Abstract
The relationship between obesity and vaccine efficacy is a serious issue. Previous studies have shown that vaccine efficacy is lower in the obese than in the non-obese. Here, we examined the influence of obesity on the efficacy of influenza vaccination using high fat diet (HFD) and regular fat diet (RFD) mice that were immunized with 2 types of influenza virus vaccines—cell culture-based vaccines and egg-based vaccines. HFD mice showed lower levels of neutralizing antibody titers as compared with RFD mice. Moreover, HFD mice showed high levels of MCP-1 in serum and adipocytes, and low level of influenza virus-specific effector memory CD8+ T cells. After challenge with influenza virus, the lungs of HFD mice showed more severe inflammatory responses as compared with the lungs of RFD mice, even after vaccination. Taken together, our data suggested that the inflammatory condition in obesity may contribute to the suppressed efficacy of influenza vaccination.
Introduction
Influenza is caused by influenza viruses and results in a severe and epidemic respiratory illness with high mortality rates, accounting globally for 500 000 deaths per year.Citation1,Citation2 To protect against infection, inactivated or attenuated influenza virus vaccines are produced and are widely considered the most effective preventative strategy. Currently, influenza vaccines are manufactured by 2 methodsCitation3; one method is the egg-based vaccine (EBV), which has some disadvantages such as the long culture period and the contamination of egg proteins that can cause allergic reactions in egg-allergic recipients.Citation4,Citation5 Another method is the cell culture-based vaccine (CBV), which is produced more quickly than the EBV without allergic elements.Citation6 Due to its safety and stability, CBV production is currently in the spotlight.Citation7
Obesity is a metabolic disorder characterized by the accumulation of excessive body fat and low-grade chronic inflammation.Citation8 Obesity has become a major health concern in many countries.Citation9-Citation13 It is well known that obesity is associated with many physiological abnormalities and diseases such as heart disease, type 2 diabetes, certain types of cancer, and osteoarthritis.Citation13 Recent studies indicated that obese individuals are more susceptible to infectious diseases, including influenza, as compared with non-obese individuals,Citation12 which is referred to as the “burden of obesity on infectious disease”. Interestingly, it was found that obese people had increased morbidity and mortality during the pandemic of the A/California/04/2009 (H1N1) virus and that obese patients had higher mortality rates than non-obese patients who were admitted to the hospital.Citation14,Citation15 Furthermore, it has been reported that obesity is associated with an impaired immune response and the poor efficacy of the influenza vaccine.Citation16-Citation18 However, it is not clear why these impaired immune responses against an immunological pathogen occur in an obese state.
In this study, we investigated neutralizing antibody production against the influenza virus in high fat diet-induced obese mice immunized by inactivated vaccines. We observed that the production of neutralizing antibody and effector memory T cells are decreased, whereas virus induced-inflammation is increased in obese mice as compared with normal mice.
Results
Obese mice have an impaired humoral immune response to influenza vaccine
Vaccines induce the antiviral responses of T cells and the production of neutralizing antibodies against the influenza virus. To investigate vaccine efficacy in obese mice, we created obese mice with HFD for 13 wk. Body weight and fat mass were increased by 1.6-fold and 9.6-fold, respectively, in HFD mice as compared with RFD mice. We analyzed several glycemic markers in both mice and we observed that the glucose, HDL, LDL, total cholesterol, and triglyceride levels were higher in the sera of HFD mice compared with the sera of RFD mice (). Whereas free fatty acids level is similar in both mouse. To determine the vaccine-induced humoral immune response in obese mice, we compared the influenza virus-specific antibodies in RFD and HFD mice. Mice were immunized 2 times with CBV or EBV (HA antigen of 1.5 ug/mouse) (). Neutralizing antibody titers (NTs) were checked 3 wk after the first vaccination and every 3 or 4 wk after the second vaccination by the plaque reduction neutralizing test (PRNT). As shown in , RFD mice showed higher NTs as compared with HFD mice (1.30 vs. 1.00, respectively). At 3 wk after the second vaccination (in ), the HFD mice showed similar neutralizing antibody titers (1.96~2.13) as compared with the RFD mice (2.05~2.13). However, the HFD mice consistently showed decreased neutralizing antibody titers (1.30~1.60) as compared with the RFD mice (1.93) at 17 wk after the second vaccination (in ). In addition, we also measured the hemagglutination inhibition (HI) titers. However, although the HI titers of HFD mice appeared to be lower compared with those of RFD mice, there was no significant difference (data not shown). Indeed, although the HI and NT titers exhibit similar trends, they are not exactly the same.Citation19 Therefore, we only used the PRNT to compare the difference in the influenza-specific neutralizing antibody titers of HFD and RFD mice. Overall, based on , these results suggest that the humoral immune response is impaired in vaccinated obese mice.
Figure 1. High fat diet (HFD)-induced obese mice on body weight, body fat mass, and glycemic markers. (A) Body weight was determined for 13 wk with RFD and HFD (mean ± SD; n = 3). *P < 0.05. (B) Glycemic markers (glucose, LDL-cholesterol, HDL-cholesterol, free fatty acid [NEFA], total cholesterol, and triglyceride) in serum and body fat mass were determined at 13 wk in RFD and HFD mice (mean ± SD; n = 3). *P < 0.05. **P < 0.01.
![Figure 1. High fat diet (HFD)-induced obese mice on body weight, body fat mass, and glycemic markers. (A) Body weight was determined for 13 wk with RFD and HFD (mean ± SD; n = 3). *P < 0.05. (B) Glycemic markers (glucose, LDL-cholesterol, HDL-cholesterol, free fatty acid [NEFA], total cholesterol, and triglyceride) in serum and body fat mass were determined at 13 wk in RFD and HFD mice (mean ± SD; n = 3). *P < 0.05. **P < 0.01.](/cms/asset/2b61ebdd-ec5a-4a1e-ae81-6a4dc72cdabd/khvi_a_10928332_f0001.gif)
Table 1. Specific immune response induced by cell culture- or egg-based vaccine
Chronic inflammation in obese mice is associated with the defective generation of effector memory CD8+ T cells
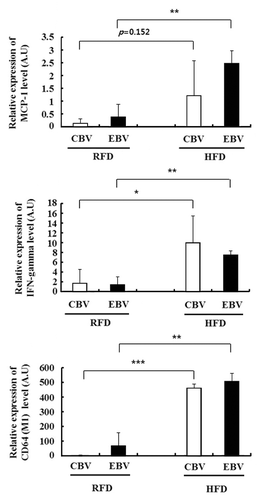
We next examined the association of inflammation and the memory response to influenza vaccination in obese mice. At 17 wk after the second vaccination (in ), monocyte chemoattractant protein-1 (MCP-1) was expressed at higher levels in the serum and fat tissue of HFD mice than in those of RFD mice (). In addition, the percentage of influenza-specific effector memory CD8+ T cells (CD62L-CCR7-) were significantly decreased in the influenza virus-stimulated SVF of HFD mice as compared with that of RFD mice (48.4% vs. 87.4% in CBV and 53.8% vs. 81.5% in EBV, respectively) (). To assess the relationship between inflammation status and pathology in obese mice, we challenged the mice with the H1N1 virus at 3 wk after the second vaccination. At 3 d after the challenge, we measured the mRNA expression levels of cytokines in the lung tissues of HFD mice and RFD mice. Inflammatory markers such as MCP-1, IFN-γ, and CD64 (a M1 macrophage marker) were expressed at significantly higher levels in the lung tissue of influenza-infected HFD mice as compared with that of influenza-infected RFD mice, despite 2 vaccinations ().
Figure 2. Chronic inflammation in obesity results in defective effector memory CD8+ T cells. (A and B) The protein and mRNA expression levels of cytokine (MCP-1) was measured from serum (A) and epididymal fat (B) of regular fat diet (RFD) and high fat diet (HFD) mice at 17 wk (in ) after their second immunization with cell culture-based vaccine (CBV) and egg-based vaccine (EBV) (mean ± SD; n = 3). (C) Influenza-specific effector memory CD8+ T cells (CD62-CCR7-) were detected by flow cytometry. Stromal vascular fraction (SVF) was isolated from the epididymal fat of RFD and HFD mice at 17 wk (in ) after the second immunization. Fat cells from SVF were infected with A/California/04/2009 (H1N1) virus ex vivo (mean ± SD; n = 3).
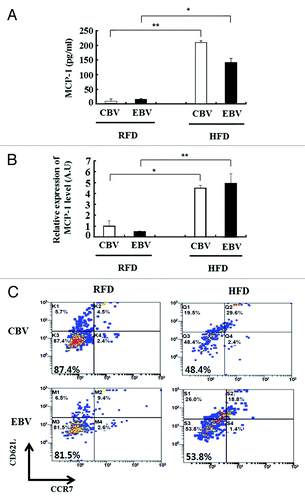
Figure 3. Chronic inflammation in high fat diet (HFD) mice showed higher inflammatory cytokines in the lung than those in regular fat diet (RFD) mice after challenge with influenza A/California/04/2009 (H1N1) virus, even with 2 vaccinations. The mRNA expression levels of cytokines (MCP-1 and IFN-r) and M1 macrophage marker (CD64) were measured from the lung tissues of mice. The data were normalized with respect to the 18S mRNA values (mean ± SD; n = 3). *P < 0.05.
Discussion
Although vaccination is believed to be the best strategy against influenza virus infection, it is insufficient for protecting some people who are elderly or obese. Recently, obesity has been recognized as a risk factor for increased morbidity and mortality among H1N1 virus-infected patients.Citation3 In this study, we attempted to analyze the influence of obesity on the induction of neutralizing antibody by vaccines and the factors that are responsible for the decreased efficacy of vaccination in the obese.
In general, prophylactic vaccines induce virus-specific memory T cell immune responses and functional neutralizing antibody production, leading to the prevention of virus entry and replication during viral infection. To examine the titers of neutralizing antibody under obese conditions, we established high fat diet-induced obese mice, which are physiologically relevant to human obesity.Citation17 The HFD mice exhibited increases in their body weight as well as their blood glucose and cholesterol levels (), which indicated that these changes reflected metabolic disorder in the mice. These obese mice immunized with 2 types of inactivated trivalent vaccine widely used to protect humans against pandemic influenza virus. Obese mice showed reduced neutralizing antibody over time as compared with normal mice (). These lower antibody levels may affect virus-induced clearance and pathogenesis when obese mice are challenged with influenza virus. Recent reports demonstrated that obese human and mouse model showed impaired T cell immune responses to influenza vaccination.Citation1,Citation16,Citation17 Consistent with these findings, we also observed that virus-specific CD8+ effector memory T cells were significantly decreased in obese mouse (). Interestingly, showed that obese (HFD) and normal (RFD) mice have similar neutralizing antibody titers at 3 wk after the second immunization (1st in ). This means that an early boosting effect may be functional in both mice. However, the neutralizing antibodies in obese mice were more quickly reduced as compared with the normal mice (second to fifth in ). This indicated that an impaired memory T cell response may have influenced the maintenance of neutralizing antibodies in obese mouse that received the booster vaccination. These results reflected the immune-suppressive characteristics of obesity. The data presented here are similar to that of previous studies, which reported that hepatitis B or tetanus vaccines induced poor antibody responses in obese patients.Citation20,Citation21 However, the decreased number of memory T cells did not affect the B cells, including their proportion, viability, or capacity for antibody production in HFD mice. Further research is required to determine the exact mechanisms responsible for the reduced capacity for neutralizing antibody maintenance in obese mice.
We also observed that vaccinated obese mice showed increases in a proinflammatory cytokine (IFN-γ), chemokine (MCP-1), and M1 macrophage marker (CD64) in their lung tissue as compared with normal mice after challenge with H1N1 virus (). MCP-1 and IFN-γ induce the recruitment of various immune cells into the lung, especially CD64-expressing M1 macrophages, and activate innate and adaptive immune responses.Citation22,Citation23 Consequently, viral infection amplifies the production of proinflammatory cytokines, which in turn exacerbate viral pathogenicity. Recently, it was suggested that the chronic inflammation in obesity may be associated with the induction of leptin resistance leading to the inhibited proliferation of effector memory T cells.Citation24 These results suggest that the systemic inflammatory state in obese individuals may contribute to their impaired immune responses to influenza vaccination. However, we cannot exclude the possibility that obesity-induced chronic inflammation may lead to tolerance of the function of immune cells, especially T cells. Thus, more intensive immunological studies are required to explain the reduced vaccine efficacy in the obese.
In summary, we showed that obese mice immunized and challenged with influenza virus demonstrated an impaired generation of neutralizing antibody and memory T cells through increased inflammation. These findings suggest that vaccination against influenza virus may not be sufficient to protect obese individuals from infection. Therefore, we need to develop more efficient strategies of influenza vaccination for the increasing population of obese individuals.
Materials and Methods
Virus
Influenza A/California/04/2009 (H1N1) virus originated from swine influenza H1N1 viruses was used for the challenge. It was obtained from the Korea Center for Disease Control. The virus was grown in the allantoic cavities of 10-d-old embryonated chicken eggs for 72 h at 35 °C. Plaque titration of influenza A virus stocks was performed on Madin–Darby canine kidney cells (MDCK) cells in the presence of 0.25% trypsin at 37 °C. The yield of the virus (1 × 107 plaque-forming units [pfu]/mouse) for animal experiments was determined by calculating the 50% lethal dose (LD50) per ml of viral stock .
Animal experiments
Four-week-old male C57BL/6J mice (Orient Bio Inc.) were fed for the entire length of the experiment with either the regular fat diet (5% fat diet, RFD) or the adjusted calories diet (60% high fat diet, HFD) (DooYeol Biotech). All animals were housed under specific-pathogen-free conditions and handled according to protocols for animal care approved by the Catholic University of Korea. The protocol was approved by the International Animal Care and Use Committee, Sungsim Campus, Catholic University of Korea. At 13 wk after HFD and RFD, the first vaccination was performed with 1.5 μg of 2 types of influenza vaccines (A/H1N1). The cell culture-based vaccine (CBV) and the egg-based vaccine (EBV) were kindly provided by SK Chemical and Korea Green Cross, respectively. To investigate the boosting effect, the second vaccination was performed 3 wk after the first vaccination. The serum was collected by retro-orbital venous plexus puncture at the proper times. The mice were challenged with 1 × 107 pfu/50 ul of H1N1 virus by intranasal injection at 3 wk after the second immunization. Their organs were harvested at 3 d after the challenge.
Plaque reduction neutralization tests (PRNTs)
The virus–antibody mixture was prepared by mixing 100 pfu of influenza virus with the serially diluted serum samples, and then incubating the mixture at 37 °C for 1 h. The mixtures were then inoculated onto MDCK cell monolayers for 1 h at 37 °C in 5% CO2. After virus adsorption, the cells were covered with the overlay medium (serum-free DMEM supplemented with 0.25% trypsin and 2% agar). The plates were incubated at 37 °C in 5% CO2 for 3 d. The neutralizing-antibody titer (NT) was expressed as the reciprocal of the highest dilution that reduced the number of plaques to 50% or less of the control value and was calculated as previously described.Citation25
Quantification of lung mRNA cytokine levels
Total RNA was extracted from the lungs and then RT–PCR was performed as previously described.Citation16 Real-time PCR was performed on a MyiQ-single iCycler (Bio-Rad) using the SYBR Green PCR Master Mix (Takara Bio Inc.). The expression of a given gene was quantified with the comparative threshold cycle (ΔCT) method and normalized to 18S rRNA. The primer set (Cosmo Genetech) for MCP-1 was: sense, 5′-CAGCAAGATG ATCCCAATGA GTAG-3′ and antisense, 5′-CTCCTTGAGT TGGTGACAAA AAC-3′; the primer set for IFNγ mRNA was: sense, 5′-AGCGGCTGAC TGAACTCAGA TTGTAG-3′ and antisense, 5′-GTCACAGTTT TCAGCTGTAT AGGG-3′.
Isolation of the stromal vascular fraction (SVF) of adipose tissue and flow cytometry
SVF from normal and obese mice were obtained from mouse epididymal fat pads as previously described.Citation26 SVF were stimulated by 10 hemagglutination units of H1N1 virus for 120 h. Stimulated cells were stained with fluorochrome-conjugated antibodies (BD Biosciences) for CD8, CD62L, and CCR7, and analyzed using a FACsan flow cytometer (Beckman Coulter).
Statistical analysis
All data are expressed as means ± SD. The data were analyzed using the Student’s t-test. Differences of P < 0.05 were regarded as significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by a grant from the Korean Healthcare Technology R&D project of the Ministry of Health and Welfare (A103001), and by a grant from Gyunggi Regional Research Center (GRRC) of the Catholic University of Korea.
References
- Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Holland LA, Weir S, Noah TL, Beck MA. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2012; 36:1072 - 7; http://dx.doi.org/10.1038/ijo.2011.208; PMID: 22024641
- Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, Matyas BT, California Pandemic (H1N1) Working Group. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011; 52:301 - 12; http://dx.doi.org/10.1093/cid/ciq152; PMID: 21208911
- Kemble G, Greenberg H. Novel generations of influenza vaccines. Vaccine 2003; 21:1789 - 95; http://dx.doi.org/10.1016/S0264-410X(03)00074-4; PMID: 12686096
- Davies RJ, Wells ID. Influenza virus vaccine and egg allergy. Proc R Soc Med 1975; 68:218; PMID: 1239018
- Wright PF, Webster RG. Orthomyxoviruses. In: Field virology. 4th edition. Philadelphia: Lippincott Williams & Wilkins 2001; 1533-79.
- Lee IS, Kim JI, Park MS. Cell culture-based influenza vaccines as alternatives to egg-based vaccines. Journal of Bacteriology and Virology 2013; 43:9 - 17; http://dx.doi.org/10.4167/jbv.2013.43.1.9
- Jang YH, Seong BL. Toward a universal influenza vaccine: from the perspective of protective efficacy. Clin Exp Vaccine Res 2013; 2:71 - 3; http://dx.doi.org/10.7774/cevr.2013.2.2.71; PMID: 23858395
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444:881 - 7; http://dx.doi.org/10.1038/nature05488; PMID: 17167477
- World Health Organization. 2006. Fact sheet 311: Obesity and overweight, Vol. 2009. Geneva, Switzerland. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- Bellisari A. Evolutionary origins of obesity. Obes Rev 2008; 9:165 - 80; http://dx.doi.org/10.1111/j.1467-789X.2007.00392.x; PMID: 18257754
- Haslam DW, James WP. Obesity. Lancet 2005; 366:1197 - 209; http://dx.doi.org/10.1016/S0140-6736(05)67483-1; PMID: 16198769
- Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 2010; 235:1412 - 24; http://dx.doi.org/10.1258/ebm.2010.010227; PMID: 21127339
- Rubenstein AH. Obesity: a modern epidemic. Trans Am Clin Climatol Assoc 2005; 116:103 - 11, discussion 112-3; PMID: 16555609
- Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis 2011; 53:413 - 21; http://dx.doi.org/10.1093/cid/cir442; PMID: 21844024
- Jain S, Chaves SS. Obesity and influenza. Clin Infect Dis 2011; 53:422 - 4; http://dx.doi.org/10.1093/cid/cir448; PMID: 21844025
- Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol 2010; 184:3127 - 33; http://dx.doi.org/10.4049/jimmunol.0903220; PMID: 20173021
- Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr 2007; 137:1236 - 43; PMID: 17449587
- Kim YH, Kim JK, Kim DJ, Nam JH, Shim SM, Choi YK, Lee CH, Poo H. Diet-induced obesity dramatically reduces the efficacy of a 2009 pandemic H1N1 vaccine in a mouse model. J Infect Dis 2012; 205:244 - 51; http://dx.doi.org/10.1093/infdis/jir731; PMID: 22147801
- Kim JI, Lee I, Park S, Hwang MW, Bae JY, Lee S, Heo J, Park MS, García-Sastre A, Park MS. Genetic requirement for hemagglutinin glycosylation and its implications for influenza A H1N1 virus evolution. J Virol 2013; 87:7539 - 49; http://dx.doi.org/10.1128/JVI.00373-13; PMID: 23637398
- Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA 1985; 254:3187 - 9; http://dx.doi.org/10.1001/jama.1985.03360220053027; PMID: 2933532
- Eliakim A, Schwindt C, Zaldivar F, Casali P, Cooper DM. Reduced tetanus antibody titers in overweight children. Autoimmunity 2006; 39:137 - 41; http://dx.doi.org/10.1080/08916930600597326; PMID: 16698670
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 2004; 25:280 - 8; http://dx.doi.org/10.1016/j.it.2004.03.008; PMID: 15145317
- Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 2007; 96:41 - 101; http://dx.doi.org/10.1016/S0065-2776(07)96002-2; PMID: 17981204
- Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity in mice reduces the maintenance of influenza-specific CD8+ memory T cells. J Nutr 2010; 140:1691 - 7; http://dx.doi.org/10.3945/jn.110.123653; PMID: 20592105
- Abe M, Kuzuhara S, Kino Y. Establishment of an analyzing method for a Japanese encephalitis virus neutralization test in Vero cells. Vaccine 2003; 21:1989 - 94; http://dx.doi.org/10.1016/S0264-410X(02)00772-7; PMID: 12706688
- Varma MJ, Breuls RG, Schouten TE, Jurgens WJ, Bontkes HJ, Schuurhuis GJ, van Ham SM, van Milligen FJ. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev 2007; 16:91 - 104; http://dx.doi.org/10.1089/scd.2006.0026; PMID: 17348807
