Abstract
The basophil activation test (BAT) is an in vitro assay where the activation of basophils upon exposure to various IgE-challenging molecules is measured by flow cytometry. It is a cellular test able to investigate basophil behavior during allergy and allergy immunotherapy. A panoply of critical issues and suggestive advances have rendered this assay a promising yet puzzling tool to endeavor a full comprehension of innate immunity of allergy desensitization and manage allergen or monoclonal anti-IgE therapy. In this review a brief state of art of BAT in immunotherapy is described focusing onto the analytical issue pertaining BAT performance in allergy specific therapy.
Introduction
The concept of immunotherapy has changed deeply in recent years. Immunotherapy in allergy has reached a renewed interest among clinicians due to the development of new therapeutic approaches based on standardized allergens taken into account after pivotal Phase III clinical trials.Citation1 Meta-analyses are powerful tool to elucidate the efficacy of immunotherapy against allergy and also provide for a glimpse on the role covered by recombinant allergens used to desensitize cellular response to allergy.Citation2-Citation5 Numerous comparative studies with specific immunotherapy vs placebo or pharmacological treatment have demonstrated the efficacy of this approach and its advantage in control of the disease. Allergen specific immunotherapy (AIT) induces favorable clinical, biological, and functional modifications in the course of allergic asthma. Actually, significant improvement in clinical manifestations has been demonstrated, even with levels of allergen exposure higher than those at the beginning of treatment.Citation6-Citation8 This improvement is associated with a reduced need for anti-inflammatory and bronchodilator treatment, with a decrease in healthcare expenditures.Citation9,Citation10 Specific bronchial reactivity shows a clear improvement with disappearance of delayed response and a clear increase in the threshold for immediate response to the allergen.Citation11
AIT includes a wide panoply of approaches. It consists of the administration of allergen extracts to patients with allergic disease to achieve clinical tolerance to the causative allergens. A World Health Organization (WHO) leading paper, published in the late nineties, defined immunotherapy as “the only form of treatment able to modify the natural course of allergic diseases”Citation12. In patients with allergic rhinitis, for example, some studies suggested that immunotherapy can modify the natural history of respiratory allergy by preventing the development of asthma in children with this disease.Citation13,Citation14 Sublingual immunotherapy (SLIT), namely a practice of administering sublingual allergens often used for respiratory allergy, is gaining an increasing diffusion worldwide as a consequence of the robust demonstration of clinical efficacy and safety provided by recent high-powered and well-designed studies.Citation15,Citation16 The recent and emerging research suggested that SLIT may have a future role in other allergic conditions such as atopic dermatitis, food, latex, and venom allergy.Citation16 The oral route has recently overwhelmed parenteral administration as occurred in sub-cutaneous immunotherapy (SCIT), due to the supposed better efficacy, safety, and cost evalutation, albeit with some controversial opinion.Citation17,Citation18 Oral immunotherapy has been reviewed for peanut, milk, and egg allergy.Citation19 The World Allergy Organization guidelines for the management of anaphylaxis have reviewed the current literature about the management of atopic hypersensitivity reactions and chronic allergy through AIT, recently reaching also interesting protocol suggestions and recommendations.Citation20,Citation21 However, these guidelines lack of further and detailed information about the use of diagnostic tools such as basophil activation tests (BATs). BAT is the only cellular test that is more frequently adopted in allergy investigation.Citation22,Citation23 Although different commercial kits and protocol approaches are currently available (), the increasing development of new immunotherapy strategies has compelled producers to evaluate new incoming and optimized BAT protocols (). However, the role of cellular test in AIT yet appears quite marginal or even paltry, respect to the supposed predictive potential of hallmarks such as the absence of symptomatic, skin reactive, or serological markers following an AIT protocol. The mechanism of desensitization or tolerance induction following AIT, involves directly innate immune cells,Citation24 thus suggesting that a cellular test may provide fundamental insights in the comprehension of the individual immune response to allergy therapy. BAT is being increasingly considered to test the in vitro efficacy of new recombinant desensitizing allergens and, moreover, it is considered also the only way to study the cell biology of peripheral blood basophils with an easily handling procedure.Citation23 This would mean that BAT further provides to be a cellular test able to dissect basophil response mechanisms to allergy onset and downregulation, a feature that should reappraise BAT as an unavoidable diagnostic tool, even in AIT. Basophil response to allergens is a complex mechanism on which polychromatic flow cytometry may shed a light and enhance the potential of BAT itself as a diagnostic tool, also during allergy therapy and AIT follow up. This review tries to address BAT application and feasibility in the context of AIT.
Table 1. Recent allergy immunotherapy reports and associated basophil activation tests (BATs)
Table 2. Basophil activation tests (BATs): potential, handling, cost-effectiveness, and reliability
Role of Basophils in Immune System Following Immunotherapy: Introduction
During immunotherapy, a cellular assay such as BAT might give important insights about AIT efficacy and maintenance dose protocol. Basophils are easily to obtain by a simple venous peripheral blood withdrawal and flow cytometry proved to be a reliable approach to evaluate in vitro response of these cells to many allergens, including food, inhalants, or chemical compounds such as latex.Citation22,Citation25 Notwithstanding, very few reports exist dealing with the role of BATs in AIT, while most of them regards immunotherapy of Hymenoptera venom allergy.Citation26-Citation30 The effect of A. mellifera venom on basophils has a nonlinear behavior, namely the Hymenoptera venom allergens used in immunotherapy (VIT) showed a biphasic or hormetic pattern on basophil IgE-mediated response,Citation31 then making difficult to use BAT as a simple allergy-non allergy probing test. Low doses of honey bee venom (HoBV) can diffuse into the systemic circulation and induce an activated state, while high doses elicit a primed state, but they induce IgE-FcεRIβ desensitization, as allergen/IgE cross-linking increases, a mechanism that allows many researchers to suggest that the higher the priming is, the greater the desensitization.Citation31 This occurred in all stung subjects, both sensitized asymptomatic and hymenoptera allergic subjects, raising issues about VIT maintenance time and doses.Citation31,Citation32 VIT effectiveness is strictly dependent on allergy desensitization mechanisms, and an understanding of this represents a major goal of immunotherapy.Citation33-Citation35 This would mean that performing a BAT by simply investigating membrane upregulation of CD63 and CD203c, must take into account the complexity of HoBV interaction with cell response to VIT and the BAT gating protocol employed in the test, in order to better optimize its performance.Citation23 Such a consideration raised from AIT with hymenoptera venom, may fit to many other immunotherapy protocols with defined allergens, such as dust mites.Citation36
The usefulness of a cellular test likewise BAT has been demonstrated for immune vaccination besides allergy diagnosis.Citation37-Citation39 Notwithstanding, basophil response to allergens used in immunotherapy does not seem to fulfil all the expectations raised from a commonly accepted model of the basophil behavior toward an IgE-mediated mechanism.Citation31,Citation40 For example, recent evidence showed that treatment with omalizumab results in a markedly increased sensitivity of basophils to an IgE-mediated stimulation in terms of the number of IgE molecules required to produce a given response.Citation41 Treatment with omalizumab in patients suffering from cat or peanut allergy enhanced the intrinsic sensitivity of circulating basophils as evaluated by histamine and LTC4 release.Citation41 The mechanism underlying this increase in IgE-mediated response is yet unclear. During the formation of omalizumab-IgE heterodimers in the serum,Citation42 the monoclonal IgG1-anti-Cε3(IgE) complex, can be recognized by FcγRs in immune and dendritic cells and removed from systemic circulation.Citation43 This evidence suggests that omalizumab may interact with basophil FcγRs and regulate cell response to surface IgE-FcεRI complexes. Although basophil response to further allergens during anti-IgE immunotherapy has not been yet investigated, the accepted model suggests that clearance of allergens by immunocomplexes (ICs)-mediated competition or trap mechanism, elicits also a downregulation mechanism of FcεRI on basophil membrane, then reducing basophil IgE-mediated response and allergy symptoms.Citation41,Citation46 The use of humanized anti-IgE antibodies, such as omalizumab,Citation44,Citation45 may raise some concern about the role of mast cell and basophil Fc-gamma receptors in resolving allergy by an anti-IgE immunotherapy approach.Citation46 Mast cells, as like as basophils, compete for FcRβ in the allergy IgE-mediated mechanism.Citation47 The subunit FcRβ is shared both by FcεRI and FcγRIIIs, and functions as an amplifier of allergy response, able to increase Syk phosphorylation and activity.Citation48 FcγRIII are downregulated by high density surface FcεRI-IgE and in atopic subjects, it is presumable therefore that a decrease in FcεRI may enhance basophil FcγRIII function.Citation49 Although basophils may express CD16b (FcγRIII) besides to CD32 (FcγRII), no BAT has been planned or performed to evaluate this issue to date. At the same time, this evidence appears quite paradoxical respect to previous reports, showing a basophil desensitization following omalizumab immunotherapy.Citation50 Certainly, immunotherapy may affect basophils directly and their expression of surface markers, such as CD63, CD193, CD203c, CD69, CD164, usually upregulated during cellular activation, may be significantly modified during treatment. Despite to the many difficulties in interpretating BAT,Citation23 this consideration prompted researchers to evaluate BAT as a promising tool during immunotherapy follow up. In a mastocytosis model, BAT showed a sensitivity of 87% and specificity of 100% in diagnosing wasp venom allergy and an ability in tracing immunotherapy against hymenoptera culprit.Citation51 Previous reports suggested the routine use of BAT to facilitate prescription of immunotherapy.Citation52-Citation54 Basophils might exert a quite different task in progressing the hypersensitivity reaction than mast cells and eosinophils, as probably they play a modulating and regulating action in allergy rather than ruling an effector mechanism.Citation55 Both the innate and acquired immune networks play an outstanding role in AIT but may hinder a full comprehension of which role basophils exert in the field. Actually, from an immune network perspective, AIT involves an early suppression of innate effector cells of allergic inflammation, including mast cells, eosinophils, and basophils, besides to affect the regulation of pro-allergic T helper 2 type (Th2) and IgE+ B cell responses in the tissue and in peripheral blood.Citation56 The role of basophil during AIT is yet far to be fully understood when one onsiders this complex pathway, fundamentally because of the many puzzling and unsolved issues still existing about the biology of basophils within the immune network.Citation57 Frequently, the allergen-tolerant state is associated with local and systemic induction of distinct populations of allergen-specific T regulatory cells, including IL-10 expressing Tregs (Tr1 cells), TGF-β+ Tregs, and FoxP3+ memory Tregs. B lymphocytes are switched in favor of producing IgG (particularly IgG4) antibodies and associated blocking activity for IgE-dependent events, including basophil activation and IgE-facilitated allergen binding to B cells. Furthermore, an induction of IL-10+ B regulatory cells and alterations in dendritic cell subsets have also recently been described.Citation58 These events are followed by the induction of T regulatory cells, suppression of allergen-specific T cell proliferation and immune deviation from Th2 in favor of Th1 responses. Alternative mechanisms of tolerance include apoptosis/deletion of antigen-specific memory Th2 cells and/or a failure of co-stimulation leading to T cell anergy. The role of T-regs in allergy and immunotherapy has been elucidated quite recently. When the allergen is taken up by regional APCs, such as dendritic cells, as occurs during AIT, it leads to the induction of T-regs.Citation58 CD4+ naïve T cells differentiate into distinct T cell subsets such as Th1, Th2, Th9, Th17, and Th22 type memory and effector cells, depending on the cytokines, other molecules and cells present in the micro-environment.Citation59. The involvement of acquired immunity and regulatory T-cells in allergy and in immnotherapy with allergens or anti-IgE moAbs, has been reviewed elsewhere,Citation56,Citation60-Citation63 while a thorough role of basophils appears yet unclear. Immune tolerance induced by AIT should involve these mechanisms and dampen basophil response to allergen by a T-cell mediated mechanism.Citation58 However, very few reports have addressed the active participation of basophil in allergy tolerance following AIT.Citation56,Citation64,Citation65
Basophil Activation Test in Immunotherapy: Allergen/Fcεri Regulation as a Possible Pitfall
Although encouraging evidence has been reported, basophil interaction with innate and acquired immunity appears highly complex to endeavor a comprehensive functional model able to highlight BAT performance during AIT. The first concern is about the allergen dose/FcεRI membrane expression ratio and its regulation on sensitized and/or activated basophils. A sub-threshold desensitization of basophils following the induction of allergy tolerance may lead to loss of Syk and cell surface FcεRI.Citation66,Citation67 Both FcεRI membrane recycling and the downstream receptor associated tyrosine kinase signaling, depend on sub-stimulatory or long lasting allergen doses. Kinetics of antigen binding on FcεRI/IgE complex has been reviewed in the pastCitation68-Citation74 and may give an interpretation to the complex responsive pattern expressed by basophil during AIT. Past reports showed that the complexity of FcεRI/IgE-allergen interaction, where the allergen may be replaced by an anti-IgE antibody able to cross link the high affinity receptor, should imply that early activation events may be not altered by the process of desensitization and could be maintained at an increased level of activity indefinitely with the maintenance of IgE crosslinks.Citation75 Therefore, AIT not necessarily exerts a tout court desensitization effect on basophil but a more complex receptor-mediated signaling probably occurs. Single basophils responded in a graded manner,Citation76,Citation77 leading to a differential CD63 intracellular endowment and membrane expression mechanism, a fact that may affect the reliability of BATs.Citation78 Furthermore, a percentage of basophils show anergy or a non responding phenotype, probably due to mechanisms related to calcium influx,Citation79 where basophils showed marked oscillations whose magnitudes were partially dependent on the strength of stimulus,Citation80 or intracellular receptor-associated signaling.Citation81 The mechanism of basophil desensitization should involve a panoply of different factors, which in turn may hamper a full elucidation of the issue. Probably, several forms of downregulation appear to occur during IgE-mediated stimulation of human basophils and, actually, previous studies reported downregulation in 2 regions of signaling; involving the molecular microenvironment of Syk kinase and PIP3, with a slight involvement of SHIP, which undergoes modest non-specific desensitization.Citation82 These results demonstrated a further process that accompanies nonspecific desensitization besides Syk and FcεRI, a persistent mechanism, which may be related to the loss of signaling components such as Syk kinase. These kind of considerations proved to be very important to esplicate the effect of anti-IgE immunotherapy, more than AIT. However, one should expand the debate about the role of basophil response to AIT when a BAT is performed.
Immunotherapy with Allergens and BAT in Sub-Cutaneous (SCIT) and Sub-Lingual (SLIT) Immunotherapy
Immunotherapy with allergens represents a promising way to dampen allergy response against molecules eliciting an hypersensitivity mechanism, although some controversy still exists regarding the potential role of specific immunotherapy (SIT) as a therapeutic intervention for patients with atopic dermatitis, eczema, and asthma.Citation83-Citation86 A 3-y sub-cutaneous immunotherapy treatment (SCIT), combined with inhaled corticosteroids, resulted as an effective immunotherapy for children with allergic asthma, showing also a reduction of the required corticosteroids dose.Citation7 SCIT or sub-lingual immunotherapy (SLIT) approaches were successfully reported also for D. pteronyssinus or house dust mite allergy,Citation87-Citation89 besides to rhinitisCitation90 and SLIT has been attempted with success also for rhinoconjunctivitis, using ragweed AIT.Citation91,Citation92 BAT used in sub-cutaneous immunotherapy against wasp venom allergy, showed an increase in sensitivity during the up-dosing phase of SCIT, an evidence reported by others also for humanized anti-IgE allergy therapy.Citation93,Citation94 This transient increase has not yet fully elucidated. Basophils increased their sensitivity after 3 wk of specific imunotherapy, probably due to a mechanism reflecting competing amounts of immunoglobulin on basophil membrane (IgE) and free immunoglobulin in circulationCitation93 and coincides with the observed early decrease in histamine release and increase in IgG4 and IgA immunoglobulins.Citation93,Citation95 This may reduce BAT performance as it could not predict hymenoptera allergy using an allergen challenge at the range 0.1–1.0 μg,Citation93 despite the use of reliable activation markers such as CD63 and CD203c.Citation96 More encouraging results were recently obtained by using BAT in SLIT for latex allergy,Citation97 where the cellular test proved to be useful in evaluating SCIT efficacy in this form of hypersensitivity.Citation98 BAT has been also reported to be a reliable tool to monitor immunotherapy in patients with mastocytosis.Citation51,Citation53,Citation93 Bidad and colleagues showed that basophils were less sensitive to wasp venom allergens after reaching the maintenance dose, a result apparently in agreement with previous reports.Citation53,Citation93 Encouraging results from early open-label studies have recently sparked great interest both in oral and sublingual immunotherapy, and thus several randomized controlled trials have recently been conducted to establish the safety and efficacy of these treatment strategies.Citation19 Although some contrasting opinions have been raised about SLIT or oral immunotherapy (OIT) in children,Citation99-Citation102 the methodology is gaining increasingly interest. Particularly due to its safety and tolerability,Citation103 though issues have been raised about the opportunity to use either sublingual (SLIT) or sub-cutaneous (SCIT) immunotherapy.Citation104
AIT is also included in the panel against allergic rhinitis,Citation105 with grass pollen allergens,Citation106 allergy to Dermatophagoides pteronyssinus,Citation107 asthma,Citation108,Citation109 atopic dermatitis.Citation110,Citation111 However, immunotherapy with allergens might be a still puzzling research field because of the complex cross reactivity occurring between different allergens, e.g., food or inhalatory allergens,Citation112,Citation113 which further affects the reliability of BAT in AIT.Citation114 Some interesting papers on AIT, such as egg or cow milk allergy, did not include or take into consideration BAT in diagnosis and therapy follow up,Citation115-Citation118 probably because clinical markers are still preferred than in vitro assays. However, BAT might still give fundamental insights on the cellular involvement in allergy development and desensitizationCitation23,Citation119 and this fact may give further chances to optimize BAT for the cellular investigation of AIT.
Immunotherapy with Allergens and BAT: Oral Immunotherapy (OIT) and Food Allergy
Oral immunotherapy for food allergy was attempted for very few allergens, such as peanutCitation120,Citation121 or eggs,Citation122 still remaining controversial opinions, therefore standing on a pivotal experimental stage.Citation19,Citation123-Citation128 Systematic reviews have tried to focus onto the issue and shed a light about current approaches in diagnosing and managing food allergy.Citation129 In peanut allergy, for example, many efforts have been recently made,Citation120,Citation123,Citation130 albeit doubts remain about reliability and clinical effectiveness of peanut allergy oral immunotherapy (OIT).Citation127 Concern about peanut allergy rises up to some years agoCitation131,Citation132 but many AIT approaches are quite recent.Citation115,Citation133 Well designed, prospective clinical trials are urgently needed to determine whether OIT is a safe, effective form of therapy for food allergy.Citation120,Citation121,Citation134,Citation135 In food allergy, immunotherapy is usually performed by oral or sublingual administration of allergens: several research studies have assessed that oral and sublingual immunotherapy for food allergy is generally well-tolerated and safe in highly controlled clinical settings.Citation136-Citation140 Reports from the NIAID-sponsored Expert Panel provided concise clinical recommendations and additional guidance on points of current controversy in patient management about food allergy and immunotherapy, yet identifying gaps in the current scientific knowledge to be addressed through future research in the field.Citation141,Citation142 Taken these reports altogether, a possible conclusion is that acquisition of oral tolerance to the diverse array of ingested food antigens and intestinal microbiota is an active immunologic process that is successfully established in the majority of individuals. In subjects who develop food allergy, there is a failure or loss of oral tolerance acquisition to a limited number of food allergens. OIT may offer a promising approach to induce specific oral tolerance to selected food allergens, though yet restricted to a small group of molecules, and can represent a potential strategy for long-term curative treatment of food allergy,Citation125 though many controversial problems about protocol and allergen dose yet exist, even for other AIT protocols.Citation143-Citation150 The application of BAT in oral immunotherapy should consider also the role of secretory IgAs and their receptor on granulocytes.Citation151-Citation153 Past reports have shown that secretory IgAs induce degranulation in IL-3 primed basophils.Citation154,Citation155 The role of secretory mucosal IgA (sIgAs) is fundamental in OIT and SLIT, both for food and inhalant allergensCitation107 and the investigation of FcαRI (CD89) expression on basophil membraneCitation156 during a BAT performance would provide important insights about SLIT or OIT efficacy.
Immunotherapy with Monoclonal Anti-IgEs and BAT
Immunotherapy with monoclonal anti-IgEs (moAbs) represents a novelty in the field of allergy therapeutics.Citation157-Citation159 The recent introduction of monoclonal anti-IgEs, such as omalizumab, allowed physicians to successfully face allergic asthma in patients with refractary asthma or severe asthma that is difficult control with conventional treatment, in which AIT is not indicated.Citation160-Citation162 Xolair® omalizumab has been investigated in various other conditions including perennial and seasonal allergic rhinitis, idiopathic anaphylaxis, mastocytosis, peanut allergy, latex allergy, atopic dermatitis, chronic urticaria, eosinophilic gastroenteritis, and nasal polyposis, with increasing success (see ref.Citation163 for a review). In this context, the analytical assessment of allergy desensitization subsequent to immunotherapy may promote BAT as an eligible tool to highlight allergy in individuals undergoing treatment.Citation40,Citation164
Particularly for omalizumab physicians and researchers have suggested this anti-IgE moAb as a reliable and promising tool against allergy, though with some raised criticism.Citation165-Citation169 Omalizumab is an IgG1 recombinant humanised monoclonal antibody which specifically binds to the Cε3 domain of immunoglobulin E, targeting the site of high-affinity IgE receptor binding.Citation170 The anti-IgE therapy has been also considered in association with specific AIT in seasonal allergic rhinitis or asthma, providing encouraging results particularly for omalizumab,Citation171,Citation172 an approach aimed at addressing atopic asthma in childhood, which still represents a big concern in allergy.Citation173 However, questions remain about the feasibility of omalizumab-related therapy, mainly because of difficulties in patient eligibility.Citation169 Committees, such as the NIH and Clinical Excellence (NICE) Appraisal Committee and the resulting NICE guidance for the Evidence Review Group (ERG), were planned and made up by Governmental foundations to assess the use of omalizumab in AIT.Citation174 This recombinant anti-IgE moAb has been investigated in several other allergic conditions, and has become a promising immunotherapeutic for several forms of allergy.Citation163,Citation175 The use of BAT in omalizumab therapy does not seem to account on a significant amount of practitioners, except for very few reports.Citation176,Citation177 Xolair®-Omalizumab is probably the most known anti-IgE moAb used in allergy therapy; however, many further attempts have been recently made to optimize immunotherapy with monoclonal antibodies.Citation178-Citation180 Monitoring omalizumab or other moAbs effect on innate immune cells involved in allergy, particularly basophils, may represent a cumbersome issue for health practitioners, due to the many reasons described below. First, the use of an anti-IgE-fluorochrome labeled to gate basophils, such as using Orpegen Basotest®, may give bias because of the dramatic effect caused by anti-IgE monoclonal antibodies on membrane FcεRIs.Citation41 Khan and colleagues used a BAT where cells were captured following the expression of IgE (anti-IgE-FITC) and CD123-PECy5 and non-expression of HLA-DR-APC, besides the absence of CD3, CD8, CD14, CD19. While they excluded subjects undergoing omalizumab treatment, their BAT was affected by a significant proportion of false-positive results, due to the anti-IgE phenotyping approach.Citation181 The possible cross-reaction with surface FcγRs on basophils by the IgG1-omalizumab may affect the upregulation of those markers related to the degranulation process, such as CD63. CD63 is actually highly sensitive to the many aspects regarding the complex pattern of response to allergens and anti-IgEs exhibited by basophils.Citation22,Citation23,Citation36,Citation40,Citation93 Granulocytes FcγRs, when activated, induce Th1 differentiation in allergy.Citation182 Furthermore, past reports showed that the expression of IgG4 is IL-4 related, likewise IgE.Citation183 These facts assess that the role of FcγRs in basophil response to omalizumab might play an utmost role also in CD63 expression and basophil releasability and influence the interpretation of the assay. The increasing diffusion of Xolair®-omalizumab in treating allergyCitation175,Citation184,Citation185 raises important issues about the opportunity to reappraise and review BAT protocols and application in subjects undergoing omalizumab treatment.
The Basophil Activation Test (BAT) During Immunotherapy: Technical Issues
Aside from some technical issue or detail and the recognized performance of CD63, BAT protocols has not yet fully optimized to date to fit as a reliable tool in evaluating immunotherapy follow up and recovery, despite to some promising discussion about.Citation40 Current BATs are usually interpreted as a simple test-bench of IgE-mediated response, without considering important effects on CD63 membrane expression and releasibility from IgAs or IgGs.Citation186 Furthermore, recent research advances have suggested the existence of at least 2 subpopulations of basophils, IL-3-elicited and TSLP-elicited basophils, rendering quite tangled the meaning and diagnostic potential of a BAT in immunotherapy, especially for CD203c.Citation57
summarizes most recent BAT performing in AIT. The response of basophils to any defined allergen is evaluated by the membrane upregulation of proteins, usually related to basophil releasability, i.e., histamine and LTC4 release, such as CD63 and CD203c.Citation22,Citation40 Most of recent BATs were performed in Hymenoptera venom AIT.Citation51,Citation52 During Hymenoptera venom immunotherapy (VIT) a rise in CD63 was previously reported.Citation187 The increase in CD63 membrane expression during VIT indicates a partial basophil degranulation with release of stored protein mediators, including IL-4, a cytokine that may cause in turn a transient upregulation of different surface antigens in an autocrine manner.Citation55 Thereafter, cytokines released by T cells, which as a result of VIT have changed from a Th2 type to a more Th1 type, downregulate the activation of the basophilic granulocytes. In a phase I clinical trial on peanut AIT, BAT, assessed at baseline and at weeks 16 and 20 following AIT with E. coli encapsulated peanut recombinant allergens (Ara h 1, Ara h 2, Ara h 3), reported a single increase of CD63% from baseline at the 0.01 μg/mL concentration (P = 0.05), while no other differences between the reactive and non reactive subjects, were shown.Citation188 In those subjects with peanut allergy, about 50% included individuals who were unable to complete the AIT protocol, leading to the conclusion that the vaccine, through a rectal route of administration, led to frequent and sometimes severe allergic reactions, in spite of the approved scientific rationale and reliable preclinical studies behind its development and the very low positive results in BAT.Citation188 MacGowan and Saini recently investigated the role of BAT in immunotherapy, reaching the conclusion that BAT is a useful in vitro tool for the diagnosis of allergy.Citation40 The main goal of a BAT during immunotherapy or allergy vaccination should be restricted to its ability in testing allergy desensitization following an AIT maintenance dose protocol or alternatively to provide instructions about the opportunity to interrupt or change the allergen dose. The many reports dealing with the issue, though some encouraging evidence, do not seem to shed a light on BAT reliability in performing an AIT follow up, however. Some concern raises from BAT protocol in itself. Following CD63 membrane up-load should be reviewed and revised taking into account the fact that this marker is highly influenced by basophil ability to respond to any allergen or anti-IgE challengeCitation78,Citation189-Citation191: BAT CD63 sensitivity ranges from 17% to 94%, with a specificity of 79–100%,Citation40 while CD203c resulted in a higher performanceCitation192: this would suggest that CD203c should be a more reliable marker to uscertain allergy desensitization but it is highly influenced by IL-3 activity and is not always directly related to degranulation.Citation55 suggests a possible approach of BAT application during an AIT protocol. Before carrying out a BAT for immunotherapy follow up, some “analitycal barriers” (indicated with capital letters) may generate bias or weakness in the analytical performance of the test. Furthermore, performers should select whether purchase or build up the assay with a non commercial protocol. This condition may enrich BAT endowment and potential, both in phenotyping and evaluation of basophil activation, but the addition of many markers might hamper the readability of the test in diagnosing allergy.Citation22 A second phase of the BAT application in a planned AIT protocol should involve an accurate investigation of basophil sensitivity to the allergen, associate to its IgE responsivity, in order to better elucidate the responsive status of basophilic cells (section D in ). Building up a new flow cytometry protocol by considering a previously reported method and adding new activation markers besides CD63, may enhance BAT reliability and diagnostic performance but the resulting BAT may be characterized by a more expensive or time-consuming pattern, respect to a simple purchasable kit. Basophil activation tests, which are routinely employed in allergy, allow cell electronic capture by targeting surface IgE in a flow cytometry protocol. During an anti-IgE immunotherapy, detect basophils by targeting high affinity FcεRI-IgE receptor complex, may result in a time-consuming procedure and low affinity FcεRII bearing cells might affect significantly basophil yield in the gating strategyCitation176; this may be the case to gate basophils using other phenotyping markers. summarizes the main hallmarks of BAT performance useful to take a decision for AIT. Most commercial kits permit to make a number of about 100 tests, obviously including controls, both negative and positive, thus yielding an average number of 30 triplicate samples in the same analytical run. This is approximatively the half amount of tests that can be performed by a home made BAT with a defined panel of fluorochrome-conjugated monoclonals, if optimizing the staining protocol.Citation189,Citation193 Phenotyping markers, likewise molecules tracing the activation mechanism of basophil, are restricted to very few molecules in available commercial kits. Each marker has its own analytical hallmark, which may affect the performance of the assay,Citation190,Citation194 therefore a BAT made up within one’s own laboratory might provide the researcher with more diagnostic advantages. Building up a CD45/CD123 gating protocol and investigating the expression of CD63 and CD203c, Subbarayal et al., showed that BAT was sensitive to IgG4 blocking immunoglobulins produced by birch pollen allergens as well as other cross reactive ones, such as Mal d 1 (hazelnut) and Cor a 1 (apple) for food allergy.Citation195 The protocol by which practitioners succeed in capturing basophils through the electronic gate in flow cytometry, plays a fundamental role in ensuring the reliability of a BAT, therefore. However, in this field, handling preventing a time-consuming approach, associated to a rapid interpretation of the result, prompts operators to select a commercial kit rather than building up a new BAT protocol. The best suggestion, if the flow cytometry method includes more than 2 markers, is to privilege the best phenotyping protocol rather than adding more than one activation markers. At the same time, basophils are hardly ever investigated about their responsiveness status, which may influence markers such as CD63Citation23 or CD203c.Citation196 Intracellular flow cytometry should address many disturbing issues, which may hamper a clear interpretation of BAT in AIT and then provide a more reliable diagnostic tool.Citation197 However, once selected the best BAT to perform for AIT, a further barrier is represented by the sensitivity to the allergen provided in the immunotherapy protocol. Depending on allergen cross-reactivity or competition with other allergy eliciting molecules, on surface IgE density and LAMP-3 (or CD63) granule-associated intracellular endowment, the response of basophils to different doses of allergens used for immunotherapy, may be significantly complex and should ask for a careful interpretation. Moreover, well conducted randomized controlled clinical trials in AIT, may use very simple and poorly predictive BATs, so possibly underestimating the role of a cellular test in the study.Citation130 The recommended use of BAT in vaccination may give possible suggestionsCitation23 but criticisms about its routine use and meaning are yet far to be fully prevented. However, most of the reported studies show BATs as simple confirmatory assay of allergen-induced desensitization,Citation198,Citation199 while cellular test should indicate fundamental parameters in innate immunity involved in allergy desensitization, even during the maintenance protocol. Furthermore, BAT is frequently used to assess in vitro allergen immunotherapeutics, often as a probe to detect AIT working.Citation199,Citation200 These circumstances might deject researchers to use BAT as a finest diagnostic tool to probe basophilic cell behavior during allergy desensitization with immunotherapy. Even the role of membrane markers, the expression of which is elicited by an anti-IgE mediated response, merits further attention.
Figure 1. Cartoon showing a possible panel of decision strategy for the optimization of BAT use during AIT. Each step, indicated by a capital letter, represent an “analytical barrier,” which the operator must address in order to reach the best quality performance of the assay. Starting from a dual choice (commercial or home made) a BAT must address “analytical barriers” represented by the proper choice of gatig markers (A) and activation/intracellular markers (B). Usually, due to limited needs regarding cost/effectiveness and feasibility/handling, practitioners select commercial one-step, dose-related 2-colors FC protocols (C). Once chosen the methodology, further barriers are represented by the allergen sensitization protocol (D), desensitization process (E), and follow up during or post maintanance (F). Home made protocols with a dose-dependent method of allergen response calibration (D) allow a better interpretation of the cellular response during AIT.
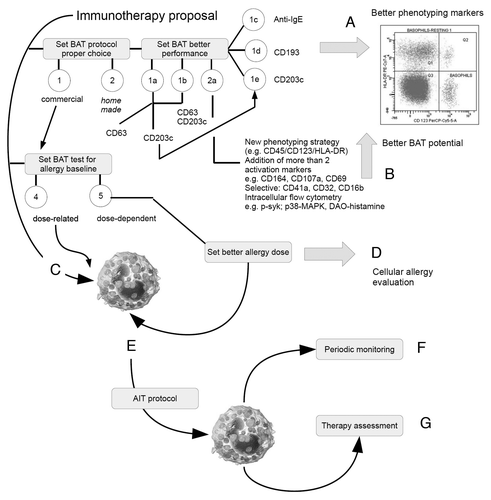
CD63 membrane expression has been reported to be associated with degranulation and releasability (histamine and LTC4) in basophils but not in mast cells.Citation193,Citation201,Citation202 Nothwithstanding, CD63 expression may be dissociated from releasability and induced by other non-IgE mediated stimuli, such as PAMPsCitation203: this should suggest that the use and interpretation of CD63 must be reviewed in light of the many issues raised from recent reports about innate immunity and allergy.Citation119 The nonlinear behavior of the main molecules used in BATs has suggested the introduction of further activation markersCitation194,Citation204,Citation205 but none of them was highlighted as molecules actually able to improve BAT in immunotherapy. CD164 was reported as a reliable marker to investigate pollen allergy diagnosisCitation206 but its evidence in immunotherapy is yet scanty. The 4-color flow cytometry protocol CD123/HLA-DR with CD63 and CD203cCitation189,Citation190 was used in peanut-allergic children with encouraging results.Citation207 BAT protocol was built within the research laboratory and allowed to assess the hypothesis that effector cell anergy could contribute to clinical desensitization.Citation207 Other protocols likewise, most introducing also CD41a, were used to diagnose and treat milk allergyCitation208,Citation209 and showed a good linearity between basophil desensitization and heat-denatured milk tolerant children. Therefore, questions remain. As BAT still remains the only cellular test that is widely used to assess allergy diagnosis and AIT follow up, further efforts have to be made to ameliorate its performing and meaning.
Though this technique has not yet been standardized, IgE, CCR3 (CD193), CRTH2 (CD294), and CD123 are commonly used to identify basophils in the peripheral blood as positive markers, often by alone or in association with negative markers such as HLA-DR, whereas CD63 and CD203c are frequently used as markers of IgE receptor activation, although many other molecules have been envisaged to probe basophil activation.
Some concluding remarks, to be kept in mind before approaching to a BAT protocol, are suggested as follows:
BATs based on a 2-colors flow cytometry (FC) are easy to handle, less expensive, and short time-consuming. They detect basophil response by targeting only CD63, which has a good relationship with cell releasability but is affected by the many issues raised in this article;
FC 2-colors BATs based on anti-IgE basophil capture may create bias in omalizumab-mediated allergy desensitization;
FC 2-colors BATs based on CD193 (CCR3), the eotaxin receptor as a phenotyping marker, have to solve possible bias related to CCR3 dependency on activation and CCR3 expression on CD3+ cells;
BATs using CD203c as a phenotyping marker should address the fact that CD203c expression is significantly related to IL-3 and moves with allergen or anti-IgE challenge on FcεRI;
Commercial FC 2–3-colors BATs are appearently cheaper but may prevent a full and thorough use of BAT as a cellular test;
Better phenotyping markers should possess most of these hallmarks: (1) highest specificity, they should be expressed only on target cells; (2) bright, highly expressed molecules; (3) not affected by activation;
Activation followed by CD63 should be optimized by adding other markers, even including intracellular FC.
A good BAT protocol should be set by considering a (bright + negative) highly specific/selective couple of phenotyping markers and 2 activation FC molecular probes (membrane markers and/or intracellular molecules).
Conclusions
Despite the encouraging role attributed to BAT, yet the behavior of basophils in allergy desensitization through allergen vaccination or immunotherapy with anti-IgEs is yet far to be elucidated. So, when performing a BAT following AIT, which is the meaning of a CD63 or CD203c membrane expression in a laboratory perspective, so to shed a light on AIT follow up and dose maintenance? The current status of our knowledge does not permit yet to find a conclusive remark and an outstanding or reliable answer to this question. The basophil activation test is considered an in vitro assay in which the activation of basophils upon exposure to various stimuli triggering allergy is measured by flow cytometry. In this sense, the basophil activation test has been validated in many IgE-mediated conditions, including drug allergy, food allergy, venom hypersensitivity, and pollen allergy. Furthermore, in recent years, the application of this test has been expanded to include quinolone and NSAID drug allergy, differentiating sensitization from true allergy, determining the allergenic potential of CCD determinants, and monitoring the success of immunotherapy. State-of-art of BATs in immunotherapy can be described as a progressing context where physicians and allergologists more frequently adopt this strategy to confirm or deny an occurred allergy desensitization. The use of BAT should give insights about how AIT is progressing, rather than focusing onto the desensitization process by an all-or-none mechanism, captured by analyzing the amount of molecules on cell membrane through flow cytometry. The basophil activation test continues to be a useful in vitro tool for the study of allergic disease but needs further insights in order to better fit its feasibilty to AIT diagnosis and follow up. Cellular tests possess the fundamental potential to focus onto the innate immunity causing allergy desensitization: in this sense, BATs represent irreplaceable tools to investigate allergy mechanism during allergen or anti-IgE immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Calderón MA, Casale TB, Togias A, Bousquet J, Durham SR, Demoly P. Allergen-specific immunotherapy for respiratory allergies: from meta-analysis to registration and beyond. J Allergy Clin Immunol 2011; 127:30 - 8; http://dx.doi.org/10.1016/j.jaci.2010.08.024; PMID: 20965551
- Calderón MA, Boyle RJ, Penagos M, Sheikh A. Immunotherapy: The meta-analyses. What have we Learned?. Immunol Allergy Clin North Am 2011; b 31:159 - 73, vii; http://dx.doi.org/10.1016/j.iac.2011.02.002; PMID: 21530812
- Wallner M, Pichler U, Ferreira F. Recombinant allergens for pollen immunotherapy. Immunotherapy 2013; 5:1323 - 38; http://dx.doi.org/10.2217/imt.13.114; PMID: 24283843
- Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy 2011; 66:775 - 83; http://dx.doi.org/10.1111/j.1398-9995.2011.02565.x; PMID: 21352238
- Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med 2012; 272:144 - 57; http://dx.doi.org/10.1111/j.1365-2796.2012.02556.x; PMID: 22640224
- Malling HJ. Comparison of the clinical efficacy and safety of subcutaneous and sublingual immunotherapy: methodological approaches and experimental results. Curr Opin Allergy Clin Immunol 2004; 4:539 - 42; http://dx.doi.org/10.1097/00130832-200412000-00011; PMID: 15640696
- Hui Y, Li L, Qian J, Guo Y, Zhang X, Zhang X. Efficacy analysis of three-year subcutaneous SQ-standardized specific immunotherapy in house dust mite-allergic children with asthma. Exp Ther Med 2014; 7:630 - 4; PMID: 24520258
- Maloney J, Bernstein DI, Nelson H, Creticos P, Hébert J, Noonan M, Skoner D, Zhou Y, Kaur A, Nolte H. Efficacy and safety of grass sublingual immunotherapy tablet, MK-7243: a large randomized controlled trial. Ann Allergy Asthma Immunol 2014; 112:146 - , e2; http://dx.doi.org/10.1016/j.anai.2013.11.018; PMID: 24468255
- Chen J, Xiang J, Wang Y, Shi Q, Tan H, Kong W. [Health economics analysis of specific immunotherapy in allergic rhinitis accompanied with asthma]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013; 27:925 - 8; PMID: 24367833
- Baena-Cagnani CE, Larenas-Linnemann D, Teijeiro A, Canonica GW, Passalacqua G. Will sublingual immunotherapy offer benefit for asthma?. Curr Allergy Asthma Rep 2013; 13:571 - 9; http://dx.doi.org/10.1007/s11882-013-0385-5; PMID: 24022465
- Martín Muñoz MF. [Efficacy of immunotherapy in the treatment of asthma]. Allergol Immunopathol (Madr) 2004; 32:133 - 41; PMID: 15120030
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol 1998; 102:558 - 62; http://dx.doi.org/10.1016/S0091-6749(98)70271-4; PMID: 9802362
- Calderón MA, Casale T, Cox L, Akdis CA, Burks AW, Nelson HS, Jutel M, Demoly P. Allergen immunotherapy: a new semantic framework from the European Academy of Allergy and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL consensus report. Allergy 2013; 68:825 - 8; http://dx.doi.org/10.1111/all.12180; PMID: 24010140
- Makatsori M, Pfaar O, Lleonart R, Calderon MA. Recombinant allergen immunotherapy: clinical evidence of efficacy--a review. Curr Allergy Asthma Rep 2013; 13:371 - 80; http://dx.doi.org/10.1007/s11882-013-0359-7; PMID: 23740287
- Larenas-Linnemann D, Blaiss M, Van Bever HP, Compalati E, Baena-Cagnani CE. Pediatric sublingual immunotherapy efficacy: evidence analysis, 2009-2012. Ann Allergy Asthma Immunol 2013; 110:402 - , e9; http://dx.doi.org/10.1016/j.anai.2013.02.017; PMID: 23706708
- Compalati E, Braido F, Walter Canonica G. Sublingual immunotherapy: recent advances. Allergol Int 2013; 62:415 - 23; http://dx.doi.org/10.2332/allergolint.13-RAI-0627; PMID: 24280671
- Dranitsaris G, Ellis AK. Sublingual or subcutaneous immunotherapy for seasonal allergic rhinitis: an indirect analysis of efficacy, safety and cost. J Eval Clin Pract 2014; Forthcoming http://dx.doi.org/10.1111/jep.12112; PMID: 24444390
- Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol 2013; 131:1361 - 6; http://dx.doi.org/10.1016/j.jaci.2013.02.013; PMID: 23557834
- Wang J, Sampson HA. Oral and sublingual immunotherapy for food allergy. Asian Pac J Allergy Immunol 2013; 31:198 - 209; PMID: 24053702
- Calabria CW. Accelerated immunotherapy schedules. Curr Allergy Asthma Rep 2013; 13:389 - 98; http://dx.doi.org/10.1007/s11882-013-0356-x; PMID: 23728613
- Esch RE, Plunkett GA. Immunotherapy preparation guidelines, rules, and regulation. Curr Allergy Asthma Rep 2013; 13:406 - 13; http://dx.doi.org/10.1007/s11882-013-0358-8; PMID: 23722699
- MacGlashan DW Jr.. Basophil activation testing. J Allergy Clin Immunol 2013; 132:777 - 87; http://dx.doi.org/10.1016/j.jaci.2013.06.038; PMID: 23958648
- Chirumbolo S. Basophil activation test to optimize the diagnosis of adverse effects following immunization to vaccines. Iran J Allergy Asthma Immunol 2013; 12:196 - 202; PMID: 23893802
- Wisniewski J, Agrawal R, Woodfolk JA. Mechanisms of tolerance induction in allergic disease: integrating current and emerging concepts. Clin Exp Allergy 2013; 43:164 - 76; http://dx.doi.org/10.1111/cea.12016; PMID: 23331558
- de Weck AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, Demoly P, Ebo DG, Mayorga L, Monneret G, et al. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. Int Arch Allergy Immunol 2008; 146:177 - 89; http://dx.doi.org/10.1159/000115885; PMID: 18268385
- Erdmann SM, Sachs B, Kwiecien R, Moll-Slodowy S, Sauer I, Merk HF. The basophil activation test in wasp venom allergy: sensitivity, specificity and monitoring specific immunotherapy. Allergy 2004; 59:1102 - 9; http://dx.doi.org/10.1111/j.1398-9995.2004.00624.x; PMID: 15355470
- Ebo DG, Hagendorens MM, Schuerwegh AJ, Beirens LM, Bridts CH, De Clerck LS, Stevens WJ. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Cytometry B Clin Cytom 2007; 72:196 - 203; http://dx.doi.org/10.1002/cyto.b.20142; PMID: 17111386
- Kucera P, Cvackova M, Hulikova K, Juzova O, Pachl J. Basophil activation can predict clinical sensitivity in patients after venom immunotherapy. J Investig Allergol Clin Immunol 2010; 20:110 - 6; PMID: 20461965
- Czarnobilska E, Gregorius A, Porebski G, Spiewak R, Sacha M. [The benefits of using basophil activation test as a diagnostic tool prior to specific immunotherapy with inhalant allergens]. Przegl Lek 2012; 69:1249 - 53; PMID: 23750433
- Žitnik SE, Vesel T, Avčin T, Šilar M, Košnik M, Korošec P. Monitoring honeybee venom immunotherapy in children with the basophil activation test. Pediatr Allergy Immunol 2012; 23:166 - 72; http://dx.doi.org/10.1111/j.1399-3038.2011.01233.x; PMID: 22136583
- Chirumbolo S, Zanoni G, Ortolani R, Vella A. In vitro Biphasic Effect of Honey Bee Venom on Basophils from Screened Healthy Blood Donors. Allergy Asthma Immunol Res 2011; 3:58 - 61; http://dx.doi.org/10.4168/aair.2011.3.1.58; PMID: 21217927
- Przybilla B, Ruëff F. Hymenoptera venom allergy. J Dtsch Dermatol Ges 2010; 8:114 - 27, quiz 128-30; http://dx.doi.org/10.1111/j.1610-0387.2009.07125_supp.x; PMID: 19751222
- Oka T, Rios EJ, Tsai M, Kalesnikoff J, Galli SJ. Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells. J Allergy Clin Immunol 2013; 132:922 - , e1-16; http://dx.doi.org/10.1016/j.jaci.2013.05.004; PMID: 23810240
- Sancho-Serra MdelC, Simarro M, Castells M. Rapid IgE desensitization is antigen specific and impairs early and late mast cell responses targeting FcεRI internalization. Eur J Immunol 2011; 41:1004 - 13; http://dx.doi.org/10.1002/eji.201040810; PMID: 21360700
- Khodoun MV, Kucuk ZY, Strait RT, Krishnamurthy D, Janek K, Lewkowich I, Morris SC, Finkelman FD. Rapid polyclonal desensitization with antibodies to IgE and FcεRIα. J Allergy Clin Immunol 2013; 131:1555 - 64; http://dx.doi.org/10.1016/j.jaci.2013.02.043; PMID: 23632296
- Sanz ML, Sánchez G, Gamboa PM, Vila L, Uasuf C, Chazot M, Diéguez I, De Weck AL. Allergen-induced basophil activation: CD63 cell expression detected by flow cytometry in patients allergic to Dermatophagoides pteronyssinus and Lolium perenne. Clin Exp Allergy 2001; 31:1007 - 13; http://dx.doi.org/10.1046/j.1365-2222.2001.01122.x; PMID: 11467990
- Chirumbolo S. Use of basophil activation test in the investigation of adverse effects to vaccines. Hum Vaccin 2011; 7:878 - 80; http://dx.doi.org/10.4161/hv.7.8.16272; PMID: 21785282
- Chirumbolo S. Low maintenance dose in honeybee venom immunotherapy and basophil-based tests. Pediatr Allergy Immunol 2011; b 22:644 - 5; http://dx.doi.org/10.1111/j.1399-3038.2011.01168.x; PMID: 21919936
- Headley A, Katelaris C, Adelstein S. Assessing basophil function. Pathology 2014; 46:Suppl 1 S39; http://dx.doi.org/10.1097/01.PAT.0000443487.35411.87
- McGowan EC, Saini S. Update on the performance and application of basophil activation tests. Curr Allergy Asthma Rep 2013; 13:101 - 9; http://dx.doi.org/10.1007/s11882-012-0324-x; PMID: 23188565
- Macglashan DW Jr., Saini SS. Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J Allergy Clin Immunol 2013; 132:906 - , e1-4; http://dx.doi.org/10.1016/j.jaci.2013.04.056; PMID: 23791510
- Demeule B, Shire SJ, Liu J. A therapeutic antibody and its antigen form different complexes in serum than in phosphate-buffered saline: a study by analytical ultracentrifugation. Anal Biochem 2009; 388:279 - 87; http://dx.doi.org/10.1016/j.ab.2009.03.012; PMID: 19289095
- Hsu CL, Shiung YY, Lin BL, Chang HY, Chang TW. Accumulated immune complexes of IgE and omalizumab trap allergens in an in vitro model. Int Immunopharmacol 2010; 10:533 - 9; http://dx.doi.org/10.1016/j.intimp.2010.02.001; PMID: 20139035
- Buhl R. Anti-IgE antibodies for the treatment of asthma. Curr Opin Pulm Med 2005; 11:27 - 34; PMID: 15591885
- Pfaar O, Klimek L. [Application of humanized Anti-IgE antibodies (omalizumab). A new principle in the treatment of allergic diseases in ENT medicine]. HNO 2007; 55:981 - 90, quiz 991-2; http://dx.doi.org/10.1007/s00106-007-1627-4; PMID: 17992493
- Chirumbolo S, Olivieri M. Increase in human basophils IgE-mediated stimulation by omalizumab: A role for membrane FcγRs?. J Allergy Clin Immunol 2014; Forthcoming http://dx.doi.org/10.1016/j.jaci.2013.12.1094; PMID: 24642141
- Dombrowicz D, Lin S, Flamand V, Brini AT, Koller BH, Kinet JP. Allergy-associated FcRbeta is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity 1998; 8:517 - 29; http://dx.doi.org/10.1016/S1074-7613(00)80556-7; PMID: 9586641
- Zaidi AK, Saini SS, Macglashan DW Jr.. Regulation of Syk kinase and FcRbeta expression in human basophils during treatment with omalizumab. J Allergy Clin Immunol 2010; 125:902 - , e7; http://dx.doi.org/10.1016/j.jaci.2009.12.996; PMID: 20236696
- Meknache N, Jönsson F, Laurent J, Guinnepain MT, Daëron M. Human basophils express the glycosylphosphatidylinositol-anchored low-affinity IgG receptor FcgammaRIIIB (CD16B). J Immunol 2009; 182:2542 - 50; http://dx.doi.org/10.4049/jimmunol.0801665; PMID: 19201911
- Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, Macglashan DW Jr., Saini SS. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol 2010; 125:889 - , e7; http://dx.doi.org/10.1016/j.jaci.2009.09.012; PMID: 19962744
- Bidad K, Nawijn MC, van Oosterhout AJ, van der Heide S, Elberink JN. Basophil Activation Test in the diagnosis and monitoring of mastocytosis patients with wasp venom allergy. Cytometry B Clin Cytom 2013; •••; http://dx.doi.org/10.1002/cytob.21148; PMID: 24327387
- Korošec P, Šilar M, Eržen R, Čelesnik N, Bajrović N, Zidarn M, Košnik M. Clinical routine utility of basophil activation testing for diagnosis of hymenoptera-allergic patients with emphasis on individuals with negative venom-specific IgE antibodies. Int Arch Allergy Immunol 2013; 161:363 - 8; http://dx.doi.org/10.1159/000348500; PMID: 23689117
- Bidad K, Nawijn MC, van Oosterhout AJ, van der Heide S, Oude Elberink JN. Basophil activation test in the diagnosis and monitoring of mastocytosis patients with wasp venom allergy on immunotherapy. Cytometry B Clin Cytom 2014; Forthcoming http://dx.doi.org/10.1002/cyto.b.21148; PMID: 24478037
- Bonadonna P, Zanotti R, Melioli G, Antonini F, Romano I, Lenzi L, Caruso B, Passalacqua G. The role of basophil activation test in special populations with mastocytosis and reactions to hymenoptera sting. Allergy 2012; 67:962 - 5; http://dx.doi.org/10.1111/j.1398-9995.2012.02849.x; PMID: 22676063
- Chirumbolo S. State-of-the-art review about basophil research in immunology and allergy: is the time right to treat these cells with the respect they deserve?. Blood Transfus 2012; 10:148 - 64; PMID: 22244003
- Matsuoka T, Shamji MH, Durham SR. Allergen immunotherapy and tolerance. Allergol Int 2013; 62:403 - 13; http://dx.doi.org/10.2332/allergolint.13-RAI-0650; PMID: 24280670
- Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol 2013; 132:789 - 801, quiz 788; http://dx.doi.org/10.1016/j.jaci.2013.07.046; PMID: 24075190
- Fujita H, Meyer N, Akdis M, Akdis CA. Mechanisms of immune tolerance to allergens. Chem Immunol Allergy 2012; 96:30 - 8; http://dx.doi.org/10.1159/000331868; PMID: 22433368
- Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O’Mahony L, Palomares O, et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol 2011; 127:701 - 21, e1-70; http://dx.doi.org/10.1016/j.jaci.2010.11.050; PMID: 21377040
- Getts DR, McCarthy DP, Miller SD. Exploiting apoptosis for therapeutic tolerance induction. J Immunol 2013; 191:5341 - 6; http://dx.doi.org/10.4049/jimmunol.1302070; PMID: 24244028
- Soyer OU, Akdis M, Ring J, Behrendt H, Crameri R, Lauener R, Akdis CA. Mechanisms of peripheral tolerance to allergens. Allergy 2013; 68:161 - 70; http://dx.doi.org/10.1111/all.12085; PMID: 23253293
- Alvaro M, Sancha J, Larramona H, Lucas JM, Mesa M, Tabar AI, Martinez-Cañavate A, Immunotherapy Working Group, Sociedad Española de Inmunología Clínica y Alergia Pediátrica (SEICAP). Allergen-specific immunotherapy: update on immunological mechanisms. Allergol Immunopathol (Madr) 2013; 41:265 - 72; http://dx.doi.org/10.1016/j.aller.2012.07.018; PMID: 23332741
- Eiwegger T, Gruber S, Szépfalusi Z, Akdis CA. Novel developments in the mechanisms of immune tolerance to allergens. Hum Vaccin Immunother 2012; 8:1485 - 91; http://dx.doi.org/10.4161/hv.20903; PMID: 23095863
- Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Mechanisms of immune tolerance to allergens in children. Korean J Pediatr 2013; 56:505 - 13; http://dx.doi.org/10.3345/kjp.2013.56.12.505; PMID: 24416044
- Jutel M, Van de Veen W, Agache I, Azkur KA, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and novel ways for vaccine development. Allergol Int 2013; 62:425 - 33; http://dx.doi.org/10.2332/allergolint.13-RAI-0608; PMID: 24153333
- Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol 2005; 138:29 - 39; http://dx.doi.org/10.1159/000087355; PMID: 16088210
- MacGlashan D Jr.. Subthreshold desensitization of human basophils re-capitulates the loss of Syk and FcεRI expression characterized by other methods of desensitization. Clin Exp Allergy 2012; 42:1060 - 70; http://dx.doi.org/10.1111/j.1365-2222.2012.04013.x; PMID: 22702505
- MacGlashan D Jr., Lichtenstein LM. Studies of antigen binding on human basophils. I. Antigen binding and functional consequences. J Immunol 1983; 130:2330 - 6; PMID: 6187850
- MacGlashan D Jr., Mogowski M, Lichtenstein LM. Studies of antigen binding on human basophils. II. Continued expression of antigen-specific IgE during antigen-induced desensitization. J Immunol 1983; 130:2337 - 42; PMID: 6187851
- MacGlashan DW Jr., Dembo M, Goldstein B. Test of a theory relating to the cross-linking of IgE antibody on the surface of human basophils. J Immunol 1985; 135:4129 - 34; PMID: 2415601
- MacGlashan DW Jr.. Releasability of human basophils: cellular sensitivity and maximal histamine release are independent variables. J Allergy Clin Immunol 1993; 91:605 - 15; http://dx.doi.org/10.1016/0091-6749(93)90266-I; PMID: 7679683
- MacGlashan D Jr., McKenzie-White J, Chichester K, Bochner BS, Davis FM, Schroeder JT, Lichtenstein LM. In vitro regulation of FcepsilonRIalpha expression on human basophils by IgE antibody. Blood 1998; 91:1633 - 43; PMID: 9473229
- MacGlashan D Jr., Lavens-Phillips S, Katsushi M. IgE-mediated desensitization in human basophils and mast cells. Front Biosci 1998; 3:d746 - 56; PMID: 9680509
- Macglashan D Jr.. IgE and FcepsilonRI regulation. Ann N Y Acad Sci 2005; 1050:73 - 88; http://dx.doi.org/10.1196/annals.1313.009; PMID: 16014522
- Warner J, MacGlashan D Jr.. Persistence of early crosslink-dependent signal transduction events in human basophils after desensitization. Immunol Lett 1988; 18:129 - 37; http://dx.doi.org/10.1016/0165-2478(88)90053-3; PMID: 2456986
- MacGlashan DW Jr., Bochner BS, Warner JA. Graded changes in the response of individual human basophils to stimulation: distributional behavior of early activation events. J Leukoc Biol 1994; 55:13 - 23; PMID: 7506746
- MacGlashan DW Jr.. Graded changes in the response of individual human basophils to stimulation: distributional behavior of events temporally coincident with degranulation. J Leukoc Biol 1995; 58:177 - 88; PMID: 7543919
- Chirumbolo S. Monitoring of CD63% in basophil activation test and suggested new parameters for allergy diagnosis. Inflamm Res 2012; 61:171 - 6; http://dx.doi.org/10.1007/s00011-011-0416-4; PMID: 22205176
- Nguyen KL, Gillis S, MacGlashan DW Jr.. A comparative study of releasing and nonreleasing human basophils: nonreleasing basophils lack an early component of the signal transduction pathway that follows IgE cross-linking. J Allergy Clin Immunol 1990; 85:1020 - 9; http://dx.doi.org/10.1016/0091-6749(90)90046-7; PMID: 1693929
- MacGlashan D Jr., Guo CB. Oscillations in free cytosolic calcium during IgE-mediated stimulation distinguish human basophils from human mast cells. J Immunol 1991; 147:2259 - 69; PMID: 1918962
- Macglashan D, Miura K. Loss of syk kinase during IgE-mediated stimulation of human basophils. J Allergy Clin Immunol 2004; 114:1317 - 24; http://dx.doi.org/10.1016/j.jaci.2004.08.037; PMID: 15577829
- MacGlashan D Jr., Vilariño N. Nonspecific desensitization, functional memory, and the characteristics of SHIP phosphorylation following IgE-mediated stimulation of human basophils. J Immunol 2006; 177:1040 - 51; http://dx.doi.org/10.4049/jimmunol.177.2.1040; PMID: 16818760
- Gendelman SR, Lang DM. Specific immunotherapy in the treatment of atopic dermatitis: a systematic review using the GRADE system. Ann Allergy Asthma Immunol 2013; 111:555 - 61; http://dx.doi.org/10.1016/j.anai.2013.08.020; PMID: 24267368
- Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol 2013; 132:110 - 7; http://dx.doi.org/10.1016/j.jaci.2013.02.044; PMID: 23647790
- Kim JM, Lin SY, Suarez-Cuervo C, Chelladurai Y, Ramanathan M, Segal JB, Erekosima N. Allergen-specific immunotherapy for pediatric asthma and rhinoconjunctivitis: a systematic review. Pediatrics 2013; 131:1155 - 67; http://dx.doi.org/10.1542/peds.2013-0343; PMID: 23650298
- Lin SY, Erekosima N, Kim JM, Ramanathan M, Suarez-Cuervo C, Chelladurai Y, Ward D, Segal JB. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA 2013; 309:1278 - 88; http://dx.doi.org/10.1001/jama.2013.2049; PMID: 23532243
- Blumberga G, Groes L, Dahl R. SQ-standardized house dust mite immunotherapy as an immunomodulatory treatment in patients with asthma. Allergy 2011; 66:178 - 85; http://dx.doi.org/10.1111/j.1398-9995.2010.02451.x; PMID: 20883456
- Chen S, Wang L, Liao F, Zeng X, Xing QB, Chen B, Lin XZ. [Efficacy of sublingual immunotherapy with Dermatophagoides farinae drops in preschool and school-age children with allergic asthma and allergic rhinitis]. Zhonghua Er Ke Za Zhi 2013; 51:831 - 5; PMID: 24484557
- Wen CJ, Zhu MF, Ren WM, Liu XY, Qian H. [Clinical efficacy and safety of sublingual immunotherapy using standardized Dermatophagoides farinae extract for children with combined allergic rhinitis and asthma syndrome]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2011; 46:393 - 6; PMID: 21781561
- Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2010; CD002893; PMID: 21154351
- Creticos PS, Esch RE, Couroux P, Gentile D, D'Angelo P, Whitlow B, Alexander M, Coyne TC. Randomized, double-blind, placebo-controlled trial of standardized ragweed sublingual-liquid immunotherapy for allergic rhinoconjunctivitis. J Allergy Clin Immunol 2013; http://dx.doi.org/10.1016/j.jaci.2012.12.1183; PMID: 24332263
- Nolte H, Hébert J, Berman G, Gawchik S, White M, Kaur A, Liu N, Lumry W, Maloney J. Randomized controlled trial of ragweed allergy immunotherapy tablet efficacy and safety in North American adults. Ann Allergy Asthma Immunol 2013; 110:450 - , e4; http://dx.doi.org/10.1016/j.anai.2013.03.013; PMID: 23706715
- Mikkelsen S, Bibby BM, Dolberg MK, Dahl R, Hoffmann HJ. Basophil sensitivity through CD63 or CD203c is a functional measure for specific immunotherapy. Clin Mol Allergy 2010; 8:2; http://dx.doi.org/10.1186/1476-7961-8-2; PMID: 20158902
- Macglashan DW Jr., Saini SS. Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J Allergy Clin Immunol 2013; 132:906 - , e1-4; http://dx.doi.org/10.1016/j.jaci.2013.04.056; PMID: 23791510
- Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR, Till SJ. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol 2008; 121:1120 - 5, e2; http://dx.doi.org/10.1016/j.jaci.2008.01.072; PMID: 18374405
- Boumiza R, Monneret G, Forissier MF, Savoye J, Gutowski MC, Powell WS, Bienvenu J. Marked improvement of the basophil activation test by detecting CD203c instead of CD63. Clin Exp Allergy 2003; 33:259 - 65; http://dx.doi.org/10.1046/j.1365-2222.2003.01594.x; PMID: 12580920
- Lasa Luaces EM, Tabar Purroy AI, García Figueroa BE, Anda Apiñaniz M, Sanz Laruga ML, Raulf-Heimsoth M, Barber Hernández D. Component-resolved immunologic modifications, efficacy, and tolerance of latex sublingual immunotherapy in children. Ann Allergy Asthma Immunol 2012; 108:367 - 72; http://dx.doi.org/10.1016/j.anai.2012.03.005; PMID: 22541410
- Nettis E, Delle Donne P, Di Leo E, Fantini P, Passalacqua G, Bernardini R, Canonica GW, Ferrannini A, Vacca A. Latex immunotherapy: state of the art. Ann Allergy Asthma Immunol 2012; 109:160 - 5; http://dx.doi.org/10.1016/j.anai.2012.07.004; PMID: 22920069
- Chini L, Monteferrario E, Graziani S, Moschese V. Novel treatments of asthma and allergic diseases. Paediatr Respir Rev 2013; PMID: 24287269
- Larenas-Linnemann D, Blaiss M, Van Bever HP, Compalati E, Baena-Cagnani CE. Pediatric sublingual immunotherapy efficacy: evidence analysis, 2009-2012. Ann Allergy Asthma Immunol 2013; 110:402 - , e9; http://dx.doi.org/10.1016/j.anai.2013.02.017; PMID: 23706708
- El-Qutob D, Mencia G, Fernandez-Caldas E. Recent Advances in Immunotherapy for Allergic Diseases. Recent Pat Inflamm Allergy Drug Discov 2013; Forthcoming PMID: 24237114
- Wu AY. Immunotherapy - Vaccines for allergic diseases. J Thorac Dis 2012; 4:198 - 202; PMID: 22833826
- Senna G, Caminati M, Canonica GW. Safety and tolerability of sublingual immunotherapy in clinical trials and real life. Curr Opin Allergy Clin Immunol 2013; 13:656 - 62; http://dx.doi.org/10.1097/ACI.0000000000000007; PMID: 24126613
- Bahceciler NN, Cobanoglu N. Subcutaneous versus sublingual immunotherapy for allergic rhinitis and/or asthma. Immunotherapy 2011; 3:747 - 56; http://dx.doi.org/10.2217/imt.11.48; PMID: 21668312
- Marrs T, Anagnostou K, Fitzsimons R, Fox AT. Optimising treatment of allergic rhinitis in children. Practitioner 2013; 257:13 - 8, 2; PMID: 23905284
- Manzotti G, Lombardi C. Allergen immunotherapy as a drug: the new deal of grass allergen tablets from clinical trials to current practice. Eur Ann Allergy Clin Immunol 2013; 45:34 - 42; PMID: 23821831
- Queirós MG, Silva DA, Siman IL, Ynoue LH, Araújo NS, Pereira FL, Almeida KC, Miranda JS, Pena JD, Cunha-Junior JP, et al. Modulation of mucosal/systemic antibody response after sublingual immunotherapy in mite-allergic children. Pediatr Allergy Immunol 2013; 24:752 - 61; http://dx.doi.org/10.1111/pai.12163; PMID: 24299565
- Compalati E, Braido F, Canonica GW. An update on allergen immunotherapy and asthma. Curr Opin Pulm Med 2014; 20:109 - 17; http://dx.doi.org/10.1097/MCP.0000000000000016; PMID: 24296688
- Passalacqua G. Specific immunotherapy in asthma: a comprehensive review. J Asthma 2014; 51:29 - 33; http://dx.doi.org/10.3109/02770903.2013.853082; PMID: 24219841
- Gendelman SR, Lang DM. Specific immunotherapy in the treatment of atopic dermatitis: a systematic review using the GRADE system. Ann Allergy Asthma Immunol 2013; 111:555 - 61; http://dx.doi.org/10.1016/j.anai.2013.08.020; PMID: 24267368
- Compalati E, Rogkakou A, Passalacqua G, Canonica GW. Evidences of efficacy of allergen immunotherapy in atopic dermatitis: an updated review. Curr Opin Allergy Clin Immunol 2012; 12:427 - 33; http://dx.doi.org/10.1097/ACI.0b013e328354e540; PMID: 22622475
- Vieths S, Scheurer S, Ballmer-Weber B. Current understanding of cross-reactivity of food allergens and pollen. Ann N Y Acad Sci 2002; 964:47 - 68; http://dx.doi.org/10.1111/j.1749-6632.2002.tb04132.x; PMID: 12023194
- Pauli G, Metz-Favre C. [Cross reactions between pollens and vegetable food allergens]. Rev Mal Respir 2013; 30:328 - 37; http://dx.doi.org/10.1016/j.rmr.2012.10.633; PMID: 23664291
- Leysen J, De Witte L, Sabato V, Faber M, Hagendorens M, Bridts C, De Clerck L, Ebo D. IgE-mediated allergy to pholcodine and cross-reactivity to neuromuscular blocking agents: Lessons from flow cytometry. Cytometry B Clin Cytom 2013; 84:65 - 70; http://dx.doi.org/10.1002/cyto.b.21074; PMID: 23355309
- Ismail IH, Tang ML. Oral immunotherapy for the treatment of food allergy. Isr Med Assoc J 2012; 14:63 - 9; PMID: 22624447
- Ojeda P, Ojeda I, Rubio G, Pineda F. Home-based oral immunotherapy protocol with pasteurized egg for children allergic to hen’s egg. Isr Med Assoc J 2012; 14:34 - 9; PMID: 22624440
- Sánchez-García S, Rodríguez del Río P, Escudero C, García-Fernández C, Ramirez A, Ibáñez MD. Efficacy of oral immunotherapy protocol for specific oral tolerance induction in children with cow’s milk allergy. Isr Med Assoc J 2012; 14:43 - 7; PMID: 22624442
- Crisafulli G, Caminiti L, Pajno GB. Oral desensitization for immunoglobulin E-mediated milk and egg allergies. Isr Med Assoc J 2012; 14:53 - 6; PMID: 22624445
- Tammaro A, Narcisi A, Amodeo R, Portaro L, Tabacco F, Cardelli P, Persechino S. CD63 cell expression detected by flow-cytometric determination of basophil activation in allergic patients. Int J Immunopathol Pharmacol 2012; 25:1143 - 7; PMID: 23298505
- Nurmatov U, Venderbosch I, Devereux G, Simons FE, Sheikh A. Allergen-specific oral immunotherapy for peanut allergy. Cochrane Database Syst Rev 2012; 9:CD009014; PMID: 22972130
- Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, Hiegel A, Kamilaris J, Carlisle S, Yue X, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol 2011; 127:654 - 60; http://dx.doi.org/10.1016/j.jaci.2010.12.1111; PMID: 21377034
- Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, Stablein D, Henning AK, Vickery BP, Liu AH, et al, Consortium of Food Allergy Research (CoFAR). Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 2012; 367:233 - 43; http://dx.doi.org/10.1056/NEJMoa1200435; PMID: 22808958
- Narisety SD, Keet CA. Sublingual vs oral immunotherapy for food allergy: identifying the right approach. Drugs 2012; 72:1977 - 89; http://dx.doi.org/10.2165/11640800-000000000-00000; PMID: 23009174
- Sampson HA. Peanut oral immunotherapy: is it ready for clinical practice?. J Allergy Clin Immunol Pract 2013; 1:15 - 21; http://dx.doi.org/10.1016/j.jaip.2012.10.009; PMID: 24229817
- Tang ML. Oral immunotherapy for food allergy. Curr Allergy Asthma Rep 2009; 9:43 - 9; http://dx.doi.org/10.1007/s11882-009-0007-4; PMID: 19063824
- Virkud YV, Vickery BP. Advances in immunotherapy for food allergy. Discov Med 2012; 14:159 - 65; PMID: 23021370
- Sampson HA. Peanut oral immunotherapy: is it ready for clinical practice?. J Allergy Clin Immunol Pract 2013; 1:15 - 21; http://dx.doi.org/10.1016/j.jaip.2012.10.009; PMID: 24229817
- Henson M, Burks AW. The future of food allergy therapeutics. Semin Immunopathol 2012; 34:703 - 14; http://dx.doi.org/10.1007/s00281-012-0319-7; PMID: 22735939
- De Silva D, Geromi M, Panesar SS, Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Cardona V, Dubois AE, Halken S, et al. the EAACI Food Allergy and Anaphylaxis Group. Acute and long-term management of food allergy: systematic review. Allergy 2013; Forthcoming PMID: 24215577
- Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, Sicherer SH, Liu AH, Stablein D, Henning AK, et al, Consortium of Food Allergy Research (CoFAR). Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol 2013; 131:119 - , e1-7; http://dx.doi.org/10.1016/j.jaci.2012.11.011; PMID: 23265698
- Hourihane JO. Recent advances in peanut allergy. Curr Opin Allergy Clin Immunol 2002; 2:227 - 31; http://dx.doi.org/10.1097/00130832-200206000-00012; PMID: 12045419
- Al-Muhsen S, Clarke AE, Kagan RS. Peanut allergy: an overview. [Review. Erratum in: CMAJ. 2003 Jun 10;168] [12] CMAJ 2003; 168:1279 - 85; PMID: 12743075
- Yu GP, Weldon B, Neale-May S, Nadeau KC. The safety of peanut oral immunotherapy in peanut-allergic subjects in a single-center trial. Int Arch Allergy Immunol 2012; 159:179 - 82; http://dx.doi.org/10.1159/000336391; PMID: 22678151
- Kostadinova AI, Willemsen LE, Knippels LM, Garssen J. Immunotherapy - risk/benefit in food allergy. Pediatr Allergy Immunol 2013; 24:633 - 44; PMID: 24112425
- Mitsias DI, Kalogiros LA, Papadopoulos NG. Conference Scene: novelties in immunotherapy. Immunotherapy 2013; 5:1033 - 7; http://dx.doi.org/10.2217/imt.13.109; PMID: 24088073
- Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy 2007; 62:1261 - 9; http://dx.doi.org/10.1111/j.1398-9995.2007.01501.x; PMID: 17919140
- Morisset M, Moneret-Vautrin DA, Guenard L, Cuny JM, Frentz P, Hatahet R, Hanss Ch, Beaudouin E, Petit N, Kanny G. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow’s milk allergy and 90 children with egg allergy. Eur Ann Allergy Clin Immunol 2007; 39:12 - 9; PMID: 17375736
- Patriarca G, Nucera E, Pollastrini E, Roncallo C, De Pasquale T, Lombardo C, Pedone C, Gasbarrini G, Buonomo A, Schiavino D. Oral specific desensitization in food-allergic children. Dig Dis Sci 2007; 52:1662 - 72; http://dx.doi.org/10.1007/s10620-006-9245-7; PMID: 17245630
- Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, Matsui EC, Burks AW, Wood RA. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol 2008; 122:1154 - 60; http://dx.doi.org/10.1016/j.jaci.2008.09.030; PMID: 18951617
- Longo G, Berti I, Burks AW, Krauss B, Barbi E. IgE-mediated food allergy in children. Lancet 2013; 382:1656 - 64; http://dx.doi.org/10.1016/S0140-6736(13)60309-8; PMID: 23845860
- Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, et al, NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126:Suppl S1 - 58; http://dx.doi.org/10.1016/j.jaci.2010.10.008; PMID: 21134576
- Burks AW, Jones SM, Boyce JA, Sicherer SH, Wood RA, Assa’ad A, Sampson HA. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics 2011; 128:955 - 65; http://dx.doi.org/10.1542/peds.2011-0539; PMID: 21987705
- Kukkonen AK, Mäkelä M, Pelkonen A. [Nut allergy - a difficult problem for the clinician]. Duodecim 2013; 129:1263 - 70; PMID: 23847912
- Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, Ventura A. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J Allergy Clin Immunol 2008; 121:343 - 7; http://dx.doi.org/10.1016/j.jaci.2007.10.029; PMID: 18158176
- Martinolli F, Carraro S, Berardi M, Ferraro V, Baraldi E, Zanconato S. Immunotherapy For Food Allergies In Children. Curr Pharm Des 2013; Forthcoming PMID: 23701567
- Mansfield LE. Oral immunotherapy for peanut allergy in clinical practice is ready. Allergy Asthma Proc 2013; 34:205 - 9; http://dx.doi.org/10.2500/aap.2013.34.3666; PMID: 23676569
- Greenhawt MJ. Oral and sublingual peanut immunotherapy is not ready for general use. Allergy Asthma Proc 2013; 34:197 - 204; http://dx.doi.org/10.2500/aap.2013.34.3661; PMID: 23676568
- Calderón MA, Frankland AW, Demoly P. Allergen immunotherapy and allergic rhinitis: false beliefs. BMC Med 2013; 11:255; http://dx.doi.org/10.1186/1741-7015-11-255; PMID: 24314210
- Calderon MA, Gerth van Wijk R, Eichler I, Matricardi PM, Varga EM, Kopp MV, Eng P, Niggemann B, Nieto A, Valovirta E, et al, European Academy of Allergy and Clinical Immunology. Perspectives on allergen-specific immunotherapy in childhood: an EAACI position statement. Pediatr Allergy Immunol 2012; 23:300 - 6; http://dx.doi.org/10.1111/j.1399-3038.2012.01313.x; PMID: 22594930
- Cox L, Compalati E, Kundig T, Larche M. New directions in immunotherapy. Curr Allergy Asthma Rep 2013; 13:178 - 95; http://dx.doi.org/10.1007/s11882-012-0335-7; PMID: 23315329
- Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol 2003; 21:177 - 204; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141011; PMID: 12524384
- Kanamaru Y, Arcos-Fajardo M, Moura IC, Tsuge T, Cohen H, Essig M, Vrtovsnik F, Loirat C, Peuchmaur M, Beaudoin L, et al. Fc alpha receptor I activation induces leukocyte recruitment and promotes aggravation of glomerulonephritis through the FcR gamma adaptor. Eur J Immunol 2007; 37:1116 - 28; http://dx.doi.org/10.1002/eji.200636826; PMID: 17393381
- van der Steen LP, Bakema JE, Sesarman A, Florea F, Tuk CW, Kirtschig G, Hage JJ, Sitaru C, van Egmond M. Blocking Fcα receptor I on granulocytes prevents tissue damage induced by IgA autoantibodies. J Immunol 2012; 189:1594 - 601; http://dx.doi.org/10.4049/jimmunol.1101763; PMID: 22802416
- Iikura M, Yamaguchi M, Fujisawa T, Miyamasu M, Takaishi T, Morita Y, Iwase T, Moro I, Yamamoto K, Hirai K. Secretory IgA induces degranulation of IL-3-primed basophils. J Immunol 1998; 161:1510 - 5; PMID: 9686618
- Iikura M, Yamaguchi M, Miyamasu M, Morita Y, Iwase T, Moro I, Yamamoto K, Hirai K. Secretory IgA-mediated basophil activation. II. Roles of GTP-binding regulatory proteins and phosphatidylinositol 3-kinase. Biochem Biophys Res Commun 1999; 264:575 - 9; http://dx.doi.org/10.1006/bbrc.1999.1543; PMID: 10529404
- Matsui T, Nunomura S, Shimokawa T, Yoshimaru T, Ra C. Functionality of the IgA Fc receptor (FcalphaR, CD89) is down-regulated by extensive engagement of FcepsilonRI. Clin Immunol 2008; 129:155 - 62; http://dx.doi.org/10.1016/j.clim.2008.07.003; PMID: 18700185
- Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, Taylor AF, Rohane P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics 2001; 108:E36; http://dx.doi.org/10.1542/peds.108.2.e36; PMID: 11483846
- Pichler WJ. [Specific and nonspecific (anti-IgE) immunotherapy of allergic diseases]. Ther Umsch 2001; 58:329 - 36; http://dx.doi.org/10.1024/0040-5930.58.5.329; PMID: 11407237
- Peng Z. Vaccines targeting IgE in the treatment of asthma and allergy. Hum Vaccin 2009; 5:302 - 9; http://dx.doi.org/10.4161/hv.5.5.7442; PMID: 19221515
- Pite H, Gaspar Â, Paiva M, Leiria-Pinto P. Omalizumab under 12 years old: real-life practice. Allergol Immunopathol (Madr) 2013; 41:133 - 6; http://dx.doi.org/10.1016/j.aller.2012.01.008; PMID: 22560014
- Hambly N, Nair P. Monoclonal antibodies for the treatment of refractory asthma. Curr Opin Pulm Med 2014; 20:87 - 94; http://dx.doi.org/10.1097/MCP.0000000000000007; PMID: 24275927
- Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, Clifton I, Paton J, Woolacott N, McKenna C. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess 2013; 17:1 - 342; PMID: 24267198
- Babu KS, Polosa R, Morjaria JB. Anti-IgE--emerging opportunities for Omalizumab. Expert Opin Biol Ther 2013; 13:765 - 77; http://dx.doi.org/10.1517/14712598.2013.782391; PMID: 23517576
- Gokmen NM, Ersoy R, Gulbahar O, Ardeniz O, Sin A, Unsel M, Kokuludag A. Desensitization effect of preseasonal seven-injection allergoid immunotherapy with olive pollen on basophil activation: the efficacy of olive pollen-specific preseasonal allergoid immunotherapy on basophils. Int Arch Allergy Immunol 2012; 159:75 - 82; http://dx.doi.org/10.1159/000335251; PMID: 22572984
- Belliveau PP. Omalizumab: a monoclonal anti-IgE antibody. MedGenMed 2005; 7:27; PMID: 16369332
- Belliveau PP, Lahoz MR. Evaluation of omalizumab from a health plan perspective. J Manag Care Pharm 2005; b 11:735 - 45; PMID: 16300417
- Bang LM, Plosker GL. Spotlight on omalizumab in allergic asthma. BioDrugs 2004; 18:415 - 8; http://dx.doi.org/10.2165/00063030-200418060-00007; PMID: 15571425
- Bang LM, Plosker GL. Omalizumab: a review of its use in the management of allergic asthma. Treat Respir Med 2004; 3:183 - 99; http://dx.doi.org/10.2165/00151829-200403030-00006; PMID: 15219177
- Buhl R, Marco AG, Cohen D, Canonica GW. Eligibility for treatment with omalizumab in Italy and Germany. Respir Med 2013; PMID: 24315468
- Easthope S, Jarvis B. Omalizumab. Drugs 2001; 61:253 - 60, discussion 261; http://dx.doi.org/10.2165/00003495-200161020-00008; PMID: 11270941
- Ridolo E, Montagni M, Melli V, Braido F, Incorvaia C, Canonica GW. Pharmacotherapy of allergic rhinitis: current options and future perspectives. Expert Opin Pharmacother 2014; 15:73 - 83; http://dx.doi.org/10.1517/14656566.2014.860445; PMID: 24219793
- Kopp MV, Hamelmann E, Bendiks M, Zielen S, Kamin W, Bergmann KC, Klein C, Wahn U, DUAL study group. Transient impact of omalizumab in pollen allergic patients undergoing specific immunotherapy. Pediatr Allergy Immunol 2013; 24:427 - 33; http://dx.doi.org/10.1111/pai.12098; PMID: 23799935
- Aryan Z, Compalati E, Canonica GW, Rezaei N. Allergen-specific immunotherapy in asthmatic children: from the basis to clinical applications. Expert Rev Vaccines 2013; 12:639 - 59; http://dx.doi.org/10.1586/erv.13.45; PMID: 23750794
- Burch J, Griffin S, McKenna C, Walker S, Paton J, Wright K, Woolacott N. Omalizumab for the treatment of severe persistent allergic asthma in children aged 6-11 years: a NICE single technology appraisal. Pharmacoeconomics 2012; 30:991 - 1004; http://dx.doi.org/10.2165/11597160-000000000-00000; PMID: 22950547
- Lieberman JA, Chehade M. Use of omalizumab in the treatment of food allergy and anaphylaxis. Curr Allergy Asthma Rep 2013; 13:78 - 84; http://dx.doi.org/10.1007/s11882-012-0316-x; PMID: 23065311
- Rodríguez-Trabado A, Fernández Pereira LM, Romero-Chala S, García-Trujillo JA, Cámara Hijón C. Monitoring omalizumab treatment efficacy in chronic urticaria by the basophil activation test. Allergol Immunopathol (Madr) 2012; 40:390 - 2; http://dx.doi.org/10.1016/j.aller.2011.09.009; PMID: 22178123
- Jandus P, Hausmann O, Haeberli G, Gentinetta T, Mueller U, Helbling A. Unpredicted adverse reaction to omalizumab. J Investig Allergol Clin Immunol 2011; 21:563 - 6; PMID: 22312942
- Vousden KA, Clarke DL, Lowe DC. Engineering approaches to develop the next generation of antibodies to respiratory targets. Inflamm Allergy Drug Targets 2013; 12:99 - 108; http://dx.doi.org/10.2174/1871528111312020004; PMID: 23517646
- O’Byrne PM. Role of monoclonal antibodies in the treatment of asthma. Can Respir J 2013; 20:23 - 5; PMID: 23457670
- Mortensen DL, Prabhu S, Stefanich EG, Kadkhodayan-Fischer S, Gelzleichter TR, Baker D, Jiang J, Wallace K, Iyer S, Fielder PJ, et al. Effect of antigen binding affinity and effector function on the pharmacokinetics and pharmacodynamics of anti-IgE monoclonal antibodies. MAbs 2012; 4:724 - 31; http://dx.doi.org/10.4161/mabs.22216; PMID: 23778267
- Khan FM, Ueno-Yamanouchi A, Serushago B, Bowen T, Lyon AW, Lu C, Storek J. Basophil activation test compared to skin prick test and fluorescence enzyme immunoassay for aeroallergen-specific Immunoglobulin-E. Allergy Asthma Clin Immunol 2012; 8:1; http://dx.doi.org/10.1186/1710-1492-8-1; PMID: 22264407
- Park HJ, Kim HS, Chung DH. Fcgamma receptors modulate pulmonary inflammation by activating innate immune cells in murine hypersensitivity pneumonitis. Immune Netw 2010; 10:26 - 34; http://dx.doi.org/10.4110/in.2010.10.1.26; PMID: 20228933
- Vercelli D, De Monte L, Monticelli S, Di Bartolo C, Agresti A. To E or not to E? Can an IL-4-induced B cell choose between IgE and IgG4?. Int Arch Allergy Immunol 1998; 116:1 - 4; http://dx.doi.org/10.1159/000023918; PMID: 9623503
- Vashisht P, Casale T. Omalizumab for treatment of allergic rhinitis. Expert Opin Biol Ther 2013; 13:933 - 45; http://dx.doi.org/10.1517/14712598.2013.795943; PMID: 23621175
- Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Gimenéz-Arnau A, Agarwal S, Doyle R, Canvin J, Kaplan A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 2013; 368:924 - 35; http://dx.doi.org/10.1056/NEJMoa1215372; PMID: 23432142
- Bruhns P, Frémont S, Daëron M. Regulation of allergy by Fc receptors. Curr Opin Immunol 2005; 17:662 - 9; http://dx.doi.org/10.1016/j.coi.2005.09.012; PMID: 16214316
- Siegmund R, Vogelsang H, Machnik A, Herrmann D. Surface membrane antigen alteration on blood basophils in patients with Hymenoptera venom allergy under immunotherapy. J Allergy Clin Immunol 2000; 106:1190 - 5; http://dx.doi.org/10.1067/mai.2000.110928; PMID: 11112905
- Wood RA, Sicherer SH, Burks AW, Grishin A, Henning AK, Lindblad R, Stablein D, Sampson HA. A phase 1 study of heat/phenol-killed, E. coli-encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP-123) for the treatment of peanut allergy. Allergy 2013; 68:803 - 8; http://dx.doi.org/10.1111/all.12158; PMID: 23621498
- Chirumbolo S, Vella A, Ortolani R, De Gironcoli M, Solero P, Tridente G, Bellavite P. Differential response of human basophil activation markers: a multi-parameter flow cytometry approach. Clin Mol Allergy 2008; 6:12; http://dx.doi.org/10.1186/1476-7961-6-12; PMID: 18925959
- Chirumbolo S, Ortolani R, Vella A. CCR3 as a single selection marker compared to CD123/HLADR to isolate basophils in flow cytometry: some comments. Cytometry A 2011; 79:102 - 6; http://dx.doi.org/10.1002/cyto.a.21008; PMID: 21265004
- Sainte-Laudy J, Boumediene A, Touraine F, Orsel I, Brianchon C, Bonnaud F, Cogné M. Use of both CD63 up regulation and IgE down regulation for the flow cytometric analysis of allergen induced basophil activation. Definition of an activation index. Inflamm Res 2007; 56:291 - 6; http://dx.doi.org/10.1007/s00011-007-7014-5; PMID: 17659434
- Özdemir SK, Güloğlu D, Sin BA, Elhan AH, Ikincioğulları A, Mısırlıgil Z. Reliability of basophil activation test using CD203c expression in diagnosis of pollen allergy. Am J Rhinol Allergy 2011; 25:e225 - 31; http://dx.doi.org/10.2500/ajra.2011.25.3723; PMID: 22185730
- De Week AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, Demoly P, Ebo DG, Mayorga L, Monneret G, et al. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. II. Technical issues. J Investig Allergol Clin Immunol 2008; 18:143 - 55; PMID: 18564624
- Chirumbolo S. CD164 and other recently discovered activation markers as promising tools for allergy diagnosis: what’s new?. Clin Exp Med 2011; 11:255 - 7; http://dx.doi.org/10.1007/s10238-011-0132-y; PMID: 21461759
- Subbarayal B, Schiller D, Möbs C, de Jong NW, Ebner C, Reider N, Bartel D, Lidholm J, Pfützner W, Gerth van Wijk R, et al. Kinetics, cross-reactivity, and specificity of Bet v 1-specific IgG4 antibodies induced by immunotherapy with birch pollen. Allergy 2013; 68:1377 - 86; http://dx.doi.org/10.1111/all.12236; PMID: 24053565
- Sturm EM, Kranzelbinder B, Heinemann A, Groselj-Strele A, Aberer W, Sturm GJ. CD203c-based basophil activation test in allergy diagnosis: characteristics and differences to CD63 upregulation. Cytometry B Clin Cytom 2010; 78:308 - 18; http://dx.doi.org/10.1002/cyto.b.20526; PMID: 20533392
- Nullens S, Sabato V, Faber M, Leysen J, Bridts CH, De Clerck LS, Falcone FH, Maurer M, Ebo DG. Basophilic histamine content and release during venom immunotherapy: insights by flow cytometry. Cytometry B Clin Cytom 2013; 84:173 - 8; http://dx.doi.org/10.1002/cyto.b.21084; PMID: 23450838
- Gadermaier E, Flicker S, Blatt K, Valent P, Valenta R. Possible therapeutic potential of a recombinant group 2 grass pollen allergen-specific antibody fragment. Allergy 2013; Forthcoming PMID: 24251384
- Pellaton C, Perrin Y, Boudousquié C, Barbier N, Wassenberg J, Corradin G, Thierry AC, Audran R, Reymond C, Spertini F. Novel birch pollen specific immunotherapy formulation based on contiguous overlapping peptides. Clin Transl Allergy 2013; 3:17; http://dx.doi.org/10.1186/2045-7022-3-17; PMID: 23725004
- Prickett SR, Voskamp AL, Phan T, Dacumos-Hill A, Mannering SI, Rolland JM, O’Hehir RE. Ara h 1 CD4+ T cell epitope-based peptides: candidates for a peanut allergy therapeutic. Clin Exp Allergy 2013; 43:684 - 97; PMID: 23711131
- Grützkau A, Smorodchenko A, Lippert U, Kirchhof L, Artuc M, Henz BM. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytometry A 2004; 61:62 - 8; http://dx.doi.org/10.1002/cyto.a.20068; PMID: 15351990
- Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol 1991; 88:328 - 38; http://dx.doi.org/10.1016/0091-6749(91)90094-5; PMID: 1716273
- Gyimesi E, Gönczi F, Szilasi M, Pál G, Baráth S, Sipka S. The effects of various doses of bacterial lipopolysaccharide on the expression of CD63 and the release of histamine by basophils of atopic and non-atopic patients. Inflamm Res 2013; 62:213 - 8; http://dx.doi.org/10.1007/s00011-012-0569-9; PMID: 23109053
- Boumiza R, Debard AL, Monneret G. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin Mol Allergy 2005; 3:9; http://dx.doi.org/10.1186/1476-7961-3-9; PMID: 15989690
- Eberlein B, León Suárez I, Darsow U, Ruëff F, Behrendt H, Ring J. A new basophil activation test using CD63 and CCR3 in allergy to antibiotics. Clin Exp Allergy 2010; 40:411 - 8; http://dx.doi.org/10.1111/j.1365-2222.2009.03426.x; PMID: 20082620
- Wolanczyk-Medrala A, Barg W, Liebhart J, Panaszek B, Nadobna G, Litwa M, Gogolewski G, Medrala W. Validation of basophil CD164 upregulation for pollen allergy diagnosis. Arch Immunol Ther Exp (Warsz) 2010; 58:459 - 65; http://dx.doi.org/10.1007/s00005-010-0104-z; PMID: 20872290
- Thyagarajan A, Jones SM, Calatroni A, Pons L, Kulis M, Woo CS, Kamalakannan M, Vickery BP, Scurlock AM, Wesley Burks A, et al. Evidence of pathway-specific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clin Exp Allergy 2012; 42:1197 - 205; http://dx.doi.org/10.1111/j.1365-2222.2012.04028.x; PMID: 22805467
- Ford LS, Bloom KA, Nowak-Węgrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. J Allergy Clin Immunol 2013; 131:180 - , e1-3; http://dx.doi.org/10.1016/j.jaci.2012.06.003; PMID: 22819512
- Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J Allergy Clin Immunol 2009; 123:789 - , e20; http://dx.doi.org/10.1016/j.jaci.2008.12.1128; PMID: 19348919
