Abstract
Cholera toxin B subunit (CTB) was investigated as a classical mucosal adjuvant that can increase vaccine immunogenicity. In this study, we found out the in vitro efficacy of cholera toxin B subunit (CTB) in activating mice bone marrow-derived dendritic cells (BMDCs) through Toll-like receptor signaling pathways. In vitro RNA and transcriptional level profiling arrays revealed that CTB guides high levels of Th1 and Th2 type cytokines, inflammatory cytokines, and chemokines. Based on the robustness of these profiling results, we examined the induction of HIV Env-specific immunity by CTB co-inoculated with HIV Env DNA vaccine intramuscularly in vivo. CTB enhanced HIV-Env specific cellular immune responses in Env-specific IFN-γ ELISPOT, compared with DNA vaccine alone. Moreover, CTB induced high levels of Env specific humoral response and promoted antibody maturation after the third round of vaccination. This combination immunization strategy induced a Th2-type bias response which is indicative of a high ratio of IgG1/IgG2a. This study reports that CTB as a classical mucosal adjuvant could enhance HIV-1 DNA-based vaccine immunogenicity intramuscularly; therefore, these findings suggest that CTB could serve as an effective candidate adjuvant for DNA vaccination.
Introduction
It is known that vaccine adjuvants can increase antigen-induced antibody titers,Citation1 reduce vaccine doses,Citation2,Citation3 increase the differentiation of memory cells,Citation4 accelerate initial immune responses,Citation5 and transform the affinity, magnitude, breadth, and specificity of the specific immune responses.Citation5,Citation6 However, the molecular mechanisms and characteristics of most adjuvants determine the application in different species of vaccines and immunization routes, notably, influenced by the adjuvant effects described above. For instance, CpG-ODN is used in DNA vaccines and stimulates TLR9 pathways,Citation7 aluminum is used in protein vaccines to induces potent humoral immune responses, cholera toxin B subunit (CTB)Citation8,Citation9, or E. coli heat-labile toxin (LT)Citation10 are mostly applied in mucosal immunization strategies.
Cholera toxin B subunit is a powerful mucosal adjuvant for the generation of mucosal antibody responses and specific immunity. The CTB subunit was shown to bind with a high affinity specifically to the cellular receptor GM1-ganglisoside(GM1).Citation11 GM1 is a receptor molecule commonly expressed embedded in the plasma membrane of a wide range of nucleated cells, including epithelial cells, lymphocytes and antigen-presenting cells. CTB exhibits differential consequences in stimulated DCs or conjugation to antigens. It has been shown to significantly enhance the immunogenicity of linked exogenous antigensCitation12 through induction of DC activation,Citation13,Citation14 enhanced B-cell and T-cell responsesCitation15, and decrease the dose of antigen.Citation12
Based on our previous studies, we found that the efficacy of immune responses induced by vaccine antigen depended on the level of inflammation or the strength of innate immunity induced by co-vaccinated adjuvants. CTB is a kind of bacterial toxin protein that could be a powerful immune agonist that stimulates innate immunity and antigen-mediated acquired immune responses.
CTB has been exploited as a DNA vaccine adjuvant, for instance, genetically fused CTB gene to a DNA vaccineCitation16 or co-administered in intradermal with a DNA vaccine.Citation17 However, CTB has rarely been reported as an adjuvant co-inoculated with DNA vaccine intramuscularly. Studies in mice model on the transfection efficiency of injected DNA have confirmed that muscle is hundreds to thousand times more permissive than other tissues for the uptake and expression of plasmid.Citation18-Citation20 However, some mucosal tissue, such as the skin, the respiratory tract, and the gut, that serve as barriers against the invasion of pathogens, have associated lymphoid tissues that provide high levels of local immune surveillance,Citation21 therefore, more opportunities to present pathogens to innate immune tissues and cells. Moreover, obviously, in inducing mucosal immunity as sIgA, the intranasal route of DNA immunization is more efficient than the intramuscular route.Citation22 It’s hard to identify which administration route is better; however, the intramuscular route shows more convenience in actual vaccination process. Hence, we want to test whether CTB also presents adjuvant activation in intramuscular administration route co-formulated with DNA vaccine.
In this study, we investigated whether CTB can be used as an effective intramuscular adjuvant for HIV-1 DNA vaccine according to the ability of CTB in activating bone-marrow derived dendritic cells through Toll-like receptors pathway, which play a critical role in linking innate immune response and adaptive immunity. CTB was capable of activating Toll-like receptor signaling pathways in BMDCs, and consequently promoted dendritic cell maturation and secretion of pro-inflammatory cytokines or chemokines in immune profiling in vitro. Moreover, CTB induced HIV Env specific strong cellular and humoral immune responses after immunization intramuscularly, compared with DNA alone. Notably CTB shifted the immune response toward a Th2-type response and enhanced specific antibody avidity. These results suggest that CTB may be a promising new systemic adjuvant to enhance immune responses to HIV and other DNA vaccines.
Results
Gene expression profiling reveals CTB activated TLR signaling pathway
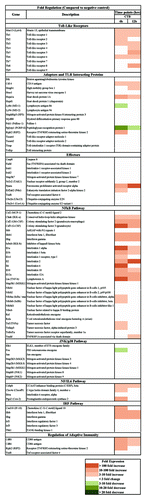
To analyze the activation of the TLR pathway in BMDCs by CTB stimulation, we assessed CTB activated BMDCs through TLRs in vitro. CTB was added to BMDCs at a final concentration of 1 μg/mL. Gene expression profiling studies detected TLR signaling pathway activation within 12 h after stimulation. Of the 84 genes included in the RTCitation2 Profiler Mouse Toll-Like Receptor Signaling array, 50% of the genes were differentially expressed (more than 3-fold change) in the stimulated BMDCs in at least one time point. Gene expression heat maps () show that genes with increased expression (≥3-fold) include Toll-like receptors (especially TLR1, TLR2 TLR3, TLR4, TLR5, TLR6, and TLR8), adaptors, interacting molecules, effectors, and critical molecules involved in the NF-κB, NF/IL6, and IRF signaling pathways. Genes with markedly increased expression (≥10-fold) included Th1-type cytokines (IL-2, IFN-γ, IL-12, TNF-α, TNF-β), Th2-bias cytokines (IL-6, IL-10) and pro-inflammatory molecules (IL-1, GM-CSF, G-CSF). BMDCs stimulation by CTB resulted in stronger, more consistent upregulation in components of the TLR pathway.
Figure 1. Heat map showed the expression of TLR pathway genes after stimulation with 1 μg/mL CTB at 6 and 12 h. Upregulation was defined as a ≥3-fold increase as compared with negative control, and markedly differentially regulated if the difference was ≥10-fold, and downregulation as a ≤3-fold decrease.
CTB induced of multiple chemokines and cytokines in BMDCs
To characterize the soluble factors released during the inflammatory response, we also identified the gene profiling results in protein levels induced by CTB. A multiplex membrane bound ELISA was used to test the supernatant of BMDC cultures with or without adjuvant stimulation. CTB stimulation resulted in increased levels of Th1-type cytokines IL-12, IL-27 IFN-γ, and Th2-associated cytokine IL-5. In addition, several chemokines such as keratinocyte-derived chemokine (KC) and eotaxin, as well as cell-attractive proteins including C5a, monokine-induced by γ-IFN (MIG), 10-kDa IFN-induced protein (IP-10), and MIP2 were upregulated ().
Figure 2. The immune stimulation effect of CTB identified on BMDCs at multiplex levels. (A) Cytokines and chemokines induced 24 h after exposure to CTB. BMDCs were stimulated with either medium or 1μg/mL CTB. Supernatant was harvested 24 h later and analyzed for the presence of cytokines and chemokines according to the manufacturers’ instructions. Expression levels elevated by more than 1.5-fold relative to control were identified as upregulated. Moreover, the time-course and quantitative analysis of cytokines secreted by BMDCs post adjuvants stimulation was evaluated. The concentration levels of IL-6 (B), IL-10 (C), and IL-12 (D) at different time points were evaluated using quantitative ELISA, data from triplicate cultures are shown as mean ± SD *indicates significant difference (P < 0.05) when comparison CTB used with negative or positive control groups.
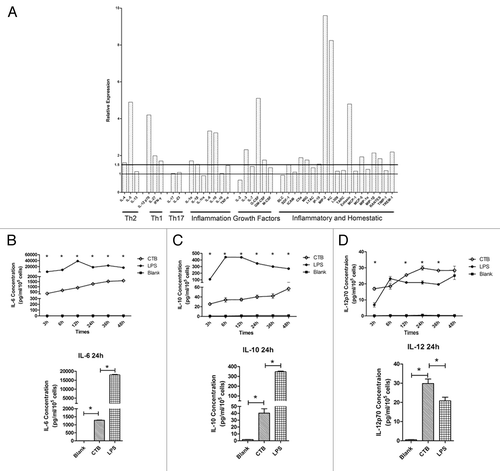
To assess the secretion levels of IL-6, IL-10, and IL-12p70 following stimulation, the supernatants of BMDC cultures were collected at increasing time intervals between 3 and 48 h after stimulation and analyzed by quantitative ELISA. Nearly all cytokines analyzed showed a time-dependent effect. CTB induced not only high level of inflammation cytokine IL-6 but also promoted Th1 cytokine IL-12p70 secretion.
All of these assays demonstrate that CTB presents the capability of immune stimulation, and promotes multiplex biological responses not only in RNA transcription levels but also in protein levels.
CTB enhanced HIV Env-specific cellular and humoral immunological response after intramuscular immunization
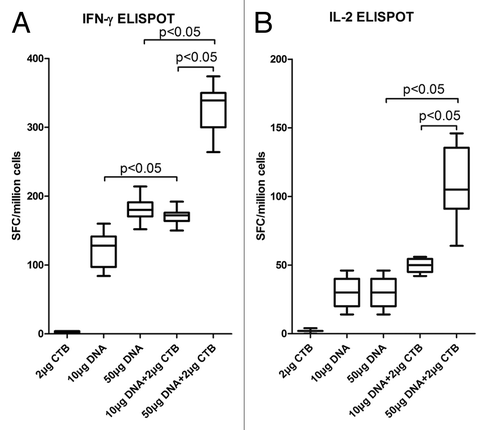
To assess the capacity of CTB to enhance cellular immune responses to pDRVI1.0-gp1455m, mice were immunized 3 times with 10 μg or 50 μg DNA alone or co-formulated with CTB intramuscularly. The results revealed that CTB co-inoculated with DNA vaccine were associated with significant increases in the ELISPOT assay when compared with responses following the same dosage with DNA alone (). Moreover, CTB exhibited more robust immune responses than other adjuvants used in our lab previously in the 50 μg DNA regimen (data not shown).Citation23 To test the feasibility of reducing immunization dosages of the DNA vaccines, we designed high dose (50 μg) and low dose (10 μg) DNA vaccine co-immunization with adjuvants. The data suggested that vaccination with 10 μg DNA co-formulation adjuvant induced similar immune response levels to 50 μg DNA alone.
Figure 3. CTB enhanced HIV-1 DNA vaccine cellular immune responses. The immunization regimen is described in . After the last immunization, IFN-γ (A) and IL-2 (B) production of splenic lymphocyte was determined by ELISPOT assay. Notably, CTB strongly enhances the DNA vaccine immunogenicity (P < 0.05). Low dose DNA vaccine co-immunized with adjuvant induced similar Env-specific cell-mediated immune responses level compared with high dose DNA vaccine alone.
To determine the role of adjuvants in inducing antibodies, an assessment of HIV Env-specific IgG titers was made. Although CTB co-immunized with low dose DNA (10 μg) did not appear to enhance the tendency of antibody titers, the high dose (50 μg) groups exhibited significant differences in comparison with 50 μg DNA alone ().
Figure 4. CTB increased HIV-1 Env specific antibody responses at the high DNA dosage. Following the immunization scheme and procedure, the sera were collected to detect HIV-1 Env-specific IgG responses by ELISA assay (A). Anti HIV-1 Env IgG responses induced by 50 μg DNA vaccine co-immunization with CTB were significantly enhanced compared with induction by DNA vaccine alone. (B) IgG isotype analyzed by detecting IgG1 and IgG2a antibody responses in each sample. (C) Avidity of the anti-Env IgG raised by high dose DNA co-administrated with CTB. Sera were analyzed in an HIV Env-specific NaSCN-displacement ELISA. Assays used each serum sample from each group at a dilution of 1:100.
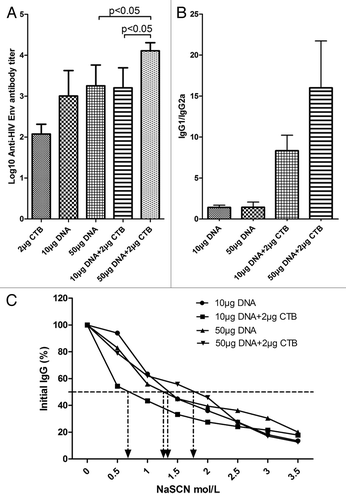
CTB has been associated with a shift in the immune response toward Th2-type responses.Citation8,Citation24 The use of CTB as an adjuvant induced a shift in the Env-specific IgG subtype response toward IgG1, which is associated with Th2-cell responses compared with DNA alone (). The higher dose group (50 μg DNA plus 2 μg CTB) displayed a stronger bias to IgG1.
Moreover, an antibody avidity assay revealed that CTB can promote Env antigen-specific antibody maturation in the 50 μg DNA group, compared with either the same dose DNA alone or lower DNA plus CTB group (). It was also confirmed that CTB advanced the humoral immune responses in the high DNA dosage group as described above.
The immune response data showed that CTB co-formulated with HIV DNA vaccine could enhance gp145 antigen specific cellular responses and high level antibody titer, promote antibody maturation and induce a Th2-skewed immune response.
CTB induced high level expressions of IL-6 and IL-10 in vivo
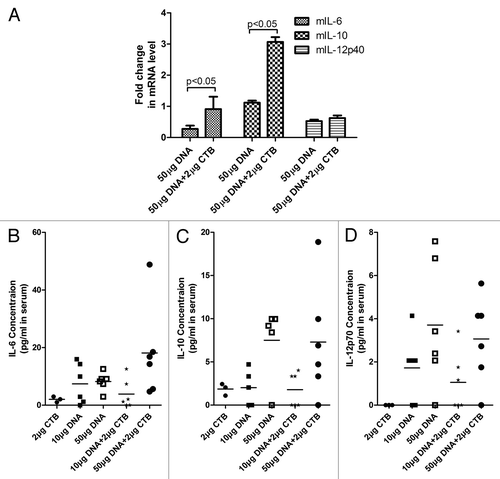
To confirm pro-inflammation cytokines presented in in vitro studies, we assessed the expression level of IL-6, IL-10, and IL-12 both in RNA transcription and in serum administrated in mice of Group 3 and Group 5. The RT-PCR results showed that CTB-inoculated mice still produced high expression levels of IL-6 and IL-10 after their last immunization at 14 d, contrasted to the mice vaccinated with DNA alone (). However, IL-12p40 did not show a significant difference between these groups. Although the concentrations of these cytokines in serum are very limited, we also found a slight raised tendency of IL-6 at CTB co-administrated group (Group 5) ().
Figure 5. CTB increased the expression level of inflammation cytokines in vivo. (A) IL-6, IL-10, and IL-12 expressions were quantified from administrated mice splenic lymphocyte from group 3 and group 5 mice as detailed in Immunization scheme () and expressed as relative units normalized to GAPDH expression. The reverse transcription reaction was incubated at 48 °C for 30 min, subjected to 10 min initial hot-start activation of the polymerase at 95 °C followed by 40 cycles at 95 °C for 15 s, 60 °C for 1 min. (B), (C), and (D) presents IL-6, IL-10, and IL-12 concentrations in mouse serum by using quantitative ELISA, respectively.
Discussion
CTB is a subunit of cholera toxin and is a powerful parenteral and mucosal immunogen; usually low doses of CTB can induce strong mucosal IgA antibody responses to overcome limitations of routine vaccines in inducing mucosal immunity.Citation25 In addition, immunization with antigen in the presence of CTB via parenteral, mucosal, and transcutaneous routes or coupled CTB to antigens results in substantial enhancement of mucosal and serum immunoglobulin responses that targeted to immunogens.
The most previous reports have demonstrated CTB is a successful and effective mucosal adjuvant when co-formulated with various types of vaccines, especially protein vaccines.Citation26-Citation28 However, in the HIV vaccine field, DNA vaccines are widely used and have some potential advantages in HIV preventive vaccination.Citation29,Citation30 Although DNA intranasal inoculation has been used in immunization strategies to promote mucosal and systemic immunity,Citation31-Citation33 but according to routine immunization procedures, most HIV-1 DNA vaccines are administrated intramuscularly. Based on our previous studies (data not shown), in combination with preliminary immune response data in vivo, investigation of one potential adjuvant in DNA vaccines, could evaluate the capacity of stimulating dendritic cell activation in vitro. We hypothesize that the ability to augment innate immunity of potential adjuvants may influence the efficacy of immune stimulation in vivo. Therefore, the focus of this study was to determine whether CTB could activate innate immunity in vitro and then enhance HIV-1 DNA vaccine immunogenicity intramuscularly.
In this study, we determined the immune stimulatory effects of CTB in vitro and in vivo. According to our observations, we conclude that CTB can serve as an effective candidate adjuvant in intramuscular route, based on the characteristics of stimulation TLR pathway activation.
In vitro gene profiling and proteome array, these results show that CTB alone can promote DC activation and significantly induce the expression of cytokines and pro-inflammatory species. In addition, several chemokines upregulated by CTB (such as TREM-1, RANTES, and IP-10), may involve the promotion of effector T-cell functions. Based on the results of Toll-like receptor pathway activation after CTB stimulation, it could not be accurately determined which kind of TLR is involved in CTB-mediated BMDC activation. We assumed that CTB may induce the robustness of immunity not only through GM1-ganglisoside receptor mediated mucosal activation, but also exploited by Toll-like receptors in the systemic immune response.
To identify the immune activation efficacy, immunization regimens in mouse models in vivo that augmentation of Env-specific immune responses following CTB were investigated. Cellular immune responses, targeted by DNA vaccines which were co-administered with CTB were significantly stronger than vaccines alone (). CTB formulated with 10 μg DNA induced cellular immune responses that were not strong enough but still significantly augment and up to the same strength as 50 μg DNA alone () in an IFN-γ ELISPOT assay. However, 10 μg DNA could not increase Env-specific antibody titer in the DNA co-inoculated with the CTB group. It may involve the weak immunogenicity of DNA vaccine; our data also demonstrated the low dose (1–10 μg) DNA vaccine cannot promote Env specific immune response without the assistance of the electroporation technique (data not shown). The IgG isotype assay indicated CTB promoted Th2-biased type response (), same as previously published studies, limited strength but significant enhancement of humoral immunity in CTB co-formulated groups (). Consistent with the tendency of antibody immune responses, CTB promoted antibody maturation when co-administrated with high dose DNA vaccine ().
Since IgG1/IgG2a isotype assay indicated CTB induced Th2-biased immune response, Env antibody titer promoted more than 5-fold change, however, cellular immunological responses also showed that immune system produced T cells secreted high levels of classical Th1 cytokines (IFN-γ and IL-2). In the classical theory of immunology, IFN-γ as typical Th1 cytokine had been confirmed to inhibit Th2-biased immune response, but not suppress or block. Some studies evidenced that, DNA vaccine co-administrated with Th1 cytokines plasmids (IFN-γ, IL-12, and IL-2 et al.) also could induced slight or higher IgG1(Th2-type) antibody response, but not suppress Th2(IgG1) immunity completely.Citation34,Citation35 IgG1/IgG2a ratio used to indicate Th1/Th2 bias and the status of immunity, but in some studies had inconsistency results.Citation36,Citation37 The more direct evidence is to detect Th1 (as IFN-γ, IL-2 et al.) and Th2 (as IL-4 et al.) cytokines concentrations in serum. Furthermore, a report has shown that one species CpG-ODN can directly stimulate mice B cells to switch isotype independence on Th1 or Th2 cytokines,Citation38 moreover, BCR ligation represents a novel pathway for Ag-induced IgG switch, that does not require additional cytokine signals.Citation39 It would be a hypothesis to explain why Th1 cytokines do not influence IgG antibody switching.
In our study, CTB induced robust immune responses, both cellular and humoral immunity. In combination with previous in vitro results, we propose the levels of innate response activation (based on TLR pathway) and inflammation responses induced by CTB might correlate with the strength of antigen-specific immune responses following CTB co-inoculated DNA vaccine. On the other hand, previous observations suggest that some chemokines and pro-inflammatory cytokines have adjuvant properties in the initiation of immune responses. Proteome profiling assays also demonstrate that the expression levels of several chemokines, such as MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), and IP-10 (CXCL10), increased in BMDCs by stimulation with CTB. We assume that the enhancement of antigen specific cellular immune responses in vivo might be due to the benefit of these chemokines’ adjuvant properties.Citation40,Citation41
Additionally, consistent with proteome profiling study, we found high levels of mRNA IL-6 and IL-10 following exposure to CTB both in vitro and in vivo, however, the IL-6, IL-10, and IL-12 concentrations in serum didn’t upregulation as in vitro assay. It could be due to very limited and undetectable concentrations of cytokines in serum, especially several days post last immunization.
In this study, we develop the adjuvant effect of CTB in DNA-formulated vaccine and expend its application to intramuscular vaccination route. So, it’ll make the administration procedure more advantage and convenient when CTB co-formulated DNA vaccine intramuscularly. In addition, due to the instability of the CTB protein, it’s better to reconstitute lyophilized CTB immediate vaccination. The characteristic of CTB adjuvant intramuscular are reflected by making bold hypotheses in vitro following by the meticulous search for evidence in vivo. We found CTB could stimulate and activate BMDC in vitro through several critical molecules and receptors involved TLR pathway, but we still need some sophisticated approaches to identify which TLR receptor might be bound by CTB specifically and explore the mechanisms underlying. In future studies, we’d like to detect Th1 and Th2 cytokines concentrations and IgG isotype concentrations instead of IgG sub-class relative levels in serum, which should be more accuracy to evaluate Th1/Th2 immune response and reflect the status or features of immune systems. Moreover, in adjuvant assessment approach, the breadth and longitude of antigen induced immune responses are also critical elements. We could set up ELISPOT assay to investigate peptide-stimulated cellular response by epitope mapping strategy, and also to monitor the dynamic change of binding antibody titer in long-term follow-up study. Furthermore, as a pivotal target, neutralizing antibody should be detected to assess the potential protective effect in future clinical trials.
This study extends the current knowledge on the adjuvant effects of CTB and further demonstrates the inflammation response induced by CTB benefit the strength and persistence of antigen specific immunological responses.
Materials and Methods
Vaccine and adjuvants
Cholera toxin B subunit was purchased from Sigma-Aldrich (C9903), and LPS contamination was removed using Detoxi-Gel Endotoxin Removing Gel (Pierce), and residual LPS content was determined using Limulus Amebocyte Lysate assay (Associates of Cape Cod). Endotoxin values of re-purified CTB were less than 0.06 EU/mg. Plasmid pGp1455m was constructed by Division of Research on Virology and Immunology (National Center for AIDS/STD Control and Prevention, China CDC), with the encoding envelope gp1455m gene from HIV-1 CN54 strain as the DNA vaccine.
Generation of bone marrow-derived dendritic cells (BMDCs) and activation in vitro
Generation of BMDC from mouse bone marrow was performed as described previously.Citation23,Citation42 Briefly, 5-wk-old male C57BL/6 mice bone marrow was flushed from the tibias and femurs and the DC cells were seeded into 6-well flat-bottom plates in RPMI-1640 complete medium containing 10% fetus bovine serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine, and 1 mM HEEPS, 20 ng/mL murine granulocyte-macrophage colony stimulating factor (GM-CSF, R&D Systems), and 20 ng/mL recombinant murine interleukin-4 (IL-4, R&D Systems). Cells were incubated in a humidified 5% CO2 incubator at 37 °C. After 48 h, non-adherent cells were gently removed and medium containing GM-CSF and IL-4 was replenished. Medium was refreshed every 2 d; on day 6 of culture, non-adherent and loosely adherent cells were collected as BMDCs placed in a 6-well culture plate with a concentration of 1.5 × 106 cells per mL. CTB and LPS were added to cultures at the final concentration of 1 μg/mL and 10 ng/mL, respectively. Buffer alone was used as a negative control. Depending on which assessment was performed, BMDCs were harvested and washed extensively after various time points. All assays were done in triplicate.
Gene expression profiling of TLR signaling pathway in vitro
BMDCs were prepared as described previously.Citation23 BMDCs were seeded in 6-well plates at a density of 1 × 106 cells/well and treated with 1 μg/mL CTB for 6 and 12 h. Medium alone was used as negative control. After exposure, total RNA was extracted from the cells using RTCitation2 qPCR-Grade RNA Isolation Kit (SABioscience, Qiagen, catalog no.PA-001) following the manufacture’s protocol. RNA quality and quantity were assessed using a NanoDrop spectrophotometer by measurement of A260/A280, and 1μg RNA was reverse transcribed to cDNA using RTCitation2 First Strand Kit (SABioscience, Qiagen).
To detect and quantify the expression of genes involved in innate immune response, we used RTCitation2 Profiler™ PCR Array Mouse Toll-Like Receptor Signaling Pathway Kit (SABioscience, Qiagen).
Quantitative real-time RT-PCR was performed using an ABI Prism 7500 series RT-PCR thermocycler (Applied Biosystems). The threshold cycle (CT) was calculated for each gene using the Sequence Detection Software (version 1.2.2, Applied Biosystems). The threshold and baseline were set manually according to the manufacturer’s instructions. According to a previous study,Citation43 CT data was uploaded into the data analysis template on the manufacturer’s website (http://www.sabiosciences.com/pcr/arrayanalysis.php). The relative expression of each gene compared with the expression in control animals was calculated on the website using the ΔΔ CT method with 5 housekeeping genes as controls (GUSB, HPRT1, HSP90AB1, GAPDH, and ACTB).
Screening cytokines in medium supernatant by proteome profiler arrays
Mouse-specific cytokines secreted by BMDCs in the presence or the absence of CTB (1 μg/mL) were determined using a mouse cytokine array panel A kit (40 cytokines) (R&D Systems) according to the manufacturer’s protocols provided. Approximately 3 mg total protein from BMDCs culture supernatant was applied on each array. Spot densities were quantified with Image J software and exported to Microsoft Excel. Spot density images were corrected for background to account for inter-array variance.
Cytokines quantitative ELISA
BMDCs were placed in 24-well plates at a density of 1 × 105 cells/well and incubated at 37 °C in the presence or absence of CTB (1 μg/mL) or LPS (10 ng/mL). Supernatant was harvested at 3 h, 6 h, 12 h, 24 h, 36 h, and 48 h post stimulation, and cytokine concentrations (IL-6, IL-10, and IL-12p70) in supernatants were determined using Quantikine cytokine ELISA kits (R&D Systems) according to the manufacturer’s protocol.
Immunization regimens
Groups of 6-wk-old female BALB/C mice (n = 6) (Vital River Laboratories) were administrated thrice intramuscularly at 3 wk intervals with different doses of pGP1455m DNA vaccine alone or co-formulated with CTB as delineation in .
Table 1. Immunization scheme
Ten days following the last immunizations, mice were euthanized. The spleens and sera were collected for analysis of cell-mediated immune responses by ELISPOT assay, HIV Env-specific antibody titers, IgG isotype and antibody avidity were measured by ELISA. Cytokine concentrations were detected by quantitative ELISA. All procedures for animal use and care were approved by the National Center for AIDS/STD Control and Prevention Institutional Committee on Laboratory Animals.
Determination of T cell response by IFN-γ and IL-2 ELISPOT assays
The ELISPOT assay described by BD ELISPOT Mouse IFN-γ ELISPOT Set and IL-2 ELISPOT Set (BD, San Diego, CA) protocol was modified to detect HIV-1 Env-specific T-cell responses.Citation44 96-well plates were coated at 4 °C overnight with 10 μg/mL of anti-mouse IFN-γ or IL-2 in sterile PBS. The plates were washed and blocked with RPMI 1640 containing 10% FBS at room temperature for 2 h. Mouse splenocytes were isolated and seeded into wells with 100 μL/well (5 × 105 cells/well) in addition to 100 μL Env peptides (Amino acid sequence: C0604200005: CKEVHNVWAT HACVPTDPNP, C060420006: SELYKYKVVE IKPLGIAPTA, C0604200007: QQSNLLRAIE AQQHLLQLTV) and incubated in a humidified 5% CO2 incubator at 37 °C for 24 h. After incubation, the ELISPOT plates were developed according to the kit instructions. Finally, plates were air-dried, and the spot-forming cells (SFC) were quantified by a Bioreader-4000 automated ELISPOT reader (BioSys) and normalized for 1 × 106 splenocytes.
Determination of Env-specific antibody by ELISA assay
96-well flat-bottom plates were coated with purified recombinant gp120 protein (a recombinant protein of HIV-1 CN54 strain expressed in 293T cells and purified to 95% purity) at a concentration of 0.5 μg/mL in coating buffer (0.012 M Na2CO3, 0.038 M NaHCO3, pH 9.6) at 4 °C overnight. Plates were washed 5 times with PBST, and blocked with 3% BSA in PBST at 37 °C for 1 h. Mouse sera were serially diluted 2-fold in block solution (starting at 1:100), and 100 μL was added to each well. After incubation at 37 °C for 2 h, the plates were washed 5 times with PBST and then incubated with HRP-labeled goat anti-mouse IgG (Santa Cruz Biotechnology), IgG1, and IgG2a (Santa Cruz Biotechnology) at 37 °C for 1 h. After the final wash, 100 μL of fresh TMB substrate (Sigma-Aldrich) was added per well, and plates were incubated for 10 min. The reaction was stopped by adding 50 μL of 2 M H2SO4, and the optical density of the plate was measured at 450 nm by Multi scan enzyme-linked immunosorbent assay (ELISA) plate reader (Thermo Life Sciences).
Avidity ELISA was performed similarly to that of serum antibody ELISA assay. Samples were diluted to optimized concentrations (starting at 1:10–1:100) and incubated at 37 °C for 2 h. Different concentrations of sodium thiocyanate (NaSCN) (Sigma-Aldrich)in PBS, were then added (0, 1, 1.5, 2, 2.5, 3, and 3.5 M NaSCN). Plates were incubated at room temperature for 15 min and then washed 6 times with PBST. All assays were done in duplicate.
Real-time RT-PCR
Total RNA was isolated with RNeasy Plus Kit (Qiagen) from mouse splenocytes according to the manufacturer’s instructions. Real-time RT-PCR was performed using an ABI Prism 7500 series RT-PCR thermocycler (Applied Biosystems) and Power SYBR Green RNA-to-CT One-Step Kit (Applied Biosystems) according to the manufacturer’s protocols. The primer pairs used were: GAPDH forward (5′-CTCATGACCA CAGTCCATGC-3′) and GAPDH reverse (5′-CACATTGGGG GTAGGAACAC-3′), mIL-6 forward (5′-CATCCAGTTG CCTTCTTG-3′) and mIL-6 reverse (5′-TCAATAGGCA AATTTCCTG-3′), mIL-10 forward (5′-ATCGATTTCT CCCCTGTG-3′) and mIL-10 reverse (5′-AATGGGAACT GAGGTATCAG-3′), mIL-12p40 forward (5′-CGTGCTCATG GCTGGTGCAA AG-3′) and mIL-12p40 reverse (5′-GATGAAGAAG CTGGTGCTG-3′).The threshold cycle (CT) was calculated for each gene using the Sequence Detection Software (version 1.2.2, Applied Biosystems). Threshold cycle (CT) values and baselines were calculated automatically, the levels of gene expression were normalized to GAPDH by using the 2ΔΔ CT method.Citation45
Statistical analysis
Statistical comparisons were performed using Prism 5.0 Software (GraphPad Inc.). One-way ANOVA analysis was used to compare experimental groups and was followed by un-pairwise multiple comparisons using a Newman-Keuls test. A P value < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Dr Wenjun Zhang, Dr Zheng Liu, Dawei Ling, and Ying Liu for their assistance in animal experiments, and Dr Lena Yao for their critical reading and correcting of the manuscript. This work was supported by National Major projects for Infectious Diseases Control and Prevention (2012ZX10001008); National Natural Science Foundation of China (81020108030); SKLID Development grant (2008SKLID101).
References
- Beran J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert Opin Biol Ther 2008; 8:235 - 47; http://dx.doi.org/10.1517/14712598.8.2.235; PMID: 18194079
- Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, Capecchi PL, di Giovanni P, Sticchi L, Gentile C, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 2009; 4:e4384; http://dx.doi.org/10.1371/journal.pone.0004384; PMID: 19197383
- Schwarz TF, Horacek T, Knuf M, Damman HG, Roman F, Dramé M, Gillard P, Jilg W. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine 2009; 27:6284 - 90; http://dx.doi.org/10.1016/j.vaccine.2009.01.040; PMID: 19856521
- Leroux-Roels I, Roman F, Forgus S, Maes C, De Boever F, Dramé M, Gillard P, van der Most R, Van Mechelen M, Hanon E, et al. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine 2010; 28:849 - 57; http://dx.doi.org/10.1016/j.vaccine.2009.10.017; PMID: 19835828
- Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med 2010; 2:ra5; http://dx.doi.org/10.1126/scitranslmed.3000624; PMID: 20371470
- Malherbe L, Mark L, Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Vaccine adjuvants alter TCR-based selection thresholds. Immunity 2008; 28:698 - 709; http://dx.doi.org/10.1016/j.immuni.2008.03.014; PMID: 18450485
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000; 408:740 - 5; http://dx.doi.org/10.1038/35047123; PMID: 11130078
- Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol 1995; 155:4621 - 9; PMID: 7594461
- Lycke N. The mechanism of cholera toxin adjuvanticity. Res Immunol 1997; 148:504 - 20; http://dx.doi.org/10.1016/S0923-2494(98)80144-2; PMID: 9588829
- Dickinson BL, Clements JD. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun 1995; 63:1617 - 23; PMID: 7729864
- Sack DA, Huda S, Neogi PK, Daniel RR, Spira WM. Microtiter ganglioside enzyme-linked immunosorbent assay for vibrio and Escherichia coli heat-labile enterotoxins and antitoxin. J Clin Microbiol 1980; 11:35 - 40; PMID: 6986402
- George-Chandy A, Eriksson K, Lebens M, Nordström I, Schön E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun 2001; 69:5716 - 25; http://dx.doi.org/10.1128/IAI.69.9.5716-5725.2001; PMID: 11500448
- Isomura I, Yasuda Y, Tsujimura K, Takahashi T, Tochikubo K, Morita A. Recombinant cholera toxin B subunit activates dendritic cells and enhances antitumor immunity. Microbiol Immunol 2005; 49:79 - 87; http://dx.doi.org/10.1111/j.1348-0421.2005.tb03632.x; PMID: 15665457
- Luci C, Hervouet C, Rousseau D, Holmgren J, Czerkinsky C, Anjuère F. Dendritic cell-mediated induction of mucosal cytotoxic responses following intravaginal immunization with the nontoxic B subunit of cholera toxin. J Immunol 2006; 176:2749 - 57; PMID: 16493030
- Eriksson K, Fredriksson M, Nordström I, Holmgren J. Cholera toxin and its B subunit promote dendritic cell vaccination with different influences on Th1 and Th2 development. Infect Immun 2003; 71:1740 - 7; http://dx.doi.org/10.1128/IAI.71.4.1740-1747.2003; PMID: 12654787
- Harakuni T, Sugawa H, Komesu A, Tadano M, Arakawa T. Heteropentameric cholera toxin B subunit chimeric molecules genetically fused to a vaccine antigen induce systemic and mucosal immune responses: a potential new strategy to target recombinant vaccine antigens to mucosal immune systems. Infect Immun 2005; 73:5654 - 65; http://dx.doi.org/10.1128/IAI.73.9.5654-5665.2005; PMID: 16113283
- Sanchez AE, Aquino G, Ostoa-Saloma P, Laclette JP, Rocha-Zavaleta L. Cholera toxin B-subunit gene enhances mucosal immunoglobulin A, Th1-type, and CD8+ cytotoxic responses when coadministered intradermally with a DNA vaccine. Clin Diagn Lab Immunol 2004; 11:711 - 9; PMID: 15242946
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science 1990; 247:1465 - 8; http://dx.doi.org/10.1126/science.1690918; PMID: 1690918
- Wolff JA, Williams P, Acsadi G, Jiao S, Jani A, Chong W. Conditions affecting direct gene transfer into rodent muscle in vivo. Biotechniques 1991; 11:474 - 85; PMID: 1793583
- Acsadi G, Jiao SS, Jani A, Duke D, Williams P, Chong W, Wolff JA. Direct gene transfer and expression into rat heart in vivo. New Biol 1991; 3:71 - 81; PMID: 1903987
- McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 1992; 10:75 - 88; http://dx.doi.org/10.1016/0264-410X(92)90021-B; PMID: 1539467
- Sasaki S, Sumino K, Hamajima K, Fukushima J, Ishii N, Kawamoto S, Mohri H, Kensil CR, Okuda K. Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with QS-21 saponin adjuvant via intramuscular and intranasal routes. J Virol 1998; 72:4931 - 9; PMID: 9573261
- Hou J, Liu Y, Liu Y, Shao Y. The MSHA strain of Pseudomonas aeruginosa activated TLR pathway and enhanced HIV-1 DNA vaccine immunoreactivity. PLoS One 2012; 7:e47724; http://dx.doi.org/10.1371/journal.pone.0047724; PMID: 23077664
- Braun MC, He J, Wu CY, Kelsall BL. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J Exp Med 1999; 189:541 - 52; http://dx.doi.org/10.1084/jem.189.3.541; PMID: 9927516
- Lavelle EC, Grant G, Pusztai A, Pfüller U, O’Hagan DT. Mucosal immunogenicity of plant lectins in mice. Immunology 2000; 99:30 - 7; http://dx.doi.org/10.1046/j.1365-2567.2000.00932.x; PMID: 10651938
- Pinkhasov J, Alvarez ML, Pathangey LB, Tinder TL, Mason HS, Walmsley AM, Gendler SJ, Mukherjee P. Analysis of a cholera toxin B subunit (CTB) and human mucin 1 (MUC1) conjugate protein in a MUC1-tolerant mouse model. Cancer Immunol Immunother 2010; 59:1801 - 11; http://dx.doi.org/10.1007/s00262-010-0906-1; PMID: 20824430
- Tamura S, Hasegawa H, Kurata T. Estimation of the effective doses of nasal-inactivated influenza vaccine in humans from mouse-model experiments. Jpn J Infect Dis 2010; 63:8 - 15; PMID: 20093755
- Price GA, Holmes RK. Evaluation of TcpF-A2-CTB chimera and evidence of additive protective efficacy of immunizing with TcpF and CTB in the suckling mouse model of cholera. PLoS One 2012; 7:e42434; http://dx.doi.org/10.1371/journal.pone.0042434; PMID: 22879984
- Doria-Rose NA, Haigwood NL. DNA vaccine strategies: candidates for immune modulation and immunization regimens. Methods 2003; 31:207 - 16; http://dx.doi.org/10.1016/S1046-2023(03)00135-X; PMID: 14511953
- Letvin NL. Progress toward an HIV vaccine. Annu Rev Med 2005; 56:213 - 23; http://dx.doi.org/10.1146/annurev.med.54.101601.152349; PMID: 15660510
- Hamajima K, Sasaki S, Fukushima J, Kaneko T, Xin KQ, Kudoh I, Okuda K. Intranasal administration of HIV-DNA vaccine formulated with a polymer, carboxymethylcellulose, augments mucosal antibody production and cell-mediated immune response. Clin Immunol Immunopathol 1998; 88:205 - 10; http://dx.doi.org/10.1006/clin.1998.4566; PMID: 9714699
- Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Miyazaki J, Wahren B, Okuda K. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J Immunol 1997; 159:3638 - 47; PMID: 9317164
- Xin KQ, Hamajima K, Sasaki S, Honsho A, Tsuji T, Ishii N, Cao XR, Lu Y, Fukushima J, Shapshak P, et al. Intranasal administration of human immunodeficiency virus type-1 (HIV-1) DNA vaccine with interleukin-2 expression plasmid enhances cell-mediated immunity against HIV-1. Immunology 1998; 94:438 - 44; http://dx.doi.org/10.1046/j.1365-2567.1998.00533.x; PMID: 9767429
- Chow YH, Chiang BL, Lee YL, Chi WK, Lin WC, Chen YT, Tao MH. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol 1998; 160:1320 - 9; PMID: 9570550
- Sin JI, Kim JJ, Boyer JD, Ciccarelli RB, Higgins TJ, Weiner DB. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J Virol 1999; 73:501 - 9; PMID: 9847356
- Boyer JD, Kim J, Ugen K, Cohen AD, Ahn L, Schumann K, Lacy K, Bagarazzi ML, Javadian A, Ciccarelli RB, et al. HIV-1 DNA vaccines and chemokines. Vaccine 1999; 17:Suppl 2 S53 - 64; http://dx.doi.org/10.1016/S0264-410X(99)00235-2; PMID: 10506409
- Lu Y, Xin KQ, Hamajima K, Tsuji T, Aoki I, Yang J, Sasaki S, Fukushima J, Yoshimura T, Toda S, et al. Macrophage inflammatory protein-1alpha (MIP-1alpha) expression plasmid enhances DNA vaccine-induced immune response against HIV-1. Clin Exp Immunol 1999; 115:335 - 41; http://dx.doi.org/10.1046/j.1365-2249.1999.00793.x; PMID: 9933462
- Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol 2007; 178:2415 - 20; PMID: 17277148
- Turner ML, Corcoran LM, Brink R, Hodgkin PD. High-affinity B cell receptor ligation by cognate antigen induces cytokine-independent isotype switching. J Immunol 2010; 184:6592 - 9; http://dx.doi.org/10.4049/jimmunol.0903437; PMID: 20483733
- Pinto AR, Reyes-Sandoval A, Ertl HC. Chemokines and TRANCE as genetic adjuvants for a DNA vaccine to rabies virus. Cell Immunol 2003; 224:106 - 13; http://dx.doi.org/10.1016/j.cellimm.2003.08.006; PMID: 14609576
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000; 12:121 - 7; http://dx.doi.org/10.1016/S1074-7613(00)80165-X; PMID: 10714678
- Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, Brinster C. Isolation of dendritic cells. Current protocols in immunology / edited by John E Coligan [et al] 2009; Chapter 3:Unit 3 7.
- Montgomery CP, Daum RS. Transcription of inflammatory genes in the lung after infection with community-associated methicillin-resistant Staphylococcus aureus: a role for panton-valentine leukocidin?. Infect Immun 2009; 77:2159 - 67; http://dx.doi.org/10.1128/IAI.00021-09; PMID: 19237525
- Sun J, Li D, Hao Y, Zhang Y, Fan W, Fu J, Hu Y, Liu Y, Shao Y. Posttranscriptional regulatory elements enhance antigen expression and DNA vaccine efficacy. DNA Cell Biol 2009; 28:233 - 40; http://dx.doi.org/10.1089/dna.2009.0862; PMID: 19388846
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
