Abstract
Irritable bowel syndrome (IBS) is a gastrointestinal disorder characterized by chronic abdominal pain, discomfort, and bloating. Interestingly, there is now evidence of the presence of a low-grade inflammatory status in many IBS patients, including histopathological and mucosal cytokine levels in the colon, as well as the presence of IBS-like symptoms in quiescent inflammatory bowel disease (IBD). The use of a genetically engineered food-grade bacterium, such as Lactococcus lactis, secreting the anti-inflammatory cytokine IL-10 has been proven by many pre-clinical studies to be a successful therapy to treat colon inflammation. In this study, we first reproduced the recovery-recurrence periods observed in IBS-patients in a new chronic model characterized by 2 episodes of DiNitro-BenzeneSulfonic-acid (DNBS)-challenge and we tested the effects of a recombinant strain of L. lactis secreting IL-10 under a Stress-Inducible Controlled Expression (SICE) system. In vivo gut permeability, colonic serotonin levels, cytokine profiles, and spleen cell populations were then measured as readouts of a low-grade inflammation. In addition, since there is increasing evidence that gut microbiota tightly regulates gut barrier function, tight junction proteins were also measured by qRT-PCR after administration of recombinant L. lactis in DNBS-treated mice. Strikingly, oral administration of L. lactis secreting active IL-10 in mice resulted in significant protective effects in terms of permeability, immune activation, and gut-function parameters. Although genetically engineered bacteria are, for now, used only as a “proof-of-concept,” our study validates the interest in the use of the novel SICE system in L. lactis to express therapeutic molecules, such as IL-10, locally at mucosal surfaces.
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder, heterogeneous in terms of clinical symptoms and pathogenesis.Citation1,Citation2 It is characterized by chronic abdominal pain and gut dysfunction.Citation3 Although IBS has been traditionally associated with psychological distress, altered gut motility, visceral hypersensitivity,Citation4 and increased gut permeability,Citation5 recent evidence support the presence of a low grade inflammation in the gut mucosa of IBS patients.Citation4 Notably, a modulation of mucosal cytokine levels in the colon of IBS patients,Citation3,Citation6 as well as the presence of IBS-like symptoms in quiescent inflammatory bowel disease (IBD) has been observed.Citation7-Citation9 The link between inflammation and functional gastrointestinal symptoms is based on the interaction between different potential pathogenic factors involved in the development of IBS.Citation4 Many of them are also related to the dysfunction of epithelial barrier which implies a permeable gut. Indeed, impaired gut barrier permeability is associated with several diseases and functional gastrointestinal syndromes.
The use of live vectors to deliver therapeutic molecules at the mucosal level has been extensively described before.Citation10 They are based on the use of recombinant bacteria producing and delivering in situ the molecule of interest. For this purpose, there are 2 basic types of bacterial vectors commonly used to deliver compounds at the mucosal level: attenuated pathogens and non-pathogenic bacteria. Gram-positive commensal or food-grade lactic acid bacteria (LAB) constitute good alternatives to pathogenic ones:Citation11,Citation12 they have been used for centuries in the fermentation and preservation of food, and are thus considered to be safe organisms with a GRAS (Generally Recognized As Safe) status. Most important, mucosal administration of genetic engineered LAB for immunization purposes have been shown to elicit both systemic and mucosal immunity.Citation13-Citation18 These encouraging results confirm the potential use of LAB as live vectors for either mucosal immunization and/or therapy.
The use LAB to prevent and treat inflammatory related diseases was performed for the first time with a recombinant strain of Lactococcus lactis, the model LAB, producing and delivering the interleukin-10 (IL-10, an anti-inflammatory cytokine) in situ, in different mouse models of colitis. Dairy mucosal administration of recombinant L. lactis secreting IL-10 reduces 50% of the colitis induced by the administration of dextran sodium sulfate (DSS) in mice.Citation19,Citation20 This beneficial effect was dependent on the secretion in situ of IL-10 by live recombinant lactococci.Citation21 The rationale in the use of recombinant L. lactis for a localized delivery of IL-10 in IBD is due to the large number of scientific studies proving that the topical treatment with this cytokine has clinic benefits.Citation20 Moreover, local delivery of IL-10 requires much lower doses than systemic treatments (intravenous injection) thus avoiding secondary side-effects.
Recently, we described a new Stress-Inducible Controlled Expression (SICE) system for the expression of therapeutic molecules (such as IL-10) in L. lactis.Citation22 This system, based on a stress-inducible promoter, allows the production and secretion of heterologous proteins (otherwise toxic for the bacterium itself such as some cytokines) at a good rate. Moreover, a recombinant strain of L. lactis secreting biologically active murine IL-10 under the control of SICE system (LL-IL10) has been successfully tested recently in different chronic colitis mouse models which mimic the relapsing pattern observed in patients with IBD.Citation23 The aim of this work is to test the effect of this LL-IL10 strain in a murine model of chronic low inflammation, which mimic the symptoms observed in IBS patients.
Results
Validation of a low-grade inflammation model in DNBS-treated mice
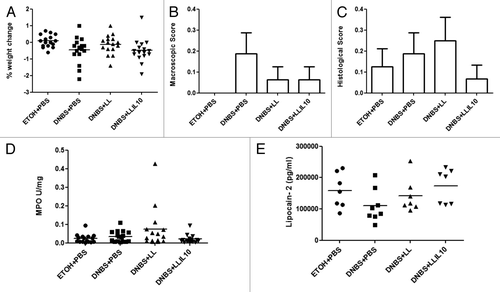
The protocol used to develop a model of low-grade inflammation in mouse is detailed in . To validate the low-grade inflammatory status after the second DNBS-administration, several parameters, directly related to inflammation, were measured. Notably, changes in weight (, A), macroscopic scores, including ulcers, hyperemia, thickening of the colon wall, the presence of adhesions between the colon and other intra-abdominal organs and the consistency of fecal material (, B), microscopic scores, including architectural derangements, goblet cell depletion, edema/ulceration, and degree of inflammatory cell infiltrate were also determined (, C), the degree of infiltration by polymorphonuclear neutrophils (measured by the colon MPO activity) (, D), and the systemic inflammatory immune response (determined by serum Lipocalin-2 [Lcn-2], an early marker of inflammation, concentrations) (, E) were recorded. As shown in and as expected, no significant differences were observed in any of the parameters tested, thus confirming the presence of a low-grade inflammatory status in DNBS-challenged mice.
Figure 2. Validation of micro-inflammation status. Severity of the colitis reactivation was assessed by body weight change (A), macroscopic scores (B), histological scores (C) MPO activity, and (D) lipocalin-2 levels in: control non-inflamed (EtOH+PBS), micro-inflamed mice (DNBS+PBS), LL-treated mice (DNBS+PBS), and LL-IL10-treated mice (DNBS+LL-IL10) (n = 16).
Recombinant LL-IL10 regulates DNBS-induced hyperpermeability
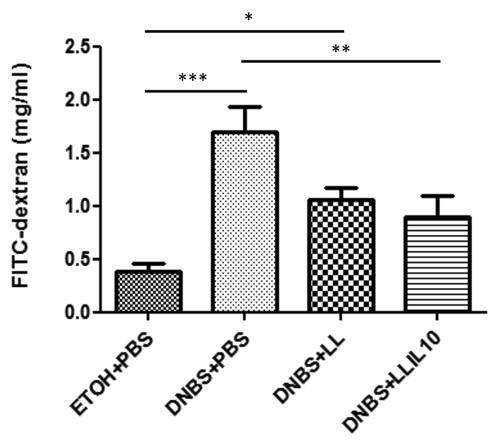
To assess barrier function, permeability to the paracellular tracer FITC-dextran was then evaluated in DNBS-treated mice. For this, mice received FICT-dextran orally 3.5 h before sacrifice and the concentration of this compound was determined in serum samples at the endpoint. As shown in , mice treated with DNBS showed higher permeability (P < 0.05), confirming an alteration in the barrier function in terms of permeability. Strikingly, administration of recombinant L. lactis secreting IL-10 significant decreases (P < 0.05) intestinal hyperpermeability. Surprisingly, administration of the wild-type strain also results in a decrease in the intestinal hyperpermeability; however, this decrease was not statistically significant vs. the DNBS-treated group (P < 0.05) ().
LL-L10 tends to counterbalance alterations in DNBS-induced apical junction proteins
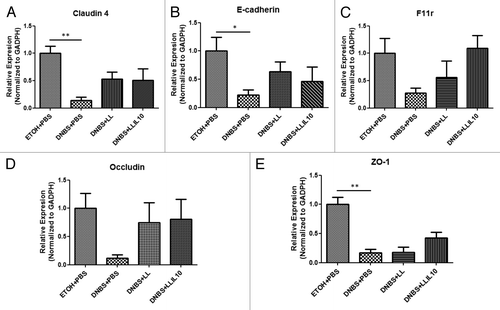
To further analyze the effect on the barrier function, the expression of relevant mRNAs of both adherens junction (AJ) and tight junction (TJ) proteins were studied by qRT-PCR. As shown in , all mRNAs: Claudin-4 (P = 0.0059), E-cadherin (P = 0.049), F11r (JAM1) (P = 0.078), Occludin (P = 0.692), and ZO-1 (P = 0.034) were less abundant in DNBS-treated mice than in control mice. This downregulation tends to be normalized by both LL and LL-IL10; however, a higher specific effect was observed when using LL-IL10 for both F11r and ZO-1 mRNAs. Expression of Claudin 2, 3, and 15 were not modulated (data no shown).
A systemic and mucosal decrease in pro-inflammatory cytokine production correlates with the protective effects of LL-IL10 after DNBS challenge
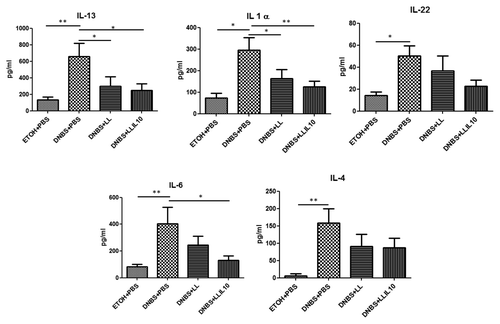
We then investigated the cytokines involved in this low-grade inflammation model by Flow Cytometry using CBA analysis. We assayed 13 cytokines (including those involved in Th1, Th2, Th17, and Th22 pathways) in both colonic () and serum () samples after the second DNBS injection.
Figure 5. Colon cytokine concentrations in DNBS micro-inflammation model. Control non-inflamed (EtOH+PBS), control inflamed (DNBS+PBS), LL-treated mice (DNBS+PBS), and LL-IL10-treated mice (DNBS+LL-IL10) (n = 16). *P < 0.05, **P < 0.001, ***P < 0.0001.
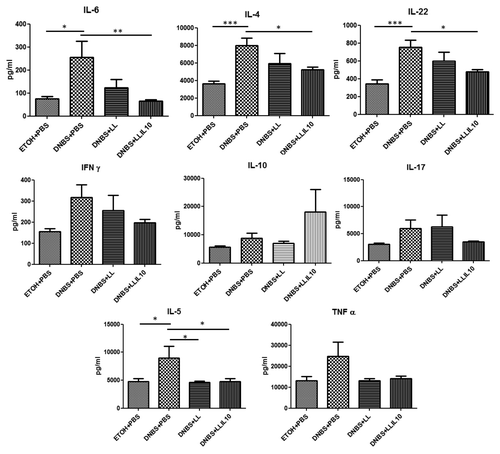
Figure 6. Serum (B) cytokine concentrations in DNBS micro-inflammation model. Control non-inflamed (EtOH+PBS), control inflamed (DNBS+PBS), LL-treated mice (DNBS+LL), and LL-IL10-treated mice (DNBS+LL-IL10) (n = 16). *P < 0.05, **P < 0.001, ***P < 0.0001.
As shown in , concentrations of IL-6, IL-22, IL-5, IL-4, IL-17, IFN-γ, and TNF-α in colon samples were higher in DNBS-treated mice than in healthy controls, which is consistent with a local inflammation. Markedly, IL-6, IL-22, and IL-4 concentrations were lower (statistically significant, P < 0.05) after LL-IL10 treatment, as well as IL-5 after administration of both LL and LL-IL10 (P < 0.05, ). Although a clear tendency was observed for IL-17, IFN-γ and TNF-α after LL-IL10 administration this diminution was not statistically significant. Of note, IL-10 results obtained by CBA confirm either the local production of this cytokine LL-IL10 or a modulation of IL-10 expression by the recombinant L. lactis strain.
Concerning cytokine concentrations in serum samples, our results confirm the low grade inflammatory status since no significant modulation was observed for most of the cytokines tested (data not shown). Nevertheless, IL-13, IL-1α, IL-22, IL-4, and IL-6 levels were significantly higher in DNBS-treated mice compared with the control group (). Interestingly, IL-13, IL-1α, and IL-6 cytokines were significantly reduced after treatment with LL-IL10 (P < 0.05) and a tendency was observed for IL-22 and IL-4. As for the results obtained in the AJ and TJ proteins by qRT-PCR (), the wild-type L. lactis strain displays some modulatory effects for some cytokines in serum samples (ie. diminution of IL-13 and IL-1α).
IL-IL10 treatment restores T-cell variations observed on DNBS-treated mice
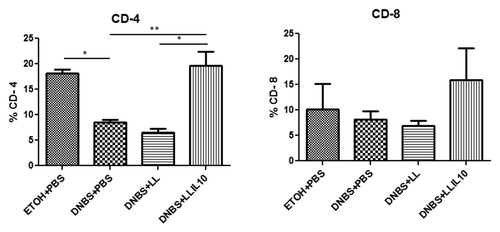
To further determine the mechanism by which LL-IL10 exert its beneficial effects, T-cells were isolated from spleens of DNBS-treated mice and analyzed by Flow cytometry after reactivation. Compared with healthy controls, DNBS-treated mice showed lower CD4+ T cells percentages (). Interestingly, these variations were completely restored in mice treated with LL-IL10 (P < 0.05) (). In contrast, no significant differences were observed in CD8+ T cells, although a weak diminution was observed in DNBS-treated mice, which was restored by LL-IL10; however, these results were not statistically significant ().
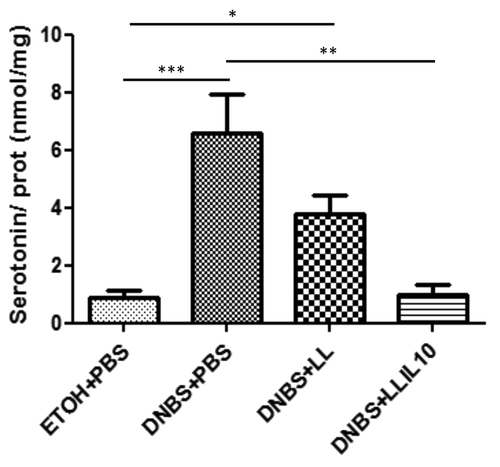
LL-IL10 restores serotonin (5-HT) variations observed in DNBS-treated mice
Serotonin is an important neurotransmitter in the gastrointestinal tract, thus, we decided to determine serotonin levels in colon samples by ELISA. As shown in , colonic serotonin concentrations were higher in DNBS-treated mice than EtOH-treated controls (P < 0.05). Strikingly LL-IL10 treatment significant restores this increase (P < 0.05) (). A decrease was also observed when using the LL strain; however this decrease was not statistically significant when compared with DNBS-treated mice ().
Discussion
Currently, there is an increasing interest in the use of genetically modified microorganisms to deliver therapeutic molecules at mucosal surfaces.Citation10,Citation24 Furthermore, this approach, in addition of being an ideal proof-of-concept method to validate new target molecules to either treat or prevent human diseases in pre-clinical models, provides additional clues of the potential mechanisms of action of the molecule of interest delivered by live bacteria. In this sense, several studies have been performed today to better decipher the effect of the delivered molecule.Citation10 For instance, the effect of the Elafin, a natural protease inhibitor that is normally expressed by the human intestine, has been deeply analyzed using recombinant lactococci.Citation25,Citation26
In this study we decided to test a L. lactis strain delivering the anti-inflammatory cytokine IL-10 locally.Citation22 Strikingly, the use of SICE system for heterologous expression of IL-10 might allow a specifically and locally production and delivery of the protein when L. lactis is subjected to stressful conditions, such as those present in the gastrointestinal tract of the host, due to an acid stress after passage through the stomach (where the pH is about 1.5–2.0) along with a bile stress in the duodenum.Citation22 Indeed, recombinant L. lactis strains secreting IL-10 (but using other expression systems) have previously shown protective effects in severe and moderate murine chronic inflammation models.Citation19,Citation20,Citation25,Citation27 One of these previous strains used a cassette based on a constitutive promoter, integrated into the chromosome for IL-10 expression and secretion. Nevertheless, it is sometimes preferable to benefit from an inducible expression system, in order to target the delivery of the protein of interest (eg. IL-10) only at the right time and place. As an example, a constitutive expression of interleukins and its accumulation in the cytoplasm could be deleterious for L. lactis,Citation20 whereas an induced expression could favor a high and localized delivery, leading to an improved efficiency. In this context, one of the most commonly used inducible system, called NICE (NIsin Controlled Expression system), responds to some of these needs and has been previously used successfully to deliver IL-10 in a murine colitis model.Citation25 However, this system suffers from several drawbacks such as the presence of regulatory genes (provided by plasmids or cloned and integrated into the bacterial chromosome), which limits the choice of appropriate production conditions in both biotechnological and laboratory applications, and the induction of recombinant strain cultures prior to use in vivo. Therefore, one alternative is the use of expression systems that do not require either the presence of regulatory genes or a previous induction of the cultures before apply such as the SICE systemCitation22 used in this study.
Interestingly, in some models of colitis and in some of the read-outs used to determine the degree of the inflammation the use of the wild-type L. lactis strain was not totally neutral.Citation23 These results, together with the recent observations made by Smelt et al.Citation28 who have found immunomodulation capacities of wild-type L. lactis, suggest a beneficial role (ie. probiotic) of this bacterium itself when used as delivery vector and confirm thus the interest in the use of this LAB as a live vector. However, the use of an immunomodulatory carrier to deliver therapeutic proteins to the mucosa could represent an additional advantage. Indeed, based on the extension of the commensal-probiotic hypothesis (which postulates the use of either beneficial commensal or probiotic bacteria as live delivery vectors for target compounds),Citation24 we can expect that their use could improve the desired beneficial effects: synergy bacteria-delivery compound. However, caution has to be made in the choice of an immunomodulatory strain because its intrinsic capacities and potential beneficial effects would largely depend of the mouse model (eg. IBS, IBD, obesity, vaccination, etc). For instance, L. lactis MG1363 (the strain used in this study and by Smelt et al. 2012Citation28) have shown a neutral behavior in several murine models of inflammation and vaccination.Citation25,Citation27,Citation29
To confirm the protective anti-inflammatory effects of LL-IL-10 strain in vivo, a murine chronic low-dose inflammation model was used to mimic the relapsing character of the symptoms of micro-inflammation linked diseases such as IBS patients. Our results showed that our model of DNBS administration results in the absence of an active inflammation status in the colon of mice as it was demonstrated by the lack of macro and microscopic damages as well as of granulocytes infiltrate. The lack of systemic inflammation was also confirmed by measuring the Lcn-2 levels (an early inflammation maker in immune complex mediated inflammatory or autoimmune disorders) in serum samples.Citation30,Citation31 Interestingly, DNBS-treated mice showed gut low-grade inflammation as confirmed by analysis of the immune response. Indeed, colon and serum cytokine levels in DNBS-treated mice as well as CD4+ and CD8+ T-cells were modulated compared with the EtOH group. Strikingly, both lactococci strains were able to counterbalance cytokine levels, and only LL-IL10 T-cell levels. These results are in agreement with the observation of Smelt et al.Citation28 who also found immunomodulation capacities of wild-type L. lactis, both in vitro and in vivo assays.
Experiments, with the paracellular tracer FITC-dextran demonstrate the presence of gut hypermeability in DNBS-inflamed mice. Treatment with both LL and LL-IL10 strains restored in vivo permeability measurements, suggesting that overall total permeability was improved with both bacteria. Increased permeability after reactivation was accompanied by a reduction in specific apical junction protein expression. In this context, LL and LL-IL10 treatment showed tendencies to restore the expression of the transmembrane proteins Occludin, E-cadherin, and Claudin 4. Moreover, specific-LL-IL10 tendencies were observed in the junctional adhesion molecule JAM/F11r and Zonula Occludens (ZO)-1 protein. Although, to our knowledge there is no study about L. lactis effect on gut permeability up to day, this result is in agreement with previous studies where a modulation of the expression of TJ proteins by probiotics has been observed.Citation32,Citation33 Furthermore, an extra positive effect was observed with the strain LL-IL10 confirming the interest of IL-10 and the selected carrier strain for our model.
Administration of probiotic bacteria is a relatively new approach to improve immune homeostasis in order to maintain host health. Indeed, oral probiotic administration modulates both T-cell and dentritic cells distribution in spleen and mesenteric lymphoid nodes (MLN).Citation28 In our model, where immune homeostasis is strongly perturbed and the intestinal barrier is compromised dysfunctional parameters may be also altered. For this reason, colonic levels of serotonin (5-HT) where determined. Serotonin initiates peristalsis, secretion, vasodilatation, and sensory signaling in the gut,Citation34 it also activates immune cells to produce pro-inflammatory mediatorsCitation35 being a link between inflammation and functional symptoms. 5-HT containing enterochromaffin cells (EC) release 5-HT,Citation36 while serotonin selective reuptake transporter (SERT), expressed on intestinal enterocytes, terminates the action of 5-HT by eliminating it from the interstitial space.Citation34 Our results demonstrated that colonic levels of 5-HT were increased in DNBS-challenged mice. This result agrees with previous studies that have found altered levels of this hormone in murine models of colitis induced by TriNitro-Benzene Sulfonic acid (TNBS),Citation37 as DNBS-induce colitis in a similar way from that induced by TNBS in terms of macroscopic appearance, histological appearance, and degree of granulocytes infiltration.Citation38,Citation39
In conclusion, in this study we have demonstrated the beneficial effects of a recombinant strain of L. lactis secreting and delivering in situ (eg. colon) IL-10 cytokine under a stress-inducible system, SICE, in a new chronic model characterized by 2 episodes of DNBS-challenge. We also demonstrated the beneficial effects of the carrier strain by its-self. In this sense, some specific effects were observed in term of TJ proteins, cytokine, and T-cells and an overall improvement has been detected for almost all the parameters tested. Besides, the use of a controlled system for locally delivery of therapeutic molecules was validated since no systemic side-effects were observed in mice after administration of recombinant L. lactis expressing IL-10 using SICE system. Finally, the use of an IL-10-producing strain to treat inflammation mediated colitisCitation20,Citation23,Citation27 has been extended to a micro-inflammation status, suggesting the possible role of this type of therapy in patients suffering from a functional disorder linked to a micro-inflammation such as IBS. However, although this study is a first proof-of-concept on the use of modified microorganisms to treat a subset of IBS, more studies should be performed (such as the use of non-disseminable L. lactis strains)Citation19 before considering this type of treatment as a feasible therapy to be tested in humans.
Material and Methods
Bacterial strains and growth conditions
L. lactis MG1363 strain secreting biologically active murine IL-10 (LL-IL10) under the control of a stress-inducible system (SICE)Citation22 and L. lactis MG1363 strain transformed with an empty plasmid (strain LL) were grown in GM17 medium plus 10 µg/mL of chloramphenicol at 30 °C without agitation. ELISA and western blot experiments with total protein extracts and culture SNs from strain LL-IL10 confirmed that it produced and secreted large amounts of IL-10 (data not shown).
Animals
Male C57BL/6 mice (6–8 wk old) (Janvier) were maintained at the animal care facilities of the National Institute of Agricultural Research (IERP, INRA, Jouy-en-Josas, France) under specific pathogen-free conditions. Mice were housed under standard conditions for a minimum of 1 wk before experimentation. All experiments were performed in accordance with European Community rules for animal care and were approved by the relevant local committee.
Induction of DNBS micro-inflammation and bacteria administration
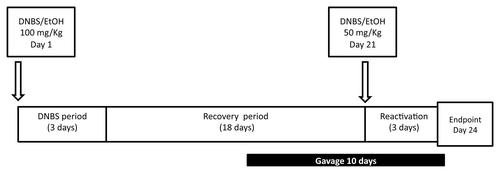
The protocol for inducing DNBS colitis is detailed in . Briefly, mice of approximately 20 g were fully anesthetized by intraperitoneal injection of 150 μL of 0.1% ketamine (Imalgene 1000, Merial) and 0.06% xylazine (Rompun). Then, a 3.5 catheter (French catheter, Solomon Scientific) was attached to a tuberculin syringe, inserted into the colon and a dose of 100 mg/Kg of DNBS solution (ICN, Biomedical Inc.) in 30% ethanol (EtOH) was injected intrarectally to induce the colitis. Control mice (without colitis) received only 30% EtOH. Ten days after the so-called DNBS period, 200 µL of 1 × 10Citation9 CFU of bacteria in PBS, or PBS alone were administered intragastrically daily for 10 d (gavage period). The inflammation was reactivated 21 d after the first DNBS injection (recovery period) with a second injection of 50 mg/Kg of DNBS solution. Weight loss was monitored during 3 d following the second DNBS injection to assess the severity of colitis.
Macroscopic damage scores
Mice were sacrificed by cervical dislocation and the abdominal cavity was opened, the colon was removed and opened longitudinally and damage was immediately assessed macroscopically. Macroscopic scores were recorded using a previously described system.Citation40 Briefly, the macroscopic criteria (assessed on a scale from 0 to 9) include macroscopic mucosal damages such as ulcers, thickening of the colon wall, the presence of adhesions between the colon and other intra-abdominal organs, the consistency of fecal material (as an indicator of diarrhea), and the presence of hyperemia.
Colonic histology and myeloperoxidase (MPO) activity
Formalin-fixed colon samples were paraffin-embedded and 3 µm-thick sections were stained with hematoxylin and eosin (H&E). Colonic damage was scored according to architectural derangement, goblet cell depletion, edema/ulceration, and degree of inflammatory cell infiltration, as previously described.Citation41 Myeloperoxidase (MPO) activity (used as a marker of neutrophil infiltration) was measured using a modified version of the protocol described by Bradley et al.Citation42 One centimeter samples of the distal colon were recovered and homogenized (50 mg/mL) in ice-cold 50 mM potassium phosphate buffer (pH 6) containing 5% hexadecyl trimethyl ammonium bromide (Sigma-Aldrich) and hydrogen peroxide (H2O2). The absorbance of the colorimetric reaction was measured using a spectrophotometer. MPO activity is expressed as units per milligram of wet tissue, 1 unit representing the conversion of 1 µM of H2O2 to water in 1 min at room temperature.
Lipocalin-2 (Lcn-2) levels
Before the mice were killed, blood samples were obtained from the retro-orbital venous plexus, and centrifuged for 10 min at 12 000 rpm and 4 °C. Clear supernatants (serum) were collected and stored at −20 °C until analysis. Serum samples were diluted in the kit-recommended diluent (1% BSA in PBS), and Lcn-2 was assayed using Duoset murine Lcn-2 ELISA kit (R&D Systems, Minneapolis, MN).Citation30
Intestinal permeability in vitro and in vivo
Three days after the final DNBS challenge, permeability in vivo was assessed using fluoroscein-conjugated dextran (FITC-dextran 3000–5000 Da, Sigma-Aldrich) as previously described.Citation43 Briefly, 3 d after the second DNBS injection, 0.6 mg/g body weight of FITC-dextran was administered to mice by oral gavage and 3.5 h later, blood samples were obtained from the retro-orbital venous plexus. Plasma FITC levels were determined by fluorometry using a microplate reader (Tecan).
Apical junctional analysis by quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from 20–30 mg samples of colon with an RNeasy Mini Kit (Qiagen). Column DNase treatment (Qiagen) was used to eliminate potential DNA contamination. RNA quantity and quality were checked with a NanoDrop apparatus (Thermo Scientific) and by agarose gel electrophoresis. Only samples with intact RNA were used for subsequent cDNA synthesis with iScript reverse transcriptase (Bio-Rad): 500 μg of the total RNA preparation was used for each sample. Quantitative real-time PCR (qRT-PCR) was performed with diluted cDNA (tenfold) in triplicate and with an iQ5 Real-Time Detection System (Bio-Rad). The reaction mix consisted of Ssofast Evagreen Supermix (Bio-Rad), primers at 0.5 μM (), and 2 μL of diluted cDNA. Values are expressed as relative fold differences normalized to a housekeeping gene, Gapdh, by the 2-ΔΔCTmethod. All procedures were performed according to the manufacturers’ instructions.
Table 1. Primers used in this study
Cytokine assays
One centimeter samples of distal colon were recovered and homogenized in an appropriate volume (to give 50 mg/mL) of TRIS-HCl buffer containing protease inhibitors (Sigma-Aldrich) in a Tissue Lyser (Qiagen). The samples were centrifuged for 20 min and the supernatants collected and frozen at -80 °C until analysis. Blood samples were obtained from the retro-orbital venous plexus before the mice were killed and centrifuged, and the sera stored at –80 °C until analysis. IL-6, IL-10, IFN-γ, TNF-α, IL-5, IL-2, IL-22, IL-1α, IL-13, IL-17, IL-4, IL-27, and IL-12p70 were assayed by Flow Cytometry using Cytometric Bead Array analysis (CBA) (Mouse Th1/Th2/Th17/Th22 13plex Flowcytomix) (eBioscience).
Analyses of lymphoid populations present in the spleen
Mononuclear cells were isolated from spleens by mechanical dissociation with a GentleMax disruptor (Miltenyi Biotech) followed by a lymphocyte specific ficoll density gradient centrifugation (Mouse Lympholyte-Cedarlane, France-Biotech). Cells were resuspended in RPMI medium supplemented with 10% of fetal calf serum (FCS), 2 mM L-glutamine, 50 U/mg penicillin, and 50 U/mg streptomycin (Lonza). For flow cytometry analysis, we use 106–107 cells per sample were used. Cells were labeled with anti-CD4-FITC and anti-CD8-PerCP, (Miltenyi Biotech). The stained cells were tested by flow cytometry (Accuri, BD) and analyzed by using the CFlowSampler software (BD).
Colonic serotonin assays
One centimeter-long sections of colon were recovered and homogenized (50 mg/mL) in PBS buffer containing protease inhibitors and Serotonin Stabilizer (LDN) in a Tissue Lyser (Qiagen). The colon contents were recovered by washing with 1 mL of PBS buffer containing protease inhibitors and LDN and centrifuged; the supernatant were stored at –80°C until further analysis. Serotonin concentrations were determined using a Serotonin Research ELISA (LDN) according to the manufacturer’s instructions.
Statistical Analysis
GraphPad software (GraphPad Sofware) was used for statistical analysis. Results are presented as bar graphs or dot plots with means +/− SEM. Most comparisons were performed using one-way analysis of variance followed by the Student-Newman-Keuls multiple comparison post hoc analysis. For data sets that were non-Gaussian or based on a score or on a percentage, the non-parametric Kruskal-Wallis test was used followed by a Dunn's Multiple Comparison test. A P value of less than 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was a part of FPARIS collaborative project selected and supported by the Vitagora Competitive Cluster and funded by the French FUI (Fond Unique Interministériel; FUI: n°F1010012D), the FEDER (Fonds Européen de Développement Régional; Bourgogne: 34606), the Burgundy Region, the Conseil Général 21, and the Grand Dijon. This work was also supported by Merck Médication Familiale (Dijon, France) and Biovitis (Saint Etienne de Chomeil, France). R.M. receives a salary from the same grants. E.F.V. holds a Canada Research Chair. The work was partially funded by a CCFC grant to P.L. and E.F.V. Authors would like to thank Jean Jacques Gratadoux and all UEAR personal for their technical help.
References
- Collins SM. Is the irritable gut an inflamed gut?. Scand J Gastroenterol Suppl 1992; 192:102 - 5; http://dx.doi.org/10.3109/00365529209095988; PMID: 1439559
- Khan S, Chang L. Diagnosis and management of IBS. Nat Rev Gastroenterol Hepatol 2010; 7:565 - 81; http://dx.doi.org/10.1038/nrgastro.2010.137; PMID: 20890316
- Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol 2011; 46:421 - 31; http://dx.doi.org/10.1007/s00535-011-0379-9; PMID: 21331765
- Ohman L, Simrén M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis 2007; 39:201 - 15; http://dx.doi.org/10.1016/j.dld.2006.10.014; PMID: 17267314
- Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SM, WEL Investigators. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther 2004; 20:1317 - 22; http://dx.doi.org/10.1111/j.1365-2036.2004.02284.x; PMID: 15606393
- Akiho H, Ihara E, Nakamura K. Low-grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol 2010; 1:97 - 105; http://dx.doi.org/10.4291/wjgp.v1.i3.97; PMID: 21607147
- Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, Dainese R, Bernard G, Anty R, Filippi J, Saint-Paul MC, Tulic MK, Verhasselt V, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut 2013; In press http://dx.doi.org/10.1136/gutjnl-2012-304066; PMID: 23878165
- Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2012; 107:1474 - 82; http://dx.doi.org/10.1038/ajg.2012.260; PMID: 22929759
- Simrén M, Axelsson J, Gillberg R, Abrahamsson H, Svedlund J, Björnsson ES. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol 2002; 97:389 - 96; http://dx.doi.org/10.1111/j.1572-0241.2002.05475.x; PMID: 11866278
- Bermúdez-Humarán LG, Aubry C, Motta JP, Deraison C, Steidler L, Vergnolle N, Chatel JM, Langella P. Engineering lactococci and lactobacilli for human health. Curr Opin Microbiol 2013; 16:278 - 83; http://dx.doi.org/10.1016/j.mib.2013.06.002; PMID: 23850097
- Seegers JF. Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol 2002; 20:508 - 15; http://dx.doi.org/10.1016/S0167-7799(02)02075-9; PMID: 12443872
- Lee SF, Halperin SA, Salloum DF, MacMillan A, Morris A. Mucosal immunization with a genetically engineered pertussis toxin S1 fragment-cholera toxin subunit B chimeric protein. Infect Immun 2003; 71:2272 - 5; http://dx.doi.org/10.1128/IAI.71.4.2272-2275.2003; PMID: 12654855
- Cortes-Perez NG, Bermúdez-Humarán LG, Le Loir Y, Rodriguez-Padilla C, Gruss A, Saucedo-Cárdenas O, Langella P, Montes-de-Oca-Luna R. Mice immunization with live lactococci displaying a surface anchored HPV-16 E7 oncoprotein. FEMS Microbiol Lett 2003; 229:37 - 42; http://dx.doi.org/10.1016/S0378-1097(03)00778-X; PMID: 14659540
- Bermúdez-Humarán LG, Langella P, Cortes-Perez NG, Gruss A, Tamez-Guerra RS, Oliveira SC, Saucedo-Cardenas O, Montes de Oca-Luna R, Le Loir Y. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect Immun 2003; 71:1887 - 96; http://dx.doi.org/10.1128/IAI.71.4.1887-1896.2003; PMID: 12654805
- Bermúdez-Humarán LG, Cortes-Perez NG, Le Loir Y, Alcocer-González JM, Tamez-Guerra RS, de Oca-Luna RM, Langella P. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J Med Microbiol 2004; 53:427 - 33; http://dx.doi.org/10.1099/jmm.0.05472-0; PMID: 15096553
- Grangette C, Müller-Alouf H, Goudercourt D, Geoffroy MC, Turneer M, Mercenier A. Mucosal immune responses and protection against tetanus toxin after intranasal immunization with recombinant Lactobacillus plantarum. Infect Immun 2001; 69:1547 - 53; http://dx.doi.org/10.1128/IAI.69.3.1547-1553.2001; PMID: 11179325
- Krüger C, Hu Y, Pan Q, Marcotte H, Hultberg A, Delwar D, van Dalen PJ, Pouwels PH, Leer RJ, Kelly CG, et al. In situ delivery of passive immunity by lactobacilli producing single-chain antibodies. Nat Biotechnol 2002; 20:702 - 6; http://dx.doi.org/10.1038/nbt0702-702; PMID: 12089555
- Robinson K, Chamberlain LM, Schofield KM, Wells JM, Le Page RW. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol 1997; 15:653 - 7; http://dx.doi.org/10.1038/nbt0797-653; PMID: 9219268
- Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, Cox E, Remon JP, Remaut E. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol 2003; 21:785 - 9; http://dx.doi.org/10.1038/nbt840; PMID: 12808464
- Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000; 289:1352 - 5; http://dx.doi.org/10.1126/science.289.5483.1352; PMID: 10958782
- Schotte L, Steidler L, Vandekerckhove J, Remaut E. Secretion of biologically active murine interleukin-10 by Lactococcus lactis. Enzyme Microb Technol 2000; 27:761 - 5; http://dx.doi.org/10.1016/S0141-0229(00)00297-0; PMID: 11118583
- Benbouziane B, Ribelles P, Aubry C, Martin R, Kharrat P, Riazi A, Langella P, Bermúdez-Humarán LG. Development of a Stress-Inducible Controlled Expression (SICE) system in Lactococcus lactis for the production and delivery of therapeutic molecules at mucosal surfaces. J Biotechnol 2013; 168:120 - 9; http://dx.doi.org/10.1016/j.jbiotec.2013.04.019; PMID: 23664884
- Martín R, Chain F, Miquel S, Lu J, Gratadoux JJ, Sokol H, Verdu EF, Bercik P, Bermúdez-Humarán LG, Langella P. The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm Bowel Dis 2014; 20:417 - 30; http://dx.doi.org/10.1097/01.MIB.0000440815.76627.64; PMID: 24418903
- Martín R, Miquel S, Ulmer J, Kechaou N, Langella P, Bermúdez-Humarán LG. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microb Cell Fact 2013; 12:71; http://dx.doi.org/10.1186/1475-2859-12-71; PMID: 23876056
- Motta JP, Bermúdez-Humarán LG, Deraison C, Martin L, Rolland C, Rousset P, Boue J, Dietrich G, Chapman K, Kharrat P, et al. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med 2012; 4:ra144; http://dx.doi.org/10.1126/scitranslmed.3004212; PMID: 23115353
- Galipeau HJ, Wiepjes M, Motta JP, Schulz JD, Jury J, Natividad JM, Pinto-Sanchez I, Sinclair D, Rousset P, Martin-Rosique R, et al. Novel Role of the Serine Protease Inhibitor Elafin in Gluten-Related Disorders. Am J Gastroenterol 2014; Forthcoming http://dx.doi.org/10.1038/ajg.2014.48; PMID: 24710505
- Foligne B, Dessein R, Marceau M, Poiret S, Chamaillard M, Pot B, Simonet M, Daniel C. Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology 2007; 133:862 - 74; http://dx.doi.org/10.1053/j.gastro.2007.06.018; PMID: 17678918
- Smelt MJ, de Haan BJ, Bron PA, van Swam I, Meijerink M, Wells JM, Faas MM, de Vos P. L. plantarum, L. salivarius, and L. lactis attenuate Th2 responses and increase Treg frequencies in healthy mice in a strain dependent manner. PLoS One 2012; 7:e47244; http://dx.doi.org/10.1371/journal.pone.0047244; PMID: 23056616
- Bermúdez-Humarán LG, Cortes-Perez NG, Lefèvre F, Guimarães V, Rabot S, Alcocer-Gonzalez JM, Gratadoux JJ, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier G, et al. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J Immunol 2005; 175:7297 - 302; http://dx.doi.org/10.4049/jimmunol.175.11.7297; PMID: 16301635
- Shashidharamurthy R, Machiah D, Aitken JD, Putty K, Srinivasan G, Chassaing B, Parkos CA, Selvaraj P, Vijay-Kumar M. Differential role of lipocalin 2 during immune complex-mediated acute and chronic inflammation in mice. Arthritis Rheum 2013; 65:1064 - 73; http://dx.doi.org/10.1002/art.37840; PMID: 23280250
- Vijay-Kumar M, Wu H, Jones R, Grant G, Babbin B, King TP, Kelly D, Gewirtz AT, Neish AS. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am J Pathol 2006; 169:1686 - 700; http://dx.doi.org/10.2353/ajpath.2006.060345; PMID: 17071592
- Miyauchi EMH, Morita H, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci 2009; 92:2400 - 8; http://dx.doi.org/10.3168/jds.2008-1698; PMID: 19447972
- Mennigen RNK, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 2009; 296:G1140 - 9; http://dx.doi.org/10.1152/ajpgi.90534.2008; PMID: 19221015
- Coates MD, Johnson AC, Greenwood-Van Meerveld B, Mawe GM. Effects of serotonin transporter inhibition on gastrointestinal motility and colonic sensitivity in the mouse. Neurogastroenterol Motil 2006; 18:464 - 71; http://dx.doi.org/10.1111/j.1365-2982.2006.00792.x; PMID: 16700726
- Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol 2010; 161:19 - 27; PMID: 20408856
- Bertrand PP, Barajas-Espinosa A, Neshat S, Bertrand RL, Lomax AE. Analysis of real-time serotonin (5-HT) availability during experimental colitis in mouse. Am J Physiol Gastrointest Liver Physiol 2010; 298:G446 - 55; http://dx.doi.org/10.1152/ajpgi.00318.2009; PMID: 20019165
- Qin HY, Xiao HT, Wu JC, Berman BM, Sung JJ, Bian ZX. Key factors in developing the trinitrobenzene sulfonic acid-induced post-inflammatory irritable bowel syndrome model in rats. World J Gastroenterol 2012; 18:2481 - 92; http://dx.doi.org/10.3748/wjg.v18.i20.2481; PMID: 22654445
- Wallace JL, Le T, Carter L, Appleyard CB, Beck PL. Hapten-induced chronic colitis in the rat: alternatives to trinitrobenzene sulfonic acid. J Pharmacol Toxicol Methods 1995; 33:237 - 9; http://dx.doi.org/10.1016/1056-8719(95)00001-X; PMID: 8527832
- Appleyard CB, Wallace JL. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am J Physiol 1995; 269:G119 - 25; PMID: 7631788
- Qiu BS, Vallance BA, Blennerhassett PA, Collins SM. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med 1999; 5:1178 - 82; http://dx.doi.org/10.1038/8328; PMID: 10502822
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993; 69:238 - 49; PMID: 8350599
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982; 78:206 - 9; http://dx.doi.org/10.1111/1523-1747.ep12506462; PMID: 6276474
- Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 2010; 139:2093 - 101; http://dx.doi.org/10.1053/j.gastro.2010.06.068; PMID: 20600011