Abstract
Lactobacilli are able to induce upregulation of co-stimulatory molecules in DCs with Th1 cytokines production and increase in Treg activity. This could explain the observed effectiveness of the prolonged administration of lactobacilli in the prevention of allergic disorders in infants and envisage the possible use of bacteria expressing the allergen for the specific immunotherapy of allergic diseases. Hence, we evaluated Streptococcus thermophilus (ST) expressing rBet v 1 as allergen delivery tool and adjuvant factor for immunotherapy in Betv1-sensitized mice. rBet v 1 gene was introduced and expressed in ST (ST[rBet v 1]). BALB/c mice were sensitized with rBet v 1 and then treated with either ST alone, ST[rBet v 1], or the combination of ST and rBet v 1, for 20 days. After 2 aerosol challenges, Treg frequency, in vitro allergen-induced cytokines, rBet v 1-specific IgE and IgG2a, and bronchial histology were made in harvested spleen, sera, and lung. Results were compared with those obtained from not-treated/sensitized mice. ST[rBet v 1] induced immunological and histological changes typical of successful SIT: increased frequency of Tregs and expression of Foxp3; decreased allergen-specific IgE/IgG2a ratio; decrease of in vitro rBet v 1-induced IL-4 from spleen cells; increased allergen-induced IL-10 and IFN-γ; drop of bronchial eosinophilia. ST and ST+rBet v 1 combination, even though induced a slight increase in the frequency of Tregs and moderate allergen-induced IL-10, were ineffective in reducing bronchial eosinophilia, allergen induced IL-4 and rBet v 1-specific IgE/IgG2a ratio. ST[rBet v 1] has tolerogenic and Th-1 skewing properties and efficiently delivers the allergen to the gut immune-system restraining and readdressing the established specific Th2 response toward the allergen in mice.
Introduction
Scientific efforts to ameliorate the effectiveness of allergen specific immunotherapy (SIT) are focused on use of hypoallergenic antigens,Citation1,Citation2 new adjuvants,Citation3 or vehicles for an optimal antigen presentation to the immune system.Citation4 Notably, few studies reported the development of SIT strategies based on the construction of live recombinant bacteria expressing the allergen, to be used as mucosal vaccines.Citation5-Citation7 Mucosal allergy vaccines based on live bacterial cells, in fact, have several advantages, such as a non-invasive route of administration, the use of defined recombinant allergens instead of extract-derived vaccines, an easier production, and the effective delivery to mucosal sites by microbial carriers with intrinsic immunomodulatory properties.Citation5-Citation7
In this context, the gram+ lactic acid bacteria (LABs) have been demonstrated to act as adjuvant factors in allergen-SIT, since they have been reported to prevent or suppress the harmful Th2 responseCitation5-Citation8 and to potentiate allergoid-SIT in mouse models.Citation9 Moreover, human studies gave promising outcomes in allergy prevention.Citation10 According to the above mentioned considerations, in our study, we developed a novel SIT strategy based on a food-grade bacterium, which (1) possesses the ability to induce a Th1 skewing of the immune response, (2) produces intracellularly the allergen, and (3) can release the allergen at intestinal level. The airborne allergen Bet v 1, the major pollen antigen from white birch (Betula verrucosa), was used as allergy model.
The LAB Streptococcus thermophilus stimulates IL-12, IFN-γ, and TGF-β expression by PBMC,Citation11,Citation12 induces the suppressor of cytokine signaling 3 (SOCS3) in macrophagesCitation13 and suppresses Th17 cells.Citation14 Moreover, oligodeoxynucleotide sequences of ST could augment the Treg response via TLR-9Citation15 and their cell wall components stimulate Th1 cells and induce IL-10 production via a TRL-2 dependent mechanism.Citation16
In earlier unpublished experiments (Supplementary data on the Online Repository), we selected, among 14 probiotic strains, the Streptococcus thermophilus DSM 20617T (ST), the principal thermophilic dairy starter for the production of yogurt and cheese, as the most effective in inducing Th1 responses in vitro (Table S1). ST induced a strong release of IL-2, TNF-α (Fig. S1), and IL-10, IL-12 by huDCs, and the upregulation of CD40 and B7.2 by BMDCs (Fig. S2). Interestingly, ST is strongly autolytic for the presence of a lysogenic phage encoding the peptidoglycan (PGN) lyase enzyme in the cell wall (Fig. S3). The autolytic phenotype of strain DSM 20617 can be of particular benefit in the context of SIT, because bacterial cells can get to autolysis once the intestine is reached, releasing intracellular molecules and enzymes, including the recombinant allergen that has been synthetized by the same strain. Therefore, ST can be considered a suitable expression system in which stably expressing foreign genesCitation17 to be used for antigen delivery to the gut-associated immune system (GALT).
Hence, we generated a ST strain stably expressing Bet v 1. The final purpose of the study was to verify (1) the effectiveness of this ST strain as delivery system of the native allergen and (2) its adjuvant properties in improving the efficacy of specific immunotherapy of Bet v 1-sensitized BALB/c mice.
Results
Expression and characterization of recombinant Bet v 1
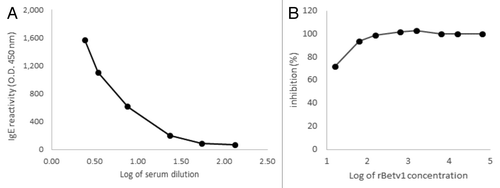
Effective transformation and translation of vector carrying the Bet v 1 gene () was confirmed by detection of a protein band of the expected molecular size of 15kDa by polyclonal mouse antibody anti-Betv1 (). The recognition of rBet v 1 by specific human IgE antibodies raised against natural Betv1 was confirmed by titration ELISA with serial dilution of the sera pool () and ELISA inhibition with increasing rBet v 1 concentration (). As expected by specific Ab-Ag binding, the reactivity of human IgE with rBet v 1 was proportional to the dilution factor (); accordingly, the pre-incubation of the undiluted sera pool with 100 ng of purified rBet v 1 lead to 100% inhibition of the binding ().
Figure 1. Production and characterization of recombinant Bet v 1 by Streptococcus thermophilus DSM 20617. (A) Map of vector pGh-Betv1; eryR, erythromycin resistance gene from Enterococcus faecalis; repA, plasmid replication protein; PT5, phage T5 promoter. (B) Western blot analysis of total cells extracts (30 µg of protein content) from rBet v 1-producing E. coli (lane 1) and Streptococcus thermophilus DSM 20617 (lane 2), and from wild type DSM 20617 (lane 3) detecting the 15 kDa rBet v 1 protein band.
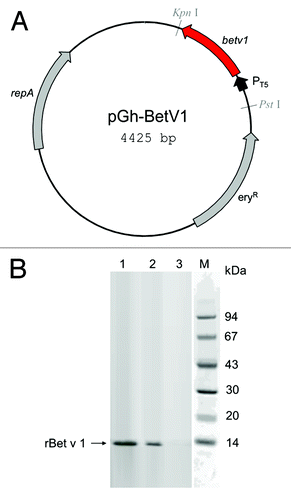
Figure 2. Immunoreactivity of recombinant Bet v 1 (rBet v 1) expressed in Streptococcus thermophilus DSM 20617. (A) Recognition of rBet v 1 expressed in Streptococcus thermophilus with pooled sera from 5 birch pollen-allergic patients (ELISA assay). IgE reactivity was tested as a function of different dilutions of sera. (B) Different concentrations of rBet v 1 were pre-incubated with sera in order to assess inhibition activity.
Allergen-specific serum IgE and IgG2a
As expected, the sensitization treatment induced a significant increase of the rBet v 1-specific IgE levels, compared with the naïve group (P < 0.001) (), which did not change significantly following the therapeutic administration of either ST or ST+rBet v 1. On the contrary, the ST[rBet v 1] treatment was associated with significantly lower levels of specific IgE, compared with the other sensitized and sensitized/treated groups (P < 0.001) (). Moreover, similar levels of antigen-specific IgG2a were found in the 3 groups of mice submitted to therapy, but only the ST[rBet v 1] group showed a significant reduction of the IgE/IgG2a ratio, compared with the sensitized/not treated group (P < 0.001) ().
Figure 3. Serum Bet v 1-specific IgE levels and IgE/IgG2a ratios. (A) Significantly higher rBet v 1-specific IgE level are found in sensitized/not treated or treated mice compared with naïve (P < 0.001). Among treated, ST[rBet v 1] group is associated with significantly lower levels of allergen-specific IgE (P < 0.001 vs ST and ST+rBet v 1). (B) Only the group treated with ST[rBet v 1] showed a significant reduction of the IgE/IgG2a ratio, compared with the sensitized/not treated group (P < 0.001). Data are the mean ± standard deviation. ANOVA: § = P < 0.001 naïve vs all other groups; **P < 0.01; ***P < 0.001. naïve: not sensitized, not treated mice; sensitized: sensitized, not treated mice; ST: wild type Streptococcus thermophilus-treated mice; ST[rBet v 1]: mice treated with recombinant Streptococcus thermophilus expressing rBet v 1; ST+rBet v 1: mice treated with recombinant wild type Streptococcus thermophilus and rBet v 1.
![Figure 3. Serum Bet v 1-specific IgE levels and IgE/IgG2a ratios. (A) Significantly higher rBet v 1-specific IgE level are found in sensitized/not treated or treated mice compared with naïve (P < 0.001). Among treated, ST[rBet v 1] group is associated with significantly lower levels of allergen-specific IgE (P < 0.001 vs ST and ST+rBet v 1). (B) Only the group treated with ST[rBet v 1] showed a significant reduction of the IgE/IgG2a ratio, compared with the sensitized/not treated group (P < 0.001). Data are the mean ± standard deviation. ANOVA: § = P < 0.001 naïve vs all other groups; **P < 0.01; ***P < 0.001. naïve: not sensitized, not treated mice; sensitized: sensitized, not treated mice; ST: wild type Streptococcus thermophilus-treated mice; ST[rBet v 1]: mice treated with recombinant Streptococcus thermophilus expressing rBet v 1; ST+rBet v 1: mice treated with recombinant wild type Streptococcus thermophilus and rBet v 1.](/cms/asset/1ef084df-61f1-4035-8ea3-276c17daabd5/khvi_a_10928155_f0003.gif)
In vitro allergen-induced cytokines
The levels of spontaneous cytokine release by unstimulated spleen cells were of similar value among the various groups with no statistically significant differences. Cytokine levels of the naïve group were: IL-4 = 15.32 ± 4.29 pg·mL−1; IL-10 = 261.23 ± 27.58 pg·mL−1; IFN-γ = 473.38 ± 71.05 pg·mL−1, without changes upon in vitro allergen-stimulation. Spleen cells from the sensitized/not treated group and the groups receiving ST and ST+rBet v 1 produced similar levels of rBet v 1-driven IL-4, significantly higher than the naïve group (P ˂ 0.001); on the contrary, the spleen cells from the group treated with the recombinant ST[rBet v 1] released significantly lower levels of IL-4, compared with the other 2 treated groups (P ˂ 0.001, vs both), although significantly higher respect to the naïve group (P ˂ 0.001) ().
Figure 4. In vitro cytokine release by spleen cells after after 48 h stimulation with rBet v 1. (A) Higher levels of IL-4 were found in sensitized (both treated and not treated) groups respect to naïve mice. ST[rBet v 1] released lower IL-4, compared with ST and ST+rBet v 1 (P < 0.001 vs both). (B) IL-10 increased in all the 3 therapy groups respect to sensitized (P < 0.05). Among treated groups, the highest levels were found in the ST[rBet v 1] group (P < 0.001). (C) ST[rBet v 1] and ST+rBet v 1 groups produced similar amount of INF-γ, significantly higher than sensitized (P < 0.001), naïve and ST groups. Data are mean ± standard deviation. Statistical significance (ANOVA): § = P < 0.001 naïve vs all other groups; *P < 0.05 ST vs sensitized; ***P < 0.001 vs all other groups, except for IFN-γ, where (#) ST+Bet v 1 has values similar to ST[rBetv1]. naïve: not sensitized, not treated mice; sensitized: sensitized, not treated mice; ST: wild type Streptococcus thermophilus-treated mice; ST[rBet v 1]: mice treated with recombinant Streptococcus thermophilus expressing rBet v 1; ST+rBet v 1: mice treated with recombinant wild type Streptococcus thermophilus and rBet v 1.
![Figure 4. In vitro cytokine release by spleen cells after after 48 h stimulation with rBet v 1. (A) Higher levels of IL-4 were found in sensitized (both treated and not treated) groups respect to naïve mice. ST[rBet v 1] released lower IL-4, compared with ST and ST+rBet v 1 (P < 0.001 vs both). (B) IL-10 increased in all the 3 therapy groups respect to sensitized (P < 0.05). Among treated groups, the highest levels were found in the ST[rBet v 1] group (P < 0.001). (C) ST[rBet v 1] and ST+rBet v 1 groups produced similar amount of INF-γ, significantly higher than sensitized (P < 0.001), naïve and ST groups. Data are mean ± standard deviation. Statistical significance (ANOVA): § = P < 0.001 naïve vs all other groups; *P < 0.05 ST vs sensitized; ***P < 0.001 vs all other groups, except for IFN-γ, where (#) ST+Bet v 1 has values similar to ST[rBetv1]. naïve: not sensitized, not treated mice; sensitized: sensitized, not treated mice; ST: wild type Streptococcus thermophilus-treated mice; ST[rBet v 1]: mice treated with recombinant Streptococcus thermophilus expressing rBet v 1; ST+rBet v 1: mice treated with recombinant wild type Streptococcus thermophilus and rBet v 1.](/cms/asset/b2396e92-b503-451f-8953-746608842fb6/khvi_a_10928155_f0004.gif)
The in vitro stimulation of spleen cells with rBet v 1 was associated with an increment of IL-10 release in all 3 groups of mice undergone to therapy, compared with the sensitized (and naïve) group. ST[rBet v 1] was the treatment inducing the highest amount of this cytokine (P < 0.001 vs all groups) ().
rBet v 1 stimulation of spleen cells was associated with similar level of INF-γ in the ST[rBet v 1] and ST+rBet v 1, higher in both cases than the sensitized, naïve, and ST groups (P < 0. 001) (). Finally, the 3 treated groups also produced similar high levels of IL-12 (data not shown).
T regulatory cells
The frequency of the CD4+ T cells population did not significantly differ among the different groups (data not shown). Whereas, the proportion of CD25+Foxp3+ cells within the CD4+ T cell subset (T reg cells) was significantly higher for the ST[rBet v 1] group, in comparison to all other groups (P < 0.001) (). As an example, typical dot plots from a single mouse of each experimental group are shown in .
Figure 5. (A) Statistical analysis results from FACS data showing the Frequency of CD25+Foxp3+ (T reg cells) within the CD4+ subpopulation in each experimental group (mean ± standard deviation). ANOVA: ***P < 0.001(ANOVA) vs. all other groups; (B) Original FACS dot plots are shown for one representative mouse in each group. naïve: not sensitized, not treated mice; sensitized: sensitized, not treated mice; ST: wild type Streptococcus thermophilus-treated mice; ST[rBet v 1]: mice treated with recombinant Streptococcus thermophilus expressing rBet v 1; ST+rBet v 1: mice treated with recombinant wild type Streptococcus thermophilus and rBet v 1.
![Figure 5. (A) Statistical analysis results from FACS data showing the Frequency of CD25+Foxp3+ (T reg cells) within the CD4+ subpopulation in each experimental group (mean ± standard deviation). ANOVA: ***P < 0.001(ANOVA) vs. all other groups; (B) Original FACS dot plots are shown for one representative mouse in each group. naïve: not sensitized, not treated mice; sensitized: sensitized, not treated mice; ST: wild type Streptococcus thermophilus-treated mice; ST[rBet v 1]: mice treated with recombinant Streptococcus thermophilus expressing rBet v 1; ST+rBet v 1: mice treated with recombinant wild type Streptococcus thermophilus and rBet v 1.](/cms/asset/7eff6ae8-808d-4710-babe-d6449091450c/khvi_a_10928155_f0005.gif)
Lung histology
Sensitized mice showed a clear peribronchial and perivascular eosinophilic infiltration of the lungs (). The group of mice who received the ST treatment exhibited mild eosinophilic inflammation surrounding the blood vessels and bronchi; the group treated with the co-administration ST+rBet v 1 presented moderate/severe degree of perivascular and peribronchial eosinophilia. The group that had been treated with ST[rBet v 1] showed restricted distribution and limited number of eosinophils (). All data are summarized in .
Figure 6. Representative hematoxylin and eosin stained lung sections of WBP challenged mice only sensitized toward Bet v 1 protein (A) or sensitized and then treated with ST[rBet v 1]. (B) Mature eosinophils are visible as small cells with donut-shaped blue nucleus of compact chromatin surrounding the pink cytoplasm (original magnification 40×). WBP: White Birch Pollen extract.
![Figure 6. Representative hematoxylin and eosin stained lung sections of WBP challenged mice only sensitized toward Bet v 1 protein (A) or sensitized and then treated with ST[rBet v 1]. (B) Mature eosinophils are visible as small cells with donut-shaped blue nucleus of compact chromatin surrounding the pink cytoplasm (original magnification 40×). WBP: White Birch Pollen extract.](/cms/asset/ed3f50ea-1e48-49c8-86e5-fb95ac221fef/khvi_a_10928155_f0006.gif)
Table 1. Eosinophilic infiltration in lung tissue after the second respiratory challenge
Discussion
The study shows that orally administered Streptococcus thermophilus expressing rBet v 1 induces a significant reduction of the Th2 allergic inflammation in sensitized mice with a shift to Th1 and Treg immune responses. These immune modulations appear to be more intense compared with ST plus Bet v 1 and ST alone. This might be due to the combination of its immunomodulatory properties with an effective allergen delivery to the GALT.
Immunomodulatory potential of probiotics, including lactobacilli, are well known as they are able to induce the production of Th1 cytokines in vitro,Citation11 with reduction of Th2-responses by means of the components of the cell wall.Citation16 They also favor DCs maturation with production of IL-12 and IL-10.Citation18 It has been shown that various strains are also able to stimulate the differentiation of T regulatory cellsCitation19 increasing the production of their regulatory cytokines IL-10 and TGF-β in murine models of allergy.Citation20,Citation21 The immunomodulatory activities of lactobacilli justifies their use as adjuvant for allergen-SIT. Furthermore, gut microbiota has a major role in favoring the natural development of tolerance toward innocuous antigens through the induction of regulatory immune responses.Citation22 Hence, the microbiota represents a possible target for treatment of allergic diseases since it provides the stimuli for tolerance acquisition:Citation10 probiotics might be suitable for this purpose.Citation16 However, the clinical application of lactobacilli in allergic diseases is controversial. In fact, some clinical trials showed that lactobacilli prevent the development of allergic diseases in early childhood,Citation23,Citation24 whereas they report short-term or no effect.Citation23-Citation25 In adults affected by atopic dermatitis and allergic rhinitis, lactobacilli induced modulation of the immune responsesCitation26-Citation29 and, in one study, also reduction of nasal symptoms.Citation28 However, there were no observations of effects on allergic asthma.Citation29 Notably, a recent study evidenced a clear Th2-Th1 shift and Treg induction in allergic patients treated with lactobacilli without improvement in allergic symptoms.Citation30 One possible explanation for the lack of clinical remission might be that lactobacilli evoke immune cellular responses that are allergen-independent and, therefore, not allergen-specific. This may hamper the clinical efficacy of the treatment. On the contrary, in our study treatment with ST[rBet v 1] produces specific systemic and local effects with reduction of allergen-induced IL-4 and increment of allergen-induced IFN-γ produced by spleen cells. The treatment also causes a reduction of allergen-dependent bronchial eosinophilia that characterizes the inflammatory response of allergic asthma in mice.Citation31 In comparison with ST and ST+rBet v 1, the treatment ST[rBet v 1] elicited the strongest allergen-induced IL-10 response. Actually, even ST alone induced a certain degree of regulatory activity and IFN-γ production, but only the recombinant ST[rBet v 1] was able to significantly increase the allergen-induced cytokines compared with sensitized/not treated mice.
In the protocol applied in the present study a bias could be represented by the shorter sensitization/challenge protocol used for sensitized/not treated mice compared with sensitized/treated groups. Usually, such type of protocol leads to an acute experimental disease and for this reason it might not represent an appropriate control group. However, in our study, the sensitized group shows an allergic response (rBet v 1-specific IgE, allergen-driven IL-4, and lung eosinophilia) similar to that observed in ST and ST+rBet v 1 treated groups receiving the prolonged protocol. Therefore, the possible bias could be considered of low importance. In any way, the most important result is that the recombinant ST[rBet v 1] shows significantly greater effects on the immune status compared with the other 2 treated groups of mice, the latter and recombinant all treated according to the same sensitization/challenge schedule.
The partial failure of the treatment with the combination of ST and rBet v 1 compared with ST[rBet v 1] can be also explained by the fact that, in the stomach and duodenum, the rBet v 1 protein can be degraded by acid and enzymatic milieu, thus losing its immunogenic potential. Actually, it has been proposed that such occurrence might explain the failure of orally administered allergen-SIT observed in clinical settings.Citation32 As to demonstrating the importance of preserving immunogenicity of the allergen for the success of the oral allergen-SIT, we previously showed that chemically modified allergens (carbamylated ovalbumin, Par j 1, and ragweed allergoids), resistant to acid and enzymatic degradation,Citation33 were effective in restoring allergen-specific tolerance and in inducing an immunoglobulin isotype switch in sensitized BALB/c mice.Citation34 Similarly, we hypnotize that our autolytic recombinant probiotic ST[rBet v 1] protects the allergen from gastric and pancreatic digestion. It releases an immunologically active allergen directly in the intestine where it stimulates resident immune cells, thus determining the observed Th2-Th1 deviation of cytokines pattern, and differentiation and proliferation of specific regulatory T cells. Therefore, recombinant ST expressing rBet v 1 acts as a vehicle, a dispenser, and an adjuvant for the allergen. The latter, in turn, provides specificity to the evoked effector and regulatory immune responses that are essential for achieving therapeutic effect.
The results of the present study are promising but should be considered preliminary. They need to be completed with a better characterization of the effects of ST[rBet v 1] on lung local immunity (cell and cytokine analysis in bronchoalveolar lavage and cytokine expression in tissue) and a demonstration of the improvement of allergic symptoms as measured by airway hyper-reactivity. Such characterizations will be the object of a further study.
In conclusion, data of the present study reinforce the concept that lactobacilli expressing allergens may be candidates for the treatment of allergic disorders in humans. Probiotic-based SIT would probably ensure higher rates of adherence to therapy because of lower production costs and ease of therapy administration.
Materials and Methods
Production of rBet v 1 in Escherichia coli used for sensitization and in vitro tests
Bet v 1-encoding cDNA flanked by a sequence coding for 6 histidines was cloned in a prokaryotic vector (pEt 3c, Stratagene) and expressed in Escherichia coli cells (BL21[DE3]pLysS, Stratagene) according to manufacturer’s protocols. Clones obtained from single bacterial colonies were sequenced according to Sanger to verify the correct cDNA sequence.
The cells were harvested by centrifugation, resuspended in a 50 mM NaH2PO4, 300 mM NaCl buffer, pH 8, and lysed by sonication. The recombinant proteins were separated by centrifugation. The pellet containing an insoluble protein aggregate was resuspended in denaturing buffer (100 mM NaH2PO4, 10 mM TRIS-HCl, 8 M urea, pH 8) and stirred for 60 min. The solubilized recombinant proteins were separated from insoluble debris by centrifugation and purified by affinity chromatography under denaturing conditions using nickel-nitrilotriacetic acid (Ni-NTA) agarose columns (Qiagen SpA). The purified proteins were refolded by dialysis for 16 h at 4 °C in 0.68% NaCl, 0.275% NaHCO3 solution, and stored at –20 °C. Determination of protein concentration of purified rBet v 1 was performed according to BradfordCitation35 using the commercial BioRad Protein Assay Dye Reagent (Bio Rad).
Endotoxin removal was obtained by processing the purified proteins in the Amicon Ultra-15 100K centrifugal filter device (Merck Millipore Ltd, Co. Cork, IRL). The content of lipopolysaccharide in the ultrafiltrated rBet v 1 preparations was determined by the Limulus amebocyte lysate test according to the instructions of the manufacturer.
After purification, rBet v1 was electrophoresed on a denaturing 4–12% polyacrilamide precast Nupage Bis-Tris gel according to manufacturer’s instructions (Novex, Life Technologies), migrating as a single band and displaying more than 98% purity.
Recombinant Bet v 1 was recognized in ELISA assays by IgE antibodies from allergic patients’ sera and by monoclonal and polyclonal antibody raised against natural Bet v 1 as described in the following paragraphs.
The rBet v 1 protein produced in E. coli was used as sensitizing antigen, recall antigen (to evaluate the secondary responses of spleen cells in vitro), and as competing antigen (in ELISA) and for airways challenge in vivo.
Construction of recombinant vector and electrotransformation of ST
A DNA fragment containing the Betv1 gene was amplified by PCR and cloned in a pUC18 vector under the constitutively active phage T5 promoter (PT5).Citation36 The PT5-Betv1 fragment was then ligated between the PstI and KpnI restriction sites of the vector pG+host (pGh-Betv1) and introduced by electroporation in Streptococcus salivarius subsp. thermophilus DSM 20617T (ST), according to Buckley et al.Citation37 All reagents were from Fermentas.
Streptococcus thermophilus growth and preparation for therapy
The ST DSM 20617 strain was chosen on the basis of its capacity to induce Th1 responses in vitro (1), induce upregulation of CD40 and B7.2 by BMDCs and release of IL-2, IL-10 and IL-12, and TNF-α by huDCs (2), and its autolytic behavior (see Supplemental Materials for details). ST was grown in M17 medium (Difco) supplemented with 2% lactose (LM17). In order to maintain plasmid-containing ST strains, LM17 medium was supplemented with 4 μg·mL−1 erythromycin.
Quantification of Betv1 by two-site sandwich ELISA
Quantification of Bet v 1 was performed using Bet v 1 ELISA kit according to manufacturer’s instructions (INDOOR Biotechnologies Ltd). In the same assay, the whole cell lysate of the rBet v 1-expressing ST strain and rBet v 1 mixed with whole cell lysate from wild type ST strain were measured.
Authentication of rBet v 1 protein
Cell extracts (30 μg) of recombinant or control strain were analyzed in western blotting according to Towbin et al.Citation38 using a rabbit polyclonal serum raised against rBet v 1 protein (10 μg × mL−1) followed by peroxidase-conjugated goat anti-rabbit IgG (1:2000) (Sigma-Aldrich).
The immunoreactivity of rBet v 1 produced by ST to human specific IgE was analyzed by ELISA and inhibition experiments using a pool of sera from birch pollen-allergic patients and goat anti-human IgE (1:4000; Biospacific) as detection antibody.
For inhibition experiments, the birch pollen specific serum pool was diluted 1:3 in saturating buffer and preincubated for 4 h at room temperature with serial dilutions of rBet v 1 produced by E. coli.
The inhibition percentage was calculated as follows: 100 × [(A–B)/A], wherein A is the adsorbance at 450 nm in the absence of inhibitor while B is the adsorbance in the presence of inhibitor.
Preparation and administration of recombinant and wild type ST cells
Wild-type and recombinant ST, grown in LM17 broth for 18 h a 42 °C, were collected, resuspended in 200 µL of 10% Skim Milk (Difco), freeze-dried, and stored at 5 °C. Just before administration, one aliquot was dissolved in 150 µl sterile pyrogen-free water and gavaged to a mouse.
Sensitization and treatment protocols
Thirty pathogen-free BALB/c mice (Charles River), aged 8 wk, were cared in accordance with the European Convention No. 123. Experiments were approved by the local Ethical Committee (Prot. 18/2011/CEISA/COM). Groups of 6 mice were treated as summarized in . Briefly, 6 mice remained naïve (not sensitized/not treated) and the others were immunized by 3 subcutaneous (s.c.) injections, 14 d apart, of 1 μg purified rBet v 1 pre-adsorbed onto 2 mg Al(OH)3 in 50 μL saline (sensitized). One group was sensitized and not treated. The other 3 sensitized groups were administered by oral gavage (after 7 days from the last s.c., 5 consecutive days a week for 4 weeks) alternatively with: 2 × 109 cfu ST; 2 × 109 cfu ST expressing 2 µg rBet v 1 (ST[rBet v 1]); or 2 × 109 cfu ST in association with 2 µg rBet v 1 (ST+rBet v 1). The day after the last treatment (for the sensitized/not treated group the day after the last sensitizing injection), all sensitized/treated groups of mice were challenged twice, one day apart, with aerosolized WBP extract (4 mL at 10 mg⋅mL−1). The day after, blood, lung, and spleens were rapidly harvested for analysis.
Figure 7. Experimental design. The study involved 5 groups of 6 mice, in particular (1) the naïve group that did not receive any treatment, (2) the sensitized group that was immunized with purified rBet v 1, and (3) 3 groups of “Sensitized/treated” that after the sensitization received one of 3 alternative treatments by oral gavage. In particular, ST group received 2 × 109 cfu of Streptococcus thermophilus the ST[rBet v 1] that received 2 × 109 cfu Streptococcus thermophilus expressing 2 µg rBet v 1, and the ST+rBet v 1 that received 2 × 109 cfu ST in association with 2 µg rBet v 1. Symbols indicate the treatment modality: vertical line, s.c. injection; triangle, aerosol; asterisk, intragastric gavage; cross, sacrifice; times sign, no treatment.
![Figure 7. Experimental design. The study involved 5 groups of 6 mice, in particular (1) the naïve group that did not receive any treatment, (2) the sensitized group that was immunized with purified rBet v 1, and (3) 3 groups of “Sensitized/treated” that after the sensitization received one of 3 alternative treatments by oral gavage. In particular, ST group received 2 × 109 cfu of Streptococcus thermophilus the ST[rBet v 1] that received 2 × 109 cfu Streptococcus thermophilus expressing 2 µg rBet v 1, and the ST+rBet v 1 that received 2 × 109 cfu ST in association with 2 µg rBet v 1. Symbols indicate the treatment modality: vertical line, s.c. injection; triangle, aerosol; asterisk, intragastric gavage; cross, sacrifice; times sign, no treatment.](/cms/asset/c56a12ed-aa79-4ca2-a8f2-1fc51fed45db/khvi_a_10928155_f0007.gif)
Allergen-specific serum immunoglobulins
Mouse serum was obtained as described elsewhere.Citation9 Allergen-specific IgE and IgG2a levels in mouse sera were determined by ELISA. In brief, microtiter plates were coated with rBet v 1 (0.1 μg × mL−1). Serum samples diluted 1/500 for IgG2a and 1/100 for IgE antibodies were applied in 100 μL volumes. After washing, anti-mouse IgG2a and IgE antibodies (1 μg⋅mL−1, Pierce antibodies) were applied overnight at 4 °C. Detection of bound antibodies was performed with peroxidase-conjugated mouse anti-rat IgG antibodies (1/1000, Betyl Laboratories). TMB (Sigma-Aldrich) was used for color development and the absorbance was measured at 405 nm with a microplate reader. The antibody levels were expressed in optical density units. Experiments were performed in duplicate.
Cell cultures and cytokine assays
Single cell suspensions of spleen were prepared by squeezing through 70-µm strainers (BD Labware) and, after erythrocytes osmotic lysis with Hybri-Max™ (Sigma-Aldrich), 5 × 106 cells were incubated in 48-well plates in 500 µL complete medium. They were either not stimulated or stimulated with 10 µg⋅mL−1 rBet v 1 endotoxin-free purified protein for 48 h. Levels of IL-4, IL-10, IL-12, and IFN-γ were determined using the multiplex assay Searchlight (Thermoscientific). Experiments were performed in triplicate.
Evaluation of T regulatory cells frequency
Regulatory T cells in single cell suspensions of spleens, prepared as described above, were stained for flow cytometry analysis using the Mouse Treg detection kit (CD4/CD25/FoxP3) (Miltenyi Biotec), according to manufacturer instruction, as previously described.Citation34 A FACSCanto II flow cytometer with FACSDiva Software 6.0 (BD Biosciences) was used for data acquisition and analysis. Single-stained and FMO samples were used as compensation and gating controls. The following gating hierarchy was applied: autofluorescent cell debris were excluded by setting a first gate in a FL-2 vs. FL-3 (mock channel) dot plot; second, a forward light scatter/side light scatter (FSC/SSC) gate was applied on lympho-monocytes; third, a gate on CD4+ T cells was set and the percentage of CD25+Foxp3+ T cells within the CD4+ population was determined. For each sample, twenty-thousand events were acquired within the lymphocyte gate. Experiments were performed in duplicate.
Lung histology
Lungs were dissected, fixed in formalin, embedded in paraffin, cut, and stained with hematoxylin and eosin, according to standard methods.Citation39
Statistical analysis
GraphPad Prism Software 6.0 (GraphPad) was used for statistical analysis. Single pairs of groups (stimulated vs unstimulated cytokine levels) were compared by the Student t test. Comparison of more than 2 groups (serum Ig, in vitro baseline-corrected cytokine levels, T reg frequency) were performed by ANOVA with Bonferroni post hoc test. Values for all measurements are expressed as mean ± SD. Probability values of P ˂ 0.05 were considered statistically significant.
| Abbreviations: | ||
| SIT | = | specific immunotherapy |
| LAB | = | lactobacillus |
| ST | = | streptococcus |
| GALT | = | gut-associated lymphoid tissue |
Additional material
Download Zip (347.3 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by Fondazione Cariplo (grant 2010–0678) (Milan, Italy), Lofarma S.p.A. (Milan, Italy), and Fondazione Università “G. d’Annunzio” (Chieti, Italy).
References
- Di Gioacchino M, Cavallucci E, Ballone E, Cervone M, Di Rocco P, Piunti E, Filardo GS, Turi MC, Mangifesta R, Quecchia C, et al. Dose-dependent clinical and immunological efficacy of sublingual immunotherapy with mite monomeric allergoid. Int J Immunopathol Pharmacol 2012; 25:671 - 9; PMID: 23058017
- Passalacqua G, Albano M, Fregonese L, Riccio A, Pronzato C, Mela GS, Canonica GW. Randomised controlled trial of local allergoid immunotherapy on allergic inflammation in mite-induced rhinoconjunctivitis. Lancet 1998; 351:629 - 32; http://dx.doi.org/10.1016/S0140-6736(97)07055-4; PMID: 9500318
- Krishna MT, Huissoon AP. Clinical immunology review series: an approach to desensitization. Clin Exp Immunol 2011; 163:131 - 46; http://dx.doi.org/10.1111/j.1365-2249.2010.04296.x; PMID: 21175592
- Kendall M, Mitchell TJ, Costigan G, Armitage M, Lenzo JC, Thomas JA, von Garnier C, Zosky GR, Turner DJ, Stumbles PA, et al. Downregulation of IgE antibody and allergic responses in the lung by epidermal biolistic microparticle delivery. J Allergy Clin Immunol 2006; 117:275 - 82; http://dx.doi.org/10.1016/j.jaci.2005.09.049; PMID: 16461127
- Daniel C, Repa A, Wild C, Pollak A, Pot B, Breiteneder H, Wiedermann U, Mercenier A. Modulation of allergic immune responses by mucosal application of recombinant lactic acid bacteria producing the major birch pollen allergen Bet v 1. Allergy 2006; 61:812 - 9; http://dx.doi.org/10.1111/j.1398-9995.2006.01071.x; PMID: 16792578
- Schabussova I, Wiedermann U. Lactic acid bacteria as novel adjuvant systems for prevention and treatment of atopic diseases. Curr Opin Allergy Clin Immunol 2008; 8:557 - 64; http://dx.doi.org/10.1097/ACI.0b013e328317b88b; PMID: 18978472
- Rigaux P, Daniel C, Hisbergues M, Muraille E, Hols P, Pot B, Pestel J, Jacquet A. Immunomodulatory properties of Lactobacillus plantarum and its use as a recombinant vaccine against mite allergy. Allergy 2009; 64:406 - 14; http://dx.doi.org/10.1111/j.1398-9995.2008.01825.x; PMID: 19120072
- Nonaka Y, Izumo T, Izumi F, Maekawa T, Shibata H, Nakano A, Kishi A, Akatani K, Kiso Y. Antiallergic effects of Lactobacillus pentosus strain S-PT84 mediated by modulation of Th1/Th2 immunobalance and induction of IL-10 production. Int Arch Allergy Immunol 2008; 145:249 - 57; http://dx.doi.org/10.1159/000109294; PMID: 17914277
- Petrarca C, Lazzarin F, Lanuti P, Marchisio M, Miscia S, Rossi C, Braga M, Mistrello G, Di Gioacchino M. Lactobacillus paracasei Lp6 favors immune modulation induced by allergoid treatment in ragweed sensitized mice. Int J Immunopathol Pharmacol 2011; 24:881 - 93; PMID: 22230395
- Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy 2007; 62:1223 - 36; http://dx.doi.org/10.1111/j.1398-9995.2007.01462.x; PMID: 17711557
- Kekkonen RA, Kajasto E, Miettinen M, Veckman V, Korpela R, Julkunen I. Probiotic Leuconostoc mesenteroides ssp. cremoris and Streptococcus thermophilus induce IL-12 and IFN-gamma production. World J Gastroenterol 2008; 14:1192 - 203; http://dx.doi.org/10.3748/wjg.14.1192; PMID: 18300344
- Donkor ON, Ravikumar M, Proudfoot O, Day SL, Apostolopoulos V, Paukovics G, Vasiljevic T, Nutt SL, Gill H. Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin Exp Immunol 2012; 167:282 - 95; http://dx.doi.org/10.1111/j.1365-2249.2011.04496.x; PMID: 22236005
- Latvala S, Miettinen M, Kekkonen RA, Korpela R, Julkunen I. Lactobacillus rhamnosus GG and Streptococcus thermophilus induce suppressor of cytokine signalling 3 (SOCS3) gene expression directly and indirectly via interleukin-10 in human primary macrophages. Clin Exp Immunol 2011; 165:94 - 103; http://dx.doi.org/10.1111/j.1365-2249.2011.04408.x; PMID: 21545585
- Ogita T, Nakashima M, Morita H, Saito Y, Suzuki T, Tanabe S. Streptococcus thermophilus ST28 ameliorates colitis in mice partially by suppression of inflammatory Th17 cells. J Biomed Biotechnol 2011; 2011:378417; http://dx.doi.org/10.1155/2011/378417; PMID: 22013382
- Shimosato T, Tohno M, Sato T, Nishimura J, Kawai Y, Saito T, Kitazawa H. Identification of a potent immunostimulatory oligodeoxynucleotide from Streptococcus thermophilus lacZ. Anim Sci J 2009; 80:597 - 604; http://dx.doi.org/10.1111/j.1740-0929.2009.00680.x; PMID: 20163626
- Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 2012; 10:66 - 78; PMID: 22101918
- Mollet B, Knol J, Poolman B, Marciset O, Delley M. Directed genomic integration, gene replacement, and integrative gene expression in Streptococcus thermophilus. J Bacteriol 1993; 175:4315 - 24; PMID: 8331064
- Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol 2002; 168:171 - 8; PMID: 11751960
- Lyons A, O’Mahony D, O’Brien F, MacSharry J, Sheil B, Ceddia M, Russell WM, Forsythe P, Bienenstock J, Kiely B, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy 2010; 40:811 - 9; PMID: 20067483
- Niers LE, Timmerman HM, Rijkers GT, van Bleek GM, van Uden NO, Knol EF, Kapsenberg ML, Kimpen JL, Hoekstra MO. Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type 2 cytokines. Clin Exp Allergy 2005; 35:1481 - 9; http://dx.doi.org/10.1111/j.1365-2222.2005.02375.x; PMID: 16297146
- von der Weid T, Bulliard C, Schiffrin EJ. Induction by a lactic acid bacterium of a population of CD4(+) T cells with low proliferative capacity that produce transforming growth factor beta and interleukin-10. Clin Diagn Lab Immunol 2001; 8:695 - 701; PMID: 11427413
- Veenbergen S, Samsom JN. Maintenance of small intestinal and colonic tolerance by IL-10-producing regulatory T cell subsets. Curr Opin Immunol 2012; 24:269 - 76; http://dx.doi.org/10.1016/j.coi.2012.03.004; PMID: 22503960
- West CE, Hammarström ML, Hernell O. Probiotics in primary prevention of allergic disease--follow-up at 8-9 years of age. Allergy 2013; 68:1015 - 20; http://dx.doi.org/10.1111/all.12191; PMID: 23895631
- Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 2003; 361:1869 - 71; http://dx.doi.org/10.1016/S0140-6736(03)13490-3; PMID: 12788576
- Gore C, Custovic A, Tannock GW, Munro K, Kerry G, Johnson K, Peterson C, Morris J, Chaloner C, Murray CS, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy 2012; 42:112 - 22; http://dx.doi.org/10.1111/j.1365-2222.2011.03885.x; PMID: 22092692
- Ivory K, Chambers SJ, Pin C, Prieto E, Arqués JL, Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin Exp Allergy 2008; 38:1282 - 9; http://dx.doi.org/10.1111/j.1365-2222.2008.03025.x; PMID: 18510694
- Drago L, Toscano M, De Vecchi E, Piconi S, Iemoli E. Changing of fecal flora and clinical effect of L. salivarius LS01 in adults with atopic dermatitis. J Clin Gastroenterol 2012; 46:Suppl S56 - 63; http://dx.doi.org/10.1097/MCG.0b013e318265ef38; PMID: 22955359
- Wassenberg J, Nutten S, Audran R, Barbier N, Aubert V, Moulin J, Mercenier A, Spertini F. Effect of Lactobacillus paracasei ST11 on a nasal provocation test with grass pollen in allergic rhinitis. Clin Exp Allergy 2011; 41:565 - 73; http://dx.doi.org/10.1111/j.1365-2222.2011.03695.x; PMID: 21395878
- Helin T, Haahtela S, Haahtela T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC 53103), on birch-pollen allergy: a placebo-controlled double-blind study. Allergy 2002; 57:243 - 6; http://dx.doi.org/10.1034/j.1398-9995.2002.1s3299.x; PMID: 11906339
- Perrin Y, Nutten S, Audran R, Berger B, Bibiloni R, Wassenberg J, Barbier N, Aubert V, Moulin J, Singh A, et al. Comparison of two oral probiotic preparations in a randomized crossover trial highlights a potentially beneficial effect of Lactobacillus paracasei NCC2461 in patients with allergic rhinitis. Clin Transl Allergy 2014; 4:1; http://dx.doi.org/10.1186/2045-7022-4-1; PMID: 24393277
- Jacobsen EA, Lesuer WE, Willetts L, Zellner KR, Mazzolini K, Antonios N, Beck B, Protheroe C, Ochkur SI, Colbert D, et al. Eosinophil activities modulate the immune/inflammatory character of allergic respiratory responses in mice. Allergy 2013; 69:315 - 27; http://dx.doi.org/10.1111/all.12321; PMID: 24266710
- Igea JM, Cuevas M, Lázaro M, Quirce S, Cuesta J. Susceptibility of a grass-pollen oral immunotherapy extract to the saliva and gastric fluid digestive process. Allergol Immunopathol (Madr) 1994; 22:55 - 9; PMID: 8059676
- Bagnasco M, Passalacqua G, Villa G, Augeri C, Flamigni G, Borini E, Falagiani P, Mistrello G, Canonica GW, Mariani G. Pharmacokinetics of an allergen and a monomeric allergoid for oromucosal immunotherapy in allergic volunteers. Clin Exp Allergy 2001; 31:54 - 60; http://dx.doi.org/10.1046/j.1365-2222.2001.00999.x; PMID: 11167951
- Petrarca C, Lazzarin F, Pannellini T, Iezzi M, Braga M, Mistrello G, Falagiani P, Di Giampaolo L, Di Gioacchino M. Monomeric allergoid intragastric administration induces local and systemic tolerogenic response involving IL-10-producing CD4(+)CD25(+) T regulatory cells in mice. Int J Immunopathol Pharmacol 2010; 23:1021 - 31; PMID: 21244752
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248 - 54; http://dx.doi.org/10.1016/0003-2697(76)90527-3; PMID: 942051
- Guglielmetti S, Ciranna A, Mora D, Parini C, Karp M. Construction, characterization and exemplificative application of bioluminescent Bifidobacterium longum biovar longum. Int J Food Microbiol 2008; 124:285 - 90; http://dx.doi.org/10.1016/j.ijfoodmicro.2008.03.033; PMID: 18471916
- Buckley ND, Vadeboncoeur C, LeBlanc DJ, Lee LN, Frenette M. An effective strategy, applicable to Streptococcus salivarius and related bacteria, to enhance or confer electroporation competence. Appl Environ Microbiol 1999; 65:3800 - 4; PMID: 10473378
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 1979; 76:4350 - 4; http://dx.doi.org/10.1073/pnas.76.9.4350; PMID: 388439
- Kiernan JA. Histological and Histochemical Methods: Theory and Practice. 4th ed. Bloxham, UK: Scion, 2008.
