 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A combined process of ammonium sulfate precipitation (salting out) and ion-exchange chromatography on DEAE-Sepharose CL-6B was used to prepare camel antivenom (IgG) against Naja Naja Oxiana for therapy. In the ammonium sulfate precipitation, the best condition for fractionation of IgG from the other proteins in camel serum was 55% precipitate. The camel IgG presented as 2 bands with molecular masses of 250 and 100 kDa, the latter corresponding to heavy chain IgG, on 10% gel electrophoresis. A trace amount of non-IgG proteins was not isolated and remained in this precipitate. Therefore in order to effectively separate albumin and the other nonspecific proteins from the IgG, the 25% precipitate of ammonium sulfate precipitation of serum was subjected to DEAE-Sepharose CL-6B column chromatography. A peak of antibody (IgG) could be obtained by elution with sodium phosphate buffer. In this stage, 2 bands of molecular masses of 150 and 75 kDa were observed on 7% gel electrophoresis. A comparative study was performed between camel IgG and conventional horse F(ab)2 antivenoms in term of potency (serum neutralization test and ELISA). Our results showed that the potency of camel antivenom was 4-fold higher than that of horse. It is suggested the combined ammonium sulfate precipitation and ion-exchange chromatography process effectively removed residual proteins in the final camel IgG preparation and can be a suitable method for large-scale refinement of therapeutic camel antivenoms.
Introduction
Antivenoms have been used successfully for more than a century and up to now constitute the only effective treatment for snakebites and envenomations by other poisonous animals.Citation1,Citation2 Currently, a great variety of antivenoms are produced in many laboratories from the serum of animals, mostly horses and sheep, that are immunized with single or multiple venoms. The 3 main types of antivenom preparations are IgG, F(ab)2, and Fab.Citation3,Citation4 All the preparations are ineffective in the local venom effects because of their inability to penetrate into the blood/tissue barrier.Citation5 Moreover, early and delayed adverse reactions are often associated with parenteral antivenom administrationCitation6 and their incidence, depending upon the administrated product, is highly different.Citation7,Citation8 Some causes of adverse reactions to antivenoms are immunogenicity of antivenom proteinsCitation3,Citation8,Citation9 and complement activation due to the presence of Fc in the whole IgG preparations or protein aggregates in 3 types of antivenoms.Citation3,Citation10,Citation11 Immunogenicity of antivenom proteins which would elicit an anti-IgG antibody response associated with delayed reactions, i.e., serum sickness.Citation3,Citation8,Citation9
Studies on camelid immunoglobulin represent an attractive alternative in developing an antibody-based approach to the treatment of envenoming. Special physicochemical properties of camelied IgG offer intriguing possibilities to improving the clinical effectiveness of antivenom treatment.Citation12 Over 50% of camelid immunoglobulin lack light chainsCitation13—these unique Ab isotypes interact with the antigen by virtue of only one single V domain. Single domain Abs isolated from camel H-chain Abs are known as VHH or nanobody and are adaptable to screening techniques such as phage display which provides the fast and easy isolation of specific Ab.Citation14,Citation15 Therefore, VHH is considerably smaller than Fab fragment (produced by papain effect) of conventional IgG.Citation5 Immunogenicity of camel IgG is low and less likely to activate the complement cascade than ovine or equine IgGCitation16; therefore, the anaphilactoid and serum sickness adverse effects in the patients treated with camelid IgG antivenom would be less. These effects are often associated with the current antivenom treatment.Citation16 The unusual thermostability of camelid IgGCitation17 can be exploited to prepare antivenom that remains efficacious after maintenance at room temperature.Citation16 It is supposed that the major clinical advance will be achieved by IgG from venom-immunized camelids to provide antivenom which is capable of treating snake envenoming. In addition, this animal is facile in terms of handling, immunization, and bleeding like horses—the yield of blood is similar to that of the horse.Citation16
Technologies for snake antivenom production vary according to the production laboratory, even in the same country. Classically, the protocols that yield the F(ab)2fragment based on pepsin digestion followed by ammonium sulfate fractionation have been used worldwide.Citation1 This method routinely is applied for the production of antivenom in horse against the Naja Naja Oxiana snake venom in Razi Institute, Iran.
The aim of this work was 2-folds, (1) to adapt the methodology used for ammonium sulfate purification (without pepsin) of horse F(ab)2 to camel IgG, in order to develop an alternative method for antivenom preparation for therapy, and (2) to compare the proposed method with conventional one in terms of potency.
Results
Antibody production
In this work, the camel immunized with different concentrations of Naja Naja Oxiana snake venom. The antivenom antibodies appeared in the serum during the third time of immunization (first bleeding) and reached the hyperimmune level in the eighth immunization step (fourth bleeding). This immunity was revealed by a double immunodiffusion method (data not shown). The routinely horse F(ab)2 antivenom used as a positive control. The hyper- immunity of both animals (camel and horse) was necessary for processing in the next steps and comparative studies.
Ammonium sulfate purification of IgG
The procedure which was used for the purification of horse F(ab)2 based on ammonium sulfate precipitation of non-IgG F(ab)2 plasma proteins was not successfully applied to the camel IgG serum. Electrophoretic analysis of the prepared supernatants and precipitations on 10% polyacrylamide gel showed different amounts of non-IgG unwanted proteins and 2 broad predominant bands of 250 and 100 kDa camel IgG (). The protein bands of 250 and 100 kDa belonged to the conventional IgG with 4 chains and heavy chain IgG devoid of light chains, respectively. In the best state of purification (i.e., 55% ammonium sulfate precipitate) (), there was the trace amount of non-IgG proteins. Therefore, to improve the purification of camel IgG, chromatography procedure was followed.
Figure 1. SDS-PAGEs of ammonium sulfate prepared IgG fractions from 25% precipitate. Aliquots of each IgG preparation were separated by electrophoresis in 10% acrylamide gels under non-reducing conditions. Proteins were stained with Coomassie brilliant blue R-250. (A–D) Correspond to precipitates 100–30%, precipitates 95–35%, supernatants100–30%, and supernatants 95–35% respectively. Numbers on the horizontal axis indicate the ammonium sulfate percent which 25% precipitate reached to it. Standard molecular masses are depicted to the left. The upper and lower arrows correspond to 250 and 100 kDa bands respectively.
IgG purification by ion-exchange chromatography
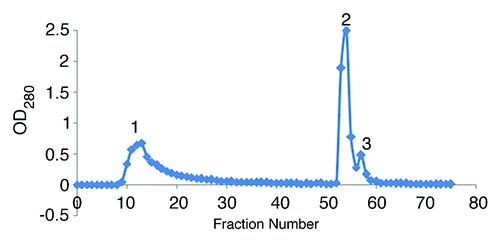
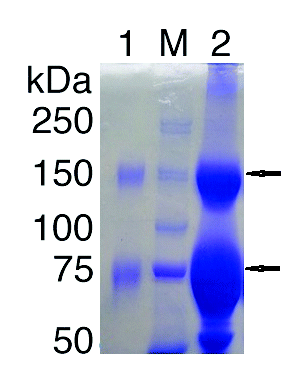
In the present study, the ion-exchange chromatography of 25% precipitated hyper immunized serum was performed on DEAE-Sepharose CL-6B column and 3 major protein peaks were obtained (). These protein peaks were run on the 10% SDS-PAGE (data not shown). The only first peak showed purified IgG with 2 molecular weights (conventional and heavy chain IgG), and the other peaks revealed non-IgG proteins. The first fraction was run on the 7% SDS PAGE, showing 2 major bands of 150 and 75 kDa ().
Figure 2. Ion-exchange chromatography of 25% ammonium sulfate precipitate on DEAE-Sepharose CL-6B column with phosphate buffer.
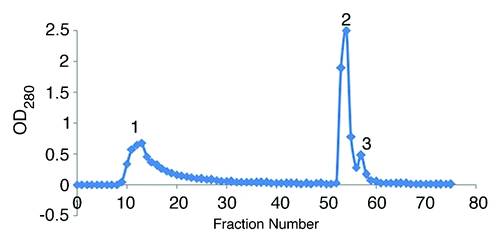
Figure 3. SDS-PAGE of camel IgG from DEAE-Sepharose CL-6B column. This electrophoresis was performed on 7.5% acrylamide gels under non-reducing conditions. Lane1, peak number 1 from DEAE-Sepharose CL-6B; M, molecular weight marker; lane2, 25% ammonium sulfate precipitate. The upper and lower arrows correspond to 150 and 75 kDa bands respectively.
Comparative ion-exchange purified IgG and routinely produced F(ab)2
In order to evaluate the potency of antivenoms prepared by the proposed method and the conventional procedure, the serum neutralization and ELISA tests were performed and the results were compared. In terms of ELISA test, the absorbance of camel IgG was 1.26 vs. 1.18 for the conventional horse F(ab)2and the difference was not significant. In terms of serum neutralization test, potency was estimated to be 8.8 LD50 and 2.2 LD50 for IgG and F(ab)2 antivenoms respectively (). The present results indicating that the potency of the camel IgG preparation is ~4-fold higher than that of the F(ab)2 preparation.
Table 1. The comparative potency test for Camel IgG and F(ab)2 Antivenoms (invivo)
Discussion
Production of antivenoms against snakes can be increased by the low-dose, low-volume, and multi-site CFA immunization. This protocol uses significantly less venom in antivenom production, which not only is economically important, i.e., reducing the cost of antivenom, but also saves the lives of captured snakes, many of which die under captivity.Citation18 Therefore, this venom immunization protocol was adapted for the preparation of camel antivenom that showed highly encouraging preclinical effectiveness in Naja Naja Oxiana. The venom toxins with low molecular weight are generally considered to be weakly immunogenic, but, the considerable antibody titer in the hyperimmunized animal (camel) was noted as was shown in double immunodiffusion test.
In the previous studies, a very simple and convenient method, based on caprylic acid precipitation of non-IgG proteins in serum or plasma, has been adapted in various laboratories for the production of equine-derived antivenoms.Citation19-Citation21 Therefore in this work attempts were made to demonstrate whether ammonium sulfate fractionation (without pepsin digestion) used for F(ab)2 preparation can be applied in the purification of IgGs from camel serum or not. This adaptation would open the possibility for manufacturing of this type of antivenom as an alternative in the antisera production centers in the world, such as Razi Institute. The conventional method for preparing F(ab)2 antivenom is expensive and time consuming and also has high risk of contamination with endotoxins due to extensive dialysis.Citation22 In terms of safety, camelid IgG is an attractive candidate, because of its reported lower potential for inducing adverse effects.Citation3 Reports of the venom-neutralizing efficacy of IgG from camels immunized with scorpion toxinsCitation23,Citation24 and llamas immunized with South American snake venomCitation25 have indicated the growing awareness of the potential advantages of camelid IgG antivenom. Moreover, it has been suggested that, owing to the reported thermostability of camelid IgG and low structural complexity of the antigen binding sites of heavy chain camelid antibodies that may become useful inhibitory molecules,Citation26 they may be valuable in terms of neutralization of toxins.Citation3 In this study, 2 bands of molecular masses of 250 and 100 kDa were observed on 10% gel, which was probably in correspondence to the classical IgG with 2 light and 2 heavy chains, and IgG constituted only by 2 heavy chains, i.e., IgG devoid of light chains. The use of 10% gel electrophoresis instead of 7% (that is commonly applied for antibody studies), is for the purpose of determining, whether the nonspecific proteins are present in the supernatants or precipitates of 30–100% ammonium sulfate fractionation or not. Electropheretic analysis showed that use of only ammonium sulfate was not sufficient for preparing camel IgG antivenom; in the best condition (i.e., 55% precipitate), the trace amount of nonspecific proteins was observed. Therefore for further purification, an additional step should be performed.
It has been reported that IgG may be purified from serum by a simple one-step ion-exchange chromatography procedure. The method is widely used and works on the principle that IgG has a higher or more basic isoelectric point than most serum proteins.Citation27 Therefore, if pH is kept below the isoelectric point of most antibodies, immunoglobulins do not bind to an anion exchanger and are separated from the majority of serum proteins bound to the column matrix. The anion-exchange reactive group, DEAE covalently linked to Sepharose is useful for this purpose.Citation27 The camel IgG antivenom purified by the above-mentioned method, and the present results showed 2 obvious bands with molecular masses of 150 and 75 on 7% PAGE. The above 2 bands of 150 and 75 kDa were the same as 250 and 100 kDa on 10% gel electrophoresis respectively. The actual reason for this shift is not clear, but it seems that gel concentration can affect the movement of antibodies throughout the gel.
There was no correlation between the in vitro and in vivo tests found in this work. The ELISA may be used to evaluate direct antibody binding to the venoms adsorbed on the microtiter plateCitation28, however all the positive in vitro binding results again did not correlate with protective in vivo activityCitation29 and vice versa. There may be several explanations for this issue, but the lack of correlation illustrates a general principle—immunoassays cannot always distinguish between neutralizing and non-neutralizing antibodies. Good correlations are usually achieved when the immunodominant epitope of the antigen coincides with the neutralizing epitope to which the protective antibody binds. Correct orientation of the antigen during the coating of the microtiter plate for exposing reactive epitopes is also an important consideration in the sensitivity of an assay.Citation30 In fact, the above explanation about the correlation between in vitro and in vivo tests confirmed the results of the present work. As it was mentioned, the camel IgG antivenom showed higher potency than the traditional horse F(ab)2 antivenom. There are 2 main factors which could cause higher potency of camel IgG antivenom than horse F(ab)2 antivenom, (1) fractionation manner—i.e., using ammonium sulfate and pepsin digestion for horse F(ab)2 and chromatography column for camel IgG—and (2) characteristic nature of camel IgGs. If the camel IgG is introduced as therapeutic sera, it can have several advantages as compare with the heterologous antivenoms, (1) less amount of camel antivenom required for treatment, (2) limited number of camel for antivenom production, and (3) low risk of contamination with endotoxin. As the number of steps in the purification of F(ab)2, exposure of the product, and duration of purification is more, the chance of contamination in the F(ab)2 is higher than that of IgG purification method.
It can be suggested that the antivenom production on the camel is a suitable alternative for therapeutic sera.
Materials and Methods
Immunization
One adult healthy camel was immunized with the venom of Naja Naja Oxiana. The lyophilized venom (with median lethal dose [LD50] of 7.8 μg venom/17–21 g of Razi mice) was provided by Razi Vaccine and Research Institute and resuspended in saline. Immunization of the camel was performed under some modifications of a multi-site, low-dose protocol.Citation18 The animal was subcutaneously immunized with 0.5, 0.5, 1.0, 2.0, 4.0, and 4.0 mg venom at 2 wk intervals—after 1 mo of resting, additional 2 steps of immunization were performed with 4 mg of venom. Each 2 mL immunization was released to 4–5 sites (0.5 mL each) in the neck. The first immunization was administrated with Freund's complete adjuvant (Razi Institute) and the remaining booster immunization was given using Freund's incomplete adjuvant. Four bleedings occurred and the final blood was taken from the jugular vein (after reaching the hyperimmune stage). Then it was left at room temperature to clot. The clear serum was collected by centrifugation and stored at −20 °C.
IgG purification
Ammonium sulfate fractionation
The camel serum was precipitated by the addition of 25% saturated ammonium sulfate (Merck) (13.6gr/100 mL) for the initial preparation of IgG. The powder was added slowly but steadily, under thorough mixing and allowed precipitate to form for 2 h at 4 °C with constant stirring. The precipitate was obtained by centrifugation at 3500 rpm for 20 min, dissolved in phosphate buffered saline (PBS) at a pH of 7.2, dialyzed against PBS overnight, and stored at −20 °C until used. The above solution was then treated with different concentrations of saturated ammonium sulfate (30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, and 100%) at 4 °C and both the precipitate and supernatant were collected after centrifugation and dialysis in the same conditions.
Chromatography on anion-exchange column
The further purification of IgG was performed by ion-exchange chromatography.Citation27 The sample (25% ammonium sulfate precipitate) was dialyzed overnight at 4 °C against 0.07 M sodium phosphate buffer at a pH of 6.3 and loaded on chromatography column (DEAE-Sepharose). The sample Elution was affected by sodium phosphate buffer and column regenerated by passing through 2–3 column volumes of phosphate buffer containing 1 M NaCl. Fractions were collected and protein concentrations were determined.
Sodium dodecyl sulfate PAGE (SDS-PAGE)
Purity of IgG was determined by SDS-PAGE. The IgG purity in ammonium sulfate precipitates and supernatants was analyzed in vertical non-reducing SDS-PAGE mini gels of 10% polyacrylamide, as described by Laemmli.Citation31
The 3 major fractions from ion-exchange separation were tested for identity by 10% polyacrylamide gel (data not shown) and then pure IgG fraction electrophoresis was done on 7% of gel.
Protein determinations
The protein concentrations were determined by the Lowry et al. methodCitation32 using bovine serum albumin as a standard.
The purity index was calculated according to this formula:
The purity of IgG as a function of total protein in this purified IgG preparation was 8–14.
Assessing reactivity of purified camel IgG and horse F(ab)2 antivenoms by enzyme-linked immunosorbent assay (ELISA)
Reactivity of ion-exchange purified IgG antivenom of camel and horse F(ab)2 antivenom (routinely produced in Razi Institute) against the venom of Naja Naja Oxiana was compared in an ELISA method. Microtiter ELISA strips (NUNC) were coated overnight at 4 °C using venom of Naja Naja Oxiana at the concentration of 10 ng/well using carbonate buffer (pH 9.6) and blocked with blocking buffer (5% skim milk+0.05% tween 20 in PBS) for 1.5 h at 37 °C. Crude camel IgG antivenom and horse F(ab)2 antivenom were added and incubated at 37 °C for 1.5 h, followed by the addition of appropriately diluted horseradish peroxidase (HRP) conjugated goat anti-camelid IgG (Kent Laboratories Inc.) and HRP conjugated goat anti-horse IgG F(ab)2 (Rockland), respectively, and then incubated at 37 °C for 1.5 h. Between each step, the strips were washed 4 times with washing buffer (0.05% tween 20 in PBS). The strips were developed with 3,3,5,5 tetramethylbenzidine (TMB) as substrate chromogen and read at OD 450 nm in an ELISA reader.
In vivo potency test of antivenoms (serum neutralization test)
Mixtures containing the constant amount of venom and varying dilutions of antivenoms were prepared in distilled water and incubated at 37 °C for 1 h. Aliquots (0.5 mL) of the mixtures containing an amount of venom corresponding to 5 LD50 were injected intravenously into the groups of 4 mice (17–21 g). Deaths were recorded over 96 h and the potency was estimated. Potency of the tested antivenom was expressed in terms of LD50. The weight in mg equivalent to the LD50 of the venom should be neutralized by a specific quantity of the antivenom based on the protection of a stated proportion of animals (e.g., 100%). The serum potency is expressed as the largest amount of venom neutralized by 1 mL of serum.Citation33
| Abbreviations: | ||
| VHH | = | heavy chain antibody |
| SDS-PAGE | = | sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| LD50 | = | lethal dose, 50% |
| ELISA | = | enzyme-linked immunosorbent assay |
| PBS | = | phosphate buffered saline |
| TMB | = | 3,3′,5,5′-tetramethylbenzidine |
| HRP | = | horseradish peroxidase |
| CFA | = | complete Freund's adjuvant |
| PI | = | purification index |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grant from the Razi Vaccine and Serum Research Institute, Karaj, Iran.
We thank Dr Abolfazl Akbari (Department of venomous animals and antivenom in Razi Institute) for his cooperation in this study.
References
- Morais VM, Massaldi H. Snake antivenoms: adverse reactions and production technology. J Venom Anim Toxins incl Trop Dis [online] 2003; 15:2 18
- Leong PK, Sim SM, Fung SY, Sumana K, Sitprija V, Tan NH. Cross neutralization of Afro-Asian cobra and Asian krait venoms by a Thai polyvalent snake antivenom (Neuro Polyvalent Snake Antivenom). PLoS Negl Trop Dis 2012; 6:e1672; http://dx.doi.org/10.1371/journal.pntd.0001672; PMID: 22679522
- Herrera M, León G, Segura A, Meneses F, Lomonte B, Chippaux JP, Gutiérrez JM. Factors associated with adverse reactions induced by caprylic acid-fractionated whole IgG preparations: comparison between horse, sheep and camel IgGs. Toxicon 2005; 46:775 - 81; http://dx.doi.org/10.1016/j.toxicon.2005.08.004; PMID: 16183094
- Zolfagharian H, Mohammadpour-Dounighi N. Progress and improvement of the manufacturing process of snake antivenom: a review. Arch Razi Inst 2013; 68:1 - 10
- Harrison RA, Hasson SS, Harmsen M, Laing GD, Conrath K, Theakston RDG. Neutralisation of venom-induced haemorrhage by IgG from camels and llamas immunised with viper venom and also by endogenous, non-IgG components in camelid sera. Toxicon 2006; 47:364 - 8; http://dx.doi.org/10.1016/j.toxicon.2005.10.017; PMID: 16359717
- Warrell DA. Clinical toxicology of snakebite in Africa and the Middle East/Arabian peninsula. In: Meier J, White J (Eds.), Handbook of Clinical Toxicology of Animal Venoms and Poisons. CRC Press: Florida, 1995: 433–92.
- Cardoso JLC, Fan HW, França FOS, Jorge MT, Leite RP, Nishioka SA, Avila A, Sano-Martins IS, Tomy SC, Santoro ML, et al. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q J Med 1993; 86:315 - 25; PMID: 8327649
- Lalloo DG, Theakston RDG. Snake antivenoms. J Toxicol Clin Toxicol 2003; 41:277 - 90, 317-27; http://dx.doi.org/10.1081/CLT-120021113; PMID: 12807311
- León G, Monge M, Rojas E, Lomonte B, Gutiérrez JM. Comparison between IgG and F(ab’)(2) polyvalent antivenoms: neutralization of systemic effects induced by Bothrops asper venom in mice, extravasation to muscle tissue, and potential for induction of adverse reactions. Toxicon 2001; 39:793 - 801; http://dx.doi.org/10.1016/S0041-0101(00)00209-9; PMID: 11137538
- León G, Lomonte B, Gutiérrez JM. Anticomplementary activity of equine whole IgG antivenoms: comparison of three fractionation protocols. Toxicon 2005; 45:123 - 8; http://dx.doi.org/10.1016/j.toxicon.2004.07.025; PMID: 15581691
- Otero R, Gutiérrez JM, Rojas G, Núñez V, Díaz A, Miranda E, Uribe AF, Silva JF, Ospina JG, Medina Y, et al. A randomized blinded clinical trial of two antivenoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: correlation between safety and biochemical characteristics of antivenoms. Toxicon 1999; 37:895 - 908; http://dx.doi.org/10.1016/S0041-0101(98)00220-7; PMID: 10340829
- Harrison RA, Wernery U. The unique properties of camelid IgG have potential to improve the treatment of snake bite. J Camel Pract Res 2007; 14:15 - 6
- Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature 1993; 363:446 - 8; http://dx.doi.org/10.1038/363446a0; PMID: 8502296
- Muyldermans S. Single domain camel antibodies: current status. J Biotechnol 2001; 74:277 - 302; PMID: 11526908
- Richard G, Meyers AJ, McLean MD, Arbabi-Ghahroudi M, MacKenzie R, Hall JC. In vivo neutralization of a-Cobratoxin with high-affinity llama single-domain antibodies (VHHs) and a VHH-Fc antibody. PLoS ONE 2013; 8:e69495; http://dx.doi.org/10.1371/journal.pone.0069495; PMID: 23894495
- Cook DAN, Owen T, Wagstaff SC, Kinne J, Wernery U, Harrison RA. Analysis of camelid IgG for antivenom development: Serological responses of venom-immunised camels to prepare either monospecific or polyspecific antivenoms for West Africa. Toxicon 2010; 56:363 - 72; http://dx.doi.org/10.1016/j.toxicon.2010.03.025; PMID: 20362604
- Omidfar K, Rasaee MJ, Kashanian S, Paknejad M, Bathaie Z. Studies of thermostability in Camelus bactrianus (Bactrian camel) single-domain antibody specific for the mutant epidermal-growth-factor receptor expressed by Pichia. Biotechnol Appl Biochem 2007; 46:41 - 9; http://dx.doi.org/10.1042/BA20060104; PMID: 16848761
- Sriprapat S, Aeksowan S, Sapsutthipas S, Chotwiwatthanakun C, Suttijitpaisal P, Pratanaphon R, Khow O, Sitprija V, Ratanabanangkoon K. The impact of a low dose, low volume, multi-site immunization on the production of therapeutic antivenoms in Thailand. Toxicon 2003; 41:57 - 64; http://dx.doi.org/10.1016/S0041-0101(02)00209-X; PMID: 12467662
- dos Santos MC, D’Império Lima MR, Furtado GC, Colletto GMDD, Kipnis TL, Dias da Silva W. Purification of F(ab’)2 anti-snake venom by caprylic acid: a fast method for obtaining IgG fragments with high neutralization activity, purity and yield. Toxicon 1989; 27:297 - 303; http://dx.doi.org/10.1016/0041-0101(89)90177-3; PMID: 2728022
- Rojas G, Jiménez JM, Gutiérrez JM. Caprylic acid fractionation of hyperimmune horse plasma: description of a simple procedure for antivenom production. Toxicon 1994; 32:351 - 63; http://dx.doi.org/10.1016/0041-0101(94)90087-6; PMID: 8016856
- Gutiérrez JM, Rojas E, Quesada L, León G, Núñez J, Laing GD, Sasa M, Renjifo JM, Nasidi A, Warrell DA, et al. Pan-African polyspecific antivenom produced by caprylic acid purification of horse IgG: an alternative to the antivenom crisis in Africa. Trans R Soc Trop Med Hyg 2005; 99:468 - 75; http://dx.doi.org/10.1016/j.trstmh.2004.09.014; PMID: 15837359
- Raweerith R, Ratanabanangkoon K. Fractionation of equine antivenom using caprylic acid precipitation in combination with cationic ion-exchange chromatography. J Immunol Methods 2003; 282:63 - 72; http://dx.doi.org/10.1016/j.jim.2003.07.014; PMID: 14604541
- Meddeb-Mouelhi F, Bouhaouala-Zahar B, Benlasfar Z, Hammadi M, Mejri T, Moslah M, Karoui H, Khorchani T, El Ayeb M. Immunized camel sera and derived immunoglobulin subclasses neutralizing Androctonus australis hector scorpion toxins. Toxicon 2003; 42:785 - 91; http://dx.doi.org/10.1016/j.toxicon.2003.10.021; PMID: 14757210
- Hmila I, Abdallah R BA, Saerens D, Benlasfar Z, Conrath K, Ayeb ME, Muyldermans S, Bouhaouala-Zahar B. VHH, bivalent domains and chimeric Heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI’. Mol Immunol 2008; 45:3847 - 56; http://dx.doi.org/10.1016/j.molimm.2008.04.011; PMID: 18614235
- Fernández GP, Segura A, Herrera M, Velasco W, Solano G, Gutiérrez JM, León G. Neutralization of Bothrops mattogrossensis snake venom from Bolivia: experimental evaluation of llama and donkey antivenoms produced by caprylic acid precipitation. Toxicon 2010; 55:642 - 5; http://dx.doi.org/10.1016/j.toxicon.2009.07.031; PMID: 19647761
- Lauwereys M, Arbabi Ghahroudi M, Desmyter A, Kinne J, Hölzer W, De Genst E, Wyns L, Muyldermans S. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J 1998; 17:3512 - 20; http://dx.doi.org/10.1093/emboj/17.13.3512; PMID: 9649422
- Page M, Thorpe R. Purification of IgG using DEAE-sepharose chromatography. In: Walker JM (Ed.), The Protein Protocols Handbook. 2nd Edition ed. Humana Press: Totowa NJ, 2002: 987–8.
- Sells PG, Theakston RDG, Warrell DA. Development of α-neurotoxin antibodies in patients envenomed by the monocellate Thai cobra (Naja kaouthia). Toxicon 1994; a 32:1667 - 71; http://dx.doi.org/10.1016/0041-0101(94)90325-5; PMID: 7725334
- Sells PG, Jones RGA, Laing GD, Smith DC, Theakston RDG. Experimental evaluation of ovine antisera to Thai cobra (Naja kaouthia) venom and its α-neurotoxin. Toxicon 1994; 32:1657 - 65; http://dx.doi.org/10.1016/0041-0101(94)90324-7; PMID: 7725333
- Sells PG. Animal experimentation in snake venom research and in vitro alternatives. Toxicon 2003; 42:115 - 33; http://dx.doi.org/10.1016/S0041-0101(03)00125-9; PMID: 12906883
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680 - 5; http://dx.doi.org/10.1038/227680a0; PMID: 5432063
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265 - 75; PMID: 14907713
- Latifi M, Manhouri H. [Antivenin production]. Mem Inst Butantan 1966; 33:893 - 7; PMID: 6002969
