Abstract
The development of a safe and effective preventive HIV-1 vaccine remains a public health priority. Despite scientific difficulties and disappointing results, HIV-1 vaccine clinical development has, for the first time, established proof-of-concept efficacy against HIV-1 acquisition and identified vaccine-associated immune correlates of risk. The correlate of risk analysis showed that IgG antibodies against the gp120 V2 loop correlated with decreased risk of HIV infection, while Env-specific IgA directly correlated with increased risk. The development of vaccine strategies such as improved envelope proteins formulated with potent adjuvants and DNA and vectors expressing mosaics, or conserved sequences, capable of eliciting greater breadth and depth of potentially relevant immune responses including neutralizing and non-neutralizing antibodies, CD4+ and CD8+ cell-mediated immune responses, mucosal immune responses, and immunological memory, is now proceeding quickly. Additional human efficacy trials combined with other prevention modalities along with sustained funding and international collaboration remain key to bring an HIV-1 vaccine to licensure.
Introduction
According to UNAIDS, 35.3 million people were living with HIV-1 at the end of 2012, the vast majority being in sub-Saharan Africa. Worldwide the number of people including children with new HIV-1 infection fell by 33% since 2001.Citation1 This fragile although uneven success in prevention programs can be ascribed to the strengthening and scaling-up of antiretroviral treatment along with existing and new prevention methods such as behavioral interventions,Citation2 treatment of sexually transmitted diseases, harm reduction,Citation3 male circumcision,Citation4 and prevention of mother-to-child transmission.Citation5 New prevention strategies including pre-exposure prophylaxis (PrEP),Citation6 antiretroviral treatment (ART) for prevention,Citation7 topical microbicides,Citation8 and HIV-1 preventive vaccines must be further explored and their access ensured. A mathematical modeling analysis showed that the implementation of combined strategies such as medical male circumcision, earlier ART and PrEP could lead to dramatic declines in HIV-1 incidence, but will not stop transmission completely.Citation9 A preventive HIV-1 vaccine as part of a comprehensive prevention packageCitation10 remains therefore among the best hopes for controlling the HIV/AIDS pandemic.Citation11
Goals of an HIV-1 Vaccine
One would expect that an HIV-1 vaccine would bring both individual and public health benefits. The first goal of an HIV-1 vaccine would be to prevent HIV-1 infection and provide sterilizing immunity. A Phase III community-based efficacy trial conducted in Thailand (RV144) in mostly heterosexual populations showed that the first goal was indeed achievable. The vaccine regimen conferred an estimated efficacy of 31% against HIV-1 acquisition after 42 mo of follow-up, with a vaccine efficacy of 60% at month 12 and declining thereafter.Citation12,Citation13 It provided the first opportunity to study immune correlations with vaccine efficacy against HIV-1,Citation14 which may permit rational vaccine design and iterative improvement.
A second goal would be to reduce peak and set point viral load by controlling viral replication and to stop progression to clinical disease in vaccine recipients who became infected. These 2 goals are complementary. However, the acceptance of a preventive vaccine that would only reduce viral load seems unlikely, as the validation of immune markers that counter viral replicationCitation15 would need to be supported by the demonstration of clinical benefit against progression to disease. In an era where the benefits of early highly active antiretroviral treatment are well established, this might be difficult ethically.
Scientific Challenges
The development of HIV-1 vaccines faces multiple scientific challenges inherent to the biological properties of HIV-1. HIV-1 integrates as a provirus into the chromosomes of long-lived reservoir memory T-cells where it can persist in a latent state.Citation16 HIV-1 globally presents extraordinary sequence diversity within and between subtypes and multiple circulating recombinant forms have been generated.Citation17,Citation18 Natural infection does not in general induce protective immunity that eradicates (sterilizing) the virus or prevents progression to disease. The trimeric HIV-1 envelope glycoprotein is composed of variable regions that are immunodominant and induce type-specific neutralizing antibodies of limited breadth, while the conserved regions such as the CD4 binding site are cryptic and poorly accessible to the immune system.Citation19 Broadly neutralizing antibodies (bNAb) develop in roughly 20% of HIV-infected after 2–3 y, but these bNAb do not appear to limit disease progression.Citation20 HIV-1 Env is covered with glycans that shield conserved epitopes from antibody recognition and evade the neutralizing antibody response.Citation21 An effective HIV-1 vaccine should therefore induce responses that differ qualitatively and quantitatively from that induced by natural infection, and able to cross-protect against various HIV-1 clades.Citation22
The limited although increasing knowledge of immune correlates of protection in humans and the poor positive predictive value of animal models mean that further empirical proof-of-concept trials are justified.
Human Efficacy Trials—What Works and What Doesn’t for Protection
Six HIV-1 vaccine efficacy trials had been conducted, which, put in perspective offer some insights into possible immune correlates for protection ().
Table 1. Phase IIb and III efficacy trials conducted in humans
Vax003 and Vax004
Monomeric gp120 HIV-1 envelope proteins failed to protect high-risk volunteers in 2 efficacy trials. Vax004 tested a bivalent recombinant HIV-1 subtype B (GN08 and MN) envelope glycoprotein subunit vaccine in US, Canada, and Netherlands men who have sex with men (MSM) and women at high risk for heterosexual transmission of HIV-1.Citation23 The vaccine did not prevent HIV-1 acquisition nor did it affect HIV-1 disease progression.Citation24 High neutralizing antibody levels against easy-to-neutralize MN strain were however significantly inversely correlated with HIV-1 incidence while low levels against more-difficult-to-neutralize viruses suggests that level and breadth were not sufficient for protection.Citation25 Whether the final level of efficacy reflects positive and negative effects on acquisition remains speculation.Citation26 The level of vaccine-induced antibody-dependent cellular virus inhibition activity (ADCVI) correlated inversely with the rate of acquiring HIV-1 infection following vaccination, However, ADCVI activity correlated poorly with neutralizing or CD4-gp120-blocking antibody activity measured against laboratory strains and was modulated by FcR polymorphisms.Citation27 Vax003 tested a bivalent recombinant HIV-1 subtype B/E (A244 CRF01_AE and MN subtype B) envelope glycoprotein subunit vaccine in injecting drug users (IDU) in Bangkok, Thailand.Citation28 The vaccine did not prevent HIV-1 acquisition nor did it affect HIV-1 disease progression. The failure of these 2 antibody-inducing vaccines led to developing and testing vaccines inducing cell-mediated immune responses.
Step and Phambili
The Step (HVTN 502/Merck 023) and Phambili (HVTN 503) vaccine trials explored whether cell-mediated immune response-inducing vaccines could prevent infection or reduce post-infection plasma viral load. The Merck vaccine (MRKAd5 HIV-1) was a mixture of replication-defective Ad5 vectors expressing HIV-1–1 gag, pol, and nef subtype B genes. The Step study enrolled predominantly high-risk populations including MSM as well as heterosexual women and men.Citation29,Citation30 The Phambili study enrolled heterosexual men and women in South Africa.Citation31 The Step trial was halted after a pre-specified interim analysis (when 30 per-protocol events had arisen in the group with Ad5 antibody titer 200 or less) that showed no protection against HIV-1 acquisition. This finding prompted the Phambili trial Ethics Committee to halt the study. There were excess HIV infections in the vaccine group but statistical significance was not seen in the primary study; post-hoc follow-up of data from both Step and Phambili rAd5 trials has suggested increased risk of HIV infection in vaccine recipients, though methodologic problems are significant. There was no significant decrease in HIV-1 viral load in the vaccine group compared with the placebo recipients in Step or Phambili. However, Phambili was unblinded prior to complete enrollment and vaccination, and interpretation of results is subject to multiple biases.Citation32 Post-hoc multivariate analysis of the Step study suggested that risk was greatest in uncircumcised men with pre-existing Ad5-NAb were at greatest increased risk for HIV-1 acquisition,Citation33 waned with time from vaccination,Citation34 and was not explained by behavioral factors.Citation35 The presence of Ad5-NAb was not linked to the risk of HIV-1 acquisition among unvaccinated populations at elevated risk of HIV-1 infection.Citation36 In addition, subjects infected during the Step trial seemed to have qualitative immune differences that increased their risk of HIV-1 infection independent of vaccination.Citation37 A sieve analysis showed evidence of vaccine-elicited immune pressure on the founder virus though no specific CD8+ CTL recognizing that epitope could be identified.Citation38 Moreover vaccinees with HLA alleles associated with HIV-1 control had a significantly lower mean viral load over time.Citation39 Interestingly, the most highly conserved epitopes were detected at a lower frequency, suggesting that stronger responses to conserved sequences may be as important as breadth for protection.Citation40
Similar Ad5 vector–based vaccines did not protect macaques from infection with SHIV89.6P but reduced viral load and preserved CD4+ T cell post-infection, findings that were not reproduced in the human trials.Citation41 The outcome of the Step trial was recapitulated in an Indian rhesus macaque study where animals vaccinated with a regimen similar to that employed in the Step trial were not protected against a SIVE660 challenge.Citation42 Rhesus macaques chronically infected with a host-range mutant Ad5 (Ad5hr) and immunized with a rAd5 SIVmac239 gag/pol/nef vaccine were challenged with a series of escalating dose penile exposures to SIVmac251. Despite inducing CD8+ T-cell responses in 70% of the monkeys the vaccine did not protect vaccinated animals from penile SIV challenge.Citation43
HVTN 505
Aiming at inducing both functional antibodies and cell-mediated responses,Citation44 a regimen with DNA vaccine prime composed of DNA plasmids encoding Gag, Pol, and Nef from HIV-1 subtype B and Env from subtypes A, B, and C and replication-defective rAd5-HIV-1 vaccine boost containing a mixture of 4 rAd5 vectors encoding the HIV-1 subtype B Gag-Pol and Env matching the DNA Env components was tested in Phase ICitation45-Citation47 and IIaCitation48 clinical trials. As opposed to the MRKAd5 HIV-1 vaccine that did not contain an envelope gene, the HVTN 505 vaccine contained 3 envelope genes. The vaccine regimen induced polyfunctional CD4+ and CD8+ T-cells, multi-clade anti-Env binding antibodies, and Nab against easy to neutralize Tier 1 viruses. The Phase IIB trial (HVTN 505) was recently stopped for futility, showing no efficacy and no statistically significant effect on viral load and a non-significant excess of HIV infection in the vaccinated group.Citation49 Further analysis is ongoing.
This prime-boost vaccine regimen failed to protect NHP against SIVmac251 infection, but 50% of vaccinated monkeys were protected from infection with SIVsmE660 with about a one-log reduction in peak plasma virus RNA in Mamu-A*01-positive animals, suggested a role of cytotoxic T lymphocytes in the control of SIV replication. However, low levels of neutralizing antibodies and an envelope-specific CD4+ T-cell response were associated with vaccine protection in these monkeys.Citation50 SIV-specific CD8+ T cells of effector memory phenotype showed strong virus-inhibitory activity (VIA) and correlated with high levels of CD107a mobilization and perforin expression.Citation51
RV144
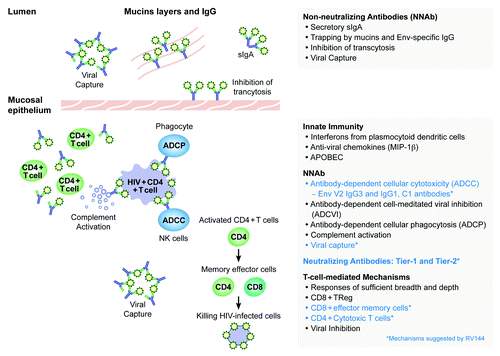
A Phase III community-based trial conducted in Thailand (RV144) provided the first evidence that an HIV-1 vaccine could confer protective efficacy against HIV-1 acquisition. The prime-boost vaccine regimen consisted of a recombinant canarypox vector, ALVAC-HIV prime (vCP1521, expressing gag, protease subtype B [LAI] and env gp120 CRF01_AE with a gp41 subtype B [LAI] transmembrane anchor) and a bivalent AIDSVAX® gp120 B/E MN and CRF01_AE (A244) boost. The vaccine regimen was safe and well tolerated.Citation52 The modified intent-to-treat analysis showed an estimated 31.2% efficacy after 42 mo of follow-up post first vaccination.Citation12,Citation53 Post hoc analysis of the interaction of risk status and acquisition efficacy was significant with greater benefit in low-risk individuals.Citation13 Vaccine efficacy appeared to be higher (60%) at 12 mo post first vaccination, suggesting an early, but nondurable, vaccine effect. There was no effect on early post-infection HIV-1 RNA VL or CD4+ T-cell count. Vaccination did not affect the clinical course of HIV-1 disease after infection, though there was evidence of reduction in seminal fluid viral load.Citation54
IFN-γ ELISPOT positive responses were detected in 41% of the vaccinees and predominantly CD4+ T cell-mediated. Responses were targeted within the HIV-1 Env region, with up to 60% of vaccinees recognizing peptides derived from the Env V2 region, which includes the α4β7 integrin binding site. Intracellular cytokine staining confirmed that Env responses predominated and were mediated by polyfunctional effector memory CD4+ T cells displaying a cytolytic phenotype.Citation55
Binding antibody against Env was nearly uniformly present to the MN and A244 vaccine antigens, but dropped 15-fold after 6 mo; p24 responses were less frequent. Antibody-dependent cell-mediated cytotoxicity (ADCC) in vaccine recipients and mediated by monoclonal (mAb) antibodies from vaccine recipients were described.Citation56,Citation57 Neutralization of Tier 1 viruses was detected in both RV144 and Vax003. The RV144 regimen was superior to 2 gp120 protein administrations alone, confirming a priming effect for ALVAC-HIV, but was inferior to a 12-mo regimen of 4 AIDSVAX® B/E inoculations.Citation58 Further analysis suggested that the lack of response to a vaccine designed to induce clade-specific HIV-1 NAb is associated with the presence of certain HLA class II alleles in Southeast Asians.Citation59
Correlates of protection—new perspectives
The RV144 trial provided a unique opportunity to perform a case control study of correlates of risk. Plasma IgG binding antibody to scaffolded gp70 V1V2 envelope proteins correlated inversely with risk of infection (higher antibody levels correlated with lower rates of infection.), while Env plasma IgA correlated directly with a higher rate of infection, raising the hypotheses that IgA responses against Env and IgG responses directed against V1V2 may be mechanistically associated with protection. Neither low levels of V1V2 antibodies nor high levels of Env-specific IgA antibodies were associated with higher rates of infection than in the placebo group. In vaccinees with low levels of Env-specific IgA antibodies, IgG avidity, ADCC, neutralizing antibodies, and Env-specific CD4+ T cells, correlated inversely with risk of infection.Citation14,Citation60-Citation62 Two weeks post last vaccination 97% of RV144 studied plasma samples from vaccine recipients contained antibodies to V2 region synthetic peptides, falling to 19% at 48 wk, suggesting that waning vaccine efficacy may be correlated with waning V2 antibody response. Interestingly, gp70 V1V2 antibodies were lower in HVTN 505 compared with RV144.Citation63 The response to V3 CRF01_AE also correlated inversely with the risk of HIV infection in vaccine recipients with lower levels of Env-specific plasma IgA and neutralizing antibodies. In Vax003 and Vax004 (no protection), serum IgG responses targeted the same epitopes as in RV144 with the exception of an additional C1 reactivity in Vax003 and infrequent V2 reactivity in Vax004. These results along with a recent sieve analysisCitation64 generate the hypothesis that IgG to linear epitopes in the V2 and V3 regions of gp120 are part of a complex interplay of immune responses that contributed to protection in RV144.Citation65
Approximately 90% of incident infections in RV144 were CRF01_AE, the predominant circulating strain in much of South East Asia. A sieve analysis identified 2 vaccine-associated genetic signatures in V2 corresponding to sites 169 and 181, further supporting the hypothesis that vaccination-induced immune responses directed against the V2 loop were associated with protection.Citation66 Monoclonal antibodies from RV144 vaccine recipients contact the V2 K169 residue, providing further evidence that vaccine-induced antibodies correspond to the observed sieve effect. These V2-specific antibodies can mediate ADCC, neutralization, and low-level virus capture.Citation67,Citation68 These findings generate the hypothesis that V2 IgG may play a role in protection against HIV-1 acquisition but do not provide evidence of a mechanistic or non-mechanistic correlate of protection.Citation69
Sequences in gp70 V1V2 antigens other than V2, such as C1 and V1, may significantly contribute to the binding responses. Some light has recently been shed on the role of plasma IgA in RV144. In the presence of low anti-Env IgA, both ADCC and NAb responses correlated with decreased risk of infection. ADCC responses were predominantly directed to the C1 conformational region of gp120.Citation57,Citation70,Citation71 IgA antibodies elicited by RV144 block C1 region-specific IgG-mediated ADCC.Citation72 Whether V2 antibodies might block the gp120-α4β7 interactionCitation73,Citation74 and contribute at least partially to the protective effect against HIV-1 sexual transmission remains to be demonstrated.Citation75 In future trials, assessing IgG and IgA to V1V2 binding antibody immune responses in the mucosal compartments will be key.
In previous clinical studies, monomeric gp120 induced high levels of Env-specific IgG4 antibodiesCitation76 while ALVAC (vCP1452) prime and gp120 MN in alum boost elicited lower IgG4 relative to IgG1 and IgG3 antibodies.Citation77 Antigen-specific IgG3 antibodies are associated with long-term control of Plasmodium falciparumCitation78 and monocyte-mediated cellular inhibition of parasite growth in vitro.Citation79 Similarly, early appearance of chikungunya virus-specific IgG3 neutralizing antibodies is associated with clearance of the virus and long-term clinical protection.Citation80 Conversely, IgG4 have been associated with progression to AIDS.Citation81 IgG3 can fix the complement and has high affinity for FcγR. A recent comparison of RV144 and Vax003 showed that Env-specific IgG3 and V1/V2 IgG3 response rates were higher in recipients of the RV144 vaccine compared with those of Vax003 vaccinees and conversely that IgG4 were considerably lower in RV144. V1/V2 IgG3 responses and IgG3 responses specific for V1/V2 169K correlated with decreased risk of HIV-1 infection after IgA adjustment.Citation82 It is speculated that ALVAC priming due to its unique proinflammatory cytokine and chemokine response following vaccination in rhesus monkeys and infection in human PBMCCitation83 may shape the IgG subclass response to IgG3 in response to envelope protein boost in humans compared with envelope vaccination alone. The contribution of Fc–FcγR interaction-mediated antibody function through mechanisms including ADCC, antibody-dependent cell mediated viral inhibition (ADCVI), and antibody-dependent cellular phagocytosis (ADCP) remains to be explored.Citation84,Citation85 A recent post hoc analysis of RV144 showed an association between the FcγRIIC polymorphism and vaccine efficacy and correlates of risk, emphasizing the potential role of FcR genetics in predicting vaccine efficacy.Citation86
Lessons learned from non-human primate challenge studies
Several NHP studies support the RV144 findings. ALVAC-SIV conferred protection from infection in neonates macaques exposed to repeated low-dose challenge.Citation87 An immunization regimen recapitulating the RV144 regimen protected against mucosal challenge of SIVmac251 acquisition in 30% of the vaccinated animals. Protected animals had a higher avidity antibodies to gp120, recognized the V2 variable envelope region, and reduced SIVmac251 infectivity in cells expressing high level of α4β7, suggesting a functional role of V2 antibodies.Citation88 Microarray analysis showed that NK cell-associated genes were upregulated after the first protein boost with increased frequency of NK22 cells expressing CCR6 (a gut homing marker) at mucosal sites and of NKG2A+ cells expressing either CD107a or IFN-γ.Citation89
Similarly, an Ad26 prime and MVA boost regimen using vaccines expressing gag-pol and env from SIVsme543 conferred 80–83% reduction in the per-exposure probability of infection against repeated low dose intrarectal inoculations of the heterologous neutralization-resistant SIVmac251. Post-infection set point viremia was reduced of 2.3 log by vaccination and was correlated with magnitude and breadth of T-cell responses to Gag. Protection against SIV acquisition correlated with Env and V2-specific binding and Tier 1 strain-neutralizing antibodies. Responses to Env are critical to prevent acquisition, as monkeys vaccinated with an Ad35/Ad26 prime-boost regimen expressing either Gag-Pol and Env or only Gag-Pol showed significantly greater protection when Env was present. Both vaccine regimens resulted in significant reductions of set point viral loads compared with controls. Immunological correlates of protection were consistent with the first experiment.Citation90 A recent intrarectal SIVsmE660 NHP challenge study where animals were vaccinated with DNA/Ad5 expressing mosaic envelopes confirmed that Env-elicited immune responses are necessary and sufficient to provide protection from acquisition.Citation91 The availability of pathogenic SHIV constructs with HIV-1 E, C, or B envelopes remains critically needed for assessing the efficacy of the ALVAC-AIDSVAX combination, other new HIV vaccines, passive HIV-1-specific immunoglobulin studies (e.g., V2-specific mAb from RV144 vaccine recipients, bNAb).
The predictive value of NHP studies is challenged by the results of the Step studyCitation41 and more recently of HVTN 505. Several rhesus macaque studies support the role of CD8+ T cells in preventing HIV-1 infection and diseaseCitation92-Citation94 but results widely differ depending on the modes of administration, virus challenge, and immunological endpoints used.Citation95-Citation98 Rhesus macaques were generally immunologically more responsive to vaccination than humans while the hierarchy in potency of single-modality (same vaccine product) prime–boost regimens using several vector approaches seem well predicted. In contrast, prime–boost vaccine regimens and vaccines using adjuvant formulations did not correlate between rhesus macaques and humans.Citation99
An emerging understanding of the early events in mucosal SIV, SHIV, and HIV-1 infections has been recently reviewedCitation100,Citation101 justifying the need to develop vaccines inducing both humoral (either local produced or resulting from transudation of plasma antibodies) and cell-mediated mucosal immune responses. A relatively small number of immune effectors at the mucosal site of entry might be at the right place at the right time to be “enough and soon enough” to clear infection. T cell-inducing HIV-1 and SIV vaccines using non-replicating vectors classically induce CD8+ central memory T-cell (TCM) responses whose protective ability depends on an anamnestic expansion to combat infection. In contrast, a replication-competent Rhesus cytomegalovirus (RhCMV)-based vaccine expressing SIV proteins was able to induce and maintain high frequency of SIV-specific CD4+ and CD8+ T-cell effector memory (TEM) responses at extra-lymphoid sites without measurable antibody responses to SIV. Fifty percent of vaccinated monkeys showed a stringent control of intra-rectally administered highly pathogenic SIVmac239 for more than a year. The outcome of challenge in RhCMV vector-vaccinated monkeys was predicted by peak SIV-specific CD8+ TEM frequencies in peripheral blood pre-challenge.Citation102,Citation103 RhCMV vectors are unaffected by pre-existing CMV-specific immunity and can repeatedly super-infect RhCMV-positive monkeys and elicit high frequency SIV-specific CD4+ and CD8+ TEM responses.Citation104
Prior infection of rhesus macaques with an attenuated SHIV conferred protection against vaginal challenge associated with SIV-specific CTL in cervical vaginal tissues,Citation105 suggesting that a modest vaccine-induced CD8+ T-cell response in the context of immunoregulatory suppression of T-cell activation may protect against vaginal HIV-1 transmission.Citation106 Supporting this hypothesis, macaques immunized with an oral vaccine comprised of Lactobacillus plantarum, a commensal bacterium that favors immune tolerance, and inactivated SIVmac239 induced CD8+ regulatory T cells (Tregs) completely protected 15 of 16 animals without inducing SIV-specific antibodies or CTL. Infection was seen after re-challenge but viral load was undetectable. Infusion of CD8 antibodies confirmed the role of CD8+ Tregs in preventing/suppressing SIV in vivo in the absence of vaccine-induced antibodies in mucosal secretions. These findings suggest a new avenue of research toward developing an HIV-1 vaccine.Citation107
Interestingly, human dimeric IgA1 mAb-treated rhesus macaques remained free of virus after intrarectal SHIV challenge while treatment with dimeric IgA2 was much less effective. Protection was correlated with virus capture and inhibition of transcytosis of cell-free virus.Citation108
New vaccine concepts
The various vaccine concepts tested in humans and lessons learned have recently been reviewed.Citation109 Countering HIV-1 variability remains one of the main hurdles for HIV-1 vaccines. Although considerable efforts are deployed to better understand the mechanisms of neutralization and develop a vaccine capable of inducing broadly neutralizing antibodies,Citation110,Citation111 these concepts have not yet been evaluated in human clinical trial. Other Env subunit protein approaches aim at improving the results observed in RV144. The analysis of A244 gp120 used in RV144 demonstrated that the deletion of 11 N terminus aminoacids of gp120 (Δ11) enhanced the antigenicity to gp120 C1 region and to V2 conformational epitopes. Conformational V1/V2 mAbs gave significantly higher levels of blocking of plasma IgG from A244 Δ11 gp120 immunized animals than IgG from animals immunized with unmodified A244 gp120.Citation112 Another approach using gp41 protein and derived peptide administered by mixed intramuscular and intranasal modalities was capable of protecting immunized monkeys against SHIV challengeCitation113 and of eliciting systemic and mucosal antibodies inhibiting HIV transcytosis in the absence of neutralizing antibodies in humans.Citation114
Bypassing the immune system by intramuscular delivery of an adeno-associated virus type 1 gene transfer vector expressing HIV-1-specific broadly neutralizing antibodies is an attractive strategy and is now in clinical trial with vector-expressed bNAb PG9. HIV-1-specific antibodies are endogenously synthesized in myofibers and passively distributed to the peripheral blood. Long-lasting neutralizing activity in serum of macaques administered with a vector expressing SIV-specific antibodies conferred complete protection against SIV intravenous challenge.Citation115 The same strategy was immunogenic in humanized miceCitation116 and able to protect against SHIV challenge.Citation117
Vectors encoding conserved HIV-1 sequencesCitation118 are now tested in humans.Citation119 Vaccine-elicited responses toward conserved regions could afford partial protection against a high-dose intrarectal SIVmac251 challenge.Citation120 However, NHP immunized with full-length HIV-1 immunogens elicited increased magnitude and breadth of cellular immune responses compared with conserved-region-only HIV-1 immunogens, including against conserved sequences.Citation121 Mosaic HIV-1 antigens expressed by Ad26 vectors markedly augmented both the breadth and depth of antigen-specific CMI responses as compared with consensus or natural sequence HIV-1 antigens in rhesus monkeys.Citation122 Recently, Ad26/MVA and Ad26/Ad35 vector-based vaccines expressing HIV-1 mosaic Env, Gag, and Pol afforded a significant reduction in the per-exposure acquisition risk following repetitive, intrarectal SHIV-SF162P3 challenges. Protection against acquisition of infection correlated with vaccine-elicited binding (although not with V2 responses), neutralizing, and functional non-neutralizing antibodies, suggesting that the coordinated activity of multiple antibody functions may contribute to protection against difficult-to-neutralize viruses.Citation123 In-depth analysis of the HVTN 505 results and underlying immune responses and sieve analysis might inform the development of other non-Ad5 rare serotype adenoviruses vectors such as Ad26, Ad35,Citation124 or ChAdV63Citation125 and more generally of T-cell-inducing vaccine strategies. DNA administered intramuscularly by electroporation augmented elicited T-cell immune responses compared with needle injection.Citation126 While successful in macaques,Citation95 the adjuvant effect of added genes expressing IL-12 or IL-15 cytokines did not dramatically improve T-cell immune responses in humans.Citation127
The degree of live-attenuated SIV vaccine-mediated protection against SIVmac239 challenge strongly correlated with the magnitude and function of SIV-specific, effector-differentiated T cells in the lymph node but not with the responses of such T cells in the blood or with other cellular, humoral, and innate immune parameters. The maintenance of protective T-cell responses was associated with persistent live-attenuated vaccine replication in the lymph node, which occurred almost exclusively in Tfh cells. The maintenance of lymphoid tissue-based, effector-differentiated, SIV-specific T cells that intercept and suppress early wild-type SIV amplification and can control and perhaps clear infection, provides a rationale for the development of persistent vectors that can elicit and maintain such response.Citation128 Several replication-competent vectors are in preclinical development or early clinical development.Citation129
Research Priorities
New efficacy trials of pox-protein vaccines
The pox-protein or DNA-pox-protein vaccine strategies remain the most likely to proceed to new efficacy trials. Although RV144 protective efficacy was 31.2% 42 mo after first vaccination, the highest efficacy (60%) was observed at 6–12 mo. The Pox Protein Public Private Partnership or P5 (Sanofi Pasteur, Novartis, Bill and Melinda Gates Foundation, US National Institutes of Health, HIV Vaccine Trial Network, and US Military HIV Research Program) is dedicated to building on the RV144 result and developing pox-protein HIV-1 vaccines with the potential for broad public health impact. A vaccination regimen with ALVAC-HIV prime and gp120 Env subunit protein boost will be tested in efficacy studies in Thailand and South Africa in high risk populations (MSM and heterosexual, respectively) with different HIV-1 subtypes (CRF01_AE and subtype C, respectively).
Independently, a Phase IIB trial with subtype C DNA prime followed by another recombinant pox vector (replication-defective vaccinia, NYVAC) boost with or without additional envelope protein subunit boost is planned in high-risk heterosexual populations Southern Africa. A similar strategy using DNA prime and replicating vaccinia Tiantan boostCitation130 is under consideration for efficacy testing in a Phase IIB in MSM in China.
However, less advanced in development, it is anticipated that a next wave of efficacy trials might test vaccines expressing bNAb, mosaic antigens or conserved sequences or other less advanced vaccine strategies such as gp41 virosomes (described above) as well as full-length single chain gp120-CD4 complex (FLSC)Citation131 in prime-boost with a pox vector.
Correlates of risk
summarizes the main findings on correlates of risk identified in the different human efficacy trials while displays possible immune mechanisms involved in protection. The identification of potential immune correlates of risk in RV144 has raised numerous scientific questions. Follow-up clinical trials are planned to assess the respective roles of the RV144 vaccination regimen components (ALVAC-HIV and AIDSVAX B/E) in eliciting immune responses in the peripheral blood and in mucosal compartments, the patterns of gene activation immune signature and their prediction of immune responses, the levels of innate apolipoprotein B expression, whether HIV-specific antibody titers, in particular V2 antibodies can be increased and sustained with additional envelope boosts, and the immune memory with late (>7 y) booster injections to RV144 vaccine recipients. The role of humoral and cell-mediated immune responses in the mucosal compartments deserves further exploration including the fine specificity of HIV-1-specific antibody response in mucosal secretions (IgG subclasses, IgA, and IgG V2 antibodies) and their possible hindrance of HIV mobilityCitation132,Citation133 and virus replication ex vivo.Citation134 While V2 antibodies generated by the RV144 vaccine regimen are cross-reactive with other HIV-1 subtypes, it remains equally critical to assess whether Env subunit proteins derived from different HIV-1 subtypes can elicit cross-reactive V2 antibodies.
Table 2. Correlates of protection: lessons learned in human efficacy trials
Broadly neutralizing antibodies
The study of bNAb and their induction by immunogens remains a focus of research activity and were recently reviewed.Citation19,Citation110,Citation135 Broadly cross-reactive HIV-1 NAb selected from the bone marrow (BM) of HIV-1-infected long-term non-progressors contains numerous somatic mutations. The recent finding that a transmitted/founder Env can be the stimulator of a potent bNAb and bind optimally to that bNAb unmutated ancestor may be key for vaccine design and could allow the induction of bNAb by targeting unmutated ancestors and intermediate ancestors of bNAb clonal lineage trees.Citation136 The overall architecture of a soluble trimeric envelope, as well as the secondary, tertiary, and quaternary interactions between gp120 and gp41 involved in its assembly were recently described. In particular, the gp120 subunits are held together by association of the V1/V2/V3 regions at the apex of the trimer.Citation137 The complete definition of how neutralization epitopes are presented in the context of a trimeric envelope should help the design of new immunogens as candidate vaccines. The study of B cells in the peripheral blood, BM and gut of vaccine recipients may help elucidate the rapidly waning antibody response observed in RV144.Citation138 It remains however critical to demonstrate that bNAb may confer protection against HIV infection in humans.
T-helper cells
Immunization with soluble Env was reported to induce short-lived antibody responses with robust peak followed by rapid contraction of circulating antibody and memory B cells.Citation139 Optimizing CD4+ T-cell responses in HIV-1 vaccine development has gained renewed attention,Citation140 in particular T follicular helper cells (Tfh). Tfh play an essential role for B-cell selection and proliferation in germinal centers, for somatic hypermutations and the development of high-affinity and broadly neutralizing antibodies. The frequency of a subpopulation of circulating memory PD-1+ CXCR5+ CD4+ T cells that are resting memory cells most related to bona fide germinal center Tfh cells by gene expression profile, cytokine profile, and functional properties was correlated with the development of HIV-specific bNAbs in HIV-infected individuals.Citation141 A better understanding of CD4+ T-cell fine specificity and functions in particular the coordinating mechanisms that lead to persistent protective CD8+ T-cell and B-cell responses, is critically needed to improve the efficacy of HIV-1 vaccine candidates. Experiments of nanoparticle malaria vaccines suggest that an increased antigen deposition/retention locally in the tissue drives B-cell responses, enhancing dendritic cell antigen presentation, compared with soluble protein immunizations.Citation142 Prolonged antigen presentation mediated by nanoparticle vaccines may have also contributed to the stimulation the formation of germinal centersCitation143 with enhanced development of CD4+ Tfh cells,Citation144 which provide critical cytokines and signals required to initiate somatic hypermutation and affinity maturation for effective B cell memory.Citation145
Non-human primates studies
It has been argued that NHP challenge studies were not predictive of the outcome of HIV-1 vaccine efficacy trials and therefore should not be gatekeepers of efficacy trials in humans. However, it must be acknowledged that the methodology used varied greatly and might not have been representative of human transmission risk. Although imperfect, the recently improved repeat, low-dose mucosal NHP challenge modelCitation146 with better standardized SIV and, to a lesser extent, SHIV challenge viruses, is likely closer to recapitulating HIV-1 transmission in humans.Citation147 The availability of new SHIV constructs derived from different HIV-1 clades (C, A, and E) would allow assessing the homologous or heterologous protective efficacy of V2-specific monoclonal antibodies derived from B cells of RV144 vaccine recipients (CH58 and CH59), of new HIV-1 subtype C or A-specific vaccine regimens, in particular Env subunit proteins. Humanized mice models have been recently improved and may offer an interesting alternative to non-human primates in testing HIV vaccines.Citation148
Although non-human primate studies may help defining immunogenicity selection criteria for advancing candidate vaccines into human testing as well as correlates of protection jointly with human efficacy trials, they remain of poor positive predictive value and are in no case substitutes of human clinical trials, in particular efficacy trials.Citation149
Deployment, impact, and cost-benefits
Several models have emphasized the public health and cost-benefit advantages of HIV-1 vaccines.Citation150 According to the World Health Organization, cost-effective vaccines provide an additional year of life at a cost less than a country’s per capita Gross National Income. Vaccines that provide cost-savings are those in which the cost of vaccination multiplied by the number of infections averted by vaccination is less than the lifetime cost of treatment averted.Citation151 Immunization strategies of a safe and efficacious HIV-1 vaccine will largely depend on its acceptability by target populations, the type, level and duration of vaccine efficacy for a given mode of HIV-1 transmission within the community, stability and thermostability, and the possible need to couple vaccination with other prevention technologies. It is important to recognize that the cost of a vaccine is more than the price. It includes the logistics around vaccine deployment (transportation, storage, delivery mechanisms, and mode of administration). Local production of vaccines may alleviate the costs and contribute to better access at the regional level. Deployment strategies will require ample consultations with regulatory, scientific and health authorities, and civil society stakeholders on a country-by-country basis.
Conclusion
Knowledge of the immune correlates of protection against HIV-1 is key to accelerating HIV-1 vaccine development. RV144 and further studies of correlates of risk have opened large and unforeseen avenues of exploration and hope for the most exciting time of HIV-1 vaccine development. The still uncertain predictive value of animal models and biomarkers of immune protection against HIV-1 necessitate however that vaccines be tested in clinical efficacy trials. A long-term strategy to ultimately end the AIDS pandemic must include both scale-up of existing HIV-1 combination prevention, treatment, and care programming, and sustained investment in research and development for a preventive HIV-1 vaccine.Citation152
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The preparation of this manuscript was supported in part by an Interagency Agreement Y1-AI-2642–12 between the US Army Medical Research and Materiel Command (USAMRMC) and the National Institutes of Allergy and Infectious Diseases. In addition this work was supported by a cooperative agreement (W81XWH-07–2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the US Department of Defense (DOD). We are grateful to Stephanie Stevens, U.S. Military HIV Research Program, for helping designing the Figure.
Disclaimer
The opinions herein are those of the authors and should not be construed as official or representing the views of the US Department of Defense or Department of the Army.
References
- UNAIDS Report on the Global AIDS Epidemic 2013. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf
- McCoy SI, Kangwende RA, Padian NS. Behavior change interventions to prevent HIV infection among women living in low and middle income countries: a systematic review. AIDS Behav 2010; 14:469 - 82; http://dx.doi.org/10.1007/s10461-009-9644-9; PMID: 19949847
- Dutta A, Wirtz AL, Baral S, Beyrer C, Cleghorn FR. Key harm reduction interventions and their impact on the reduction of risky behavior and HIV incidence among people who inject drugs in low-income and middle-income countries. Curr Opin HIV AIDS 2012; 7:362 - 8; http://dx.doi.org/10.1097/COH.0b013e328354a0b5; PMID: 22647588
- Wamai RG, Morris BJ, Bailis SA, Sokal D, Klausner JD, Appleton R, Sewankambo N, Cooper DA, Bongaarts J, de Bruyn G, et al. Male circumcision for HIV prevention: current evidence and implementation in sub-Saharan Africa. J Int AIDS Soc 2011; 14:49; http://dx.doi.org/10.1186/1758-2652-14-49; PMID: 22014096
- Chi BH, Adler MR, Bolu O, Mbori-Ngacha D, Ekouevi DK, Gieselman A, Chipato T, Luo C, Phelps BR, McClure C, et al. Progress, challenges, and new opportunities for the prevention of mother-to-child transmission of HIV under the US President’s Emergency Plan for AIDS Relief. J Acquir Immune Defic Syndr 2012; 60:Suppl 3 S78 - 87; http://dx.doi.org/10.1097/QAI.0b013e31825f3284; PMID: 22797744
- Kim SC, Becker S, Dieffenbach C, Hanewall BS, Hankins C, Lo YR, Mellors JW, O’Reilly K, Paxton L, Roffenbender JS, et al. Planning for pre-exposure prophylaxis to prevent HIV transmission: challenges and opportunities. J Int AIDS Soc 2010; 13:24; http://dx.doi.org/10.1186/1758-2652-13-24; PMID: 20624303
- Cohen MS, Holmes C, Padian N, Wolf M, Hirnschall G, Lo YR, Goosby E. HIV treatment as prevention: how scientific discovery occurred and translated rapidly into policy for the global response. Health Aff (Millwood) 2012; 31:1439 - 49; http://dx.doi.org/10.1377/hlthaff.2012.0250; PMID: 22778333
- Krakower D, Mayer KH. Promising prevention approaches: tenofovir gel and prophylactic use of antiretroviral medications. Curr HIV/AIDS Rep 2011; 8:241 - 8; http://dx.doi.org/10.1007/s11904-011-0094-4; PMID: 22002729
- Cremin I, Alsallaq R, Dybul M, Piot P, Garnett G, Hallett TB. The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. AIDS 2013; 27:447 - 58; http://dx.doi.org/10.1097/QAD.0b013e32835ca2dd; PMID: 23296196
- Padian NS, McCoy SI, Karim SS, Hasen N, Kim J, Bartos M, Katabira E, Bertozzi SM, Schwartländer B, Cohen MS. HIV prevention transformed: the new prevention research agenda. Lancet 2011; 378:269 - 78; http://dx.doi.org/10.1016/S0140-6736(11)60877-5; PMID: 21763938
- O’Connell RJ, Kim JH, Corey L, Michael NL. Human immunodeficiency virus vaccine trials. Cold Spring Harb Perspect Med 2012; 2:a007351; http://dx.doi.org/10.1101/cshperspect.a007351; PMID: 23209178
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al, MOPH-TAVEG Investigators. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209 - 20; http://dx.doi.org/10.1056/NEJMoa0908492; PMID: 19843557
- Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, Khamboonruang C, Thongcharoen P, Morgan P, Benenson M, et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis 2012; 12:531 - 7; http://dx.doi.org/10.1016/S1473-3099(12)70088-9; PMID: 22652344
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275 - 86; http://dx.doi.org/10.1056/NEJMoa1113425; PMID: 22475592
- Gurunathan S, Habib RE, Baglyos L, Meric C, Plotkin S, Dodet B, Corey L, Tartaglia J. Use of predictive markers of HIV disease progression in vaccine trials. Vaccine 2009; 27:1997 - 2015; http://dx.doi.org/10.1016/j.vaccine.2009.01.039; PMID: 19200450
- Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, Chun TW, Churchill M, Di Mascio M, Katlama C, et al, International AIDS Society Scientific Working Group on HIV Cure. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol 2012; 12:607 - 14; http://dx.doi.org/10.1038/nri3262; PMID: 22814509
- McBurney SP, Ross TM. Viral sequence diversity: challenges for AIDS vaccine designs. Expert Rev Vaccines 2008; 7:1405 - 17; http://dx.doi.org/10.1586/14760584.7.9.1405; PMID: 18980542
- Hemelaar J, Gouws E, Ghys PD, Osmanov S, WHO-UNAIDS Network for HIV Isolation and Characterisation. Global trends in molecular epidemiology of HIV-1 during 2000-2007. AIDS 2011; 25:679 - 89; http://dx.doi.org/10.1097/QAD.0b013e328342ff93; PMID: 21297424
- Koff WC. HIV vaccine development: challenges and opportunities towards solving the HIV vaccine-neutralizing antibody problem. Vaccine 2012; 30:4310 - 5; http://dx.doi.org/10.1016/j.vaccine.2011.11.014; PMID: 22100891
- Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine?. Nat Med 2009; 15:866 - 70; PMID: 19525964
- Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, Hermanus T, Bajimaya S, Tumba NL, Abrahams MR, et al. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med 2012; 18:1688 - 92; http://dx.doi.org/10.1038/nm.2985; PMID: 23086475
- Nabel GJ, Fauci AS. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat Med 2010; 16:1389 - 91; http://dx.doi.org/10.1038/nm1210-1389; PMID: 21135852
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF, rgp120 HIV Vaccine Study Group. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005; 191:654 - 65; http://dx.doi.org/10.1086/428404; PMID: 15688278
- Gilbert PB, Ackers ML, Berman PW, Francis DP, Popovic V, Hu DJ, Heyward WL, Sinangil F, Shepherd BE, Gurwith M. HIV-1 virologic and immunologic progression and initiation of antiretroviral therapy among HIV-1-infected subjects in a trial of the efficacy of recombinant glycoprotein 120 vaccine. J Infect Dis 2005; 192:974 - 83; http://dx.doi.org/10.1086/432734; PMID: 16107949
- Gilbert P, Wang M, Wrin T, Petropoulos C, Gurwith M, Sinangil F, D’Souza P, Rodriguez-Chavez IR, DeCamp A, Giganti M, et al. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J Infect Dis 2010; 202:595 - 605; http://dx.doi.org/10.1086/654816; PMID: 20608874
- Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, Heyward WL, Jobes DV, Popovic V, Self SG, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis 2005; 191:666 - 77; http://dx.doi.org/10.1086/428405; PMID: 15688279
- Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol 2007; 178:6596 - 603; PMID: 17475891
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K, Bangkok Vaccine Evaluation Group. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 2006; 194:1661 - 71; http://dx.doi.org/10.1086/508748; PMID: 17109337
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al, Step Study Protocol Team. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372:1881 - 93; http://dx.doi.org/10.1016/S0140-6736(08)61591-3; PMID: 19012954
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, et al, Step Study Protocol Team. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 2008; 372:1894 - 905; http://dx.doi.org/10.1016/S0140-6736(08)61592-5; PMID: 19012957
- Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, Mlisana K, Metch B, de Bruyn G, Latka MH, et al, HVTN 503/Phambili study team. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis 2011; 11:507 - 15; http://dx.doi.org/10.1016/S1473-3099(11)70098-6; PMID: 21570355
- Gray GE, Metch B, Churchyard G, Mlisana K, Nchabeleng M, Allen M, Moodie Z, Kublin J, Bekker LG, HVTN 503 team. Does participation in an HIV vaccine efficacy trial affect risk behaviour in South Africa?. Vaccine 2013; 31:2089 - 96; http://dx.doi.org/10.1016/j.vaccine.2013.01.031; PMID: 23370155
- D’Souza MP, Frahm N. Adenovirus 5 serotype vector-specific immunity and HIV-1 infection: a tale of T cells and antibodies. AIDS 2010; 24:803 - 9; http://dx.doi.org/10.1097/QAD.0b013e3283379712; PMID: 20168203
- Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, del Rio C, Casapia M, Santiago S, Gilbert P, Corey L, et al, Step/HVTN 504 Study Team. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study). J Infect Dis 2012; 206:258 - 66; http://dx.doi.org/10.1093/infdis/jis342; PMID: 22561365
- Koblin BA, Mayer KH, Noonan E, Wang CY, Marmor M, Sanchez J, Brown SJ, Robertson MN, Buchbinder SP. Sexual risk behaviors, circumcision status, and preexisting immunity to adenovirus type 5 among men who have sex with men participating in a randomized HIV-1 vaccine efficacy trial: step study. J Acquir Immune Defic Syndr 2012; 60:405 - 13; http://dx.doi.org/10.1097/QAI.0b013e31825325aa; PMID: 22421748
- Curlin ME, Cassis-Ghavami F, Magaret AS, Spies GA, Duerr A, Celum CL, Sanchez JL, Margolick JB, Detels R, McElrath MJ, et al. Serological immunity to adenovirus serotype 5 is not associated with risk of HIV infection: a case-control study. AIDS 2011; 25:153 - 8; http://dx.doi.org/10.1097/QAD.0b013e328342115c; PMID: 21150554
- Cheng C, Wang L, Gall JG, Nason M, Schwartz RM, McElrath MJ, DeRosa SC, Hural J, Corey L, Buchbinder SP, et al. Decreased pre-existing Ad5 capsid and Ad35 neutralizing antibodies increase HIV-1 infection risk in the Step trial independent of vaccination. PLoS One 2012; 7:e33969; http://dx.doi.org/10.1371/journal.pone.0033969; PMID: 22496775
- Rolland M, Tovanabutra S, deCamp AC, Frahm N, Gilbert PB, Sanders-Buell E, Heath L, Magaret CA, Bose M, Bradfield A, et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med 2011; 17:366 - 71; http://dx.doi.org/10.1038/nm.2316; PMID: 21358627
- Fitzgerald DW, Janes H, Robertson M, Coombs R, Frank I, Gilbert P, Loufty M, Mehrotra D, Duerr A, Step Study Protocol Team. An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: results from a randomized placebo-controlled trial (the Step study). J Infect Dis 2011; 203:765 - 72; http://dx.doi.org/10.1093/infdis/jiq114; PMID: 21343146
- Li F, Finnefrock AC, Dubey SA, Korber BT, Szinger J, Cole S, McElrath MJ, Shiver JW, Casimiro DR, Corey L, et al. Mapping HIV-1 vaccine induced T-cell responses: bias towards less-conserved regions and potential impact on vaccine efficacy in the Step study. PLoS One 2011; 6:e20479; http://dx.doi.org/10.1371/journal.pone.0020479; PMID: 21695251
- Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med 2008; 14:617 - 21; http://dx.doi.org/10.1038/nm.f.1759; PMID: 18535579
- Reynolds MR, Weiler AM, Piaskowski SM, Piatak M Jr., Robertson HT, Allison DB, Bett AJ, Casimiro DR, Shiver JW, Wilson NA, et al. A trivalent recombinant Ad5 gag/pol/nef vaccine fails to protect rhesus macaques from infection or control virus replication after a limiting-dose heterologous SIV challenge. Vaccine 2012; 30:4465 - 75; http://dx.doi.org/10.1016/j.vaccine.2012.04.082; PMID: 22569124
- Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, et al. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J Virol 2012; 86:2239 - 50; http://dx.doi.org/10.1128/JVI.06175-11; PMID: 22156519
- Walker BD, Ahmed R, Plotkin S. Moving ahead an HIV vaccine: use both arms to beat HIV. Nat Med 2011; 17:1194 - 5; http://dx.doi.org/10.1038/nm.2529; PMID: 21988996
- Koup RA, Roederer M, Lamoreaux L, Fischer J, Novik L, Nason MC, Larkin BD, Enama ME, Ledgerwood JE, Bailer RT, et al, VRC 009 Study Team, VRC 010 Study Team. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS One 2010; 5:e9015; http://dx.doi.org/10.1371/journal.pone.0009015; PMID: 20126394
- Kibuuka H, Kimutai R, Maboko L, Sawe F, Schunk MS, Kroidl A, Shaffer D, Eller LA, Kibaya R, Eller MA, et al. A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-Uninfected East Africans (RV 172). J Infect Dis 2010; 201:600 - 7; http://dx.doi.org/10.1086/650299; PMID: 20078213
- Jaoko W, Karita E, Kayitenkore K, Omosa-Manyonyi G, Allen S, Than S, Adams EM, Graham BS, Koup RA, Bailer RT, et al. Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One 2010; 5:e12873; http://dx.doi.org/10.1371/journal.pone.0012873; PMID: 20877623
- Churchyard GJ, Morgan C, Adams E, Hural J, Graham BS, Moodie Z, Grove D, Gray G, Bekker LG, McElrath MJ, et al, NIAID HIV Vaccine Trials Network. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204). PLoS One 2011; 6:e21225; http://dx.doi.org/10.1371/journal.pone.0021225; PMID: 21857901
- Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, et al, HVTN 505 Study Team. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013; 369:2083 - 92; http://dx.doi.org/10.1056/NEJMoa1310566; PMID: 24099601
- Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, et al. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci Transl Med 2011; 3:81ra36; http://dx.doi.org/10.1126/scitranslmed.3002351; PMID: 21543722
- Yamamoto T, Johnson MJ, Price DA, Wolinsky DI, Almeida JR, Petrovas C, Nason M, Yeh WW, Shen L, Roederer M, et al. Virus inhibition activity of effector memory CD8(+) T cells determines simian immunodeficiency virus load in vaccinated monkeys after vaccine breakthrough infection. J Virol 2012; 86:5877 - 84; http://dx.doi.org/10.1128/JVI.00315-12; PMID: 22419810
- Pitisuttithum P, Rerks-Ngarm S, Bussaratid V, Dhitavat J, Maekanantawat W, Pungpak S, Suntharasamai P, Vanijanonta S, Nitayapan S, Kaewkungwal J, et al. Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS One 2011; 6:e27837; http://dx.doi.org/10.1371/journal.pone.0027837; PMID: 22205930
- Gilbert PB, Berger JO, Stablein D, Becker S, Essex M, Hammer SM, Kim JH, Degruttola VG. Statistical interpretation of the RV144 HIV vaccine efficacy trial in Thailand: a case study for statistical issues in efficacy trials. J Infect Dis 2011; 203:969 - 75; http://dx.doi.org/10.1093/infdis/jiq152; PMID: 21402548
- Rerks-Ngarm S, Paris RM, Chunsutthiwat S, Premsri N, Namwat C, Bowonwatanuwong C, Li SS, Kaewkungkal J, Trichavaroj R, Churikanont N, et al. Extended evaluation of the virologic, immunologic, and clinical course of volunteers who acquired HIV-1 infection in a phase III vaccine trial of ALVAC-HIV and AIDSVAX B/E. J Infect Dis 2013; 207:1195 - 205; http://dx.doi.org/10.1093/infdis/jis478; PMID: 22837492
- de Souza MS, Ratto-Kim S, Chuenarom W, Schuetz A, Chantakulkij S, Nuntapinit B, Valencia-Micolta A, Thelian D, Nitayaphan S, Pitisuttithum P, et al, Ministry of Public Health–Thai AIDS Vaccine Evaluation Group Collaborators. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol 2012; 188:5166 - 76; http://dx.doi.org/10.4049/jimmunol.1102756; PMID: 22529301
- Karnasuta C, Paris RM, Cox JH, Nitayaphan S, Pitisuttithum P, Thongcharoen P, Brown AE, Gurunathan S, Tartaglia J, Heyward WL, et al, Thai AIDS Vaccine Evaluation Group, Thailand. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine 2005; 23:2522 - 9; http://dx.doi.org/10.1016/j.vaccine.2004.10.028; PMID: 15752839
- Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 2012; 86:11521 - 32; http://dx.doi.org/10.1128/JVI.01023-12; PMID: 22896626
- Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 2012; 206:431 - 41; http://dx.doi.org/10.1093/infdis/jis367; PMID: 22634875
- Paris R, Bejrachandra S, Thongcharoen P, Nitayaphan S, Pitisuttithum P, Sambor A, Gurunathan S, Francis D, Ratto-Kim S, Karnasuta C, et al, Thai AIDS Vaccine Evaluation Group. HLA class II restriction of HIV-1 clade-specific neutralizing antibody responses in ethnic Thai recipients of the RV144 prime-boost vaccine combination of ALVAC-HIV and AIDSVAX(®) B/E. Vaccine 2012; 30:832 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.11.002; PMID: 22085554
- Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Arworn D, Shen X, et al, MOPH TAVEG Collaboration. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses 2012; 28:1444 - 57; http://dx.doi.org/10.1089/aid.2012.0103; PMID: 23035746
- Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, Morris DE, Tomaras G, Rao M, Billings E, et al. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS One 2013; 8:e53629; http://dx.doi.org/10.1371/journal.pone.0053629; PMID: 23349725
- Zolla-Pazner S, Decamp A, Gilbert PB, Williams C, Yates NL, Williams WT, Howington R, Fong Y, Morris DE, Soderberg KA, et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 2014; 9:e87572; http://dx.doi.org/10.1371/journal.pone.0087572; PMID: 24504509
- Tomaras G, Shen X, Seaton K, Janes H, Grove D, Decamp A, et al. Vaccine Induced Antibody Responses in HVTN 505, a Phase IIb HIV-1 Efficacy Trial. [PL04.04] AIDS Res Hum Retroviruses 2013; 29:A-168
- Rolland M, Edlefsen PT, Gottardo R, Montefiori DC, Zolla-Pazner S, Moody A, et al. Genetic and immunological evidence for a role of Env-V3 antibodies in the RV144 trial. [P03-73 LB] AIDS Res Hum Retroviruses 2013; 29:A-168
- Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, et al. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 2013; 8:e75665; http://dx.doi.org/10.1371/journal.pone.0075665; PMID: 24086607
- Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 2012; 490:417 - 20; http://dx.doi.org/10.1038/nature11519; PMID: 22960785
- Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 2013; 38:176 - 86; http://dx.doi.org/10.1016/j.immuni.2012.11.011; PMID: 23313589
- Liu P, Yates NL, Shen X, Bonsignori M, Moody MA, Liao HX, Fong Y, Alam SM, Overman RG, Denny T, et al. Infectious virion capture by HIV-1 gp120-specific IgG from RV144 vaccinees. J Virol 2013; 87:7828 - 36; http://dx.doi.org/10.1128/JVI.02737-12; PMID: 23658446
- Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 2012; 54:1615 - 7; http://dx.doi.org/10.1093/cid/cis238; PMID: 22437237
- Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, et al. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 2011; 85:7029 - 36; http://dx.doi.org/10.1128/JVI.00171-11; PMID: 21543485
- Moody MA, Yates NL, Amos JD, Drinker MS, Eudailey JA, Gurley TC, Marshall DJ, Whitesides JF, Chen X, Foulger A, et al. HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages. J Virol 2012; 86:7496 - 507; http://dx.doi.org/10.1128/JVI.00426-12; PMID: 22553329
- Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 2013; 110:9019 - 24; http://dx.doi.org/10.1073/pnas.1301456110; PMID: 23661056
- Nakamura GR, Fonseca DP, O’Rourke SM, Vollrath AL, Berman PW. Monoclonal antibodies to the V2 domain of MN-rgp120: fine mapping of epitopes and inhibition of α4β7 binding. PLoS One 2012; 7:e39045; http://dx.doi.org/10.1371/journal.pone.0039045; PMID: 22720026
- Mayr LM, Cohen S, Spurrier B, Kong X-P, Zolla-Pazner S. Epitope mapping of conformational V2-specific anti-HIV human monoclonal antibodies reveals an immunodominant site in V2. PLoS One 2013; 8:e70859; http://dx.doi.org/10.1371/journal.pone.0070859; PMID: 23923028
- Rao M, Peachman KK, Kim J, Gao G, Alving CR, Michael NL, Rao VB. HIV-1 variable loop 2 and its importance in HIV-1 infection and vaccine development. Curr HIV Res 2013; 11:427 - 38; http://dx.doi.org/10.2174/1570162X113116660064; PMID: 24191938
- Gorse GJ, Patel GB, Mandava M, Berman PW, Belshe RB, National Institute of Allergy and Infectious Disease Aids Vaccine Evaluation Group. MN and IIIB recombinant glycoprotein 120 vaccine-induced binding antibodies to native envelope glycoprotein of human immunodeficiency virus type 1 primary isolates. AIDS Res Hum Retroviruses 1999; 15:921 - 30; http://dx.doi.org/10.1089/088922299310638; PMID: 10408729
- Banerjee K, Klasse PJ, Sanders RW, Pereyra F, Michael E, Lu M, Walker BD, Moore JP. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res Hum Retroviruses 2010; 26:445 - 58; http://dx.doi.org/10.1089/aid.2009.0223; PMID: 20377426
- Roussilhon C, Oeuvray C, Müller-Graf C, Tall A, Rogier C, Trape JF, Theisen M, Balde A, Pérignon JL, Druilhe P. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med 2007; 4:e320; http://dx.doi.org/10.1371/journal.pmed.0040320; PMID: 18001147
- Tebo AE, Kremsner PG, Luty AJ. Plasmodium falciparum: a major role for IgG3 in antibody-dependent monocyte-mediated cellular inhibition of parasite growth in vitro. Exp Parasitol 2001; 98:20 - 8; http://dx.doi.org/10.1006/expr.2001.4619; PMID: 11426948
- Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, Rénia L, Leo YS, Ng LF. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J Infect Dis 2012; 205:1147 - 54; http://dx.doi.org/10.1093/infdis/jis033; PMID: 22389226
- Ljunggren K, Broliden PA, Morfeldt-Månson L, Jondal M, Wahren B. IgG subclass response to HIV in relation to antibody-dependent cellular cytotoxicity at different clinical stages. Clin Exp Immunol 1988; 73:343 - 7; PMID: 3208446
- Yates NL, Liao HX, Fong Y, De Camp A, Vandergrift NA, Alam MS, et al. HIV-1 Env V1/V2 IgG3 Responses Correlate with Decreased Transmission Risk in the RV144 2 Trial and Distinguish Vaccine-Elicited Humoral Responses from the VAX003 Vaccine Trial. Sci Transl Med 2014; forthcoming
- Teigler JE, Phogat S, Franchini G, Hirsch VM, Michael NL, Barouch DH. The canarypox virus vector ALVAC induces distinct cytokine responses compared to the vaccinia virus-based vectors MVA and NYVAC in rhesus monkeys. J Virol 2014; 88:1809 14; http://dx.doi.org/10.1128/JVI.02386-13; PMID: 24257612
- Forthal D, Hope TJ, Alter G. New paradigms for functional HIV-specific nonneutralizing antibodies. Curr Opin HIV AIDS 2013; 8:393 - 401; http://dx.doi.org/10.1097/COH.0b013e328363d486; PMID: 23924999
- Vargas-Inchaustegui DA, Robert-Guroff M. Fc receptor-mediated immune responses: new tools but increased complexity in HIV prevention. Curr HIV Res 2013; 11:407 - 20; http://dx.doi.org/10.2174/1570162X113116660063; PMID: 24191937
- Li SS, Gilbert PB, Tomaras GD, Kijak G, Ferrari G, Thomas R, et al. Association of FcγRIIC Polymorphism with Vaccine Efficacy and Correlates of HIV-1 Infection Risk in RV144. [P06-20 LB] AIDS Res Hum Retroviruses 2013; 29:A-168
- Van Rompay KK, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, Earl P, Harvey D, Franchini G, Tartaglia J, et al. Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J Acquir Immune Defic Syndr 2005; 38:124 - 34; http://dx.doi.org/10.1097/00126334-200502010-00002; PMID: 15671796
- Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol 2013; 87:1708 - 19; http://dx.doi.org/10.1128/JVI.02544-12; PMID: 23175374
- Liyanage NP, Pegu P, Gordon S, Cameron M, Foulds K, Doster M, et al. RV144-like trial in macaques using ALVAC-SIV & gp120 induces innate immunity and increases the frequency of NK22 & NKG2A+ cells in mucosal tissues. Retrovirology 2012; 9:Suppl 2 O14; http://dx.doi.org/10.1186/1742-4690-9-S2-O14
- Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 2012; 482:89 - 93; http://dx.doi.org/10.1038/nature10766; PMID: 22217938
- Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, et al. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 2014; 505:502 8; http://dx.doi.org/10.1038/nature12893; PMID: 24352234
- Freel SA, Saunders KO, Tomaras GD. CD8(+)T-cell-mediated control of HIV-1 and SIV infection. Immunol Res 2011; 49:135 - 46; http://dx.doi.org/10.1007/s12026-010-8177-7; PMID: 21170741
- McDermott AB, Koup RA. CD8(+) T cells in preventing HIV infection and disease. AIDS 2012; 26:1281 - 92; http://dx.doi.org/10.1097/QAD.0b013e328353bcaf; PMID: 22441256
- Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, et al. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature 2012; 491:129 - 33; http://dx.doi.org/10.1038/nature11443; PMID: 23023123
- Winstone N, Wilson AJ, Morrow G, Boggiano C, Chiuchiolo MJ, Lopez M, Kemelman M, Ginsberg AA, Mullen K, Coleman JW, et al. Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. J Virol 2011; 85:9578 - 87; http://dx.doi.org/10.1128/JVI.05060-11; PMID: 21734035
- Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 2009; 457:87 - 91; http://dx.doi.org/10.1038/nature07469; PMID: 18997770
- Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J Virol 2011; 85:11007 - 15; http://dx.doi.org/10.1128/JVI.05346-11; PMID: 21917969
- Vaccari M, Keele BF, Bosinger SE, Doster MN, Ma ZM, Pollara J, Hryniewicz A, Ferrari G, Guan Y, Forthal DN, et al. Protection afforded by an HIV vaccine candidate in macaques depends on the dose of SIVmac251 at challenge exposure. J Virol 2013; 87:3538 - 48; http://dx.doi.org/10.1128/JVI.02863-12; PMID: 23325681
- Bett AJ, Dubey SA, Mehrotra DV, Guan L, Long R, Anderson K, Collins K, Gaunt C, Fernandez R, Cole S, et al. Comparison of T cell immune responses induced by vectored HIV vaccines in non-human primates and humans. Vaccine 2010; 28:7881 - 9; http://dx.doi.org/10.1016/j.vaccine.2010.09.079; PMID: 20937317
- Belyakov IM, Ahlers JD. Mucosal immunity and HIV-1 infection: applications for mucosal AIDS vaccine development. Curr Top Microbiol Immunol 2012; 354:157 - 79; http://dx.doi.org/10.1007/82_2010_119; PMID: 21203884
- Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol 2012; 188:5811 - 7; http://dx.doi.org/10.4049/jimmunol.1102695; PMID: 22675215
- Hansen SG, Piatak M Jr., Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature 2013; 502:100 - 4; http://dx.doi.org/10.1038/nature12519; PMID: 24025770
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011; 473:523 - 7; http://dx.doi.org/10.1038/nature10003; PMID: 21562493
- Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 2010; 328:102 - 6; http://dx.doi.org/10.1126/science.1185350; PMID: 20360110
- Genescà M, Skinner PJ, Bost KM, Lu D, Wang Y, Rourke TL, Haase AT, McChesney MB, Miller CJ. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol 2008; 1:219 - 28; http://dx.doi.org/10.1038/mi.2008.6; PMID: 19079181
- Genescà M, Ma ZM, Wang Y, Assaf B, Qureshi H, Fritts L, Huang Y, McChesney MB, Miller CJ. Live-attenuated lentivirus immunization modulates innate immunity and inflammation while protecting rhesus macaques from vaginal simian immunodeficiency virus challenge. J Virol 2012; 86:9188 - 200; http://dx.doi.org/10.1128/JVI.00532-12; PMID: 22696662
- Lu W, Chen S, Lai C, Guo W, Fu L, Andrieu JM. Induction of CD8+ regulatory T cells protects macaques against SIV challenge. Cell Rep 2012; 2:1736 - 46; http://dx.doi.org/10.1016/j.celrep.2012.11.016; PMID: 23260669
- Watkins JD, Sholukh AM, Mukhtar MM, Siddappa NB, Lakhashe SK, Kim M, Reinherz EL, Gupta S, Forthal DN, Sattentau QJ, et al, CAVD Project Group. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS 2013; 27:F13 - 20; http://dx.doi.org/10.1097/QAD.0b013e328360eac6; PMID: 23775002
- Excler JL, Tomaras GD, Russell ND. Novel directions in HIV-1 vaccines revealed from clinical trials. Curr Opin HIV AIDS 2013; 8:421 - 31; http://dx.doi.org/10.1097/COH.0b013e3283632c26; PMID: 23743791
- Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, et al. A Blueprint for HIV Vaccine Discovery. Cell Host Microbe 2012; 12:396 - 407; http://dx.doi.org/10.1016/j.chom.2012.09.008; PMID: 23084910
- Saunders KO, Rudicell RS, Nabel GJ. The design and evaluation of HIV-1 vaccines. AIDS 2012; 26:1293 - 302; http://dx.doi.org/10.1097/QAD.0b013e32835474d2; PMID: 22706011
- Alam SM, Liao HX, Tomaras GD, Bonsignori M, Tsao CY, Hwang KK, Chen H, Lloyd KE, Bowman C, Sutherland L, et al. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J Virol 2013; 87:1554 - 68; http://dx.doi.org/10.1128/JVI.00718-12; PMID: 23175357
- Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 2011; 34:269 - 80; http://dx.doi.org/10.1016/j.immuni.2011.01.015; PMID: 21315623
- Leroux-Roels G, Maes C, Clement F, van Engelenburg F, van den Dobbelsteen M, Adler M, Amacker M, Lopalco L, Bomsel M, Chalifour A, et al. Randomized Phase I: Safety, Immunogenicity and Mucosal Antiviral Activity in Young Healthy Women Vaccinated with HIV-1 Gp41 P1 Peptide on Virosomes. PLoS One 2013; 8:e55438; http://dx.doi.org/10.1371/journal.pone.0055438; PMID: 23437055
- Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Clark KR. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med 2009; 15:901 - 6; http://dx.doi.org/10.1038/nm.1967; PMID: 19448633
- Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 2011; 481:81 - 4; http://dx.doi.org/10.1038/nature10660; PMID: 22139420
- Balazs AB, Ouyang Y, Hong CM, Chen J, Nguyen SM, Rao DS, An DS, Baltimore D. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med 2014; 20:296 - 300; http://dx.doi.org/10.1038/nm.3471; PMID: 24509526
- Létourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, Dorrell L, Dong T, Korber B, McMichael AJ, et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One 2007; 2:e984; http://dx.doi.org/10.1371/journal.pone.0000984; PMID: 17912361
- Borthwick N, Ahmed T, Ondondo B, Hayes P, Rose A, Ebrahimsa U, et al. Vaccine-elicited Human T Cells Recognizing Conserved Protein Regions Inhibit HIV-1. Mol Ther 2014; 22:464 75; http://dx.doi.org/10.1038/mt.2013.248; PMID: 24166483
- Koopman G, Beenhakker N, Nieuwenhuis I, Doxiadis G, Mooij P, Drijfhout JW, Koestler J, Hanke T, Fagrouch Z, Verschoor EJ, et al. DNA/long peptide vaccination against conserved regions of SIV induces partial protection against SIVmac251 challenge. AIDS 2013; forthcoming http://dx.doi.org/10.1097/QAD.0000000000000047; PMID: 24105029
- Stephenson KE, SanMiguel A, Simmons NL, Smith K, Lewis MG, Szinger JJ, Korber B, Barouch DH. Full-length HIV-1 immunogens induce greater magnitude and comparable breadth of T lymphocyte responses to conserved HIV-1 regions compared with conserved-region-only HIV-1 immunogens in rhesus monkeys. J Virol 2012; 86:11434 - 40; http://dx.doi.org/10.1128/JVI.01779-12; PMID: 22896617
- Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 2010; 16:319 - 23; http://dx.doi.org/10.1038/nm.2089; PMID: 20173752
- Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 2013; 155:531 - 9; http://dx.doi.org/10.1016/j.cell.2013.09.061; PMID: 24243013
- Penaloza-MacMaster P, Provine NM, Ra J, Borducchi EN, McNally A, Simmons NL, Iampietro MJ, Barouch DH. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J Virol 2013; 87:1373 - 84; http://dx.doi.org/10.1128/JVI.02058-12; PMID: 23152535
- Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, Hill AV, Cottingham MG. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One 2012; 7:e40385; http://dx.doi.org/10.1371/journal.pone.0040385; PMID: 22808149
- Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, Boente-Carrera M, Vittorino R, Caskey M, Andersen J, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One 2011; 6:e19252; http://dx.doi.org/10.1371/journal.pone.0019252; PMID: 21603651
- Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, Hural J, Lubeck M, Eldridge J, Cardinali M, et al, NIAID HIV Vaccine Trials Network. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One 2012; 7:e29231; http://dx.doi.org/10.1371/journal.pone.0029231; PMID: 22242162
- Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD 3rd, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med 2012; 18:1673 - 81; http://dx.doi.org/10.1038/nm.2934; PMID: 22961108
- Parks CL, Picker LJ, King CR. Development of replication-competent viral vectors for HIV vaccine delivery. Curr Opin HIV AIDS 2013; 8:402 - 11; http://dx.doi.org/10.1097/COH.0b013e328363d389; PMID: 23925000
- Liu L, Hao Y, Luo Z, Huang Y, Hu X, Liu Y, Shao Y. Broad HIV-1 neutralizing antibody response induced by heterologous gp140/gp145 DNA prime-vaccinia boost immunization. Vaccine 2012; 30:4135 - 43; http://dx.doi.org/10.1016/j.vaccine.2012.04.075; PMID: 22561314
- DeVico A, Fouts T, Lewis GK, Gallo RC, Godfrey K, Charurat M, Harris I, Galmin L, Pal R. Antibodies to CD4-induced sites in HIV gp120 correlate with the control of SHIV challenge in macaques vaccinated with subunit immunogens. Proc Natl Acad Sci U S A 2007; 104:17477 - 82; http://dx.doi.org/10.1073/pnas.0707399104; PMID: 17956985
- Shukair SA, Allen SA, Cianci GC, Stieh DJ, Anderson MR, Baig SM, Gioia CJ, Spongberg EJ, Kauffman SM, McRaven MD, et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol 2013; 6:427 - 34; http://dx.doi.org/10.1038/mi.2012.87; PMID: 22990624
- Fahrbach KM, Malykhina O, Stieh DJ, Hope TJ. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS One 2013; 8:e76176; http://dx.doi.org/10.1371/journal.pone.0076176; PMID: 24098437
- Fletcher PS, Elliott J, Grivel JC, Margolis L, Anton P, McGowan I, Shattock RJ. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 2006; 20:1237 - 45; http://dx.doi.org/10.1097/01.aids.0000232230.96134.80; PMID: 16816551
- Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev 2013; 254:225 - 44; http://dx.doi.org/10.1111/imr.12075; PMID: 23772623
- Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al, NISC Comparative Sequencing Program. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 2013; 496:469 - 76; http://dx.doi.org/10.1038/nature12053; PMID: 23552890
- Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 2013; 342:1477 - 83; http://dx.doi.org/10.1126/science.1245625; PMID: 24179159
- Haynes BF, Moody MA, Liao HX, Verkoczy L, Tomaras GD. B cell responses to HIV-1 infection and vaccination: pathways to preventing infection. Trends Mol Med 2011; 17:108 - 16; http://dx.doi.org/10.1016/j.molmed.2010.10.008; PMID: 21112250
- Sundling C, Martinez P, Soldemo M, Spångberg M, Bengtsson KL, Stertman L, Forsell MN, Karlsson Hedestam GB. Immunization of macaques with soluble HIV type 1 and influenza virus envelope glycoproteins results in a similarly rapid contraction of peripheral B-cell responses after boosting. J Infect Dis 2013; 207:426 - 31; http://dx.doi.org/10.1093/infdis/jis696; PMID: 23162135
- Streeck H, D’Souza MP, Littman DR, Crotty S. Harnessing CD4⁺ T cell responses in HIV vaccine development. Nat Med 2013; 19:143 - 9; http://dx.doi.org/10.1038/nm.3054; PMID: 23389614
- Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al, International AIDS Vaccine Initiative Protocol C Principal Investigators. Human circulating PD-⁺1CXCR3⁻CXCR5⁺ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013; 39:758 - 69; http://dx.doi.org/10.1016/j.immuni.2013.08.031; PMID: 24035365
- Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater 2011; 10:243 - 51; http://dx.doi.org/10.1038/nmat2960; PMID: 21336265
- Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A 2012; 109:1080 - 5; http://dx.doi.org/10.1073/pnas.1112648109; PMID: 22247289
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 2010; 33:241 - 53; http://dx.doi.org/10.1016/j.immuni.2010.07.015; PMID: 20691615
- Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends?. Nat Immunol 2011; 12:472 - 7; http://dx.doi.org/10.1038/ni.2019; PMID: 21739669
- Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med 2009; 206:1117 - 34; http://dx.doi.org/10.1084/jem.20082831; PMID: 19414559
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008; 105:7552 - 7; http://dx.doi.org/10.1073/pnas.0802203105; PMID: 18490657
- Tager AM, Pensiero M, Allen TM. Recent advances in humanized mice: accelerating the development of an HIV vaccine. J Infect Dis 2013; 208:Suppl 2 S121 - 4; http://dx.doi.org/10.1093/infdis/jit451; PMID: 24151317
- Esparza J. Solid vaccine protection against SIV in rhesus monkeys provides proof-of-concept for further evaluation of a novel HIV vaccine approach in humans. Expert Rev Vaccines 2012; 11:539 - 42; http://dx.doi.org/10.1586/erv.12.21; PMID: 22827240
- Schwartländer B, Stover J, Hallett T, Atun R, Avila C, Gouws E, Bartos M, Ghys PD, Opuni M, Barr D, et al, Investment Framework Study Group. Towards an improved investment approach for an effective response to HIV/AIDS. Lancet 2011; 377:2031 - 41; http://dx.doi.org/10.1016/S0140-6736(11)60702-2; PMID: 21641026
- World Health Organisation. Macroeconomics and Health: Investing in Health for Economic Development. Report of the Commission on Macroeconomics and Health. Geneva 2001.
- Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, Ahmed R, Bhan MK, Plotkin SA. Accelerating next-generation vaccine development for global disease prevention. Science 2013; 340:1232910; http://dx.doi.org/10.1126/science.1232910; PMID: 23723240