach2 was a target of miR30b using web-accessible microRNA database search programs (http://www.targetscan.org, http://www.microrna.org, and http://pictar.mdc-berlin.de) (). To test the hypothesis, the recombinant plasmids, pmirGLO-hB, and pmirGLO-hB mt, were constructed. Related fragments, pmirGLO vector, pmirGLO-hB, and pmirGLO-hB mt were as expected using agarose gel electrophoresis analysis (), and the results of sequencing showed that pmirGLO-hB and pmirGLO-hB mt were obtained successfully (). After transfection for 48 h, the transfection efficiency (Fig. S2) and the interaction of miR30b and Bach2 were analyzed. As shown in , luciferase activity in the pmirGLO-hB + miR30b group was significantly lower than that in pmirGLO + miR30b group (P < 0.05), whereas there were no significant differences in luciferase activity between pmirGLO-hB mt + miR30b group and pmirGLO + miR30b group, which demonstrated that Bach2 was a novel target of miR30b.
Figure 5. Bach2 is a novel target of miR30b. The interaction sites predicted between miR30b and Bach2 is shown in (A). Agarose gel electrophoresis assayed related fragments, plasmids involving in the recombinant plasmids, pmirGLO-hB and pmirGLO-hB mt (B); line 1, DL 1000 Marker; line 2, objective fragment of wild type; line 3, forward objective fragment of mutant type; line 4, reverse objective fragment of mutant type; line 5, completely objective fragment of mutant; line 6, pmirGLO vector; line 7, pmirGLO-hB; line 8, double enzyme digestion of pmirGLO-hB; line 9, pmirGLO-hB mt; line 10, double enzyme digestion of pmirGLO-hB mt; line 11, DL15000 Marker. Objective fragments in pmirGLO-hB and pmirGLO-hB mt was evaluated using sequencing (C). Luciferase activity was detected using a dual-luciferase assay system at 48 h after transfection (D) and repeated 3 times. The underline indicates the mutant site in this Figure.
The effect of miR30b on Bach2 in Kyn-mediated inhibition of IgM responses
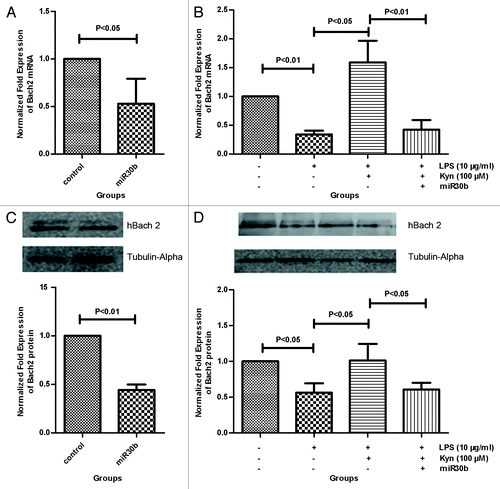
The Bach2 mRNA and protein levels were analyzed using real-time PCR and western blot, respectively. MiR30b significantly decreased the mRNA and protein levels of Bach2 compared with corresponding control group (P < 0.05, P < 0.01, respectively; ). In addition, miR30b significantly decreased the Bach2 mRNA and protein levels when co-administered with 100 μM Kyn+10 μg/mL LPS (P < 0.01, P < 0.05, respectively; ). We also found that Kyn co-administered with 10 μg/mL LPS upregulated Bach2 mRNA and protein levels compared with that induced by LPS alone (P < 0.05, P < 0.05, respectively; ). These data further suggested that miR30b is involved in Kyn-mediated suppression of IgM responses induced by LPS.
Figure 6. The effect of miR30b on Bach2 mRNA and protein levels. B cells incubated with miR30b, LPS (10 μg/mL), LPS (10 μg/mL) + Kyn (100 μM), LPS (10 μg/mL) + Kyn (100 μM) + miR30b, or without were harvested at 72 h. The non-treated B cells are considered the control. The Bach2 mRNA and protein levels were measured using real-time PCR and western blot, respectively. Kyn changed the Bach2 mRNA (A) and protein (C) levels in B cells. In the process of Kyn-mediated suppression of IgM responses induced by LPS, the effect of miR30b on Bach2 mRNA and protein levels is presented in panels (B) and (D), respectively. The results represent the mean of 3 separate experiments.
Discussion
Although the pathogenesis of autoimmune diseases remains unclear, it is related to innate and adaptive immunity, especially the dysfunction of autoimmune tolerance. Thus, immunologists have been interested in how to induce immune tolerance or suppress immune response in vivo to determine a better treatment for autoimmune diseases. Vaccination for autoimmune diseases is a potential treatment strategy because vaccines induce antigen specificity and immune memory within the immune response. However, there is no suitable adjuvant to direct the immune response toward tolerance or suppression.Citation2
Kyn, an endogenous substance, is a vaccine adjuvant that is safer than exogenous substances. In the current study, we investigated the effects of Kyn on the immunogenicity of HAV in vivo and the generation of IgM induced by LPS in B cells in vitro. We demonstrated that Kyn is a potent suppressive adjuvant. Compared with the humoral immune responses induced by the immunogen alone, Kyn co-administration elicited a weaker antigen-specific humoral immune responses () and lower IgM responses (). It is well known that Kyn can be degraded to 3-HK, QUIN, and KYNA by related enzyme. These metabolites are also capable of the immunomodulatory actions.Citation32 Therefore, their contribution to the Kyn-mediated suppression of humoral immune responses cannot be ignored. However, AHR activation is unique to Kyn in the tryptophan metabolites.Citation11 We also demonstrated that AHR was activated by Kyn by determining the level of CYP1A1 mRNA (data not shown), a biomarker of activated AHR, using real-time PCR. The activated AHR is transferred to the nucleus where it forms a dimer with aryl hydrocarbon receptor nuclear translocator.Citation33 The dimerized compound, AHR/ARNT, can act as a transcriptional factor and inhibit the activity of the transcription factor, activator protein 1 (AP-1), by interacting with the core-binding motif of the dioxin-responsive elements (DRE) located in the AP-1 promoter/enhancer regions.Citation34,Citation35 AP-1 is a dimeric complex comprised of jun and fos family members,Citation36 and triggers the differentiation switch by activating Blimp 1 under stimulation by antigens (for example, LPS).Citation35 In addition, AHR activation directly suppresses antibody secretion by binding to DRE in the IgH 3′α enhancer,Citation37 and indirectly represses the expression of Igκ and IgJ leading to impaired IgM assembly and secretion.Citation37,Citation38,Citation39 These results support the hypothesis that Kyn negatively regulates the humoral immune response. It is maybe more appropriate that the Kyn compound adjuvant has an effect on the humoral immune responses against HAV in vivo or LPS in vitro.
MicroRNAs regulate the expression levels of many genes, and it has been estimated that 30–60% of the genes in a given genome are regulated in this manner.Citation40,Citation41,Citation42 In this study, we analyzed the contribution of microRNAs to the process of humoral immune responses suppressed by Kyn, and found that, in the presence of Kyn, the level of miR30b was significantly decreased compared with that in the corresponding control group (). This result was supported by the data that 2,3,7,8-tetrachlorodibenzo-p-dioxin, a potent environmental toxicant and a well-known ligand of AHR, inhibited B cell terminal differentiation, and significantly downregulated miR30b.Citation43 In breast cancer, Ichikawa et al. found that upregulation of miR30b was favorable for the therapeutic actions produced by Trastuzumab,Citation44 which suggested that the decrease of miR30b promoted the downregulation of the immune response. Our data in this study showed a similar result, that downregulation of miR30b by its inhibitor significantly decreased the generation of IgM compared with that induced by LPS alone (). This demonstrated that negative regulation of miR30b suppresses IgM responses. Most importantly, miR30b reduced the suppression of Kyn-mediated IgM responses (), which suggested that miR30b is involved in the process where Kyn negatively regulates IgM responses induced by LPS.
MicroRNAs regulate the expression of mRNAs by inhibiting their translation into proteins by promoting sequestration and degradation. For mRNA targets that are only partially complementary, the key is the complementarity between the microRNA “seed region” (positions 2–8 from the 5′ end) and the mRNA target.Citation31,Citation45 Our results demonstrated that there is a direct interaction between Bach2 and miR30b through site-directed mutagenesis of target mRNA that was complementary to the miR30b “seed region” (), and that the mRNA and protein levels of the transcriptional repressor, Bach2, were impaired due to the action of miR30b (), which could facilitate the differentiation of B cells toward antibody-secreting plasma cells. Although there are also cases of greater specificity between microRNAs and their targets, some microRNA may target hundreds of different mRNAs.Citation46 In addition to Bach2, it was also reported that the key transcriptional factors controlling the B cell terminal differentiation, Bcl6 and Blimp1, were also the target of miR30b.Citation47,Citation48 The main function of Bcl6 is to suppress Blimp1 by direct binding to a response element in the prdm1 gene that encodes the Blimp1 protein,Citation49 and by indirectly suppressing the activation of AP-1Citation50 and signal transducer and activator of transcription 3 (STAT3),Citation51 which are both transcriptional activators of Blimp1. Bach2 suppresses B cell terminal differentiation through negatively controlling Blimp1 in B cellsCitation16 and is positively regulated by Pax5,Citation18 which also represses Blimp1 by directly binding to the prdm1 gene promoter.Citation52 By contrast, Blimp1, a master regulator of B cell terminal differentiation, also represses Bcl6 and Pax5 gene expression.Citation53,Citation54 Thus, the mutual inhibition of Blimp1 and Bcl6 or Blimp1 and Pax5 forms a double-negative feedback loop, which plays a key role in B cell terminal differentiation. The net function of Blimp1 depends on 3 separate transcriptional repressors (Bcl6, Bach2, and Pax5) and one transcriptional activator (Irf4).Citation55 In addition, De Abrew et al. showed that activated AHR bound to a region within the first intron of the bach2 gene, which suggested that Bach2 was directly regulated by AHR.Citation56 The sophisticated interaction among these factors mentioned above explains why miR30b significantly decreased Bach2 mRNA level in B cells ().
Taken together, the data in the present study demonstrate that Kyn is a novel suppressive adjuvant that attenuates the immunogenicity of HAV, a TD antigen, in vivo, and LPS, a TI antigen, in vitro. Moreover, we confirmed that miR30b is involved in the process of Kyn-mediated suppression of LPS-induced IgM responses in normal human B cells in vitro. Bach2 is also identified as a novel target of miR30b. These data suggest that Kyn could be a novel and effective suppressive adjuvant for vaccines used in the treatment of autoimmune diseases.
Materials and Methods
Animal immunization
Female ICR mice (6–8 wk of age; n = 40) and purified HAV stock (titer, 1:512) were obtained from the Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College. The mice were randomly divided into 5 groups with 8 animals per group. Mice in each group were subcutaneously immunized with physiological saline, HAV or HAV mixed with the selected dose of Kyn (K8625–100MG, Sigma-Aldrich) in 200 μL final volume, except the blank control group (). Blood samples were collected through the caudal vein at the 4th, 8th, 12th, and 16th week after immunization. Subsequently, serum samples were taken. The experimental animal protocol was approved by the Office of Laboratory Animal Management of Yunnan Province, China.
Cell culture and transfection
Normal human B cells were separated from volunteers’ peripheral blood that was obtained from the Blood Center using a kit according to the manufacturer’s instructions (Human B Cell Isolation Kit II; 130–091–151, Miltenyi Biotec). Cells were stained fluorescently with anti-human CD19-PE antibody. Cell debris and dead cells were excluded from the analysis based on scatter signals and PI fluorescence. The purity of isolated B cells was examined using a flow cytometry (FCM) (BECKMAN COULTER) analysis of CD19 expression. Normal human B cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibcal), 23.8 mM sodium bicarbonate, 2 mM L-glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin, 13.5 mM HEPES (Sigma-Aldrich), 1 mM sodium pyruvate (Sigma-Aldrich), 0.1 mM nonessential amino acids (Hyclone), and 50 μM β-mercaptoethanol. The HEK 293T cell line was obtained from the Kunming Institute of Zoology, Chinese Academy of Sciences and grown in high glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin, 100 U/mL penicillin. All cells were maintained at 37 °C in an atmosphere of 5% CO2–95% air.
All transfection experiments in this paper were performed using the LipofectamineTM 2000 reagent (11668-019, Invitrogen), according to the manufacturer’s recommendations.
Enzyme-linked immunosorbent assay (ELISA)
The Protein DetectorTM ELISA Kit (54-62-18, Kirkegaard and Perry Laboratories, Inc.) was used to measure HAV antibody titer. Briefly, 96-well flat-bottom plates were coated with purified HAV (titer, 1:512) at a concentration of 150 μL/mL in 1 × coating solution and incubated at 4 °C overnight. The coating solution was then discarded, and the plates were blocked using the 1 × BSA blocking solution, 100 μL/well, 37 °C for 1 h. Mouse serum was serially diluted 2-fold in 1 × BSA diluent solution (starting at 1:20) and 100 μL was added to each well, and then incubation at 37 °C for 1 h. After the incubation period, the plates were washed 5 times with 1 × washing solution and then incubated with HRP-labeled anti-mouse IgG antibody (diluted 1:1000) at 37 °C for 1 h. After the final wash, 100 μL of enzyme substrate was added to each well and the plates were incubated for 10 min. The reaction was stopped by adding 100 μL/well 2 M H2SO4, and the optical density (OD) was determined at 405 nm using an ELISA plate reader (SYNERGY4, BioTek [REMOVED EQ FIELD]).
After 3 d, cell culture supernatants were collected from the normal human B cells that were stimulated with 10 μg/mL LPS (L7895–1MG, Sigma-Aldrich) and/or 10 μg/mL LPS mixed with different Kyn concentrations (30 μM, 50 μM, 100 μM, 200 μM, and 1000 μM) or without, which were incubated with 10 μg/mL LPS, 10 μg/mL LPS+miR30b (GenePharma), 10 μg/mL LPS+miR30b inhibitor (GenePharma), 10 μg/mL LPS+100 μM Kyn, 10 μg/mL LPS+100 μM Kyn+miR30b, or without. The non-stimulated B cells were considered the control group. A Human ELISA Kit for IgM (E90543Hu, Uscn Life Science Inc.) was used to detect secreted IgM, according to the manufacturer’s instructions. The OD at 450 nm was read using an ELISA plate reader (SYNERGY4, BioTek).
Real-time reverse transcriptase polymerase chain reaction
Real-time PCR was performed using a CFX96TM real-time system (BIO-RAD) with qPCR software. To detect changes of microRNAs, total RNA was extracted from normal human B cells that were treated with 10 μg/mL LPS, 100 μM Kyn, 10 μg/mL LPS + 100 μM Kyn, or without for 8 h using an RNeasy Kit (Qiagen). The non-treated B cells were considered the control group. After 72 h of transfection, total RNA was isolated from normal human B cells that were stimulated with miR30b, 10 μg/mL LPS, 10 μg/mL LPS + 100 μM Kyn, 10 μg/mL LPS + 100 μM Kyn + miR30b, or without, to measure the change of Bach2 mRNA. The non-stimulated B cells were considered the control group. The RNA was reverse-transcribed into cDNA using the Reverse Transcription System (Promega), according to the manufacturer’s instructions. Amplification of cDNA was performed using the GoTaq qPCR Master Mix (Promega) as suggested by the manufacturer’s instructions. Gene names and the sequences of primers are shown in . The qPCR cycling conditions were as follows—after an initial denaturation and enzyme activation at 95 °C for 3 min, the amplification was performed by denaturation at 95 °C for 15 s, annealing at 56.5 °C for 15 s and extension at 72 °C for 30 s. Bach2 and miR30b mRNA levels were normalized to GAPDH or the small nuclear RNA U6 in order to control for differences in RNA loading, quality, and cDNA synthesis. Relative gene expression data were analyzed using the 2-ΔΔCt method.Citation57 The amplified fragments of miR30b were cloned into the pGEM-T Vector (Promega) and sequenced to evaluate the results of real-time PCR.
Table 2. Sequences of primers used in this study
Western blot analysis
Seventy-two hours after transfection, total cellular protein was harvested from normal human B cells that were incubated with miR30b, 10 μg/mL LPS, 10 μg/mL LPS + 100 μM Kyn, 10 μg/mL LPS + 100 μM Kyn + miR30b, or without, using RIPA buffer (Pierce) and PMSF (Solarbio). The non-stimulated B cells were considered the control group. They were denatured and subjected to denaturing SDS-PAGE and then preincubated for 30 min at room temperature in transfer buffer before being blotted onto polyvinylidene fluoride membranes (Millipore). The membranes were then blocked with 5% defatted milk powder/PBS containing 0.05% Tween 20, at 37 °C for 2 h. Rabbit anti-Bach2 antibody (1:1000) (ab83364, Abcam) and mouse anti-Tubulin-Alpha antibody (1:5000) (66031-1-Ig, ProteintechTM) were used as the detecting antibodies and incubated at 37 °C for 2h. Subsequently, immunoreactive bands were detected by incubation with HRP-labeled anti-rabbit/mouse IgG antibody (1:5000) (SA00001-1, SA00001-2, ProteintechTM), respectively, and strengthened by ImmobilonTM western chemiluminescent HRP substrate (Millipore). The membranes were scanned and quantified using the ChemiDoc-It Imaging System (Ultra-Violet Products Ltd). Protein expression levels were obtained from the images using the Visionworks LS Soft.
Plasmid construction and luciferase reporter assay
The 3′-UTR fragments containing the predicted Bach2-miR30b binding site were amplified using a PCR amplification kit (Takara) with human cDNA, and the mutant fragments with mutant sites located in the complementary miR30b seed region sequences were obtained using the PCR site-directed mutagenesis. Subsequently, they were cloned into the pmirGLO dual-luciferase miRNA target expression vector (Promega) using a DNA ligation kit (Takara). The recombinant plasmids—pmirGLO-human Bach2 (pmirGLO-hB) and pmirGLO-human Bach2 mutant (pmirGLO-hB mt)—and the pmirGLO vector were extracted from LB culture using the PureYield Plasmid Midiprep System (Promega). The PCR primer set sequences including the site of the restriction endonuclease Sac I (Takara), Xba I (Takara), and the mutant sites are listed in . HEK 293T cells were plated in 24-well plates at the concentration of 2 × 105 cells/well. miR30b (20 pmol) and each plasmid (0.4 μg) were co-transfected into the HEK 293T cells that were used as a vehicle cell to detect the interaction of miR30b with predicted target. Luciferase activity was measured using the dual-luciferase assay system (Promega) with SYNERGY4 (BioTek). Firefly luciferase activity was normalized to Renilla luciferase activity to adjust for variations in transfection efficiency among experiments.
Statistical analysis
All the experiments are repeated 3 times, and data are expressed as the mean ± standard error. Statistical analyses were performed using the professional statistical computer software, SPSS. Differences between groups were determined using a t test. P < 0.05 was considered statistically significant.
| Abbreviations: | ||
| Kyn | = | kynurenine |
| LPS | = | lipopolysaccharide |
| HAV | = | hepatitis A virus |
| miR30b | = | microRNA30b |
| AHR | = | aryl hydrocarbon receptor |
Additional material
Download Zip (158.4 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Ting Luo, Hongjuan Li, and Xiao Wang for their assistance in the animal experiments. This research was supported by the National High Technology Research and Development Program (863 Program) of China: Development and Application of New Types of Vaccine Adjuvant (Grant No. 2012AA02A406), and the Innovation Team Project of Yunnan Province of China “Provincial Innovation Team for Application Research on New Types of Vaccine Adjuvant, Institute of Medical Biology, Chinese Academy of Medical Sciences” (Grant No. 2011CI140, http://www.ynstc.gov.cn/kjcxdw/200911170004.htm).
References
- Vyse TJ, Todd JA. Genetic analysis of autoimmune disease. Cell 1996; 85:311 - 8; http://dx.doi.org/10.1016/S0092-8674(00)81110-1; PMID: 8616887
- Kang Y, Xu L, Wang B, Chen A, Zheng G. Cutting edge: Immunosuppressant as adjuvant for tolerogenic immunization. J Immunol 2008; 180:5172 - 6; PMID: 18390698
- Watanabe Y, Fujiwara M, Yoshida R, Hayaishi O. Stereospecificity of hepatic L-tryptophan 2,3-dioxygenase. Biochem J 1980; 189:393 - 405; PMID: 6783035
- Heyes MP, Saito K, Major EO, Milstien S, Markey SP, Vickers JH. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease. Attenuation of synthesis from L-tryptophan by 6-chlorotryptophan and 4-chloro-3-hydroxyanthranilate. Brain 1993; 116:1425 - 50; PMID: 8293279
- Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation?. Immunol Today 1999; 20:469 - 73; http://dx.doi.org/10.1016/S0167-5699(99)01520-0; PMID: 10500295
- Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem 1986; 261:3648 - 53; PMID: 2419335
- Hoshi M, Ito H, Fujigaki H, Takemura M, Takahashi T, Tomita E, Ohyama M, Tanaka R, Saito K, Seishima M. Indoleamine 2,3-dioxygenase is highly expressed in human adult T-cell leukemia/lymphoma and chemotherapy changes tryptophan catabolism in serum and reduced activity. Leuk Res 2009; 33:39 - 45; http://dx.doi.org/10.1016/j.leukres.2008.05.023; PMID: 18639341
- Song H, Park H, Kim YS, Kim KD, Lee HK, Cho DH, Yang JW, Hur DY. L-kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species. Int Immunopharmacol 2011; 11:932 - 8; http://dx.doi.org/10.1016/j.intimp.2011.02.005; PMID: 21352963
- Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 2002; 196:459 - 68; http://dx.doi.org/10.1084/jem.20020121; PMID: 12186838
- Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A 2010; 107:19961 - 6; http://dx.doi.org/10.1073/pnas.1014465107; PMID: 21041655
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011; 478:197 - 203; http://dx.doi.org/10.1038/nature10491; PMID: 21976023
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 2010; 185:3190 - 8; http://dx.doi.org/10.4049/jimmunol.0903670; PMID: 20720200
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 2004; 279:23847 - 50; http://dx.doi.org/10.1074/jbc.R400004200; PMID: 15028720
- Nebert DW, Karp CL. Endogenous functions of the aryl hydrocarbon receptor (AHR): intersection of cytochrome P450 1 (CYP1)-metabolized eicosanoids and AHR biology. J Biol Chem 2008; 283:36061 - 5; http://dx.doi.org/10.1074/jbc.R800053200; PMID: 18713746
- Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 2008; 21:102 - 16; http://dx.doi.org/10.1021/tx7001965; PMID: 18076143
- Ochiai K, Muto A, Tanaka H, Takahashi S, Igarashi K. Regulation of the plasma cell transcription factor Blimp-1 gene by Bach2 and Bcl6. Int Immunol 2008; 20:453 - 60; http://dx.doi.org/10.1093/intimm/dxn005; PMID: 18256039
- Muto A, Ochiai K, Kimura Y, Itoh-Nakadai A, Calame KL, Ikebe D, Tashiro S, Igarashi K. Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch. EMBO J 2010; 29:4048 - 61; http://dx.doi.org/10.1038/emboj.2010.257; PMID: 20953163
- Schebesta A, McManus S, Salvagiotto G, Delogu A, Busslinger GA, Busslinger M. Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity 2007; 27:49 - 63; http://dx.doi.org/10.1016/j.immuni.2007.05.019; PMID: 17658281
- Xu S, Guo K, Zeng Q, Huo J, Lam KP. The RNase III enzyme Dicer is essential for germinal center B-cell formation. Blood 2012; 119:767 - 76; http://dx.doi.org/10.1182/blood-2011-05-355412; PMID: 22117047
- Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, et al. Regulation of the germinal center response by microRNA-155. Science 2007; 316:604 - 8; http://dx.doi.org/10.1126/science.1141229; PMID: 17463289
- Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, et al. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity 2007; 27:847 - 59; http://dx.doi.org/10.1016/j.immuni.2007.10.009; PMID: 18055230
- Fragoso R, Mao T, Wang S, Schaffert S, Gong X, Yue S, Luong R, Min H, Yashiro-Ohtani Y, Davis M, et al. Modulating the strength and threshold of NOTCH oncogenic signals by mir-181a-1/b-1. PLoS Genet 2012; 8:e1002855; http://dx.doi.org/10.1371/journal.pgen.1002855; PMID: 22916024
- Ke XS, Liu CM, Liu DP, Liang CC. MicroRNAs: key participants in gene regulatory networks. Curr Opin Chem Biol 2003; 7:516 - 23; http://dx.doi.org/10.1016/S1367-5931(03)00075-9; PMID: 12941428
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281 - 97; http://dx.doi.org/10.1016/S0092-8674(04)00045-5; PMID: 14744438
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004; 5:522 - 31; http://dx.doi.org/10.1038/nrg1379; PMID: 15211354
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature 2005; 435:834 - 8; http://dx.doi.org/10.1038/nature03702; PMID: 15944708
- Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev 2005; 15:563 - 8; http://dx.doi.org/10.1016/j.gde.2005.08.005; PMID: 16099643
- Kapsimali M, Kloosterman WP, de Bruijn E, Rosa F, Plasterk RH, Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol 2007; 8:R173; http://dx.doi.org/10.1186/gb-2007-8-8-r173; PMID: 17711588
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215 - 33; http://dx.doi.org/10.1016/j.cell.2009.01.002; PMID: 19167326
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 2007; 27:91 - 105; http://dx.doi.org/10.1016/j.molcel.2007.06.017; PMID: 17612493
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005; 120:15 - 20; http://dx.doi.org/10.1016/j.cell.2004.12.035; PMID: 15652477
- Belladonna ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, Schwarcz R, Fallarino F, Puccetti P. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol 2006; 177:130 - 7; PMID: 16785507
- Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 1992; 256:1193 - 5; http://dx.doi.org/10.1126/science.256.5060.1193; PMID: 1317062
- Suh J, Jeon YJ, Kim HM, Kang JS, Kaminski NE, Yang KH. Aryl hydrocarbon receptor-dependent inhibition of AP-1 activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin in activated B cells. Toxicol Appl Pharmacol 2002; 181:116 - 23; http://dx.doi.org/10.1006/taap.2002.9403; PMID: 12051995
- Schneider D, Manzan MA, Yoo BS, Crawford RB, Kaminski N. Involvement of Blimp-1 and AP-1 dysregulation in the 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated suppression of the IgM response by B cells. Toxicol Sci 2009; 108:377 - 88; http://dx.doi.org/10.1093/toxsci/kfp028; PMID: 19237549
- Karin M, Liu Zg, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 1997; 9:240 - 6; http://dx.doi.org/10.1016/S0955-0674(97)80068-3; PMID: 9069263
- Sulentic CE, Holsapple MP, Kaminski NE. Putative link between transcriptional regulation of IgM expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin and the aryl hydrocarbon receptor/dioxin-responsive enhancer signaling pathway. J Pharmacol Exp Ther 2000; 295:705 - 16; PMID: 11046109
- Sulentic CE, Holsapple MP, Kaminski NE. Aryl hydrocarbon receptor-dependent suppression by 2,3,7, 8-tetrachlorodibenzo-p-dioxin of IgM secretion in activated B cells. Mol Pharmacol 1998; 53:623 - 9; PMID: 9547351
- Yoo BS, Boverhof DR, Shnaider D, Crawford RB, Zacharewski TR, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the regulation of Pax5 in lipopolysaccharide-activated B cells. Toxicol Sci 2004; 77:272 - 9; http://dx.doi.org/10.1093/toxsci/kfh013; PMID: 14600275
- Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett 2005; 579:5911 - 22; http://dx.doi.org/10.1016/j.febslet.2005.07.070; PMID: 16111679
- Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol 2007; 23:175 - 205; http://dx.doi.org/10.1146/annurev.cellbio.23.090506.123406; PMID: 17506695
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009; 19:92 - 105; http://dx.doi.org/10.1101/gr.082701.108; PMID: 18955434
- Moffat ID, Boutros PC, Celius T, Lindén J, Pohjanvirta R, Okey AB. microRNAs in adult rodent liver are refractory to dioxin treatment. Toxicol Sci 2007; 99:470 - 87; http://dx.doi.org/10.1093/toxsci/kfm189; PMID: 17698510
- Ichikawa T, Sato F, Terasawa K, Tsuchiya S, Toi M, Tsujimoto G, Shimizu K. Trastuzumab produces therapeutic actions by upregulating miR-26a and miR-30b in breast cancer cells. PLoS One 2012; 7:e31422; http://dx.doi.org/10.1371/journal.pone.0031422; PMID: 22384020
- Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev 2004; 18:504 - 11; http://dx.doi.org/10.1101/gad.1184404; PMID: 15014042
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005; 433:769 - 73; http://dx.doi.org/10.1038/nature03315; PMID: 15685193
- Chang CC, Zhang QY, Liu Z, Clynes RA, Suciu-Foca N, Vlad G. Downregulation of inflammatory microRNAs by Ig-like transcript 3 is essential for the differentiation of human CD8(+) T suppressor cells. J Immunol 2012; 188:3042 - 52; http://dx.doi.org/10.4049/jimmunol.1102899; PMID: 22387553
- Zhang J, Jima DD, Jacobs C, Fischer R, Gottwein E, Huang G, Lugar PL, Lagoo AS, Rizzieri DA, Friedman DR, et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood 2009; 113:4586 - 94; http://dx.doi.org/10.1182/blood-2008-09-178186; PMID: 19202128
- Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol 2004; 173:1158 - 65; PMID: 15240705
- Vasanwala FH, Kusam S, Toney LM, Dent AL. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J Immunol 2002; 169:1922 - 9; PMID: 12165517
- Reljic R, Wagner SD, Peakman LJ, Fearon DT. Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med 2000; 192:1841 - 8; http://dx.doi.org/10.1084/jem.192.12.1841; PMID: 11120780
- Mora-López F, Reales E, Brieva JA, Campos-Caro A. Human BSAP and BLIMP1 conform an autoregulatory feedback loop. Blood 2007; 110:3150 - 7; http://dx.doi.org/10.1182/blood-2007-05-092262; PMID: 17682124
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 2002; 17:51 - 62; http://dx.doi.org/10.1016/S1074-7613(02)00335-7; PMID: 12150891
- Sciammas R, Davis MM. Modular nature of Blimp-1 in the regulation of gene expression during B cell maturation. J Immunol 2004; 172:5427 - 40; PMID: 15100284
- Calame K. Activation-dependent induction of Blimp-1. Curr Opin Immunol 2008; 20:259 - 64; http://dx.doi.org/10.1016/j.coi.2008.04.010; PMID: 18554885
- De Abrew KN, Kaminski NE, Thomas RS. An integrated genomic analysis of aryl hydrocarbon receptor-mediated inhibition of B-cell differentiation. Toxicol Sci 2010; 118:454 - 69; http://dx.doi.org/10.1093/toxsci/kfq265; PMID: 20819909
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
