Abstract
Previously, we have reported that either CIM or PZQ, 2 clinical drugs, could be used to develop as adjuvants on HBV DNA vaccine to elicit both humoral and cellular immune responses. Here, we demonstrate that combinations of CIM and PZQ as adjuvants for a HBV DNA vaccine, could induce much stronger antigen specific CD4+ and CD8+ T cell responses compared either with CIM or PZQ alone. The synergistic effects of CIM plus PZQ to HBV DNA vaccine were observed on a higher IgG2a/IgG1 ratio, an increase of HBsAg-specific CD4+ T cells capable of producing IFN-γ or IL-17A and a robust IFN-γ-, IL-17A-, or TNF-α-producing CD8+ T cells to HBsAg. Most importantly, the antigen-specific CTL response was also elevated significantly, which is critical for the eradication of hepatitis B virus (HBV) infected cells. Using an HBsAg transgenic mouse model, the expression of HBsAg in the hepatic cells was also significantly reduced after immunized with pCD-S2 in the presence of 0.5% CIM and 0.25% PZQ. Further investigations demonstrated that the synergistic effects of combination of CIM and PZQ were dependent on enhanced cytotoxic CD8+ T cells, which was correlated with impaired activities of regulatory T cells. Therefore, combinations of CIM and PZQ have great potential to be used as effective adjuvants on DNA-based vaccinations for the treatment of chronic hepatitis B.
Introduction
Hepatitis B is one of the most prevalent and serious liver diseases, which was caused by the hepatitis B virus (HBV) infection. At present, there are more than 350 million people suffering from chronic hepatitis B in the world and 15–25% of these infected persons are at high risk of developing serious liver damages, including liver cirrhosis, hepatocellular carcinoma, and even death.Citation1,Citation2 Fortunately, we have safe and effective recombinant hepatitis B surface antigen (HBsAg) vaccines to protect newborns and uninfected individuals from HBV infection.Citation3 In addition, the availability of immunomodulators (interferon α, thymosin α1) and potent nucleoside analogs (such as lamivudine, adefovir, entecavir, etc.) treatments mark a new era in the control of chronic hepatitis B.Citation4-Citation6 However, the therapeutic efficacy of IFN-α is quite limited,Citation7 and long-term treatments with nucleoside analogs appears to show increased rates of resistance to antiviral drugs and other severe side effects.Citation6 In particular, the failure of traditional antiviral strategies to eradicate the HBV infected cells highlights the need for novel therapeutic vaccines development.
DNA vaccines encoding a viral protein represent an attractive strategy to fight against various infectious diseases caused by HBV, human immunodeficiency virus (HIV) and influenza virus infection.Citation8-Citation10 In contrast to commercial recombinant HBsAg vaccines, DNA-based vaccination have great advantages in the ability to induce both humoral and cellular immune responses, which is of great important for eliminating virus infected cells.Citation11,Citation12 Whereas, the relatively weak immune responses in human clinical trials have limited its application,Citation13 which attract our attention on developing potent effective adjuvants to improve the therapeutic efficacy of HBV DNA vaccines.
Because of the relative safety and mild side effects, some small chemical molecules have shown promise to be used as an adjuvants to stimulate the immune responses of DNA based vaccination.Citation14-Citation16 Praziquantel (PZQ), an effective anthelmintic drug against all forms of schistosomiasis,Citation17,Citation18 is explored as an adjuvant for HBV DNA vaccines to facilitate virus specific Tc1and Tc17 responses via the decreased expression of TGF-β from T regulatory cells.Citation19,Citation20 In addition, cimetidine (CIM), a drug that is prescribed for the treatment of heartburn and peptic ulcers,Citation21 is a H2-receptor antagonist, which also could be used as an adjuvant for HBV DNA vaccines to increase the secretion pro-inflammatory cytokines and decrease the production of anti-inflammatory cytokine, IL-10.Citation22-Citation24 Interestingly, CIM also could increase the bioavailability of PZQ due to its ability to reduce the enzyme activities of cytochrome P450, which is crucial for the metabolism of PZQ.Citation25-Citation27 These observations prompted us to investigate whether CIM could act synergistically with PZQ to further enhance the immune responses on HBV DNA vaccine.
In this study, we evaluated the adjuvant effects of CIM plus PZQ on humoral and cellular immune responses triggered by immunization with HBV DNA vaccines (pCD-S2) that encodes hepatitis B surface antigen pre-S2 and S regions. Compared with CIM or PZQ alone, combination of CIM and PZQ further impaired the suppressive abilities of Tregs and significantly enhanced HBsAg-specific CTL response, which is mainly mediated by IFN-γ and IL-17A producing CD8+ T cells. In addition, the therapeutic efficacy of DNA based vaccination in the cooperation of CIM plus PZQ was further confirmed in an HBsAg-Tg mouse model. Therefore, combination therapy with HBV DNA vaccine plus 2 or more adjuvants proposed to be potential immunotherapeutic approaches for the treatment of chronic hepatitis B.
Results
HBsAg-specific humoral immune responses
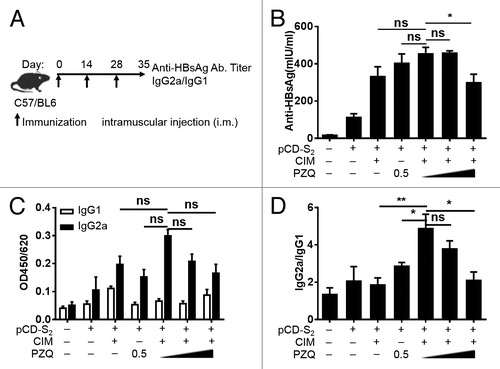
Hepatitis B surface antibodies (anti-HBs) are a key serological marker that can be used to monitor the vaccine-induced humoral immune responses. To evaluate whether combination of CIM and PZQ as adjuvants for DNA vaccine could induce better humoral response than mice immunized with pCD-S2 and either one, WT C57BL/6 mice were immunized with pCD-S2 DNA vaccine in the presence or absence of CIM, PZQ, or both (), and the immunization schedule was shown in . Seven days after the third immunization, serum IgG, IgG2a, and IgG1 antibodies against HBsAg were measured by quantitative ELISA method. Compared with the pCD-S2 immunized group, the levels of HBsAg-specific IgG were significantly increased in the presence of CIM, PZQ, or both. Although, combination of CIM and PZQ as adjuvants did not further increase the production of total IgG or IgG2a compared with mice treated with either of them (). The ratio of IgG2a/IgG1 was dramatically elevated in the group of pCD-S2 with 0.5% CIM and 0.25% PZQ, suggesting a Th1 biased response ().
Table 1. Immunization groups
Figure 1. Effects of CIM plus PZQ as adjuvants on humoral response. Naive mice were immunized (i.m.) with pCD-S2 in the presence or absence of CIM, PZQ, or combination of CIM and different concentration of PZQ (0.25%, 0.5%, and 1.0%). The immunization schedule was shown in (A). Serum samples from different groups were collected and detected with ELISA kits on day 7 after the third immunization. (B) The HBsAg-specific total IgG were measured and quantified according to the standard concentration of anti-HBsAg antibodies. (C) The HBsAg-specific IgG1 and IgG2a antibodies were also measured using the ELISA method and the OD values were shown in the results. (D) The IgG2a/IgG1 ratios were calculated and presented. The data are shown as mean ± SEM from 3 independent experiments. *P < 0.05 and **P < 0.01 (unpaired Student’s t test).
Combination of CIM and PZQ induced stronger DTH response and T cell proliferation activities
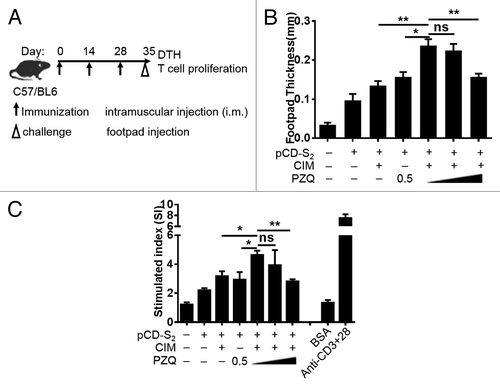
Delayed-type hypersensitivity (DTH) is a simple in vivo assay of cell-mediated immune function.Citation28 To test the effects of CIM plus PZQ on cell-mediated responses to pCD-S2 DNA vaccine, the mice were immunized the same as before and challenged with HBsAg on their footpads on day 7 after the third immunization. The thickness of footpad was measured and quantified at 48 h after the challenge (). As shown in , mice immunized with pCD-S2 with the combinations of CIM and PZQ induced stronger HBsAg specific DTH response significantly compared with mice treated with pCD-S2 in the presence of either one. Meanwhile, the proliferation capability of antigen specific T cells was also enhanced after immunized with pCD-S2 and the combinations of CIM and PZQ (). In addition, the cell-mediated immune responses were inhibited when higher concentration of PZQ was used, indicating a dose dependent response ().
Figure 2. Combination of CIM and PZQ as adjuvants on cell mediated immunity. (A) WT C57BL/6 mice were immunized 3 times with the plasmid pCD-S2 in the presence or absence of CIM or PZQ, or combination of CIM and different dosages of PZQ. On day 7 after the third immunization, mice in different groups were challenged with 10 μg HBsAg in the right footpad and the left footpad were injected with PBS as control. (B) The thickness of the footpad was measured at 48 h after challenge. (C) Single splenocytes were isolated from another 5 animals in different groups on day 7 after the third immunization and stimulated with 10 μg/mL HBsAg in the presence of 0.5 μg/mL anti-CD28 for 72 h. T cell proliferation was analyzed using MTT method. Data represent 3 independent experiments with similar results (means ± SEM), *P < 0.05 and **P < 0.01 (unpaired Student’s t test).
Co-administration of CIM and PZQ with pCD-S2 DNA vaccine enhanced HBsAg-specific CD4+ T cell response
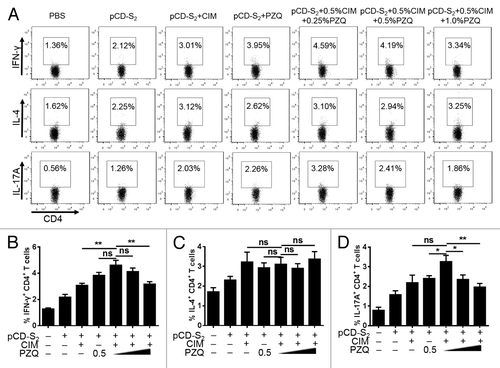
To further investigate the synergistic effects of CIM plus PZQ on HBsAg-specific CD4+ T cell responses, single suspension of splenocytes was prepared from mice on day 7 after the third immunization and re-stimulated with 10 μg/mL HBsAg in vitro. Then, intracellular stainings were performed to measure the production of IFN-γ, IL-4, and IL-17A in CD4+ T cells. As shown in , IFN-γ-producing CD4+ T cells was significantly increased after immunized with pCD-S2 DNA vaccine in the presence of 0.5% CIM and 0.25% PZQ compared with the mice treated with pCD-S2 plus CIM alone and a higher IL-17A-producing CD4+ T cells was also observed after co-administration of 0.5% CIM and 0.25% PZQ with pCD-S2 DNA vaccine in contrast to the mice treated with pCD-S2 and PZQ (), whereas, the effects on IL-4-producing CD4+ T cells were minimal (). These results demonstrated that the combinations of CIM and PZQ elicited stronger CD4+ T cell mediated immune response triggered by the pCD-S2 DNA vaccinations.
Figure 3. Effects of the combination of CIM and PZQ on CD4+ T cell responses. WT mice were immunized 3 times with the plasmid pCD-S2 in the presence or absence of CIM or PZQ, or combination of CIM and PZQ. Single splenocytes were isolated from mice in different groups on day 7 after the third immunization and stimulated with 10 μg/mL HBsAg in the presence of 0.5 μg/mL anti-CD28 for 24 h. The protein transport inhibitor Brefeldin A was added into the culture medium in the last 6 h. Intracellular staining for IFN-γ, IL-4, and IL-17A in CD4+ T cells was analyzed by flow cytometer (A). The percentage of IFN-γ (B), IL-4 (C), and IL-17A (D) in CD4+ T cells are shown. The data are the representation of 3 independent experiments (means ± SEM), *P < 0.05 and **P < 0.01 (unpaired Student’s t test).
Combination of CIM and PZQ with pCD-S2 DNA vaccine could stimulate stronger HBsAg-specific CD8+ T cell mediated cytotoxic response
CD8+ T effector cells suggested to have the ability to lyse virus infected hepatocytes, which is important for the efficient control of chronic HBV infection.Citation29 To assess if combinations of CIM and PZQ could augment HBsAg-specific CD8+ T-cell responses to pCD-S2 DNA vaccine, mice were immunized as previously described () and their productions of IFN-γ, IL-17A, and TNF-α in CD8+ T cells were examined on day 7 after the third immunization. We observed that the combination of CIM and PZQ synergistically increased the frequency of IFN-γ-producing and TNF-α-producing CD8+ T cells significantly () compared with the mice immunized with pCD-S2 with either of adjuvants. While, the production of IL-17A in CD8+ T cells was relatively enhanced in contrast to mice treated with pCD-S2 and CIM alone and no significant changes was observed between PZQ alone and the combination of CIM and PZQ ().
Figure 4. Effects of the combination of CIM and PZQ on CD8+ T cell responses. Single splenocytes were isolated from mice in different groups on day 7 after the third immunization and stimulated with 10 μg/mL S208–215 in the presence of 0.5 μg/mL anti-CD28 for 12 h. The protein transport inhibitor Brefeldin A was added into the culture medium in the last 6 h. Intracellular staining for IFN-γ, IL-17A, and TNF-α in CD8+ T cells was analyzed by flow cytometer (A). The percentage of IFN-γ (B), IL-17A (C), and TNF-α (D) in CD8+ T cells are shown. (E) Syngeneic splenocytes were prepared from naïve mice and divided into 2 parts. One was pulsed with HBsAg specific peptide S208–215, labeled with 20 μM of CFSE and termed as CFSEhigh cells, the other was loaded with non-specific peptide OVA257–264, labeled with 1 μM of CFSE and defined as CFSElow cells. The equal number of cells from the 2 parts were mixed and injected intravenously into recipient mice on day 7 after the third immunization, which were pretreated with pCD-S2 DNA vaccine in the presence or absence of CIM or PZQ, or both of CIM and PZQ. Twelve hours later, the splenocytes from recipient mice were used to examine the antigen specific cytolytic responses. One representative results is shown (E), and the percentage of specific lysis was summarized (F). Results are mean ± SEM, *P < 0.05, **P < 0.01, and ***P < 0.001 (unpaired Student’s t test).
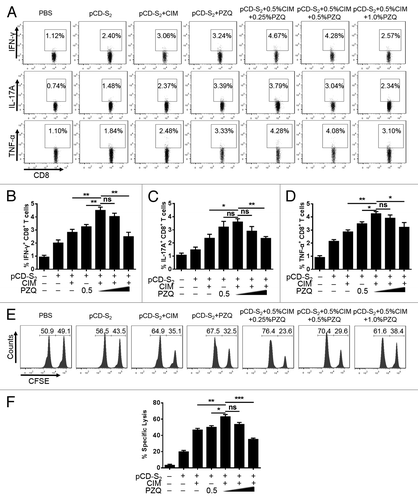
Next, we evaluated if combinations of CIM and PZQ have an effect on HBsAg-specific cytotoxic response using an in vivo CTL method. As shown in , the combination of CIM and PZQ synergistically induced stronger cytotoxic response significantly compared with mice immunized with pCD-S2 DNA vaccine in the presence of either of them. Taken together, these results indicated that CIM and PZQ could act synergistically to enhance CD8+ T cell mediated immune response triggered by the immunization of pCD-S2 DNA vaccine.
The cytotoxic response is dependent on IFN-γ- and IL-17A-producing CD8+ T cells
To further determine which subpopulations of T cell was responsible for the augmented CTL response, CD8KO mice were immunized 3 times with pCD-S2 in the combination of 0.5% CIM and 0.25% PZQ. While, the WT mice were treated under the same protocol and injected with anti-CD3 or anti-CD4 neutralizing antibodies, respectively after the final immunization (). The depletion efficacy was confirmed by cell surface staining before an in vivo CTL assay (). We found that the CTL activities were completely abolished when the CD3+ or CD8+ T cells were depleted. Lack of CD4+ T cells seemed to have a little impact on the CTL response ().
Figure 5. CD8+ T cells are critical for the elevated antigen specific cytotoxic response. As shown in (A), naïve mice were immunized 3 times the same as previously described and 100 μg specific neutralizing antibodies against CD3, CD4, or CD8 were injected intraperitoneally on day 28, 31, and 35. (B) The depletion efficacy was detected by cell surface staining and analyzed on flow cytometer. (C) The ability of antigen specific in vivo cytotoxic lysis was analyzed on day 35 after immunization using the same protocol as before. (D) CD8+ T cells were purified from mice immunized 3 times with pCD-S2 in the presence of 0.5% CIM plus 0.25% PZQ and used as effector T cells. Hepatocytes from HBsAg-Tg mice were isolated and labeled with 20 μM CFSE as target cells. The effector cells were mixed with target cells at a 10:1 ratio and co-cultured for 3 d. (E) The specific lysis was analyzed by flow cytometer. Data are representative of 3 independent experiments (mean ± SEM), *P < 0.05 and **P < 0.01 (unpaired Student’s t test).
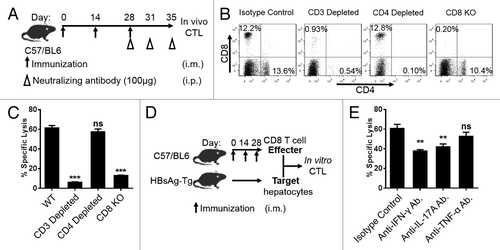
It has been demonstrated that cytokines (such as IFN-γ, IL-17A, and TNF-α) produced by activated CD8+ T cells were critical for their CTL responses. To confirm which cytokine is more essential for the above CD8+ T cells mediated cytotoxic responses, a in vitro CTL assay was performed as described in the section of Materials and Methods and . Compared with the isotype control antibody treated samples, the CTL response was reduced significantly after blocked with IFN-γ or IL-17A specific antibodies separately. Nevertheless, the cytolysis activity was not affected when treated with anti-TNF antibodies (). These results indicated that the achievement of antigen specific CTL activities by the combination of CIM and PZQ with pCD-S2 DNA vaccine are largely dependent on the activities of IFN-γ and IL-17A producing CD8+ T cells.
Combination of CIM and PZQ with pCD-S2 DNA vaccine decreased the frequency and suppressive function of Treg
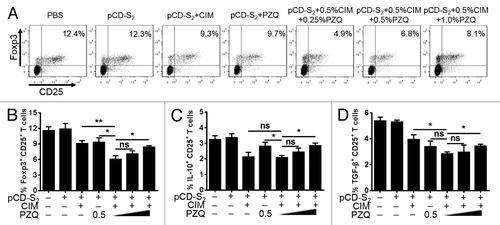
Several studies have demonstrated that CD4+CD25+ T regulatory cells (Treg) could suppress the function of effector T cells and contribute to the impaired immune response in chronic HBV infected patients.Citation30,Citation31 In addition, depletion of Tregs with anti-CD25 mAb showed enhanced HBV-specific CD8+ T cell response.Citation32 Here, we evaluated if the number and suppressive activities of Tregs were affected by the combination of CIM and PZQ with pCD-S2 DNA vaccine. As shown in , mice were divided into 7 groups and immunized 3 times with different immunization regimens. Seven days after the final immunization, single suspension splenocytes were prepared from immunized mice and used to test the frequency of CD4+CD25+Foxp3+ Tregs and the expression of IL-10 and TGF-β in CD4+CD25+ Tregs by intracellular staining assay. The results showed that the combination of CIM and PZQ could not only decreased the percentage of CD4+CD25+Foxp3+ Tregs (), but also impaired the production of IL-10 and TGF-β in CD4+CD25+ T cells, which is important for the inhibitory function of Tregs, when compared with mice treated with pCD-S2 DNA vaccine in the presence of either of them (). Moreover, the additive effects of CIM plus PZQ on Tregs were dependent on the concentration of PZQ ().
Figure 6. Effects of the combination of CIM and PZQ as adjuvants on regulatory T cells. WT C57BL/6 mice were immunized 3 times with the plasmid pCD-S2 in the presence or absence of CIM or PZQ, or both of them, respectively. Seven days after the final immunization, single splenocytes were prepared from different groups and stained with fluorescence-labeled monoclonal antibodies against CD4, CD25, Foxp3, IL-10, and TGF-β. The percentage of Foxp3 in CD4+CD25+ T cells (A, B), IL-10 in CD4+CD25+ T cells (C), and TGF-β in CD4+CD25+ T cells (D) is analyzed by flow cytometer. Data are shown means ± SEM and represent one of 3 independent experiments. *P < 0.05 and **P < 0.01 (unpaired Student’s t test).
Combination of CIM and PZQ with pCD-S2 DNA vaccine could break immune tolerance in HBsAg-Tg mice
HBsAg-transgenic mice, which were engineered to produce HBV surface antigen, have been used to mimic the features of chronic hepatitis in humans.Citation33 To investigate the therapeutic effects of the combination of CIM and PZQ as adjuvants to the pCD-S2 DNA vaccine, the HBsAg-Tg mice were immunized with pCD-S2 in the presence or absence of 0.5% CIM, 0.5% PZQ, or combination of 0.5% CIM and 0.25% PZQ. Seven days after the third immunization, the livers were isolated from different groups and lymphocyte infiltrations in the livers were examined by H&E staining method. Compared with the mice treated with pCD-S2 in the presence of either CIM or PZQ, we observed much more lymphocytes infiltrated into the livers after the combination of CIM and PZQ as adjuvants (). The infiltrated lymphocytes seem to be CD8+ T cells as detected with anti-CD8 specific antibodies using immunohistochemical (IHC) staining method (). Importantly, no obvious liver damages were observed as a normal liver morphology with these infiltrated CD8+ T cells. Significantly, the HBsAg-secretion hepatocytes were reduced after the combination of CIM and PZQ with pCD-S2 DNA vaccinations, which is consistent with the observed level of CD8+ T cell infiltrations (). However, the concentration of serum HBsAg almost had no changes in different groups ().
Figure 7. The therapeutic efficacy of combination of CIM and PZQ with pCD-S2 DNA vaccine in HBsAg-Tg mice. The transgenic mice were immunized with pCD-S2, pCD-S2/0.5%CIM, pCD-S2/0.5%PZQ or pCD-S2/0.5%CIM/0.25%PZQ. Livers from transgenic mice were prepared on day 7 after third immunization, fixed, sectioned, and stained with H&E for histological evaluation. Immunohistochemistry results demonstrated CD8-specific T cells or HBsAg-expressing hepatocytes. Representative images are showed (A). (B) The HBsAg-staining was quantified using Image-Pro Plus software and expressed by integrated option density (IOD). (C) The concentration of serum HBsAg was measured on day 7 after the third immunization. (D) The activities of ALT in the serum on day 7 after the third immunization Data were shown as mean ± SEM and analyzed by the unpaired Student t test. *P < 0.05 and **P < 0.01.
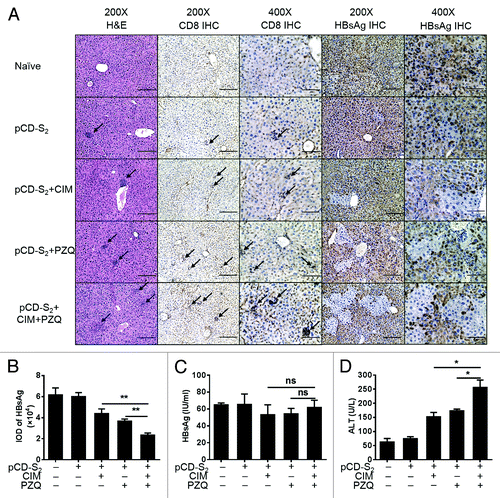
Serum alanine aminotransferase (sALT) is commonly used as a diagnostic evaluation of liver injury, which is released into the circulation by lysed hepatocytes. To confirm whether the decrease of HBsAg-producing hepatic cells attributed to the cytolytic ability of infiltrated CD8+ T cells, the level of sALT was measured on day 7 after the third immunization. Compared with mice treated with pCD-S2 in the presence of either CIM or PZQ, an elevated level of sALT was observed in mice receiving the pCD-S2 DNA vaccine in the presence of the combined CIM and PZQ ().
Discussion
HBV infected patients, especially those with chronic hepatitis B, tend to establish a largely tolerogenic environment.Citation34 It has been demonstrated that the immune response of those patients are too weak to control or eliminate the virus infected cells, resulting in persistent infection.Citation35,Citation36 Using an HBsAg-Tg mouse model, we found that immunization of a plasmid pCD-S2 in the cooperation of CIM and PZQ was sufficient to break the immune tolerance in transgenic mice (). The enhanced antiviral responses ascribe to empowered CD8+ T cells, which could induce the destruction of virus-infected cells both in vivo and in vitro ( and ). Further, these cytotoxic CD8+ T cells are also strongly associated with viral clearance in HBV infected humans.Citation37-Citation39
According to their cytokine-secretion profiles, CD8+ T cells can be categorized into distinct subsets, including IFN-γ-producing or TNF-α-producing T cells (Tc1), IL-4-producing T cells (Tc2), and IL-17A-producing T cells (Tc17).Citation40,Citation41 Recent studies have shown that IFN-γ and TNF-α contributed to the antiviral abilities of CD8+ T cells through cytolytic and noncytolytic pathways in the HBV-transgenic mouse modelCitation42,Citation43 or other virus infected diseases.Citation44 In addition, the inhibition of viral replication and the specific destruction of virus-infected cells was significantly reduced in IFN-γ knockout mice.Citation19,Citation45 The cytotoxic effect of IFN-γ was thought mainly via directly induction the secretion of Perforin and granzyme B to lyse the target cells, upregulation of Fas expression on hepatocytes and enhanced expression of death related genes or downregulation of anti-apoptotic molecules.Citation46-Citation48 While, Tc17 cells which are responsible for CD8+ T cell mediated CTL response, displayed protective roles against various infectious diseasesCitation49 and in the cancer immunotherapy.Citation50 A causal role for Tc17 in the efficient control of chronic HBV infection was also previously exhibited in HBsAg transgenic mouse model by using IL-17A knockout miceCitation51 or adoptively transfer antigen specific Tc17 cells.Citation19 Here we demonstrated that combination of CIM and PZQ as adjuvants for DNA based vaccination could significantly induce the Tc1 and Tc17 cell subsets (), which is critical for the stronger cytotoxic response. In addition, the results from in vitro CTL assay provide strong support for the concept that IFN-γ and IL-17A are mainly responsible for the CD8+ T cell mediated cytotoxic response (). However, the exact mechanisms of CD8+ T cell mediated cytolytic effect still need our further investigation.
T regulatory cells (Tregs) have been described as CD4+CD25+Foxp3+ T cells, which involves the suppression of immune responses and maintenance of peripheral tolerance.Citation52 Growing evidence reported that a higher frequency of CD4+CD25+ regulatory T cells were associate with the weak immune responses in chronic hepatitis B virus infected patientsCitation53 and depletion of Tregs could activate the function of HBV-specific CD8+ T cells.Citation32 Moreover, the suppressive activity of Treg was mainly mediated through the secretion of inhibitory cytokines including IL-10 and TGF-β, the granzymes and perforin-dependent cytolysis pathway, the metabolic disruption of the effector T cells, and modulate the maturation or function of dendritic cells.Citation54 The previous data revealed that CIM, a prescribed drug for peptic ulcer, could inhibit the production of anti-inflammatory cytokine IL-10 via modulation the interaction of histamine with H2 receptors.Citation55 While, PZQ, another prescribed drug for the treatment of Schistoma japonicum infection, have been reported to impair the function of Treg via inhibition TGF-β/Smad2,3 signaling pathway.Citation56,Citation57 Here we found that the frequency and function of CD4+CD25+ Tregs was significantly impaired in the splenocytes of mice immunized with the plasmid pCD-S2 in the presence of 0.5% CIM and 0.25% PZQ, which is responsible for the elevated adaptive immunity (). In addition, several studies have reported that decreased frequency of Treg also results in significantly higher Th17 cells in HBV-infected patients,Citation58-Citation60 which is consistant with our results (). While the biological function of these activated Th17 cells should be well characterized in future study.
Previously, we have confirmed that CIM, as an adjuvant, have the ability to induce DC activition and promote the secretion of pro-inflammatory cytokine IL-12 via the suppression of anti-inflammatory cytokine IL-10.Citation24,Citation61 While, adjuvant activity of PZQ was mainly to enhance CD8+ T cell mediated CTL response through inhibition of TGF-β.Citation57 However, in chronic HBV infection and other chronic infectious diseases, both innate and adaptive immunity are simultaneously required for their efficient therapy. The obvious advantages to combine CIM with PZQ are described as follows: (1) the combination of CIM and PZQ could develop much more cost-effective stategies to fight against chronic infectious diseases due to CIM’s ability to increase the bioavailability of PZQ and (2) they could induce better therapeutic effects by complementing each other in the way to impair the function of Tregs and enhance CD8 functions. Importantly, aluminum is still the most common adjuvant used in human and the relative safety of CIM and PZQ make them have great potential to be used as effective adjuvants for the development of therapeutic vaccines against chronic infections.
Materials and Methods
Animals and reagents
Female 6–8 wk old C57BL/6 wild type mice were purchased from the Shanghai SLAC Laboratory Animal Co. LTD. In addition, 12-wk-old transgenic mice designed as Tg (Alb-1HBV)44Bri/JCitation62 were ordered from the animal center of Shanghai Public Health Clinical Center. All mice were kept under specific pathogen-free conditions at the Fudan University and handled according to the animal welfare guidelines for experimental animals.
Cimetidine (Sigma) was initially dissolved in 0.15 M hydrochloric acid and then adjusted to neutral pH with 1M sodium hydroxide and subsequently diluted to 0.5% with phosphate-buffered saline (PBS). Praziquantel was purchased from China North Pharmaceutical Group Corporation and dissolved in ethanol to 6.7% as previously described.Citation19 Subsequently, the dissolved PZQ was diluted into 0.25%, 0.5%, and 1.0% with PBS. The purified HBsAg were purchased from Shanghai Guikang Biotechnology, Ltd. The HBsAg specific CTL epitopes S208–215 (ILSPFLPL; H-2b-restricted) and OVA derived CTL peptide OVA257–264 (SIINFEKL; H-2b-restricted) were synthesized by Scipeptide Biotechnology, Ltd.
Plasmid construction and preparations
The plasmid pcDNA3.1-S2 (pcD-S2) was constructed as previously describedCitation63 and stored in our lab. Briefly, coding sequences for HBV surface antigen preS2 and S were inserted into EcoRI/KpnI digested pcDNA3.1 vector (Invitrogen) and the identity of the plasmid was verified by sequencing. Subsequently, plasmid DNA were prepared using the EndoFree Plasmid Maxi kit (Qiagen Inc.) according to the manufacture’s instruction.
Immunization
The C57BL/6 mice were randomly divided into 7 groups (5 mice per group) and immunized intramuscularly with 100 μg pcD-S2 alone, 100 μg pCD-S2 in the presence of CIM or PZQ, or 100 μg pCD-S2 premixed with 0.5% CIM plus different concentrations of PZQ (0.25%, 0.5%, and 1.0%) on days 0, 14, and 28. As shown in .
T cell proliferation assay
Single splenocytes suspensions were prepared from mice in different groups on day 7 after the third immunization. Two × 105 cells were added to each well in 96-well, flat-bottom culture plates in 100 μL volume and stimulated with HBsAg (10 μg/mL) at 37 °C 5% CO2. And 10 μg/mL BSA (irrelevant antigen) or Anti-CD3 (1 μg/mL) + Anti-CD28 (0.5 μg/mL) was used as positive control. After 48 h stimulation, 20 μL of MTT (5mg/mL) was added to each well and incubated for another 4 h. The plates were centrifuged at 1500 rpm for 5 min, the supernatants were removed carefully by pipetting, and then 150 μL of DMSO (Sigma) was added to each well to dissolve the crystal. The absorbance was measured at 570 nm in the xMark Microplate spectrophotometer (Bio-Rad Laboratories).
Enzyme-linked immunosorbent assays (ELISA)
To detect anti-HBsAg-specific IgG, IgG2a, and IgG1 antibodies, serum samples were obtained from mice in different groups on day 7 after the third immunization and determined by the quantitative ELISA method. The 96-well plates were coated with 2 μg/mL of HBsAg in a coating buffer (50 mM carbonate-bicarbonate buffer, pH 9.6) at 4 °C overnight in 100 μL volume. Then, the plates were washed 4 times with PBST and blocked with 5% of BSA in PBST for 1 h at 37 °C. After 4 washes, the serial diluted HBsAb standards or serum samples (100 μL/well) from individual mice were incubated for 1 h at 37 °C. After 4 washes, 1:5000 diluted horseradish peroxidase conjugated goat anti-mouse IgG, IgG2a, and IgG1 antibodies was added to corresponding wells and the plates were incubated at 37 °C for another 1 h. Then, after 8 washes, 100 μL of TMB dissolved in 0.025 M phosphate-citrate buffer was added to each well and the reaction was stopped by the addition of 50 μL of 2 M H2SO4. The optical density (OD) at 450/620nm was then measured in the xMark Microplate spectrophotometer (Bio-Rad Laboratories). The anti-HBsAb titers were expressed in milli international units per mL (mIU/mL) and determined by comparison with a standard curve generated from measurement of the HBsAb standards from Beijing Kinghaw Biological Pharmacy Enterprise Co. Ltd. All serum samples were determined in triplicates.
In vivo depletion of CD3- and CD4- specific T Cells
Specific lymphocyte subsets were depleted by intraperitoneal injection of specific monoclonal antibodies. Briefly, C57BL/6 mice were injected intraperitoneally with anti-mouse CD3 (17A2), anti-mouse CD4 (GK1.5), or rat IgG2a κ chain isotype control antibody (eBR2a) in 200 μL phosphate-buffered saline (PBS) on days 0, 4, and 6 after the third immunization of pcD-S2 plus CIM and PZQ. Depletion efficiency was measured by cell surface staining with APC-Cy7 labeled anti-mouse CD3 mAb (17A2), APC labeled anti-mouse CD8 mAb (53–6.7) and FITC labeled anti-mouse CD4 mAb (GK1.5), then the stained cells were detected on LSR Fortessa (BD Biosciences). The depletion efficiencies were greater than 90%. All antibody used were purchased from eBioscience.
Delayed-type hypersensitivity assay
All mice in different groups were injected with HBsAg (10 μg/50 μL) in the right footpad as test and saline solution in the left footpad as negative control on day 7 after the third immunization. After 48 h, the thicknesses of footpads were measured with a micrometer and calculated by the following formula: Footpad Thickness = thickness of right footpad − thickness of left footpad.
Intracellular cytokines staining
Single splenocyte suspensions were prepared from mice on day 7 after the third immunization and 1 × 106 cells were added to 96-well, round-bottom culture plates in 100 μL volume. For detection cytokines secreted from CD4+ or CD8+ T cells, splenocytes were stimulated with HBsAg (10 μg/mL) or S208–215 (10 μg/mL) for 24 h at 37 °C, 5% CO2 in the presence of anti-CD28 (0.5 ug/mL). The phorbol 12-myristate 13-acetate (PMA) and ionomycin were used as a positive control. In the last 6 h of incubation, protein transport inhibitor containing 3 μg/mL Brefeldin A (BD Biosciences) was added into the culture medium. After stimulation, intracellular cytokines staining was performed as previously described. Briefly, cells were pre-stained with surface markers (such as CD3, CD4, or CD8) specific monoclonal antibodies at room temperature for 20 min. After washes, cells were fixed with 4% paraformaldehyde and then permeabilized with 0.1% saponin (Sigma-Aldrich). For immunostaining of cytoplasmic IFN-γ, IL-4, IL-17A, and TNF-α, the appropriate fluorescent-labeled anti-mouse monoclonal antibodies were added and stained on ice for 3 h. Finally, cells were washed with PBS and acquired on BD Fortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo software (Tree Star). All fluorescent-labeled antibodies used were purchased from eBioscience.
Cytotoxic lysis assay
For in vivo CTL, splenocytes from syngeneic naive C57BL/6 mice were divided into 2 parts. One part was pulsed with 10−6 M HBsAg-derived peptides S208–215 and labeled with 20 μM of CFSE (defined as CFSEhigh target cells). The other part was pulsed with 10−6 M OVA-derived peptides OVA257–264 and labeled with 1μM of CFSE (defined as CFSElow cells) as a non-target control. Cells from the 2 parts were mixed in a 1:1 ratio and injected into immunized recipient mice at 2 × 107 total cells per mouse via the tail vein on day 7 after the final immunization. Eight hours later, splenocytes were isolated from the recipients and their differential CFSE fluorescent intensities were measured with a BD FACS LSR Fortessa (BD Biosciences). Specific lysis was calculated using the following formula: Percentage specific lysis = (1 − [ratio unprimed/ratio primed] × 100), where ratio = percentage CFSElow/percentage CFSEhigh.
For in vitro CTL, the splenocytes were prepared from mice on day 7 after the third immunization and the effector CD8+ T cells were purified with MagCellect mouse CD8+ T cell isolation kit (R&D Systems) according to the manufacturer’s instructions. Subsequently, the collagenase digested hepatic cells from untreated HBsAg-Tg mice were labeled with 20 μM of CFSE and used as target cells. After washing with RPMI1640, the purified effector CD8+ T cells were co-cultured with target cells (E:T = 10:1) in the presence of different neutralizing antibodies against IFN-γ (XMG1.2), IL-17A (eBio17CK15A5), and TNF-α (MP6-XT3) or isotype control in the 96-well plate at 37 °C, 5% CO2. After 48 h co-culture, cells were washed and measured on BD Fortessa flow cytometer (BD Biosciences). Decreases in the frequency and intensity of CFSE labeled cells were used to calculate the cytolysis ability. All neutralizing antibodies were purchased from eBioscience.
Histology and immunohistochemistry (IHC)
Seven days after the final immunization, the livers from the immunized HBsAg-Tg mice were collected and fixed in 4% neutral buffered paraformaldehyde (Sigma-Aldrich). Following fixation, the liver was trimmed, embedded in paraffin, cut into about 4 µm sections, and stained with hematoxylin-eosin (H&E) for histopathological evaluations.
For IHC staining, unstained slides were prepared from formalin-fixed, paraffin-embedded tissues. Antigen retrieval using EDTA was conducted before incubation with anti-mouse CD8 mAb and anti-HBsAg mAb. Secondary antibody incubation was performed with horseradish peroxidase-conjugated goat anti-mouse IgG. The images were captured with an EVOS XL light microscope (Advance microscopy group) to determine the histological changes.
Statistical analysis
Statistics were performed using GraphPad Prism Software 6.0 (GraphPad) and presented as mean ± SEM. An unpaired student’s t test analysis was used for all data analysis. *P < 0.05 indicates statistical significance.
| Abbreviations: | ||
| CIM | = | cimetidine |
| PZQ | = | Praziquantel |
| HBV | = | hepatitis B virus |
| HBsAg | = | hepatitis B virus surface antigen |
| Treg | = | T regulatory cell |
| DTH | = | delayed-type hypersensitivity |
| CTL | = | cytotoxic T lymphocytes |
| TNF-α | = | tumor necrosis factor-α |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by the National High-Tech 863 Project of China (2012AA02A407 and 2012AA02A406) and Department of Scientific and Technology of Shanghai Municipal Government (2013QLG009) to BW. We thank Dr Jane Q.L. Yu and Mr Zhonghuai He for their assistance in this work.
References
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005; 5:215 - 29; http://dx.doi.org/10.1038/nri1573; PMID: 15738952
- Lok AS. Chronic hepatitis B. N Engl J Med 2002; 346:1682 - 3; http://dx.doi.org/10.1056/NEJM200205303462202; PMID: 12037146
- Chan CY, Lee SD, Lo KJ. Legend of hepatitis B vaccination: the Taiwan experience. J Gastroenterol Hepatol 2004; 19:121 - 6; http://dx.doi.org/10.1111/j.1440-1746.2004.03153.x; PMID: 14731119
- van Zonneveld M, Honkoop P, Hansen BE, Niesters HG, Darwish Murad S, de Man RA, Schalm SW, Janssen HL. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology 2004; 39:804 - 10; http://dx.doi.org/10.1002/hep.20128; PMID: 14999700
- Chan HL, Tang JL, Tam W, Sung JJ. The efficacy of thymosin in the treatment of chronic hepatitis B virus infection: a meta-analysis. Aliment Pharmacol Ther 2001; 15:1899 - 905; http://dx.doi.org/10.1046/j.1365-2036.2001.01135.x; PMID: 11736720
- Papatheodoridis GV, Dimou E, Papadimitropoulos V. Nucleoside analogues for chronic hepatitis B: antiviral efficacy and viral resistance. Am J Gastroenterol 2002; 97:1618 - 28; http://dx.doi.org/10.1111/j.1572-0241.2002.05819.x; PMID: 12135009
- Wong DK, Cheung AM, O’Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med 1993; 119:312 - 23; http://dx.doi.org/10.7326/0003-4819-119-4-199308150-00011; PMID: 8328741
- MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, Chattergoon MA, Baine Y, Higgins TJ, Ciccarelli RB, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis 1998; 178:92 - 100; http://dx.doi.org/10.1086/515613; PMID: 9652427
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time?. Nat Rev Genet 2008; 9:776 - 88; http://dx.doi.org/10.1038/nrg2432; PMID: 18781156
- Chow YH, Huang WL, Chi WK, Chu YD, Tao MH. Improvement of hepatitis B virus DNA vaccines by plasmids coexpressing hepatitis B surface antigen and interleukin-2. J Virol 1997; 71:169 - 78; PMID: 8985336
- Mancini M, Hadchouel M, Davis HL, Whalen RG, Tiollais P, Michel ML. DNA-mediated immunization in a transgenic mouse model of the hepatitis B surface antigen chronic carrier state. Proc Natl Acad Sci USA 1996; 93:12496 - 501; http://dx.doi.org/10.1073/pnas.93.22.12496; PMID: 8901610
- Davis HL, McCluskie MJ, Gerin JL, Purcell RH. DNA vaccine for hepatitis B: evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci U S A 1996; 93:7213 - 8; http://dx.doi.org/10.1073/pnas.93.14.7213; PMID: 8692971
- Tacket CO, Roy MJ, Widera G, Swain WF, Broome S, Edelman R. Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine 1999; 17:2826 - 9; http://dx.doi.org/10.1016/S0264-410X(99)00094-8; PMID: 10438052
- Greenland JR, Letvin NL. Chemical adjuvants for plasmid DNA vaccines. Vaccine 2007; 25:3731 - 41; http://dx.doi.org/10.1016/j.vaccine.2007.01.120; PMID: 17350735
- Geng S, Zhong Y, Wang S, Liu H, Zou Q, Xie X, Li C, Yu Q, He Z, Wang B. Amiloride enhances antigen specific CTL by faciliting HBV DNA vaccine entry into cells. PLoS One 2012; 7:e33015; http://dx.doi.org/10.1371/journal.pone.0033015; PMID: 22438887
- Jin H, Li Y, Ma Z, Zhang F, Xie Q, Gu D, Wang B. Effect of chemical adjuvants on DNA vaccination. Vaccine 2004; 22:2925 - 35; http://dx.doi.org/10.1016/j.vaccine.2003.12.026; PMID: 15246629
- Cioli D, Pica-Mattoccia L.. Praziquantel. Parasitology Research 2003; 90:Supp 1 S3 9; PMID: 12811543
- Pearson RD, Guerrant RL. Praziquantel: a major advance in anthelminthic therapy. Ann Intern Med 1983; 99:195 - 8; http://dx.doi.org/10.7326/0003-4819-99-2-195; PMID: 6881777
- Zou Q, Yao X, Feng J, Yin Z, Flavell R, Hu Y, Zheng G, Jin J, Kang Y, Wu B, et al. Praziquantel facilitates IFN-γ-producing CD8+ T cells (Tc1) and IL-17-producing CD8+ T cells (Tc17) responses to DNA vaccination in mice. PLoS One 2011; 6:e25525; http://dx.doi.org/10.1371/journal.pone.0025525; PMID: 21998665
- Zou Q, Hu Y, Xue J, Fan X, Jin Y, Shi X, Meng D, Wang X, Feng C, Xie X, et al. Use of praziquantel as an adjuvant enhances protection and Tc-17 responses to killed H5N1 virus vaccine in mice. PLoS One 2012; 7:e34865; http://dx.doi.org/10.1371/journal.pone.0034865; PMID: 22529945
- Bodemar G, Walan A. Maintenance treatment of recurrent peptic ulcer by cimetidine. Lancet 1978; 1:403 - 7; http://dx.doi.org/10.1016/S0140-6736(78)91200-X; PMID: 75439
- O’Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol 2011; 128:1153 - 62; http://dx.doi.org/10.1016/j.jaci.2011.06.051; PMID: 21824648
- Li MJ, Lei JH, Wang T, Lu SJ, Guan F, Liu WQ, Li YL. Cimetidine enhances the protective effect of GST DNA vaccine against Schistosoma japonicum. Exp Parasitol 2011; 128:427 - 32; http://dx.doi.org/10.1016/j.exppara.2011.05.012; PMID: 21624364
- Wang J, Su B, Ding Z, Du X, Wang B. Cimetidine enhances immune response of HBV DNA vaccination via impairment of the regulatory function of regulatory T cells. Biochem Biophys Res Commun 2008; 372:491 - 6; http://dx.doi.org/10.1016/j.bbrc.2008.04.191; PMID: 18502198
- Steiner K, Garbe A, Diekmann HW, Nowak H. The fate of praziquantel in the organism I. Pharmacokinetics in animals. Eur J Drug Metab Pharmacokinet 1976; 1:85 - 95; http://dx.doi.org/10.1007/BF03189262
- Knodell RG, Browne DG, Gwozdz GP, Brian WR, Guengerich FP. Differential inhibition of individual human liver cytochromes P-450 by cimetidine. Gastroenterology 1991; 101:1680 - 91; PMID: 1955133
- Dachman WD, Adubofour KO, Bikin DS, Johnson CH, Mullin PD, Winograd M. Cimetidine-induced rise in praziquantel levels in a patient with neurocysticercosis being treated with anticonvulsants. J Infect Dis 1994; 169:689 - 91; http://dx.doi.org/10.1093/infdis/169.3.689; PMID: 8158054
- Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today 1998; 19:37 - 44; http://dx.doi.org/10.1016/S0167-5699(97)01186-9; PMID: 9465487
- Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, Larrubia JR, Webster GJM, McMichael AJ, Ferrari C, et al. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology 1999; 117:1386 - 96; http://dx.doi.org/10.1016/S0016-5085(99)70289-1; PMID: 10579980
- Alatrakchi N, Koziel M. Regulatory T cells and viral liver disease. J Viral Hepat 2009; 16:223 - 9; http://dx.doi.org/10.1111/j.1365-2893.2009.01081.x; PMID: 19222744
- Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X, et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol 2006; 177:739 - 47; PMID: 16785573
- Furuichi Y, Tokuyama H, Ueha S, Kurachi M, Moriyasu F, Kakimi K. Depletion of CD25+CD4+T cells (Tregs) enhances the HBV-specific CD8+ T cell response primed by DNA immunization. World J Gastroenterol 2005; 11:3772 - 7; PMID: 15968737
- Chisari FV, Pinkert CA, Milich DR, Filippi P, McLachlan A, Palmiter RD, Brinster RL. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science 1985; 230:1157 - 60; http://dx.doi.org/10.1126/science.3865369; PMID: 3865369
- Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol 2002; 76:8609 - 20; http://dx.doi.org/10.1128/JVI.76.17.8609-8620.2002; PMID: 12163580
- Liang M, Ma S, Hu X, Zhou B, Zhang J, Chen J, Wang Z, Sun J, Zhu X, Abbott W, et al. Cellular immune responses in patients with hepatitis B surface antigen seroclearance induced by antiviral therapy. Virol J 2011; 8:69; http://dx.doi.org/10.1186/1743-422X-8-69; PMID: 21320337
- Carey I, D’Antiga L, Bansal S, Longhi MS, Ma Y, Mesa IR, Mieli-Vergani G, Vergani D. Immune and viral profile from tolerance to hepatitis B surface antigen clearance: a longitudinal study of vertically hepatitis B virus-infected children on combined therapy. J Virol 2011; 85:2416 - 28; http://dx.doi.org/10.1128/JVI.01449-10; PMID: 21147914
- Rehermann B, Lau D, Hoofnagle JH, Chisari FV. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J Clin Invest 1996; 97:1655 - 65; http://dx.doi.org/10.1172/JCI118592; PMID: 8601631
- Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003; 77:68 - 76; http://dx.doi.org/10.1128/JVI.77.1.68-76.2003; PMID: 12477811
- Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000; 191:1269 - 80; http://dx.doi.org/10.1084/jem.191.8.1269; PMID: 10770795
- Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 1995; 2:271 - 9; http://dx.doi.org/10.1016/1074-7613(95)90051-9; PMID: 7697544
- Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 2008; 321:408 - 11; http://dx.doi.org/10.1126/science.1159806; PMID: 18635804
- Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J Immunol 2010; 184:287 - 95; http://dx.doi.org/10.4049/jimmunol.0902761; PMID: 19949099
- Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 1996; 4:25 - 36; http://dx.doi.org/10.1016/S1074-7613(00)80295-2; PMID: 8574849
- Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med 2000; 192:63 - 75; http://dx.doi.org/10.1084/jem.192.1.63; PMID: 10880527
- Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-gamma(-/-) mice, but not in TNF-alpha(-/-) mice: role for IFN-gamma in activating apoptosis of hepatocytes. J Immunol 1997; 159:1418 - 28; PMID: 9233639
- Roth E, Pircher H. IFN-γ promotes Fas ligand- and perforin-mediated liver cell destruction by cytotoxic CD8 T cells. J Immunol 2004; 172:1588 - 94; PMID: 14734739
- Roth E, Pircher H. IFN-gamma promotes Fas ligand- and perforin-mediated liver cell destruction by cytotoxic CD8 T cells. J Immunol 2004; 172:1588 - 94; PMID: 14734739
- Spanaus KS, Schlapbach R, Fontana A. TNF-alpha and IFN-gamma render microglia sensitive to Fas ligand-induced apoptosis by induction of Fas expression and down-regulation of Bcl-2 and Bcl-xL. Eur J Immunol 1998; 28:4398 - 408; http://dx.doi.org/10.1002/(SICI)1521-4141(199812)28:12<4398::AID-IMMU4398>3.0.CO;2-Y; PMID: 9862377
- Yeh N, Glosson NL, Wang N, Guindon L, McKinley C, Hamada H, Li Q, Dutton RW, Shrikant P, Zhou B, et al. Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J Immunol 2010; 185:2089 - 98; http://dx.doi.org/10.4049/jimmunol.1000818; PMID: 20624947
- Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood 2009; 114:596 - 9; http://dx.doi.org/10.1182/blood-2009-02-203935; PMID: 19471017
- Wu B, Zou Q, Hu Y, Wang B. Interleukin-22 as a molecular adjuvant facilitates IL-17-producing CD8+ T cell responses against a HBV DNA vaccine in mice. Hum Vaccin Immunother 2013; 9:2133 - 41; http://dx.doi.org/10.4161/hv.26047; PMID: 23941891
- Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci 2009; 66:2603 - 22; http://dx.doi.org/10.1007/s00018-009-0026-2; PMID: 19390784
- Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005; 41:771 - 8; http://dx.doi.org/10.1002/hep.20649; PMID: 15791617
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8:523 - 32; http://dx.doi.org/10.1038/nri2343; PMID: 18566595
- Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol 1998; 161:2586 - 93; PMID: 9725260
- Watanabe K, Mwinzi PN, Black CL, Muok EM, Karanja DM, Secor WE, Colley DG. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am J Trop Med Hyg 2007; 77:676 - 82; PMID: 17978070
- Zou Q, Zhong Y, Su H, Kang Y, Jin J, Liu Q, Geng S, Zhao G, Wang B. Enhancement of humoral and cellular responses to HBsAg DNA vaccination by immunization with praziquantel through inhibition TGF-beta/Smad2,3 signaling. Vaccine 2010; 28:2032 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.10.101; PMID: 20188260
- Li J, Qiu SJ, She WM, Wang FP, Gao H, Li L, Tu CT, Wang JY, Shen XZ, Jiang W. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS One 2012; 7:e39307; http://dx.doi.org/10.1371/journal.pone.0039307; PMID: 22745730
- Li J, Wu W, Peng G, Chen F, Bai M, Zheng M, Chen Z. HBcAg induces interleukin-10 production, inhibiting HBcAg-specific Th17 responses in chronic hepatitis B patients. Immunol Cell Biol 2010; 88:834 - 41; http://dx.doi.org/10.1038/icb.2010.63; PMID: 20498672
- Zhang JY, Song CH, Shi F, Zhang Z, Fu JL, Wang FS. Decreased ratio of Treg cells to Th17 cells correlates with HBV DNA suppression in chronic hepatitis B patients undergoing entecavir treatment. PLoS One 2010; 5:e13869; http://dx.doi.org/10.1371/journal.pone.0013869; PMID: 21079784
- Zhang W, Wang J, Su B, Li R, Ding Z, Kang Y, Wang B. Cimetidine augments Th1/Th2 dual polarized immune responses to recombinant HBV antigens. Vaccine 2011; 29:4862 - 8; http://dx.doi.org/10.1016/j.vaccine.2011.03.091; PMID: 21481324
- Babinet C, Farza H, Morello D, Hadchouel M, Pourcel C. Specific expression of hepatitis B surface antigen (HBsAg) in transgenic mice. Science 1985; 230:1160 - 3; http://dx.doi.org/10.1126/science.3865370; PMID: 3865370
- Du X, Zheng G, Jin H, Kang Y, Wang J, Xiao C, Zhang S, Zhao L, Chen A, Wang B. The adjuvant effects of co-stimulatory molecules on cellular and memory responses to HBsAg DNA vaccination. J Gene Med 2007; 9:136 - 46; http://dx.doi.org/10.1002/jgm.1004; PMID: 17310492
