Abstract
Autologous tumor cell-based vaccines provide a wide range of tumor antigens and personalized neo-epitopes based on individual tumors’ unique antigenic mutanome signatures. However, tumor-derived factors may hamper in situ maturation of dendritic cells (DC) and thus interfere with the generation of effective anti-tumor immunity. As the skin is a preferred site for tumor vaccine delivery, we investigated the influence of primary colon carcinoma-derived soluble factors on the maturation state of migrating DC in a human skin explant model. Primary tumor-derived supernatants (TDSN) enhanced the phenotypic maturation state of skin-emigrated DC, resulting in an increased T-cell stimulatory ability in an allogeneic mixed leukocyte response. In case of monocyte-derived DC a similar TDSN-induced maturation induction was found to entirely depend on cyclooxygenase (COX)-regulated prostaglandins. In contrast, the increase in skin-emigrated DC maturation was completely prostaglandin-independent, as evidenced by the inability of the COX inhibitor indomethacin to abrogate this TDSN-induced effect. Although TDSN conditioning affected a drop in IL-12p70 release by the skin-emigrated DC and induced a predominant Th17/Th22 transcriptional profile in subsequently stimulated T-cells, Th cell subset differentiation, as assessed by intracellular cytokine expression upon polyclonal priming and re-stimulation, was not affected. Comparative analysis of phenotypic and transcriptional profiles suggests that the observed maturational effects in skin-derived DC may have been induced by tumor-derived GM-CSF. In conclusion, soluble factors derived from whole-cell colon tumor vaccines will not negatively impact DC migration and maturation in human skin, but rather induce DC maturation that will facilitate the priming of a poly-functional Th cell response.
Introduction
We have previously reported on the clinical efficacy of intradermally (i.d.) administered autologous whole-cell tumor vaccines for the treatment of colon cancer patients.Citation1 A moderate, though significant, increase in disease-free survival was observed in a multi-center randomized phase III trial for Duke’s B patients. A recently published retrospective analysis of this trial revealed that patients with micro-satellite-stable tumors (MSS) in particular benefited from this vaccination, with long recurrence-free survival, whereas patients with micro-satellite instable tumors (MSI) did not. The latter group however showed prolonged survival regardless of vaccination, most likely due to the intrinsic immunogenicity of MSI tumors.Citation2 Thus, intradermal vaccination with a live autologous whole-cell tumor vaccine may benefit patients with early-stage MSS tumors. Such autologous tumor-based vaccines may receive renewed interest in this era of next-generation sequencing which has demonstrated the immunogenic potential of neo-epitopes arising from unique mutations in individual tumors.Citation3-Citation5 Indeed, Segal and colleagues estimated that in each colon tumor anywhere between 40 and 60 neo-epitopes could be expected to arise with the potential to bind to MHC class-I molecules and to thus provide a target for cytotoxic T-lymphocytes (CTL).Citation6 It nevertheless remains costly and technically challenging to identify driver mutations with immunogenic potential to design targeted, personalized, and effective vaccines for each individual patient. Vaccination approaches based on autologous tumor cells would circumvent this need while encompassing all possibly relevant antigens and immunogenic neo-epitopes.
Whereas patients with Duke’s B MSS colon tumors experienced prolonged recurrence-free survival upon i.d. vaccination with an autologous tumor cell vaccine, no such increase in recurrence-free survival was observed in more advanced stages of the disease,Citation1 indicating the need for enhanced vaccination efficacy. The pivotal role of dendritic cells (DC) in the initiation and the effector phase of the immune response is under intense investigation. In established tumors the immune response often proves to be ineffective. A number of publications has correlated poor clinical outcome with low numbers and activation state of tumor-associated DC.Citation7-Citation9 This has raised interest in DC and their complex interactions with stromal, immune and tumor cells, as well as their role in the generation of antitumor immunity and the maintenance of immunosurveillance. Tumors utilize various mechanisms to subvert DC functions, e.g., by interfering with migration or proper DC maturation. To this end, tumor cells secrete a plethora of immunosuppressive soluble factors, but they may also induce their release by stromal cells or by infiltrating immune cells.Citation10,Citation11 We previously demonstrated the inhibitory effect of supernatants from primary colon tumors on the differentiation of monocyte-derived DC (MoDC) from blood monocytes.Citation10 Here, we ascertained the effect of these supernatants on the maturation state of already fully differentiated DC, as interference of tumor-derived soluble factors with the activation of DC may seriously hamper tumor vaccination approaches. More specifically, soluble factors derived from intradermally applied autologous tumor-based vaccines may interfere with local DC activation in the skin vaccination site, either directly, or indirectly through conditioning of the dermal stroma. We therefore examined the effect of colon tumor-derived soluble factors on cutaneous DC in their natural tissue context, employing single-cell suspensions of primary colon tumors collected and stored for the purpose of vaccine preparation. We made use of a previously described ex vivo human skin explant model, which allowed the study of effects on the maturation and migration of skin-resident DC under near-physiological conditions.Citation12
Rather than inhibition, we report an enhanced phenotypic maturation of skin-emigrated DC under the influence of colon tumor-derived soluble factors. While for MoDC a similarly activating effect was found to be entirely prostaglandin-dependent, for skin-emigrated DC it was not. Based on similarities in effects we propose that the observed maturation-inducing effects in skin DC may be related to colon tumor-derived GM-CSF. Encouragingly, our data suggest that soluble factors derived from whole-cell colon tumor vaccines will neither interfere with migration and maturation of DC in human skin, nor with their T-cell stimulatory ability.
Results
Primary colon tumor-derived supernatants induce DC maturation
We and others previously studied the phenotype of DC spontaneously migrating from human skin explants and found DC migration to be optimal by day 2 after the start of explant culture.Citation12,Citation13 Migrated cells harvested at this time can be roughly subdivided into 2 major phenotypes: CD1a+CD83+CD14- mature DC and CD1a±CD83-CD14+ immature macrophage-like cells. The balance between these 2 populations can be shifted in favor of the mature skin-emigrated DC by the intracutaneous conditioning of DC by such maturation-inducing agents as GM-CSF or IL-4.Citation14 I.d. injection of 50% (v/v) TDSN prior to culture, and the inclusion of 3% (v/v) TDSN in the medium during explant culture (both found to yield maximal effects in titration experiments), induced a significantly enhanced maturation state of skin-emigrated DC based on activation marker levels similar to those achieved by the known classic in vivo DC maturation inducer GM-CSF (). This maturation induction was accompanied by a shift from CD1a±CD83-CD14+ immature macrophage-like cells to CD1a+CD83+CD14- mature DC among the migrated cells (); overall, the effects of primary colon TDSN on the expression levels of CD83, CD40, CD54, and CD86 was significant (P < 0.01, n = 8). Beside the acquisition of phenotypic markers of maturation, treatment with TDSN also increased the T-cell stimulatory ability of both MoDC and skin-emigrated DC in allogeneic Mixed Leukocyte Reactions (MLR, ).
Figure 1. Primary colon tumor derived supernatants (TDSN) promote maturation of DC migrating from human skin explants. Intradermal (i.d.) injection -prior to culture- of human skin explants with 100 ng GM-CSF or 50% (v/v) TDSN (combined with 3% (v/v) TDSN added to the culture supernatant) (A and B) enhanced the maturation state of migrated DC (harvested and analyzed 2 d after the start of skin explant culture; Mean Fluorescence Intensities (MFI) of the tested markers are listed in the histograms and the histogram markers indicate fluorescence levels obtained with the isotype controls) and (C and D) shifted migrated DC from an immature CD14+ phenotype to a mature CD1a+ phenotype. (E) T-cell stimulatory capacity in an allogeneic Mixed Leukocyte Reaction (MLR) of unmodulated or TDSN-matured DC, emigrated from human skin explants. Mature DC were cocultured at the indicated stimulator: responder ratio for 4 d and pulsed with [3H]-thymidine during the last 18 h. Proliferative responses ([3H]-Thymidine incorporation) are shown as mean counts per minute (cpm) from triplicate wells. All data shown are representative or averages (± sd) of 3–8 independent experiments, *P < 0.05.
![Figure 1. Primary colon tumor derived supernatants (TDSN) promote maturation of DC migrating from human skin explants. Intradermal (i.d.) injection -prior to culture- of human skin explants with 100 ng GM-CSF or 50% (v/v) TDSN (combined with 3% (v/v) TDSN added to the culture supernatant) (A and B) enhanced the maturation state of migrated DC (harvested and analyzed 2 d after the start of skin explant culture; Mean Fluorescence Intensities (MFI) of the tested markers are listed in the histograms and the histogram markers indicate fluorescence levels obtained with the isotype controls) and (C and D) shifted migrated DC from an immature CD14+ phenotype to a mature CD1a+ phenotype. (E) T-cell stimulatory capacity in an allogeneic Mixed Leukocyte Reaction (MLR) of unmodulated or TDSN-matured DC, emigrated from human skin explants. Mature DC were cocultured at the indicated stimulator: responder ratio for 4 d and pulsed with [3H]-thymidine during the last 18 h. Proliferative responses ([3H]-Thymidine incorporation) are shown as mean counts per minute (cpm) from triplicate wells. All data shown are representative or averages (± sd) of 3–8 independent experiments, *P < 0.05.](/cms/asset/8374bd05-06e8-499b-8450-cdc367b37684/khvi_a_10928548_f0001.gif)
Addition of 30% (v/v) TDSN to 7-d immature MoDC induced a level of phenotypic maturation comparable to the addition of the DC-maturation inducer Prostaglandin-E2 (10 μM), as judged on the basis of expression levels of the DC maturation markers CD83 and CD86 (measured after 48h of maturation induction, ). Over a total of 11 experiments these maturation-enhancing effects reached significance for expression levels of both CD83 and CD86 (), and also resulted in an increased allogeneic T-cell priming capacity of the MoDC (). Interestingly, TDSN derived from colon carcinoma cell lines (A2233, Colon 320, HT 29, and WiDr, at 30% [v/v]) did not have any effect on MoDC maturation (data not shown). We previously identified prostaglandins as the factor in colon-derived TDSN responsible for the inhibition of MoDC differentiation.Citation10 Here, we show that the TDSN-mediated maturation effects in MoDC are similarly prostaglandin-dependent, as shown by abrogation of the effects of TDSN generated in the presence of the COX-inhibitor indomethacin (IM, ). In contrast, these TDSN-mediated effects in skin-emigrated DC appeared to be wholly prostaglandin-independent (). Of note, effectiveness of COX inhibition was ascertained by measuring the PGE2 content in IM-modulated and unmodulated TDSN. PGE2 concentrations were absent or strongly reduced in IM-modulated TDSN, but did not influence the levels of other cytokines (e.g., IL-10 and IL-6) present in the TDSN (assessed by ELISA, data not shown).Citation4
Figure 2. Primary colon tumor derived supernatants (TDSN) promote maturation of monocyte-derived DC (MoDC) in a prostaglandin-dependent manner. (A–C) 48 h maturation induction of 7-d immature DC, generated with GM-CSF and IL-4, by either 10 μM PGE2 or 30% (v/v) TDSN, revealed TDSN-induced maturation comparable with PGE2 induced maturation, as determined by flowcytometric analysis of CD83 and CD86 levels (, n = 11), which was entirely abrogated by the use of Indomethacin (IM)-modulated TDSN (, n = 4). Expression levels of the studied (maturation) markers are listed as Mean Fluorescence Intensities (MFI). (D) Mixed Leukocyte Reactions were performed with MoDC, either unmodulated or matured by the indicated TDSN conditions. Data shown are representative or averages (± sd) of 3–11 separate experiments, *P < 0.05.
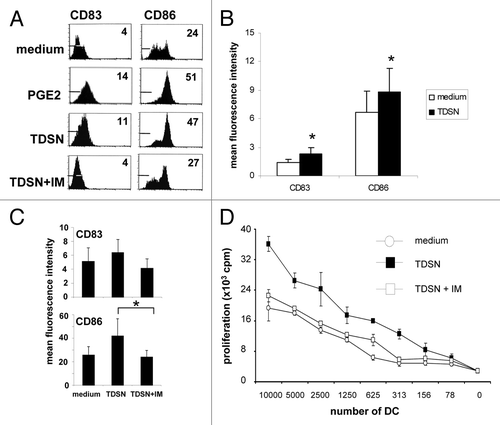
Figure 3. Maturation-inducing effects of primary colon tumor derived supernatants (TDSN) on skin-emigrating DC are not dependent on prostaglandins. Colon TDSN were i.d. injected at 50% (v/v) prior to explant culture, while 3% (v/v) was added to the culture media of the explant cultures. Skin explants were removed at day 2 of culture, after which migrated DC were immediately harvested (or 5 d later for IL-12p70 release testing). (A) Maturation induction of skin-emigrated DC by TDSN was not abrogated by indomethacin (IM) modulation of the TDSN during their generation. Expression levels of the studied (maturation) markers are listed as Mean Fluorescence Intensities (MFI). (B) Mixed Leukocyte Reactions were performed with skin-emigrated DC, either unmodulated or matured by the indicated TDSN conditions. Data shown are representative of 3–5 separate experiments. (C) DC harvested from the cultures at day 7 were stimulated by CD40L-transduced J558 cells in the presence of 1000 IU/ml IFNγ and supernatants were harvested 24h later. IL-12p70 concentrations were determined by ELISA; averages ± sd are indicated. *P < 0.05 vs medium controls. Data shown are representative of 3–5 separate experiments.
Although TDSN did not interfere in any way with the migration rate of skin-derived DC (data not shown), TDSN did significantly inhibit the ability of migrated DC to produce IL-12p70 in response to CD40L-stimulation, which, again, was not influenced by IM-mediated COX inhibition during the generation of the employed TDSN ().
Effects on Th cell subset differentiation of TDSN-conditioned skin-derived DC
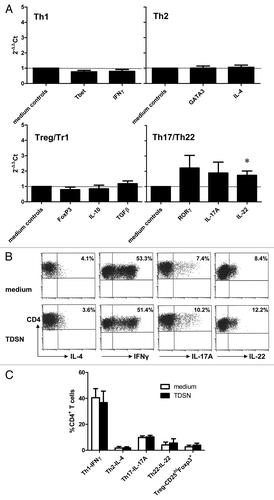
To ascertain how i.d. delivery of TDSN affected the Th cell-stimulatory ability of subsequently migrated DC, they were loaded with anti-CD3 and co-cultured with allogeneic CD4+CD25- T-cells over a period of 2 wk. After polyclonal re-stimulation the Th cells were then profiled for the presence of Th1-, Th2-, Th17/22-, and Treg-associated transcripts (). These analyses suggested a modest induction of Th17/Th22 related genes such as RoR-γt, IL-17 and IL-22 in the TDSN conditions compared with the medium control. However, testing the Th cells for their actual ability to produce Th1-, Th2-, Th17-, or Th22-associated cytokines by intracellular staining, and simultaneously for Treg frequencies, demonstrated no major shifts induced by TDSN-conditioned DC in Th subset distribution profiles (). A predominance of Th1 cells was apparent both in control cultures and in cultures with TDSN-conditioned skin DC.
Figure 4. Broad Th subset-inducing capacity of skin-emigrated DC i.d. modulated by primary colon tumor derived supernatants (TDSN). Colon TDSN were i.d. injected at 50% (v/v) prior to explant culture, while 3% (v/v) was added to the culture media of the explant cultures. Skin explants were removed at day 2 of culture, after which migrated DC were immediately harvested and loaded with anti-CD3 and co-cultured for 14 d with CD4+CD25- T-cells. (A) After 14 d the Th cells were restimulated with immobilized anti-CD3 and anti-CD28 for 24h after which mRNA was isolated and the indicated transcript levels were determined by qRT-PCR: values relative to medium controls, n = 3. (B and C) Alternatively 14 d-primed Th cells were restimulated with PMA/ionomycin for 4h after which intracellular cytokine production levels or FoxP3 expression in relation to high CD25 levels (i.e., Tregs) were assessed; representative results (B) and averaged data ± sem (C) are shown of 3 separate experiments; *P < 0.05.
GM-CSF as likely perpetrator of maturation induction in skin-emigrated DC by TDSN
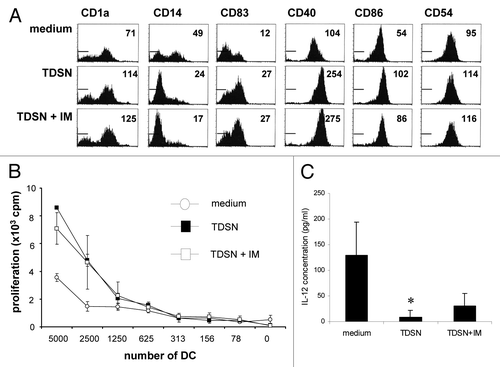
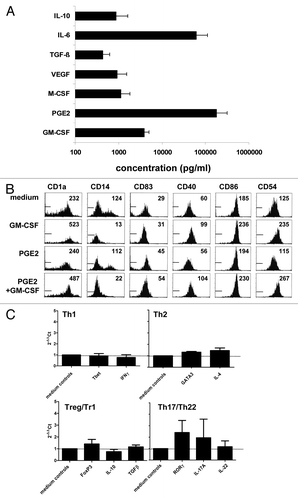
To ascertain whether the observed prostanoid-independent maturational effects on skin-emigrated DC were induced by tumor-derived soluble factors or rather by factors derived from the tumor infiltrate, tumor single cell suspensions were depleted of leukocytes by CD45-specific panning. Supernatants were subsequently generated from both the tumor and the infiltrate fractions and tested separately for maturing effects on skin-emigrating DC. As demonstrated in , tumor-derived soluble factors were clearly responsible for the observed maturation induction and not infiltrate-derived factors. The employed primary colon TDSN contained a wide variety of factors, known for their DC-modulatory abilities (n = 17, ). Among the tested factors, IL-6, GM-CSF, and PGE2 were known to have DC-maturational effects. As PGE2 was ruled out and we previously had observed no effects of IL-6 in the employed explant model,Citation15 GM-CSF remained as a possible candidate. Indeed, in terms of phenotype-modulating effects, those of GM-CSF more closely mimicked the effects of colon TDSN than of PGE2, including the loss of CD14+ cells after migration, which was not apparent upon i.d. PGE2 delivery (). Like the employed TDSN, i.d. delivery of GM-CSF led to the induction of a transcriptional Th17/Th22-like profile in primed Th cells by skin-emigrated DC (). Although these observations make GM-CSF a likely causative agent for the observed effects, the overall immune-modulatory effects of the TDSN will obviously be determined by the combined activity of the multitude of factors they comprise.
Figure 5. Soluble colon tumor factor-induced maturation of DC migrated from skin is mediated by the CD45-negative cell fraction. The CD45+ immune infiltrate was depleted from colon tumor single-cell suspensions by panning and 24 h supernatants were generated from both the CD45- and the CD45+ fractions. Unseparated and separated tumor-derived supernatants (TDSN) were i.d. injected at 50% v/v prior to explant culture, while 3% v/v was added to the culture media of the explant cultures. Skin explants were removed at day 2 of culture. Two-day migrated DC were harvested and analyzed by flow cytometry for the indicated markers. Mean Fluorescence Intensities (MFI) are listed. TDSN from 2 primary colon tumors were similarly generated with equivalent results.
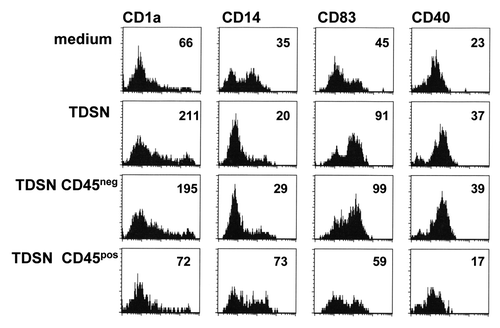
Figure 6. Phenotypic DC modulation and transcriptional effects on primed Th cells of GM-CSF-conditioned and skin-emigrated DC. (A) ELISA analysis of the cytokine contents of supernatants prepared from freshly dissociated primary colon tumors (1 x 106 tumor cells/ ml /24 hr, n = 17) revealed production of IL-10, IL-6, TGFβ1, VEGF, M-CSF, PGE2, and GM-CSF. Results shown are means ± sd. (B) Intradermal (i.d.) injection -prior to culture- of human skin explants with 100 ng GM-CSF or 10 μM PGE2 or a combination thereof enhanced the maturation state of migrated DC (harvested and analyzed 2 d after the start of skin explant culture; Mean Fluorescence Intensities (MFI) of the tested markers are listed in the histograms and the histogram markers indicate fluorescence levels obtained with the isotype controls). Data are representative of 3 experiments. (C) Alternatively, skin explants were removed at day 2 of culture, after which DC, emigrated form medium- or GM-CSF –injected skin explants, were immediately harvested and loaded with anti-CD3 and co-cultured for 14 d with CD4+CD25- T cells. After 14 d the Th cells were restimulated with immobilized anti-CD3 and anti-CD28 for 24h after which mRNA was isolated and the indicated transcript levels were determined by qRT-PCR: values relative to medium controls, n = 3.
Discussion
Tumors interact with their microenvironment to promote their own survival and to escape immune surveillance.Citation11 As part of these escape strategies tumors secrete suppressive factors that inhibit the development and functionality of DC. Intradermal vaccination with autologous colon tumor vaccines may be hampered by the same mechanisms. We previously reported that primary colon TDSN interfered with MoDC development in vitro.Citation10 This impaired differentiation was caused by tumor-related upregulation of the COX-2 enzyme with concomitant PGE2 production by the primary colon tumor suspensions. In the present study we show that primary colon TDSN enhance rather than frustrate the maturation of skin-emigrated DC, ascertained in an organotypic skin explant system.Citation12 In contrast to the previous and current in vitro MoDC studies, COX-induced prostaglandins did not play a role in the TDSN-induced enhancement of skin-emigrated DC maturation.
PGE2, the most likely culprit in the observed maturation induction of MoDC by colon TDSN, is constitutively expressed in the intestinal Lamina Propria and may serve to condition local DC to a non-inflammatory default setting, maintaining a general state of tolerance in the healthy gut.Citation16,Citation17 Further upregulation of PGE2 under the influence of tumor-associated COX-2 expression in colon tumors will reinforce this state and lock the DC in a state that is not conducive to the initiation of an anti-tumor immune response. Although PGE2 serves as a (co-)factor for phenotypic DC maturation, the functional consequences of this maturation may be detrimental in terms of anti-tumor immunity: it will skew cytokine production to a high type-2 immunity promoting IL-10 release and may lead to the expression of the tryptophan-catabolizing enzyme Indoleamine-2,3-Dioxygenase (IDO), known for its T-cell suppressive effects.Citation18-Citation21 Discrepancy in prostaglandin dependence of the TDSN-mediated maturation induction between in vitro generated MoDC and skin-resident DC may in part be explained by a difference in the intrinsic susceptibility of these DC types to the effects of prostaglandins, since PGE2-induced maturation was more robust in MoDC than in skin-emigrated DC. Of note, as transcriptional levels of PGE2 receptors between MoDC and CD1a+ dermal DC are comparable these may not account for the observed difference in PGE2 susceptibility.Citation22
In addition, a possible explanation lies in the indirect effects through modulation of the skin microenvironment by the TDSN. Factors in the colon TDSN may have induced production of DC-maturing agents by keratinocytes, which upon activation are known to release such DC-activating cytokines as GM-CSF, IL-1β, and TNFα. Particularly the IL-1β-TNFα axis has been reported to play a vital role in DC migration from the epidermis, with IL-1β inducing TNFα release which acts as a direct mediator of Langerhans cell maturation and migration.Citation23,Citation24 We studied the possible role of the IL-1β/TNFα axis in the observed skin-emigrated DC maturation induction by TDSN, but could not block this effect by the i.d. injection of TNFα neutralizing antibodies (data not shown).
What exact factors in the primary TDSN are responsible for the enhanced maturation of skin explant-emigrated DC remains to be elucidated. Future studies should focus on the identity and source of the responsible soluble factors. GM-CSF is however a likely candidate and has previously been described to be secreted by colon tumors with both direct and indirect, immune-dependent antitumor effects.Citation25 GM-CSF is known as an immune adjuvant, and potent DC-activator, especially when administered intradermally.Citation26,Citation27
The finding that colon TDSN actually induce DC maturation may seem unexpected and counterintuitive since various studies have pointed to the reduced frequency of mature CD83+ DC infiltrating colon tumor fields in vivo and have identified this as a bad prognostic factor.Citation28-Citation32 However, this depletion of local CD83+ DC may be due to the inhibition of local DC precursor differentiation into bona fide DC (e.g., by tumor-derived prostaglandins and/or IL-6), rather than the inhibition of maturation of tumor-infiltrating DC. Such a dichotomous effect of tumor-derived factors on DC differentiation and maturation (inhibiting the first, enhancing the latter) as we have now observed for colon carcinoma, has previously been described for melanoma.Citation33 Others have also described (partially) maturing effects of TDSN on DC, in keeping with our own findings.Citation34,Citation35 The concomitant block in IL-12p70 release might have been expected to prohibit the generation of an effective T-cell-mediated anti-tumor response and to rather have led to active tolerance induction. Indeed, a number of recent publications have suggested that mature CD83+ DC rather than immature DC are responsible for maintaining tolerance.Citation29,Citation36-Citation38 However, we found no evidence of hampered Th cell priming or Th cell differentiation induction by the TDSN-conditioned skin-emigrated DC, nor of increased Treg expansion. Although the modest induction of a Th17/Th22 transcriptional profile would be in keeping with recent reports relating IL-17 and IL-22 with colon cancer development,Citation39-Citation41 the maintained predominance of a Th1 response in co-cultures of TDSN-conditioned skin DC and allogeneic T-cells bodes well for their projected ability to induce effective antitumor immunity.
Results from our study contrast with those from Michielsen et al.Citation42,Citation43 who demonstrated inhibition of MoDC maturation induction by colon tumor-derived soluble factors. In that study TDSN were derived from tumor explants. It is conceivable that (1) the intact 3D colon tumor environment led to a different secretome profile – both studies focused on different secreted factors present within the supernatants, or (2) interaction of tumor derived factors with elements in the skin microenvironment led to the differential effects on DC maturation observed in our studies. Also, differences between the study results could be due to differences in methodology. In their study, Michielsen et al. pre-exposed the immature MoDC for 4 h to the tumor explant-derived supernatants prior to adding LPS to induce DC maturation, whereas we added TDSN directly to immature MoDC and assessed their maturational effects per se. Of note, when we added TDSN and LPS simultaneously to immature DC we observed enhancement of LPS-induced maturation by the TDSN (data not shown). These differences in methodologies derive from differences in the research questions for both studies: whereas Michielsen et al. aimed to study what would happen to tumor-infiltrating immature DC once they would arrive in the tumor microenvironment, our study was designed to study the effects of whole tumor cell-based vaccines on DC in the human skin microenvironment. As such the model employed in our study carries certain advantages and disadvantages. It allows for an accurate and physiologically highly relevant assessment of the effects of conditioning of the human skin microenvironment by colon tumor-derived soluble factors on the T cell activating properties of subsequently migrating DC. However, it is also a relatively ill-defined, “dirty” system that is not readily amenable to delineation of immune-modulatory factors, mechanisms, and cross-talk events in the dermis, that underlie the observed stimulatory effects on migratory DC after i.d. delivery of primary colon tumor-derived supernatants.
We conclude that colon cancer-derived soluble factors in the skin microenvironment do not recapitulate the immune suppressive tumor microenvironment but in effect induce phenotypic DC maturation, which is accompanied by increased priming of a poly-functional Th cell response. As such i.d. delivery of autologous colon cancer-based vaccines should be fully compatible with the envisioned induction of anti-tumor immunity, certainly when further combined with powerful adjuvants that will ensure a strong type-1 T-cell response and efficient cross-priming of tumor-specific CTL.
Materials and Methods
Cell lines
The CD40L-transfected J558 plasmacytoma cell line was a kind gift of Dr M Kapsenberg, Amsterdam Medical Center, Amsterdam, The Netherlands (J558-CD40L).
Monoclonal antibodies and recombinant cytokines
The following cytokines and reagents were used: Prostaglandin E2 (PGE2) (Sigma-Aldrich) and rhGM-CSF (Berlex Laboratories Inc.). The following PE- or FITC-labeled mAbs were used: CD83 (Immunotech), CD80, CD86, CD40, CD54, CD14, and HLA-DR (all from BD Biosciences).
Tumor dissociation
Cell suspensions prepared from human primary tumors (colon carcinoma Duke's B or Duke's C) were handled within 24 h of surgical removal as described.Citation1 In short, viable tumor was minced with a scalpel and dissociated for 1 to 3 h with 0.02% DNase (Boeringer Mannheim GmbH) and 0.14% Collagenase type IV (Boeringer Mannheim GmbH) in Hanks’ Balanced Salt Solution (HBSS, Lonza). The cell suspension was filtered and washed to remove tissue debris and immediately cryo-preserved using a control rate freezing system and stored in liquid nitrogen. Before use, tumor cell suspensions were rapidly thawed and diluted in HBSS containing 0.02% DNase. After 3 washes cell numbers and cell viability was determined by trypan blue exclusion and used to prepare tumor-derived supernatants (TDSN).
Preparation of tumor-derived supernatants
TDSN were prepared from freshly dissociated primary tumors as described.Citation10 Cells were seeded at a density of 1 × 106cells/ml in complete medium (IMDM containing 10% FCS (Hyclone), 50 U/ml penicillin-streptomycin, 1.6 mM L-glutamine, 0.01 mM 2-ME). After 24 h culture, tumor cells were centrifuged (1500 rpm, (530 g), 5 min), supernatants were run through a 0.22 μm Millex-GP filter (Merck Chemicals), and stored at –80 °C. Prostaglandin synthesis was blocked through COX inhibition. TDSN were prepared, as described above, through culture of primary tumor cells in the presence or absence of a non-selective COX-1/COX-2 inhibitor, indomethacin (Merck Chemicals) at a concentration of 10 μM. Immune infiltrate was separated from the colon tumor suspensions through indirect panning on the basis of CD45 positivity as described previously.Citation10 Remaining colon tumor cells were used to prepare supernatant at a concentration of 1 × 106cells/ml, while isolated CD45+ tumor-derived leukocytes were cultured at a concentration corresponding with 1 × 106colon cells/ml to obtain supernatants.
Generation and maturation of MoDC
PBMC were obtained from healthy donors by density gradient centrifugation over Lymphoprep (Nycomed AS) and cryo-preserved as previously described.Citation44 PBMC were thawed and resuspended in culture medium IMDM containing 10% FCS and allowed to adhere to 6-well tissue culture plates (3 × 106cells/ml) for 2 h at 37 °C. Non-adherent cells were removed and adherent cells were cultured in complete medium supplemented with 100 ng/ml GM-CSF (specific activity 1.11 × 106IU/mg, Schering-Plough) and 1000 U/ml IL-4 (specific activity 108U/mg, Sanquin). Immature MoDC (iMoDC) were collected at day 6 and plated at a density of 0.3 × 106cells/ml into a 12-well tissue culture plate. Maturation was induced by addition of TDSN 30% (v/v) or PGE2 at 10 μM for 48 h in the presence of 1000 U IL-4/ml and 100 ng/ml GM-CSF.
Skin preparation and explant cultures
Healthy human skin specimens were obtained after verbal informed consent at the time of hospital admission from patients undergoing corrective breast or abdominal plastic surgery at the VU University medical center (VUmc, Amsterdam, The Netherlands) or at the Tergooi hospital (Hilversum, The Netherlands) and used in an anonymous fashion. Patients that objected to this procedure signed a statement to this effect, in accordance with the “Code for Proper Use of Human Tissues” as formulated by the Dutch Federation of Medical Scientific Organizations (http://www.fmwv.nl) and following procedures approved by the IRB.Citation14 Cytokines or TDSN (50% v/v) were injected into the dermis with a Micro-Fine Insulin syringe (0.33 mm [29G] × 12.7mm needle, BD Biosciences) at the indicated amounts and in a total volume of 20μl serum-free medium (following a methodology that we previously described).Citation12 A sham control was included by injecting skin with 20 μl serum-free medium (indicated as “medium” in the text and figures). At the site of injection a 5 mm urtica appeared and an exact punch biopsy of 6 mm was taken. The biopsy was lifted from the specimen with a sterile forceps and with sterile scissors the dermis was cut at a depth of 2–3 mm to obtain skin explants. Biopsies were placed in 1 ml culture medium IMDM containing 5% inactivated and (0.2 μm) filtered Human Pool Serum (HPS, Sanquin), either pure or supplemented with 3% (v/v) TDSN. After a migration period of 48 h cells were harvested and tested for phenotype, cytokine production, or T-cell stimulatory capacity.
Flowcytometry
Immunophenotypic analysis was performed using FACS.Citation12 In short, cells were washed in PBS supplemented with 1% BSA and 0.02% NaN3 (PBA) and incubated for 30 min at room temperature in the presence of appropriate dilutions of PE- or FITC-conjugated specific mAbs or with the corresponding isotype matched mAb. Excess mAb was removed by washing in PBA. The cells were subsequently analyzed, using FACS Calibur and CellQuest-Pro software (BD Biosciences). Results were expressed either as mean fluorescence or the percentage of positive cells.
Mixed leukocyte reaction
5 × 104Responder PBMC/well, isolated from buffy coats as described above, were incubated with titrated amounts of allogeneic stimulator DC. Cells were cultured in 96-well round-bottom plates (Costar) in complete medium supplemented with 10% HPS for 5 d at 37°C and 5% CO2 in a humidified atmosphere. Cells were pulsed with [3H] Thymidine (0.5 μCi/well [18.5 × 10Citation3] Bq/well) Amersham) during the last 18h of culture. [3H] Thymidine incorporation was measured using a liquid scintillation counter (Wallac). Responses are shown as mean counts per minute (cpm) from triplicate wells.
IL-12p70 release
Mature MoDC or skin-emigrated DC were analyzed for functional IL-12p70 release as described previously.Citation10 Briefly, 4 × 104DC were incubated with 4 × 104J558-CD40L cells in the presence of 1000 U rhIFNγ/ml (Sanquin) in 200 μl complete medium. We previously found IL-12p70 release for skin-emigrated DC to be optimal when J558-CD40L cells were added to the skin-emigrated DC at day 7 after the start of explant cultureCitation15 all skin DC IL-12p70 release data were from this time point. After 24h co-culture of d7 DC and irradiated J558-CD40L cells, the supernatants were collected and stored at –20°C. IL-12p70 concentrations were determined by capture ELISA as previously described.Citation10
Th and Treg differentiation assays
40 000 skin-emigrated DC were incubated with 0.5 μg/ml anti-CD3 (OKT-3, eBioscience) in 200 μl complete medium (i.e., IMDM supplemented with 10% FCS, 100 IU/ml sodium penicillin, 100 µg/ml streptomycin sulfate, 2 mM L-glutamine, and 0.01 mM 2-ME) for 15 min at 4 °C. After incubation, the DC were co-cultured with 20 000 CD4+CD25- T-cells (isolated by magnetic bead separation using the untouched CD4 isolation kit and anti-CD25 beads from Miltenyi, according to the manufacturer’s instructions) for 14 d. At day 7, 10 U/ml IL-2 (Strathmann Biotec) was added to the cultures. On day 14, T-cells were harvested and analyzed by flowcytometry for CD3, CD4, CD25, and FoxP3 expression as previously describedCitation45 or 50 000–100 000 T-cells were stimulated for 4 h with 50 ng/ml PMA and 500 ng/ml ionomycin in the presence of 0.5 µl/ml brefeldin A (GolgiPlug, BD Biosciences), stained for CD3 and CD4, and for IFNγ, IL-4, IL-17A, or IL-22 (all from BD Biosciences) after permeabilisation using the BD Perm-Fix kit and subsequently analyzed, using a FACSCalibur and Cellquest-Pro FACS analysis software (BD Biosciences). Alternatively, Th cells were stimulated with 0.5 μg/ml anti-CD3 (OKT-3) and 0.5 μg/ml anti-CD28 (clone 15E8) overnight. At that time stimulated Th cells were harvested and RNA isolated to also assess transcript levels of Th1-, Th2-, Th17-, Th22-, or Treg-associated transcription factors and cytokines
RNA isolation and cDNA synthesis
Total RNA was isolated using the RNeasy Plus Micro kit (Qiagen). Contaminating genomic DNA was removed by using the gDNA Eliminator spin colums from the kit. The concentration and purity of the RNA was analyzed using the NanoDrop ND-1000 (Thermo Scientific). cDNA was synthesized using oligo(dT)20 primers and the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer's instructions. Input of RNA was 240 ng. After cDNA synthesis nuclease-free water was added up to a final volume of 80 µl.
Real-time qRT-PCR
Transcripts were quantified by real-time quantitative polymerase chain reaction (qPCR) using an ABIPRISM 7900 Sequence Detector and pre-designed TaqMan Gene Expression Assays, reagents and probes according to manufacturer’s instructions (Applied Biosystems), as described previously.Citation15,Citation45 We validated all primers according to protocol. Mean relative mRNA expression was calculated using Pfaffl method.Citation46
Statistical Analysis
DC subset frequencies and marker expression levels, cytokine levels, transcript levels, and T-cell frequencies and responses were compared between conditions using the paired T test. Excel or Prism 4.0 statistical software (GraphPad Software Inc.) was used. Differences were considered significant when P < 0.05 in two-sided analyses.
| Abbreviations: | ||
| MoDC | = | monocyte derived dendritic cell |
| PGE2 | = | prostaglandin E2 |
| TDSN | = | tumor derived supernatant |
| COX | = | cyclooxygenase |
| HSP | = | heat shock protein |
| CTL | = | cytotoxic T lymphocyte |
| VEGF | = | vascular epithelial growth factor |
| GM-CSF | = | granulocyte macrophage-colony stimulating factor |
| MLR | = | mixed leukocyte reaction |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We gratefully acknowledge our colleagues from the Oncopathology Unit who provided us with tumor single-cell suspensions from the Active Specific Immunisation (ASI) program.
This study was supported by a grant from the Netherlands Organization for Scientific Research (NWO) to T.D.G. (NWO VIDI grant 917-56-321)
References
- Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet 1999; 353:345 - 50; http://dx.doi.org/10.1016/S0140-6736(98)07186-4; PMID: 9950438
- de Weger VA, Turksma AW, Voorham QJ, Euler Z, Bril H, van den Eertwegh AJ, Bloemena E, Pinedo HM, Vermorken JB, van Tinteren H, et al. Clinical effects of adjuvant active specific immunotherapy differ between patients with microsatellite-stable and microsatellite-instable colon cancer. Clin Cancer Res 2012; 18:882 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-11-1716; PMID: 22156611
- Castle JC, Kreiter S, Diekmann J, Löwer M, van de Roemer N, de Graaf J, Selmi A, Diken M, Boegel S, Paret C, et al. Exploiting the mutanome for tumor vaccination. Cancer Res 2012; 72:1081 - 91; http://dx.doi.org/10.1158/0008-5472.CAN-11-3722; PMID: 22237626
- Heemskerk B, Kvistborg P, Schumacher TN. The cancer antigenome. EMBO J 2012; 32:194 - 203; http://dx.doi.org/10.1038/emboj.2012.333; PMID: 23258224
- Kreiter S, Castle JC, Türeci O, Sahin U. Targeting the tumor mutanome for personalized vaccination therapy. Oncoimmunology 2012; 1:768 - 9; http://dx.doi.org/10.4161/onci.19727; PMID: 22934277
- Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, Allison JP. Epitope landscape in breast and colorectal cancer. Cancer Res 2008; 68:889 - 92; http://dx.doi.org/10.1158/0008-5472.CAN-07-3095; PMID: 18245491
- Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med 1999; 190:1417 - 26; http://dx.doi.org/10.1084/jem.190.10.1417; PMID: 10562317
- Coventry BJ, Morton J. CD1a-positive infiltrating-dendritic cell density and 5-year survival from human breast cancer. Br J Cancer 2003; 89:533 - 8; http://dx.doi.org/10.1038/sj.bjc.6601114; PMID: 12888826
- Vermi W, Bonecchi R, Facchetti F, Bianchi D, Sozzani S, Festa S, Berenzi A, Cella M, Colonna M. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol 2003; 200:255 - 68; http://dx.doi.org/10.1002/path.1344; PMID: 12754747
- Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, Pinedo HM, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol 2002; 168:4333 - 43; PMID: 11970975
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol 2004; 4:941 - 52; http://dx.doi.org/10.1038/nri1498; PMID: 15573129
- de Gruijl TD, Luykx-de Bakker SA, Tillman BW, van den Eertwegh AJ, Buter J, Lougheed SM, van der Bij GJ, Safer AM, Haisma HJ, Curiel DT, et al. Prolonged maturation and enhanced transduction of dendritic cells migrated from human skin explants after in situ delivery of CD40-targeted adenoviral vectors. J Immunol 2002; 169:5322 - 31; PMID: 12391253
- Lukas M, Stössel H, Hefel L, Imamura S, Fritsch P, Sepp NT, Schuler G, Romani N. Human cutaneous dendritic cells migrate through dermal lymphatic vessels in a skin organ culture model. J Invest Dermatol 1996; 106:1293 - 9; http://dx.doi.org/10.1111/1523-1747.ep12349010; PMID: 8752673
- de Gruijl TD, Sombroek CC, Lougheed SM, Oosterhoff D, Buter J, van den Eertwegh AJ, Scheper RJ, Pinedo HM. A postmigrational switch among skin-derived dendritic cells to a macrophage-like phenotype is predetermined by the intracutaneous cytokine balance. J Immunol 2006; 176:7232 - 42; PMID: 16751366
- Lindenberg JJ, Oosterhoff D, Sombroek CC, Lougheed SM, Hooijberg E, Stam AG, Santegoets SJ, Tijssen HJ, Buter J, Pinedo HM, et al. IL-10 conditioning of human skin affects the distribution of migratory dendritic cell subsets and functional T cell differentiation. PLoS One 2013; 8:e70237; http://dx.doi.org/10.1371/journal.pone.0070237; PMID: 23875023
- Newberry RD, McDonough JS, Stenson WF, Lorenz RG. Spontaneous and continuous cyclooxygenase-2-dependent prostaglandin E2 production by stromal cells in the murine small intestine lamina propria: directing the tone of the intestinal immune response. J Immunol 2001; 166:4465 - 72; PMID: 11254702
- Morelli AE, Thomson AW. Dendritic cells under the spell of prostaglandins. Trends Immunol 2003; 24:108 - 11; http://dx.doi.org/10.1016/S1471-4906(03)00023-1; PMID: 12615202
- Steinbrink K, Paragnik L, Jonuleit H, Tüting T, Knop J, Enk AH. Induction of dendritic cell maturation and modulation of dendritic cell-induced immune responses by prostaglandins. Arch Dermatol Res 2000; 292:437 - 45; http://dx.doi.org/10.1007/s004030000159; PMID: 11000287
- Kaliński P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. Dendritic cells, obtained from peripheral blood precursors in the presence of PGE2, promote Th2 responses. Adv Exp Med Biol 1997; 417:363 - 7; http://dx.doi.org/10.1007/978-1-4757-9966-8_59; PMID: 9286387
- Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol 2002; 168:2255 - 63; PMID: 11859113
- Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood 2005; 106:2375 - 81; http://dx.doi.org/10.1182/blood-2005-03-0979; PMID: 15947091
- Lundberg K, Albrekt AS, Nelissen I, Santegoets S, de Gruijl TD, Gibbs S, Lindstedt M. Transcriptional profiling of human dendritic cell populations and models--unique profiles of in vitro dendritic cells and implications on functionality and applicability. PLoS One 2013; 8:e52875; http://dx.doi.org/10.1371/journal.pone.0052875; PMID: 23341914
- Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor alpha and interleukin 1 beta for migration. Adv Exp Med Biol 1997; 417:125 - 8; http://dx.doi.org/10.1007/978-1-4757-9966-8_21; PMID: 9286349
- Cumberbatch M, Bhushan M, Dearman RJ, Kimber I, Griffiths CE. IL-1beta-induced Langerhans’ cell migration and TNF-alpha production in human skin: regulation by lactoferrin. Clin Exp Immunol 2003; 132:352 - 9; http://dx.doi.org/10.1046/j.1365-2249.2003.02146.x; PMID: 12699428
- Urdinguio RG, Fernandez AF, Moncada-Pazos A, Huidobro C, Rodriguez RM, Ferrero C, Martinez-Camblor P, Obaya AJ, Bernal T, Parra-Blanco A, et al. Immune-dependent and independent antitumor activity of GM-CSF aberrantly expressed by mouse and human colorectal tumors. Cancer Res 2013; 73:395 - 405; http://dx.doi.org/10.1158/0008-5472.CAN-12-0806; PMID: 23108143
- Molenkamp BG, van Leeuwen PA, van den Eertwegh AJ, Sluijter BJ, Scheper RJ, Meijer S, de Gruijl TD. Immunomodulation of the melanoma sentinel lymph node: a novel adjuvant therapeutic option. Immunobiology 2006; 211:651 - 61; http://dx.doi.org/10.1016/j.imbio.2006.06.009; PMID: 16920504
- Vuylsteke RJ, Molenkamp BG, van Leeuwen PA, Meijer S, Wijnands PG, Haanen JB, Scheper RJ, de Gruijl TD. Tumor-specific CD8+ T cell reactivity in the sentinel lymph node of GM-CSF-treated stage I melanoma patients is associated with high myeloid dendritic cell content. Clin Cancer Res 2006; 12:2826 - 33; http://dx.doi.org/10.1158/1078-0432.CCR-05-2431; PMID: 16675577
- Dadabayev AR, Sandel MH, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn C, Ensink NG, Tollenaar RA, et al. Dendritic cells in colorectal cancer correlate with other tumor-infiltrating immune cells. Cancer Immunol Immunother 2004; 53:978 - 86; http://dx.doi.org/10.1007/s00262-004-0548-2; PMID: 15197496
- Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res 1998; 58:4245 - 9; PMID: 9766645
- Miyagawa S, Soeda J, Takagi S, Miwa S, Ichikawa E, Noike T. Prognostic significance of mature dendritic cells and factors associated with their accumulation in metastatic liver tumors from colorectal cancer. Hum Pathol 2004; 35:1392 - 6; http://dx.doi.org/10.1016/j.humpath.2004.07.018; PMID: 15668897
- Sandel MH, Dadabayev AR, Menon AG, Morreau H, Melief CJ, Offringa R, van der Burg SH, Janssen-van Rhijn CM, Ensink NG, Tollenaar RA, et al. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res 2005; 11:2576 - 82; http://dx.doi.org/10.1158/1078-0432.CCR-04-1448; PMID: 15814636
- Schwaab T, Weiss JE, Schned AR, Barth RJ Jr.. Dendritic Cell Infiltration in Colon Cancer. J Immunother 2001; 24:130 - 7; http://dx.doi.org/10.1097/00002371-200103000-00007
- Berthier-Vergnes O, Gaucherand M, Péguet-Navarro J, Plouet J, Pageaux JF, Schmitt D, Staquet MJ. Human melanoma cells inhibit the earliest differentiation steps of human Langerhans cell precursors but failed to affect the functional maturation of epidermal Langerhans cells. Br J Cancer 2001; 85:1944 - 51; http://dx.doi.org/10.1054/bjoc.2001.2183; PMID: 11747338
- Kiertscher SM, Luo J, Dubinett SM, Roth MD. Tumors promote altered maturation and early apoptosis of monocyte-derived dendritic cells. J Immunol 2000; 164:1269 - 76; PMID: 10640740
- Menetrier-Caux C, Thomachot MC, Alberti L, Montmain G, Blay JY. IL-4 prevents the blockade of dendritic cell differentiation induced by tumor cells. Cancer Res 2001; 61:3096 - 104; PMID: 11306493
- Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol 2001; 2:1010 - 7; http://dx.doi.org/10.1038/ni722; PMID: 11590405
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2002; 2:151 - 61; http://dx.doi.org/10.1038/nri746; PMID: 11913066
- Tan JK, O’Neill HC. Maturation requirements for dendritic cells in T cell stimulation leading to tolerance versus immunity. J Leukoc Biol 2005; 78:319 - 24; http://dx.doi.org/10.1189/jlb.1104664; PMID: 15809288
- Harrison C. Cancer: IL-22: linking inflammation and cancer. Nat Rev Drug Discov 2013; 12:504; http://dx.doi.org/10.1038/nrd4065; PMID: 23787957
- Jiang R, Wang H, Deng L, Hou J, Shi R, Yao M, Gao Y, Yao A, Wang X, Yu L, et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer 2013; 13:59; http://dx.doi.org/10.1186/1471-2407-13-59; PMID: 23379788
- Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Roliński J, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 2011; 186:4388 - 95; http://dx.doi.org/10.4049/jimmunol.1003251; PMID: 21357259
- Michielsen AJ, Hogan AE, Marry J, Tosetto M, Cox F, Hyland JM, Sheahan KD, O’Donoghue DP, Mulcahy HE, Ryan EJ, et al. Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer. PLoS One 2011; 6:e27944; http://dx.doi.org/10.1371/journal.pone.0027944; PMID: 22125641
- Michielsen AJ, O’Sullivan JN, Ryan EJ. Tumor conditioned media from colorectal cancer patients inhibits dendritic cell maturation. Oncoimmunology 2012; 1:751 - 3; http://dx.doi.org/10.4161/onci.19570; PMID: 22934271
- Tillman BW, de Gruijl TD, Luykx-de Bakker SA, Scheper RJ, Pinedo HM, Curiel TJ, Gerritsen WR, Curiel DT. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J Immunol 1999; 162:6378 - 83; PMID: 10352250
- Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 2008; 112:2340 - 52; http://dx.doi.org/10.1182/blood-2008-01-133967; PMID: 18617638
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29:45e; http://dx.doi.org/10.1093/nar/29.9.e45; PMID: 11328886
