Abstract
Herpes zoster (HZ) is a common disease among elderly, which may develop into a severe pain syndrome labeled postherpetic neuralgia (PHN). A live-attenuated varicella zoster virus vaccine has been shown to be effective in reducing the incidence and burden of illness of HZ and PHN, providing the opportunity to prevent significant health-related and financial consequences of HZ. In this review, we summarize the available literature on cost-effectiveness of HZ vaccination and discuss critical parameters for cost-effectiveness results. A search in PubMed and EMBASE was performed to identify full cost-effectiveness studies published before April 2013. Fourteen cost-effectiveness studies were included, all performed in western countries. All studies evaluated cost-effectiveness among elderly above 50 years and used costs per quality-adjusted life year (QALY) gained as primary outcome. The vast majority of studies showed vaccination of 60- to 75-year-old individuals to be cost-effective, when duration of vaccine efficacy was longer than 10 years. Duration of vaccine efficacy, vaccine price, HZ incidence, HZ incidence and discount rates were influential to the incremental cost-effectiveness ratio (ICER). HZ vaccination may be a worthwhile intervention from a cost-effectiveness point of view. More extensive reporting on methodology and more detailed results of sensitivity analyses would be desirable to address uncertainty and to guarantee optimal comparability between studies, for example regarding model structure, discounting, vaccine characteristics and loss of quality of life due to HZ and PHN.
Introduction
Varicella zoster virus (VZV) causes chickenpox (varicella) and shingles (herpes zoster [HZ]). Varicella commonly occurs during childhood and is regarded as a mild self-limiting disease.Citation1 After remission, however, the virus remains latent, residing in the sensory nerve ganglia of the dorsal root, and can be reactivated decades later in life.Citation2 This reactivation episode is labeled HZ and is characterized by a painful dermatomal skin rash.Citation1The lifetime risk to encounter HZ has been estimated at 20–30%Citation3 and the probability to develop HZ as well as the severity of pain increase with age.Citation4,Citation5 Besides age, other risk-factors to HZ are a compromised or suppressed immune system and the female gender.Citation6,Citation7 Although the rash heals within a month,Citation8 complications might occur. The most serious complication is postherpetic neuralgia (PHN), defined as a neuropathic pain persisting longer than three months.Citation9 It has been estimated that approximately 8–33% of HZ patients develop PHN, and the risk increases with age.Citation4,Citation10-Citation12 Pain due to PHN may remain for months or even yearsCitation13,Citation14 and available therapeutic options are only partially effective.Citation15 PHN has been shown to have a substantial impact on the patient’s quality of life and functional status. Often reported sequelae of PHN comprise sleeping problems, chronic fatigue, anorexia, weight loss and depression, resulting in substantial interference with social life and self-care.Citation4,Citation14,Citation16,Citation17
In 2006, a VZV vaccine with the tradename Zostavax® (Sanofi-Pasteur/MSD) was approved by the US Food and Drug Administration (FDA) as well as by the European Medicines Agency (EMA).Citation18,Citation19 Zostavax® contains a live attenuated strain of VZV and is thought to induce primarily T-cell-mediated immunity against VZV. A large double-blind placebo-controlled clinical trial including 38,546 immunocompetent adults > 60 y of age (Shingles Prevention Study [SPS]) has demonstrated that the vaccine reduces the incidence of HZ by 51%, the pain burden by 61%, and the incidence of PHN by 67%.Citation20,Citation21 However, the vaccine-induced protection seems to decline with age, with an efficacy against HZ of 64% among individuals of 60 to 69 y of age and 38% in individuals aged 70 y or older.Citation20,Citation21 A more recent trial additionally showed that efficacy against HZ was 70% among of 50 to 59 y of age.Citation22 Regarding safety of the vaccine, multiple studies have shown that Zostavax® is well-tolerated and that side reactions are generally mild.Citation20,Citation20,Citation23-Citation26 However, as the mean follow-up time of the SPS was limited to 3.1 y,Citation20,Citation21 the duration of the vaccine protection is still unknown. A short-term persistence substudy (STPS) of the SPS recently showed that vaccine efficacy persists for at least 7 y, but also demonstrated that protection is waning in time.Citation27 Notably, the vaccine is contraindicated for immunocompromised patients, as it comprises a live attenuated virus.Citation19
Given all this evidence, vaccination against HZ might be an interesting option for introduction into national immunization programs. Besides reducing the disease burden itself, prevention of HZ and PHN may yield a significant benefit in limiting the economic burden to the healthcare. For instance, in the US, healthcare costs per acute episode of HZ were estimated at $431 and in the UK, healthcare costs of HZ and PHN were £103 and £397 per episode, respectively.Citation28,Citation29
After the results of the SPS were published, multiple cost-effectiveness analyses for different countries have been performed. The aim of this review is to summarize and synthesize the literature on cost-effectiveness of routine vaccination against HZ and to identify those input parameters that are crucial in determining cost-effectiveness outcomes.
Methods
Search strategy
A bibliographic search was performed in MEDLINE and EMBASE for relevant papers assessing the cost-effectiveness of HZ vaccination (April 10, 2013). The search was restricted to the English language and the search algorithm was as follows: (‘herpes zoster’ OR ‘shingles’ OR ‘postherpetic neuralgia’) AND (‘vaccination’ OR ‘vaccine’ OR ‘Zostavax’ OR ‘immunization’) AND (‘cost-effectiveness’ OR ‘cost-utility’ OR ‘cost-benefit’ OR ‘economic evaluation’ OR ‘pharmacoeconomics’). The search was limited to articles with an abstract. Only cost-effectiveness studies of HZ vaccination were assessed and original full papers were considered; reviews, editorials and letters were excluded. We screened titles, abstracts and finally the full content of the articles identified and selected. Studies on varicella vaccination only were excluded. Studies combining varicella and HZ vaccination were excluded in the main analysis, but briefly discussed in a separated section. A manual examination of reference sections of included papers was performed in order to identify further material of interest (snowballing).
Synthesis of results
We focused in particular on those variables exhibiting a large impact on the cost-effectiveness and we assessed these parameters critically. Obviously, our analysis plan comprised a review of the main characteristics of the studies, including type of analysis, perspective, targeted population, time horizon, discount rates and a short description of main results. Furthermore, results were stratified by vaccination age, as incidence of HZ, risk to PHN and vaccine efficacies are highly dependent on age. Finally, we analyzed per study which parameters influenced cost-effectiveness results significantly. To improve the comparability of the selected studies, costs were standardized to 2006 euros according to country-specific harmonized consumer price indices. If the costing year was not provided in the study, we assumed a costing year of ‘publication year – 3 y’. Studies were evaluated with regards to various aspects, including model type, perspective taken and quality according to previously defined criteria.
Results
Study selection
A total of 369 studies were found in MEDLINE and EMBASE. After evaluation of titles, abstracts or full contents, 18 studies were identified that assessed the cost-effectiveness of HZ vaccination. Two studies were excluded, because full texts were not available.Citation30,Citation31 However, their main results will be briefly mentioned when cost-effectiveness results are discussed. Two studies were excluded from the main analysis, but described in a separated section, because they assessed the cost-effectiveness of HZ vaccination combined with varicella vaccination.Citation32,Citation33 Finally 14 cost-effectiveness studies of HZ vaccination remained and were systematically reviewed.Citation34-Citation47
Main study characteristics
summarizes the main characteristics of the included studies ordered by publication date. Several studies did not mention all the main features reported in , such as time horizon, costing year, sensitivity analysis and funding. In these cases, we estimated most plausible values and options and explicitly marked this in the table.
Table 1. Main characteristics and results of the included studies
Country and funding
Of the 14 studies included, 9 were conducted in European countries (UK, Belgium, The Netherlands, Switzerland and France)Citation34-Citation47 and 5 in non-European countries (US and Canada).Citation35-Citation39 Six studies were funded by the pharmaceutical industry,Citation36,Citation38,Citation41,Citation42,Citation44,Citation46 five studies by public resourcesCitation34,Citation37,Citation39,Citation40,Citation43,Citation45 and two studies were performed without external funding.Citation35,Citation47
Type of analysis
All 14 studies used the incremental cost-effectiveness ratio (ICER) as primary outcome, in which costs are expressed as monetary units and effects as quality-adjusted life years (QALY) gained. Several studies also performed a cost-effectiveness analysis, presenting results as costs per averted HZ case,Citation36,Citation41,Citation42,Citation44,Citation46 per averted PHN case,Citation36,Citation41,Citation42,Citation44,Citation46 or per life year gained.Citation34
Model design and alternatives
A total of 13 studies used a “traditional” decision analytic model to calculate the cost-effectiveness of vaccination against HZ.Citation34-Citation47 Decision models which were predominantly used concern Cohort models and Markov models. One study used discrete event simulation (DES) modeling.Citation39 DES models are able to track the process of individual patients through particular states instead of cohorts. This provides the model with a ‘memory function’ because specific attributes can be assigned to individuals and might in specific situations provide a superior alternative over adding “tunnel states” into the Markov model to artificially create memory. Within the context of the 14 studies analyzed, a total of 9 different models were usedCitation34,Citation35,Citation37-Citation40,Citation42,Citation45,Citation47 as some authors adapted already available models.Citation36,Citation41,Citation43,Citation44,Citation46 As no country already implemented HZ vaccination in its national immunization program when the analysis was performed, all studies compared routine vaccination against HZ with no such vaccination.
Perspective
The perspective that was most often used is that of the health-care payer, which only includes medical costs.Citation34,Citation36,Citation38,Citation40-Citation43,Citation45-Citation47 A total of 8 studies used the societal perspective, taking into account medical costs as well as costs due to productivity losses.Citation35-Citation37,Citation41-Citation44,Citation47 The third-party payer’s (TPP) perspective, only including reimbursed medical costs, was used by four studies.Citation39,Citation41,Citation44,Citation46 Notably, one study can provide results from multiple perspectives.
Target group and time horizon
All studies targeted on population groups of 60 y of age or higher, however, some studies also assessed vaccination ages below this age.Citation34,Citation38,Citation41,Citation42 Vaccination age was explicitly varied in sensitivity analyses by all studies. Most studies indicated that VZV vaccination was restricted to the immunocompetent population,Citation35-Citation37,Citation39-Citation44,Citation46,Citation47 as the manufacturer of the VZV vaccine states that immunocompromised patients are contraindicated for the vaccine.Citation19 Only one study considered a scenario in which also immunocompromised patients would be vaccinated.Citation45 All studies used the life-time horizon,Citation34-Citation47 which can be regarded as optimal.Citation48
Discounting
Discounting adjusts benefits and costs for the so-called ‘time preference’, since it is generally advantageous to receive a benefit earlier or to pay costs later (see Appendix A in Supplemental material). The discount rates applied are highly dependent on national guidelines of the country for which the analysis is performed. A total of 9 studies applied an equal discount rate for costs and health effectsCitation34-Citation40,Citation42,Citation46 and 5 studies discounted costs at an higher rate than QALYs (differential discounting).Citation41,Citation43-Citation45,Citation47
Sensitivity analysis
To deal with uncertainty, studies generally perform sensitivity analyses investigating the impact of varying parameters on the study results (see Appendix A in Supplemental material for detailed information on different types of sensitivity analyses). All reviewed studies performed a one-way sensitivity analysis.Citation34-Citation47 In addition, two studies performed a multiway sensitivity analysisCitation37,Citation45 and nine studies a probabilistic sensitivity analysis (PSA).Citation35,Citation37-Citation43,Citation46
Quality assessment
A review of Szucs et al.Citation49 evaluated the quality of 11 of the included studies using the British Medical Journal (BMJ)’s checklist by Drummond and JeffersonCitation50 and the “Quality of Health Economic Studies” evaluation tool by Ofman et al.Citation51 Szucs et al.Citation49 concluded that the quality of these studies varied from ‘Moderate’ 37, 38, 4337, 38, 43 to ‘Moderate-Good’Citation35,Citation36,Citation39-Citation42,Citation44. We assessed the three other included studies using the same criteria as Szucs et al.Citation49 used. The study of Bilcke et al.Citation45 was judged as ‘Good’ and the study of Bresse et al.Citation46 and de Boer et al.Citation47 were evaluated as ‘Moderate-Good’.
Main input parameters
An overview of input parameters used for the four important domains, i.e., epidemiological, QALY losses, vaccine characteristics and costs are shown in .
Table 2. Input data of main parameters of the included studies
Epidemiological input
HZ incidence is obviously an important parameter in economic evaluations of HZ vaccination given the direct relation with HZ and PHN cases potentially to be prevented. shows that the ranges of HZ incidence used in the various studies varied little between different countries. Studies performed in the US seem to use on average somewhat higher incidence rates as compared with the rates used in European studies, especially in the age range of 60–70 y.Citation35-Citation37,Citation39 Logically, the HZ incidence increases with age in all studies. Multiple studies used HZ incidence rates adjusted to a immunocompetent population to be in line with the included population of the vaccine efficacy data.Citation35,Citation36,Citation39,Citation40,Citation42,Citation43,Citation45Concerning PHN incidence, most studies quantified the number of PHN cases directly from the number of HZ cases by using proportions.Citation34-Citation47 One study used a different method by quantifying HZ on the basis of a severity of illness score, in which the burdens of HZ and PHN are combined.Citation45 The proportion of HZ cases developing PHN varied extensively between the studies. For example, in a Dutch study the proportion PHN cases out of HZ ranged between 4.7–11.7% among the different age groups,Citation47 whereas a British study used a range of 9–52%.Citation40 A compromising factor in comparing the studies was the way PHN was defined in the models. In most studies, PHN was defined as pain persisting longer than 3 mo,Citation36-Citation40,Citation43,Citation46,Citation47 but other studies used PHN proportions after 1 moCitation34,Citation41,Citation42,Citation44 or after 6 mo.Citation35 Consequently, also the duration of PHN differed among the various studies - 8.3 mo to 4.2 - years, as this is directly related to the definition of PHN used.
QALY losses
The calculation of the QALYs gained depends on two parameters, i.e., the weight of the health-related quality of life attributed to a health state of disease (utility) and the time spent in this health state (general information on utilities can be found in Appendix A in Supplemental material). For a detailed look into the health effects of HZ and PHN, we refer to a recently published paper of Drolet et al.Citation15 shows that most studies split up HZ and PHN in mild, moderate and severe pain states, according to the validated pain inventory measurement Zoster Brief Pain Inventory (ZBPI),Citation52 which was also used in the SPS. The assignment of HZ and PHN cases between the different pain states differs among the selected studies. Some studies assigned around 20% of HZ/PHN cases as moderate/severe,Citation34,Citation40 while in other studies this proportion varied between 58–83% of PHN, depending on the age of diagnosis.Citation41,Citation42,Citation44,Citation46,Citation47 Consequently, also the PHN duration varied between these studies, with studies reporting lower proportions of moderate/severe cases applying longer PHN durations (). Pain severity and pain duration were influential for the cost-effectiveness results. For example, the study of Moore et al.Citation42 showed that using data on pain severity/pain duration from a general practitioner’s database instead of the SPS study increased the ICER from €19,003 per QALY gained to €36,908 per QALY gained.
In the selected studies, some differences were present in assigning utilities to HZ and PHN. Most studies assigned equal utilities to pain caused by HZ or PHN, while two studies distinguished between these two diseases.Citation35,Citation37 One study combined QALY loss of HZ and PHN in a severity of illness measurement.Citation45 In the study of Van Hoek et al.,Citation40 a model was used to determine weights of health related quality of life from several pain severity states of HZ in time, using 6 studies of EQ-5D. EQ-5D is an generic instrument quantifying quality of life taking into account five dimensions of health status.Citation53 Regarding utilities, the weight of mild pain ranged between 0.69–0.77 and of severe pain between 0.25–0.55. Because the vaccine was regarded as safe and side effects were generally mild and restricted to local reactions at the vaccination side, only two studies included a QALY penalty for side effects.Citation35,Citation37
Vaccine characteristics
Concerning vaccine efficacy, 13 studies used data from the SPS,Citation20,Citation21 the only clinical trial supplying data on this aspect. One study was forced to assume a vaccine efficacy rate, because it was performed before the SPS was conducted.Citation34 Yet, when vaccine efficacy against HZ incidence between selected studies was compared, differences were observed (). These differences can be assigned to two main causes. First, the SPS has shown that HZ vaccine efficacy depends on the age of vaccination, being lower in the higher age groups. This trend was applied or modeled differently among the analyzed studies, resulting in differences in vaccine efficacy. Second, the duration of vaccine-induced protection is still unknown. Several studies have imposed a waning function in their base-case analysis and among them two studies have combined this parameter within the vaccine efficacy function itself.Citation40,Citation43 These different approaches hampers the comparability of the studies. The used duration of protection in the base-case analysis ranged from 7.5 yCitation40,Citation43 to a lifetime protection.Citation36,Citation38,Citation41,Citation42,Citation44 All industry-funded studies except one assumed lifelong vaccine-induced protection in their base-case analyses, while publicly funded studies were more conservative by assuming limited durations of protection. Notably, all studies explicitly varied the duration of vaccine-induced protection in the sensitivity analyses. The study of Bilcke et al.Citation45 also assessed the influence of the type of function used to model vaccine efficacy on cost-effectiveness results. This was based on previous work of the same authors,Citation54 in which was evaluated what function of time since vaccination and age at vaccination fitted the data of the SPS and STPS best. Although the results showed that models with different functions (e.g., linear, logarithmic, exponential etc.) fitted the data comparably, they differ substantially in how they estimate vaccine efficacy as a function of time and age of vaccination. Notably, the study of Bilcke et al.Citation45 demonstrated that function type influences cost-effectiveness substantially in age-cohorts > 75 y in the scenario most in favor of vaccination (includes waning of vaccine efficacy in time).
Besides efficacy against HZ incidence, most studies included additional efficacy of vaccination toward PHNCitation35,Citation36,Citation38,Citation39,Citation41,Citation42,Citation46,Citation47,Citation49 or toward the burden of disease (BOI)Citation35,Citation37,Citation40-Citation46 in the base-case scenario. Two studies imposed additional efficacy against PHN in a scenario analysis.Citation40,Citation43 A general note regarding this additional efficacy is that the SPS publications reported efficacy against BOI and incidence of PHN for the entire population, and not just for those who developed HZ.Citation20,Citation21 Thus, these measures incorporated the decreasing HZ incidence, which implies that vaccine efficacy rates against PHN and BOI should be corrected before it can be directly applied on the BOI or the risk on PHN per HZ case itself. Papers demonstrated that after this correction, additional vaccine efficacy against PHN or BOI might only be present above the age of 70.Citation37,Citation55
Costs
In general, a distinction can be made between medical costs and societal costs. All studies included the three major direct cost burdens, i.e., GP costs, hospitalization costs and drug costs. Four studies used cost data specified for the immunocompetent population, as this is the targeted population.Citation36,Citation40,Citation42,Citation47 Societal costs due to productivity losses were included in 8 of the 14 studies.Citation35-Citation37,Citation41-Citation44,Citation47 Cost parameters were not regarded as influential parameters in many studies. Just one study reported that PHN costs had a large impact on the ICER.Citation36 Since the targeted population consisted of the elderly in all studies, indirect costs did not influence the ICER to a considerable extent, as the labor participation among the vaccinated population is generally low. However, since the age of retirement will probably rise in most countries due to healthy aging, this might change in the future.
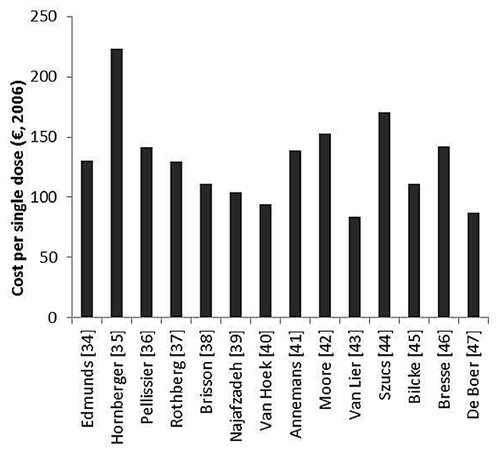
As the vaccine price was still unknown at the moment of analysis of the studies, authors had to assume the vaccine price. Although the vaccine price for the private sector is nowadays known in some countries, it is not clear which reduction can be expected when the vaccine is bought in bulk quantities. shows the vaccination costs used among the selected studies (expressed in 2006 euros). These vaccination costs include the vaccine price as well as the costs of vaccine administration. When only a range of vaccine prices was presented, vaccination costs as used in the sensitivity analysis were taken. Not all studies differentiated vaccination costs between vaccine price and administration costs. Among studies stating the separated vaccine price, the price varied between €65 and €154. The total vaccination costs ranged from €83 to €223. Highest vaccination costs were used by Hornberger et al.,Citation35 ranging from $50 to $500 and using a value of $200 in the one-way sensitivity analysis. Notably, industry-funded studies used on average higher vaccine prices in the base-case as compared with studies funded from other sources. Among the six studies with the highest vaccine price, five studies were funded by industry.Citation35,Citation36,Citation41,Citation42,Citation44,Citation46 The vaccine pricelist of the US Centers for Disease Control and prevention (CDC) of 2013 presents a price per dose of $166 and $114 for the private sector and CDC itself, respectively.Citation56 This would imply a 30% reduction for buying the vaccine in bulk quantities. As authors were forced to assume the vaccine price, this parameter was explicitly altered in the sensitivity analysis by all studies.
Cost-effectiveness results
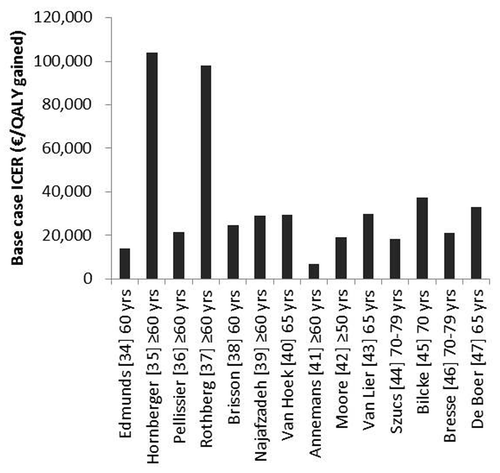
Effects of HZ vaccination on health outcomes and related QALY gains, incremental costs and cost-effectiveness results are shown in and . Generally, cost-effectiveness studies assuming a life-long duration of vaccine-induced protection showed the lowest cost-effectiveness results, in the range of €5000 – €25,000 per QALY gained.Citation36,Citation38,Citation41,Citation42,Citation44 Studies assuming a shorter duration of protection (range 7.5 and 15 y) in their base-case analysis, found higher cost-effectiveness results varying between €25,000 – €40,000 per QALY gained.Citation39,Citation40,Citation43,Citation47 Two studies reported ICERs in the range of €10,000–15,000, despite a duration of protection that was limited to 10 y.Citation34,Citation46 For Edmunds et al.Citation34 this could be explained by the fact this study was performed before the SPS results came out and therefore assuming a relatively high vaccine efficacy of 70% for all age groups. In the study of Bresse et al.Citation46 a relatively high amount of medical costs were prevented as compared with other studies. Two studies for the US showed cost-effectiveness results exceeding €50,000 per QALY gained.Citation35,Citation37 This might be explained by that both studies assigned lower QALY losses to HZ and PHN cases as compared with other studies. Moreover, the study of Hornberger et al.Citation35 assumed a relatively high vaccine price and a low risk to develop PHN. One study was difficult to compare with other studies, because only best-case and worst-case scenarios were presented in the results, representing an extremely broad range of for instance €2294 to €73,513 per QALY gained for vaccinating a cohort of 70 y olds.Citation33
Table 3. Modeled health and economic impact of vaccinating 1 million elderly against HZ
Figure 2. The incremental cost-effectiveness ratios (ICERs) of the included studies. For the study of Bilcke et al.Citation45 the median between the best case and worst case scenario was taken.
All studies, except one, stated in their conclusion that HZ vaccination may be cost-effective referring to the results of their base-case analysis. Only the Dutch study of Van Lier et al.Citation43 concluded that vaccination was marginally cost-effective from the societal perspective as well as from the health care payer’s perspective.Citation43 However, in this study a cost-effectiveness threshold of €20,000 per QALY gained was applied, which is low compared with that of other countries or compared with the GDP per capita of the Netherlands. When a threshold of €50,000 per QALY gained would be used, a value which also has been suggested for the Netherlands, the results of Van Lier et al.Citation43 would be regarded as cost-effective. This higher threshold was also used in the other Dutch study included in our analyses by de Boer et al.Citation47 Two German studies on cost-effectiveness of HZ vaccination were identified, but not included because full content was not available for evaluation.Citation30,Citation31 The study of Wasem et al.Citation30 found that vaccination of people above 60 y of age was cost-effective with an ICER of €20,139 per QALY gained from the TPP’s perspective. The study of Ultsch et al.Citation31 presented no cost-utility results, but found that vaccination of a cohort aged 50–54 y would cost €280 per HZ case prevented from the societal perspective.
Optimal vaccination age
shows that cost-effectiveness of HZ vaccination varies significantly over a range of vaccination ages. Different optimal vaccination ages were found and this was also dependent on the duration of vaccine efficacy which was assumed. Studies with limited durations of vaccine protection found an optimum vaccination age in the range of 70 to 75 y,Citation37,Citation39,Citation43,Citation46,Citation47 while studies assuming life-long protection reported ages between 60 and 69 as most beneficial.Citation35,Citation36,Citation41,Citation42,Citation44 Also inclusion of additional efficacy against PHN and BOI or not was an important factor to determine the optimal vaccination age. For instance, the study of Van Hoek et al.,Citation40 considering no additional efficacy against PHN in the base-case scenario, found an optimum age of 65 y. However, when additional protection against PHN was taken into account, the optimum vaccination age increased to 75 y.
Gender
Two studies also stratified results by gender.Citation37,Citation47 Both studies concluded that vaccination of women is more cost-effective than vaccination of men, because HZ incidence is higher among women ().
Influential parameters for cost-effectiveness
Sensitivity analyses provide information on which parameters can be regarded as most influential to the cost-effectiveness ratio. Parameters most often considered as influential are the duration of vaccine efficacy and the vaccine price (). All studies except one,Citation46 showed that duration of vaccine-induced protection or waning rate affected the ICER considerably. Only two studies came to the conclusion that vaccine price does not have a high impact on cost-effectiveness.Citation41,Citation42 Other often mentioned parameters influencing the ICER largely were vaccine efficacy,Citation34,Citation37,Citation40,Citation47 HZ incidenceCitation40,Citation44,Citation47 and discount rates.Citation37,Citation39,Citation41-Citation44,Citation46 Also utilities, pain severity split and duration of HZ and especially PHN, all involved in the calculation of the QALY losses, was reported to influence cost-effectiveness outcomes largely.Citation34,Citation35,Citation39,Citation42,Citation44,Citation46,Citation47
Combined varicella and zoster vaccination strategies
An interesting target of HZ vaccination might be the use in combination with varicella vaccination. As mentioned in the introduction, VZV is responsible for varicella as well as HZ. However, it has been hypothesized decades ago that re-exposure to circulating VZV could inhibit the reactivation of VZV.Citation2 This theory is also known as the ‘exogenous boosting’ theory and would imply that if adults come into contact with varicella infected children, their immunity against VZV is boosted and consequently the risk of developing HZ reduces. Consequently, in case universal varicella vaccination among children is implemented, a temporary increase of HZ incidence might arise due to an absence of exogenous boosting. Although the exact consequences of this theory are still under debate,Citation57-Citation63 a recently published systematic review concluded that exogenous boosting exists, however it seems not to account for all populations and all situations.Citation64
As mentioned above, two studies were found analyzing the cost-effectiveness of a combined varicella and HZ vaccination program.Citation32,Citation33 Both studies used an dynamic transmission model which accounted for passive immunity (herd immunity), age structures, gradual loss of vaccine or disease-acquired immunity and social contact mixing patterns.Citation65 The two studies also included the effect of exogenous boosting in their base-case analysis. The results showed that both models predicted an increase of HZ incidence in at least the first five decades following varicella vaccination, depending on the duration of protection of natural boosting. As HZ has a much higher disease burden than varicella, varicella vaccination was not deemed to be cost-effective within a time frame of 50 y, but might be cost-effective when an infinite time-horizon is used. Both studies demonstrated that combining varicella vaccination with HZ vaccination of the elderly is more cost-effective than varicella vaccination alone. In the study of Van Hoek et al.,Citation32 the probabilistic sensitivity analysis demonstrated that 70% of the simulations were cost-effective for the combined vaccination strategy and 50% of the simulations for varicella vaccination alone (willingness-to-pay threshold of £30,000 per QALY gained, infinite time horizon). In the study of Bilcke et al.,Citation33 the time horizon in which more than 50% of the simulations was cost-effective (threshold €35,000 per QALY gained) decreased from 99 y to 90 y, when HZ vaccination was added to varicella vaccination. The time horizon could decline further to 56 y, when the duration of protection of the HZ vaccine is extended to lifelong.
Discussion
This review assesses the available literature on health-economic evaluations of HZ vaccination. To assure that no studies examining the cost-effectiveness of HZ vaccination were missed, the search was performed within two distinct databases using an extensive range of related search-terms. Moreover, reference lists of potential articles were additionally screened. A total of 14 studies were included for extensive review. All studies concluded that HZ zoster might be cost-effective; however, this was not the case in all scenarios and at all vaccination ages. Generally, all studies showed that vaccination against HZ is cost-effective when vaccination is administered between the age of 60 and 75 y and the duration of vaccine-induced protection is longer than 10 y. These findings are consistent with other reviews summarizing evidence on the cost-effectiveness of HZ vaccination.Citation15,Citation49 However, compared with the review of Szucz et al.,Citation49 three more studies were included. Moreover, this review contains more specific data on the clinical results found in the studies, the influence of the modeling of vaccine efficacy on cost-effectiveness results and the optimum age of vaccination. Finally, results from studies combining HZ vaccination with varicella vaccination were also summarized. Differences in modeling approaches and input parameters hampered a straightforward comparison of the cost-effectiveness studies. It should however be noted that the heterogeneity between studies supports the credibility and robustness of HZ vaccination as a cost-effective intervention. The major key drivers for cost-effectiveness turn out to be duration of vaccine-induced protection, vaccine price and vaccination age.
Most studies targeted elderly above the age of 60 y, because this age-group was included in the SPS study providing evidence on vaccine efficacy.Citation20,Citation21 Later studies also included vaccination ages between 50 and 60 y, when efficacy data specifically for this age-group came available.Citation22 The optimal vaccination age from cost-effectiveness point of view ranges between 60 and 75, depending on duration of vaccine-induced protection and additional efficacy against BOI and PHN above the age of 70. To provide insight in the optimal vaccination age, modeling is needed, as several input parameters have been shown to vary during aging. On one hand, the SPS has shown that vaccine efficacy decreases during aging, which would imply that vaccinating at younger age would be more cost-effective. On the other hand the incidence of HZ and the risk to develop PHN after HZ reactivation increases with age. A crucial role in this specific research question is reserved for the duration of vaccine protection. As mentioned, some studies assumed that the duration of vaccine-induced protection is lifelong, while others assumed durations varying from 7.5 to 15 y. It seems plausible that, assuming lifelong protection, the scenario with the youngest vaccination age would be most cost-effective, because in that case the vaccinees are protected earlier. However, this is not necessarily the case. Among the six studies using lifelong protection in the base-case analysisCitation36,Citation38,Citation41,Citation42,Citation44 or almost lifelong (30 y),Citation35 five studies indeed showed that the optimum vaccination age was in the younger age groups (range 60–69 y). However, among three of these five studies also a scenario of vaccination between 50–59 y was applied, which means that the youngest vaccination age was not necessarily the most cost-effective vaccination age. The study of Brisson et al.Citation38 even found an optimum vaccination age of 70 y, unless a lifelong protection was assumed. Responsible for this potentially counterintuitive phenomenon is the discounting factor. HZ and PHN incidence is highest beyond the age of 70. Assuming lifelong protection, these cases are prevented independent of vaccination age. However, the counting of discountable years starts at the time-point of vaccination and as costs and QALYs are generally saved beyond the age of 70, outcomes are much more affected by discounting if the vaccination age is 50 y than if the age of 70 y. This explains why the youngest age is not necessarily the most optimal vaccination age even if a lifelong protection is assumed, but ages in the range of 60 to 70 y.
The major limitation within assessing the cost-effectiveness of HZ vaccination is the unknown duration of vaccine-induced protection. Up to now, protection has been shown to persist for at least 7 y.Citation27 Assumptions concerning duration of vaccine-induced protection vary from 7.5 y to lifelong. Follow-up of vaccine efficacy has to be maintained to provide more certainty about the duration of protection. To address uncertainty of duration of vaccine protection toward cost-effectiveness results, studies should vary this duration for specific age-groups as this duration might be age-dependent.Citation54 Another limitation is the unknown vaccine price when the vaccine is bought in bulk quantities. As this is generally kept confidential by pharmaceutical companies, such information unfortunately will not be available for cost-effectiveness analyses. Studies funded by pharmaceutical companies assumed in general a lifelong vaccine induced protection, which provides the opportunity to generate cost-effective results in the base-case analysis while using significant higher vaccine prices. Concerning HZ incidence, data was mainly obtained from general practitioners (GP) databases. Using such a source implies a risk for underestimation if not all HZ patients visit a GP. Incidence rates were similar between different studies, although in the United States it seemed somewhat higher. However, this might also be caused by differences in the surveillance systems. A recently published study showed that HZ incidences were similar between European countries.Citation66 Studies performed in countries which include varicella vaccination in their immunization programs should use updated data on HZ incidence, because circulation of varicella might have an impact on the occurrence of HZ in elderly. With our analysis applied to the Netherlands without a universal varicella vaccination,Citation47 one should be aware that for countries with a universal varicella vaccination, this policy might influence the cost-effectiveness of HZ vaccination. Parameters as severity of pain and duration of pain due to HZ/PHN are influential for the amount of QALYs gained and different sources estimating utilities should be used to address uncertainty into QALY losses. Moreover, studies using validated instruments to estimate quality of life should be preferred. Factors which were ignored in most studies but should be incorporated to achieve a complete view of the consequences of HZ vaccination are complications from ophthalmic manifestations of HZ and vaccine adverse events vaccination. Also productivity losses might become more important as a consequence of healthy aging and the evidence of vaccine efficacy in the younger age group between 50–59 y.
Further research will better inform on the duration of protection of the vaccine and reduce uncertainty in this area. With various initiatives to synthesize health-economic methods between countries, it might be expected that future cost-effectiveness analyses in the area will be even better comparable, enhancing this type of evidence synthesis as done here and allowing even conclusions to be drawn. With price being an important determinant of cost-effectiveness of HZ-vaccination, outcomes of price negotiations, potential tendering and price-volume deals might crucially influence the outcomes of future cost-effectiveness analyses being embarked upon.
Conclusion
In the light of current published studies, HZ vaccination of the elderly seems to be cost-effective, with the exact cost-effectiveness profile being dependent on the vaccination age, duration of vaccine efficacy and vaccine price. In general HZ vaccination was cost-effective in all studies, when vaccine protection was at least 10 y. Because of aging of the population, the burden of HZ and PHN might have a growing impact on the health-care budget and the population’s health-related quality of life. Therefore, universal HZ vaccination might present an interesting opportunity to reduce this burden. When updated information on the duration of vaccine-induced protection, HZ incidence or vaccine price becomes available, cost-effectiveness results should be updated in order to reassess vaccination recommendations and optimum vaccination age. To improve possibilities for a direct comparison of different cost-effectiveness studies, more extensive reporting on methodology and more detailed results of sensitivity analyses would be desirable.
| Abbreviations: | ||
| DES | = | Discrete event simulation |
| EMA | = | European Medicines Agency |
| FDA | = | Food and Drugs administration |
| HZ | = | Herpes zoster |
| PHN | = | Postherpetic neuralgia |
| PSA | = | Probabilistic sensitivity analysis |
| QALY | = | Quality-adjusted life year |
| SPS | = | Shingles prevention study |
| STPS | = | Short-term persistence substudy |
| TPP | = | Third-party-payer |
| VZV | = | Varicella zoster virus |
| ZBPI | = | Zoster brief pain inventory |
Additional material
Download Zip (999 KB)Disclosure of Potential Conflicts of Interest
This work was developed in the absence of any specific grants. MJP and JCW have received grants or advisory fees from various pharmaceutical companies, including grants or fees related to the subject matter of this article.
References
- Heininger U, Seward JF. Varicella. Lancet 2006; 368:1365 - 76; http://dx.doi.org/10.1016/S0140-6736(06)69561-5; PMID: 17046469
- Hope-Simpson RE. The nature of herpes zoster: A long-term study and a new hypothesis. Proc R Soc Med 1965; 58:9 - 20; PMID: 14267505
- Johnson R, McElhaney J, Pedalino B, Levin M. Prevention of herpes zoster and its painful and debilitating complications. Int J Infect Dis 2007; 11:Suppl 2 S43 - 8; http://dx.doi.org/10.1016/S1201-9712(07)60021-6; PMID: 18162246
- Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, Patrick D, Blanchette C, Mansi JA. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ 2010; 182:1731 - 6; http://dx.doi.org/10.1503/cmaj.091711; PMID: 20921251
- Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ 2000; 321:794 - 6; http://dx.doi.org/10.1136/bmj.321.7264.794; PMID: 11009518
- Wareham DW, Breuer J. Herpes zoster. BMJ 2007; 334:1211 - 5; http://dx.doi.org/10.1136/bmj.39206.571042.AE; PMID: 17556477
- Opstelten W, Van Essen GA, Schellevis F, Verheij TJM, Moons KGM. Gender as an independent risk factor for herpes zoster: a population-based prospective study. Ann Epidemiol 2006; 16:692 - 5; http://dx.doi.org/10.1016/j.annepidem.2005.12.002; PMID: 16516488
- Arvin AM. Varicella-zoster virus. Clin Microbiol Rev 1996; 9:361 - 81; PMID: 8809466
- Dworkin RH, Portenoy RK. Proposed classification of herpes zoster pain. Lancet 1994; 343:1648 - 1648; http://dx.doi.org/10.1016/S0140-6736(94)93106-2; PMID: 7911959
- Opstelten W, Zuithoff NP, van Essen GA, van Loon AM, van Wijck AJ, Kalkman CJ, Verheij TJ, Moons KG. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain 2007; 132:Suppl 1 S52 - 9; http://dx.doi.org/10.1016/j.pain.2007.02.004; PMID: 17379412
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain 2002; 18:350 - 4; http://dx.doi.org/10.1097/00002508-200211000-00002; PMID: 12441828
- Scott FT, Leedham-Green ME, Barrett-Muir WY, Hawrami K, Gallagher WJ, Johnson R, Breuer J. A study of shingles and the development of postherpetic neuralgia in East London. J Med Virol 2003; 70:Suppl 1 S24 - 30; http://dx.doi.org/10.1002/jmv.10316; PMID: 12627483
- Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract 1975; 25:571 - 5; PMID: 1195231
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6:356 - 63; http://dx.doi.org/10.1016/j.jpain.2005.01.359; PMID: 15943957
- Drolet M, Oxman MN, Levin MJ, Schmader KE, Johnson RW, Patrick D, Mansi JA, Brisson M. Vaccination against herpes zoster in developed countries: State of the evidence. Hum Vaccin Immunother 2013; •••:9; PMID: 23570049
- Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, Chan IS, Choo P, Levin MJ, Johnson G, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain 2007; 23:490 - 6; http://dx.doi.org/10.1097/AJP.0b013e318065b6c9; PMID: 17575488
- Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis 2004; 39:342 - 8; http://dx.doi.org/10.1086/421942; PMID: 15307000
- Mitka M. FDA approves shingles vaccine: herpes zoster vaccine targets older adults. JAMA 2006; 296:157 - 8; http://dx.doi.org/10.1001/jama.296.2.157; PMID: 16835412
- European Medicines Agency. Zostavax: EPAR - product information. 2013; 2013.
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al, Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271 - 84; http://dx.doi.org/10.1056/NEJMoa051016; PMID: 15930418
- Oxman MN, Levin MJ, Shingles Prevention Study Group. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis 2008; 197:Suppl 2 S228 - 36; http://dx.doi.org/10.1086/522159; PMID: 18419402
- Schmader KE, Levin MJ, Gnann JW Jr., McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922 - 8; http://dx.doi.org/10.1093/cid/cir970; PMID: 22291101
- Baxter R, Tran TN, Hansen J, Emery M, Fireman B, Bartlett J, Lewis N, Saddier P. Safety of Zostavax™--a cohort study in a managed care organization. Vaccine 2012; 30:6636 - 41; http://dx.doi.org/10.1016/j.vaccine.2012.08.070; PMID: 22963800
- Vesikari T, Hardt R, Rumke HC, Icardi G, Montero J, Thomas S, Sadorge C, Fiquet A. Immunogenicity and safety of a live attenuated shingles (herpes zoster) vaccine (zostavax ((R))) in individuals aged >/= 70 years: A randomized study of a single dose versus two different two-dose schedules. Hum Vaccin Immunother 2013; •••:9; PMID: 23570049
- Simberkoff MS, Arbeit RD, Johnson GR, Oxman MN, Boardman KD, Williams HM, Levin MJ, Schmader KE, Gelb LD, Keay S, et al, Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med 2010; 152:545 - 54; http://dx.doi.org/10.7326/0003-4819-152-9-201005040-00004; PMID: 20439572
- Tseng HF, Liu A, Sy L, Marcy SM, Fireman B, Weintraub E, Baggs J, Weinmann S, Baxter R, Nordin J, et al, Vaccine Safety Datalink (VSD) Team. Safety of zoster vaccine in adults from a large managed-care cohort: a Vaccine Safety Datalink study. J Intern Med 2012; 271:510 - 20; http://dx.doi.org/10.1111/j.1365-2796.2011.02474.x; PMID: 22026504
- Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, et al, Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55:1320 - 8; http://dx.doi.org/10.1093/cid/cis638; PMID: 22828595
- Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect 2009; 137:38 - 47; http://dx.doi.org/10.1017/S0950268808000678; PMID: 18466661
- Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics 2007; 25:155 - 69; http://dx.doi.org/10.2165/00019053-200725020-00007; PMID: 17249857
- Wasem J, Lang K, Papageorgiou M, Ultsch B, Martin M. Health economic evaluation of a new vaccine for the prevention of herpes zoster and post-herpetic neuralgia in adults a german analysis. Value Health 2009; 12:A294; http://dx.doi.org/10.1016/S1098-3015(10)74442-9
- Ultsch B, Reinhold T, Siedler A, Krause G, Wichmann O. Health economic evaluation of the vaccination against herpes zoster and postherpetic neuralgia in germany. Value Health 2012; 15:A394; http://dx.doi.org/10.1016/j.jval.2012.08.1118
- van Hoek AJ, Melegaro A, Gay N, Bilcke J, Edmunds WJ. The cost-effectiveness of varicella and combined varicella and herpes zoster vaccination programmes in the United Kingdom. Vaccine 2012; 30:1225 - 34; http://dx.doi.org/10.1016/j.vaccine.2011.11.026; PMID: 22119592
- Bilcke J, Jan van Hoek A, Beutels P. Childhood varicella-zoster virus vaccination in belgium: Cost-effective only in the long run or without exogenous boosting?. Hum Vaccin Immunother 2013; •••:9; PMID: 23570049
- Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine 2001; 19:3076 - 90; http://dx.doi.org/10.1016/S0264-410X(01)00044-5; PMID: 11312002
- Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med 2006; 145:317 - 25; http://dx.doi.org/10.7326/0003-4819-145-5-200609050-00004; PMID: 16954357
- Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007; 25:8326 - 37; http://dx.doi.org/10.1016/j.vaccine.2007.09.066; PMID: 17980938
- Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis 2007; 44:1280 - 8; http://dx.doi.org/10.1086/514342; PMID: 17443464
- Brisson M, Pellissier JM, Camden S, Quach C, De Wals P. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin 2008; 4:238 - 45; http://dx.doi.org/10.4161/hv.4.3.5686; PMID: 18382137
- Najafzadeh M, Marra CA, Galanis E, Patrick DM. Cost effectiveness of herpes zoster vaccine in Canada. Pharmacoeconomics 2009; 27:991 - 1004; http://dx.doi.org/10.2165/11314010-000000000-00000; PMID: 19908924
- van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine 2009; 27:1454 - 67; http://dx.doi.org/10.1016/j.vaccine.2008.12.024; PMID: 19135492
- Annemans L, Bresse X, Gobbo C, Papageorgiou M. Health economic evaluation of a vaccine for the prevention of herpes zoster (shingles) and post-herpetic neuralgia in adults in Belgium. J Med Econ 2010; 13:537 - 51; http://dx.doi.org/10.3111/13696998.2010.502854; PMID: 20707768
- Moore L, Remy V, Martin M, Beillat M, McGuire A. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc 2010; 8:7-7547-8-7.
- van Lier A, van Hoek AJ, Opstelten W, Boot HJ, de Melker HE. Assessing the potential effects and cost-effectiveness of programmatic herpes zoster vaccination of elderly in the netherlands. BMC Health Serv Res 2010; 10:237-6963-10-237.
- Szucs TD, Kressig RW, Papageorgiou M, Kempf W, Michel JP, Fendl A, Bresse X. Economic evaluation of a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in older adults in Switzerland. Hum Vaccin 2011; 7:749 - 56; http://dx.doi.org/10.4161/hv.7.7.15573; PMID: 21606685
- Bilcke J, Marais C, Ogunjimi B, Willem L, Hens N, Beutels P. Cost-effectiveness of vaccination against herpes zoster in adults aged over 60 years in Belgium. Vaccine 2012; 30:675 - 84; http://dx.doi.org/10.1016/j.vaccine.2011.10.036; PMID: 22120193
- Bresse X, Annemans L, Preaud E, Bloch K, Duru G, Gauthier A. Vaccination against herpes zoster and postherpetic neuralgia in france: A cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res 2013.
- de Boer PT, Pouwels KB, Cox JM, Hak E, Wilschut JC, Postma MJ. Cost-effectiveness of vaccination of the elderly against herpes zoster in The Netherlands. Vaccine 2013; 31:1276 - 83; http://dx.doi.org/10.1016/j.vaccine.2012.12.067; PMID: 23306360
- Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. New York: Oxford University Press Inc.; 2005.
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013; 31:125 - 36; http://dx.doi.org/10.1007/s40273-012-0020-7; PMID: 23335045
- Drummond MF, Jefferson TO, The BMJ Economic Evaluation Working Party. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996; 313:275 - 83; http://dx.doi.org/10.1136/bmj.313.7052.275; PMID: 8704542
- Ofman JJ, Sullivan SD, Neumann PJ, Chiou CF, Henning JM, Wade SW, Hay JW. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm 2003; 9:53 - 61; PMID: 14613362
- Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, Johnson G, Bauer M, Williams HM, Kaplan KM, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain 2004; 5:344 - 56; http://dx.doi.org/10.1016/j.jpain.2004.06.001; PMID: 15336639
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001; 33:337 - 43; http://dx.doi.org/10.3109/07853890109002087; PMID: 11491192
- Bilcke J, Ogunjimi B, Hulstaert F, Van Damme P, Hens N, Beutels P. Estimating the age-specific duration of herpes zoster vaccine protection: a matter of model choice?. Vaccine 2012; 30:2795 - 800; http://dx.doi.org/10.1016/j.vaccine.2011.09.079; PMID: 21964056
- Brisson M, Pellissier JM, Levin MJ. Cost-effectiveness of herpes zoster vaccine: flawed assumptions regarding efficacy against postherpetic neuralgia. Clin Infect Dis 2007; 45:1527 - 9; http://dx.doi.org/10.1086/523011; PMID: 17990240
- Centers for Disease Control and Prevention. CDC vaccine price list. March 7 2013; 2013.
- Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, Seward JF. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health 2005; 5:68; http://dx.doi.org/10.1186/1471-2458-5-68; PMID: 15960856
- Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis 2005; 191:2002 - 7; http://dx.doi.org/10.1086/430325; PMID: 15897984
- Grant KA, Carville KS, Kelly HA. Evidence of increasing frequency of herpes zoster management in Australian general practice since the introduction of a varicella vaccine. Med J Aust 2010; 193:483; PMID: 20955129
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332 - 40; http://dx.doi.org/10.1093/cid/ciq077; PMID: 21217180
- Tanuseputro P, Zagorski B, Chan KJ, Kwong JC. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine 2011; 29:8580 - 4; http://dx.doi.org/10.1016/j.vaccine.2011.09.024; PMID: 21939721
- Chao DY, Chien YZ, Yeh YP, Hsu PS, Lian IB. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000-2008. Epidemiol Infect 2012; 140:1131 - 40; http://dx.doi.org/10.1017/S0950268811001786; PMID: 21906410
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: Herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine 2013; 31:1680 - 94; http://dx.doi.org/10.1016/j.vaccine.2012.05.050; PMID: 22659447
- Ogunjimi B, Van Damme P, Beutels P. Herpes zoster risk reduction through exposure to chickenpox patients: A systematic multidisciplinary review. PLoS One 2013; 8:e66485; http://dx.doi.org/10.1371/journal.pone.0066485; PMID: 23805224
- Hethcote H. The mathematics of infectious diseases. SIAM Rev 2000; 42:599 - 653; http://dx.doi.org/10.1137/S0036144500371907
- Pinchinat S, Cebrian-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across europe: Results from a systematic literature review. BMC Infect Dis 2013; 13:170-2334-13-170..