Abstract
To describe the trends in serotype distribution and antimicrobial susceptibility of S. pneumoniae causing invasive pneumococcal diseases (IPD) we tested 238 pneumococci isolates from normally sterile sites between 2009 and 2012 and compared these findings with previous data collected within our network. Serotyping was performed for 15 serotypes contained in the 7-,10-, 13-, and experimental 15-valent pneumococcal conjugate vaccines (PCV). The most common serotypes found were 6B (13.9%), 19A (12.6%), 14 (8.0%), 18C (5.9%), and 6A (3.8%); and 39.9% were non-PCV15 serotypes. One of 81 patients with available data had breakthrough infection with vaccine serotype (19F). There was a significant increase of serotype 19A among children ≤5 years (5.6% in 2000–2009 vs 18.3% in 2009–2012, P = 0.003). The all-age serotype coverage was 36.4%, 41.5%, 59.3%, and 59.7% for PCV7, PCV10, PCV13, and PCV 15, respectively. The corresponding coverage in children ≤5 years were 46.4%, 48.8%, 73.2%, and 73.2% respectively. High susceptibilities to penicillin (89.7%), cefotaxime (95.7%), cefditoren (90.2% by Spanish breakpoints), ofloxacin (97.9%), and levofloxacin (100%), but low to cefdinir (50.0%), cefditoren (45.1% by US-FDA breakpoints), macrolides (<50%), clindamycin (67.7%), tetracycline (41.4%), and trimethoprim-sulfamethoxazole (32.4%) were observed. Serotype 19A was less susceptible to penicillin (80.0 vs 91.2%, P = 0.046), cefditoren (66.7 vs 95.5% by Spanish breakpoints, P = 0.004), and tetracycline (9.1 vs 45.5%, P = 0.024) than non-19A isolates. These data emphasize the need for continued surveillance to monitor changes in serotypes as well as antimicrobial susceptibilities in order to guide strategies for prevention and treatment.
Introduction
Since the introduction of heptavalent pneumococcal conjugate vaccine (PCV7) the epidemiology of pneumococcal infection has changed globally. There has been a dramatic decline in both invasive (IPD) and non-invasive pneumococcal diseases caused by vaccine serotypes following the inclusion of PCV7 in to national immunization programs. However, emergence of non-vaccine serotypes, particularly serotype 19A, has been noted in many parts of the world including North America,Citation1,Citation2 Europe,Citation3-Citation7 Oceania,Citation8 Latin America,Citation9 and Asia.Citation10-Citation12 The emergent serotype 19A was associated with a higher rate of penicillin non-susceptibity.Citation13-Citation15 Other non-vaccine serotype have been identified to increase penicillin nonsusceptibility of invasive pneumococci in the post PCV7 era. Of these serotypes, 15A, 23A, 35B, and 6C were the most common among penicillin-nonsusceptible isolates from population-based invasive pneumococcal surveillance in the United States during 2007.Citation16
The emergence of non-PCV7 serotypes led to the development and introduction of broader PCVs: a 10-valent vaccine (PCV10)—PCV7 with serotypes 1, 5, and 7F addedCitation17,Citation18; and a 13-valent vaccine (PCV13)—PCV10 with serotypes 3, 6A, and 19A added.Citation19 Recently, the 15-valent vaccine (PCV15) with serotypes 22F and 33F added to PCV13, has been tested in clinical trials.Citation20 After the introduction of PCV13 in the US in 2010, serotype 19A has started to decrease while the prevalence of other vaccine-containing or vaccine-related serotypes (3, 6C, 7F) remain unchanged. Only serotype 35B was found to increase from 2008–2009 to 2010–2011.Citation21 A recent randomized double-blind trial comparing the impact of PCV13 vs. PCV7 on nasopharyngeal (NP) colonization showed that PCV13 significantly reduced NP colonization of the 4 additional PCV13 serotypes and serotype 6C. These findings supported the vaccine effectiveness for vaccine and vaccine-related serotypes.Citation22
While data regarding burdens of pneumococcal diseases and serotype distribution in developed regions such as Europe and North America have been well described, such data in developing regions, including Asia, are limited. In 2012, Jauneikaite et al. reported that serotype distribution of invasive pneumococcal isolates was similar across countries in the Association of South East Asian Nations (ASEAN) region (Laos, Malaysia, the Philippines, Singapore, Thailand, and Vietnam). The most common serotypes were 19F (10.8%), 23F (10.7%), 14 (9.2%), 6B (8.9%), 1 (5.7%), 19A (4.3%), and 3 (4%).Citation23 A recent report from a study performed in 11 Asian countries by the Asian Network for Surveillance of Resistance Pathogen (ANSORP) showed that the PCV7 serotype coverage significantly decreased from 60.5% in 2000 to 2001 to 52.4% in 2008 to 2009 (P = 0.002), while the prevalence of serotype 19A markedly increased from 3% to 8.2%. (P < 0.001).Citation24 Contrary to North America, and Europe, most Asian countries that found increases in serotype 19A had low rates of PCV7 use. One of the reasons for the increased prevalence of 19A in Asian countries could be from widespread use of macrolides in clinical practice and clonal spread of macrolide-resistant strains. Among serotype 19A in the ANSORP study, 86.0% showed erythromycin resistance.Citation24 This association was observed in a study in southern Israel before the introduction of PCV7 that found increased rate of penicillin and erythromycin-nonsusceptible serotype 6A and 19A isolated in children with otitis media which was temporally associated with increased use of azithromycin and oral cephalosporins.Citation25
The clear benefits of PCV have led to the increased inclusion of PCV into the national immunization programs of many countries. In Thailand, PCV coverage has been low and the vaccine is not currently included in the Expanded Program on Immunization (EPI). However, it is anticipating larger scale access in both children and adults. The evaluation and monitoring of serotype coverage of new pneumococcal conjugate vaccines and antimicrobial susceptibility of pneumococci causing IPD are important to guide strategies for prevention, treatment, and future vaccine development. In 2005, we initiated a collaborative network of hospital laboratories in Bangkok to collect and share pneumococcal isolates from clinical specimens. The primary objective of the network is to monitor the trend of serotype and antimicrobial susceptibility and previously, we have reported the serotype distribution and antimicrobial susceptibilities of S. pneumoniae during 2000 to 2005Citation26 and 2006 to 2009.Citation27 In the current report, our objectives were (1) to describe the serotype distribution and antimicrobial susceptibility of S. pneumoniae causing IPD in various age groups between 2009 and 2012; (2) to evaluate the serotype coverage of the currently available pneumococcal conjugate vaccines; and (3) to compare findings with the previous publications from this network.
Results
There were 238 pneumococcal isolates studied. An amount of 224 (94.1%) isolates was from heath care centers in Bangkok. Specimens were blood (211, 88.7%), CSF (8, 3.4%), pleural fluid (6, 2.5%), abdominal fluid (1, 0.4%), joint fluid (1, 0.4%), pus from brain tissue (1, 0.4%), aortic wall tissue (1, 0.4%), and uterine cavity swab (1, 0.4%). Eight (3.4%) patients had S. pneumoniae isolated from more than one site; 6 blood and CSF, 1 blood and pleural fluid, 1 blood and ascitic fluid. Patients’ age ranged from 1 d to 89 y and the distribution of ages was ≤5, 6–49, 50–64, ≥65 y in 82 (34.5%), 66 (27.7%), 28 (11.8%), and 60 (25.2%) specimens, respectively. The age of 2 (0.8%) subjects was unknown and they were excluded from the age-specific analysis. Of the 93 patients with available clinical data, 69 (74.2%) had comorbidities. Fourteen (36.8%) of 38 children ≤5 y of age with IPD had comorbidities while 45 (81.8%) of 55 patients >5 y had underlying medical conditions (P < 0.001). Eight (9.9%) of 81 patients with available vaccination data received pneumococcal vaccines at least 2 wk prior to the onset of IPD; 7 children received PCV and 1 adult received 23-valent polysaccharide vaccine. Of the 7 children with breakthrough IPD, only 1 child (a 32 mo-old healthy infant who received 4 doses of PCV7) had PCV failure and developed vaccine-type IPD, serotype 19F. An adult with breakthrough serotype 6B IPD was 63 y old with underlying Waldenstrom's macroglobulinemia. Of the 77 patients with known clinical outcome, 10 died resulting in a case-fatality rate of 13.0% among those with IPD; 2 of 32 (6.3%) in children ≤5 y and 8 of 45 (17.8%) in older children and adults.
The serotype distribution and coverage by pneumococcal conjugate vaccine
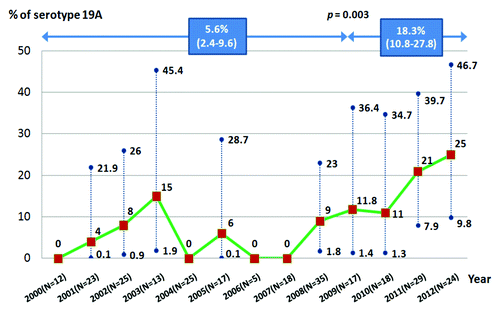
The serotype distribution of pneumococcal isolates causing IPD across the different age groups is shown in . The 5 most common vaccine serotypes were 6B (13.9%), 19A (12.6%), 14 (8.0%), 18C (5.9%), and 6A (3.8%). Non-PCV15 serotypes were found in 39.9% of all isolates. Serotype 19A was found in 18.3%, 10.6%, 3.6%, and 11.7% among patients age ≤5 y, 6–49 y, 50–64 y, and ≥65 y, respectively. In comparison with our previous reports between 2000 and early 2009, the prevalence of serotype 19A isolated from children ≤5 y significantly increased between 2009 and 2012 (5.6% vs 18.3%, P = 0.003), . Serotype 19A accounted up to 25.0% of IPD among children ≤5 y in 2012. Furthermore, we found an increase of NVT (14.5% vs 26.8%, P = 0.017) and a decrease of serotype 23F (20.7% vs 1.2%, P < 0.001).
Figure 1. The serotype distribution of pneumococcal isolates causing IPD in various age groups, central Thailand, 2009–2012 (n = 238).
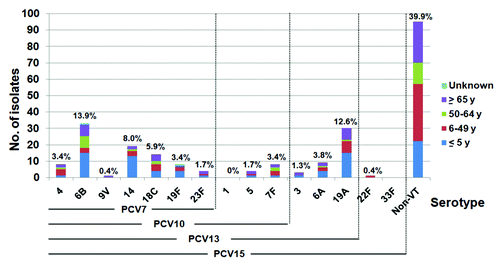
Figure 2. Prevalence of serotype 19A in children ≤5 y with IPD, Thailand, 2000–2012 (n = 261). *Present in percentage with 95% CI; The isolates included in the studies were from prospective collection from the year 2005 and the stored isolates before 2005.
The serotype coverage of PCVs in each age group is shown in . PCV7 and PCV10 provided similar coverage rates (less than 50.0%) in all age groups. PCV13 provided higher coverage rate, similar to that of PCV15 in all age groups, and the coverage rate was highest in the group of children younger than 5 y (73.2%). In comparison with those reported by our network in 2000–2005Citation26 and 2006–2009,Citation27 serotype coverage reduced by 37% and 34% for PCV7 in children less than 5 y (from 73.9% and 70.3% to 46.4%).
Table 1. Serotype coverage by 7-, 10-, 13-, and 15-valent pneumococcal conjugate vaccine in various age groups (n = 236a)
Antimicrobial susceptibility of S. pneumoniae
Antimicrobial susceptibility of S. pneumoniae isolates causing IPD is shown in . The high rates of overall susceptibilities were observed for penicillin (89.7%; 91.8% for non-meningeal isolates, and 50.0% for meningeal isolates), cefotaxime (95.7%; 96.4% for non-meningeal isolates, and 85.7% for meningeal isolates), ofloxacin (97.9%), and linezolid (97.9%); and lower rates of susceptibilities were observed for cefdinir (50.0%), macrolides (<50%), clindamycin (67.7%), tetracycline (41.4%), and TMP-SMX (32.4%). All isolates were susceptible to levofloxacin and vancomycin. Antimicrobial susceptibility for cefditoren was 90.2% using the recommended breakpoints established by the Spanish Regulatory Agency (http://www.aemps.es) for Europe, and 45.1% using the breakpoints established by US Food and Drug Administration (US FDA) (http://www.fda.gov). The MIC50 and MIC90 were 0.25 and 2 µg/mL for penicillin; and 0.125 and 1 µg/mL for cefotaxime, respectively.
Table 2. Antimicrobial susceptibility of S. pneumoniae isolates causing IPD, 2009–2012
Among the isolates of serotype 19A, the susceptibility rates were lower for penicillin (80.0 vs 91.2%, P = 0.046) and tetracycline (9.1 vs 45.5%, P = 0.024) in comparison to non-19A serotypes. In children ≤5 y, serotype 19A was significantly less susceptible to cefditoren than other serotypes (66.7 vs 95.5%, P = 0.004) ().
Table 3. Comparison of antimicrobial susceptibility of S. pneumoniae serotype 19A vs non-19A causing IPD, 2009–2012
Discussion
This report describes the changes in serotype distribution of pneumococcal isolates causing IPD in central Thailand. We found an increase in serotype 19A, especially in children ≤5 y after 2009, in spite of the low rate of PCV use in the country. We also found a decrease in serotype coverage rates for PCV7 and PCV10, which probably was an effect of increased 19A serotype and NVT, while for PCV13 the most recent coverage rate was 73.2% in children ≤5 y and 58.3% in elderly >65 y. From 2005 to 2012, the antimicrobial susceptibility rates to penicillin and cefotaxime of IPD isolates in our network were unchanged, except for the lower susceptibility rates for penicillin of meningeal isolates and for macrolides, which are alarming.
The increased incidence of serotype 19A IPD has been documented in several countries after a widespread use of PCV7.Citation1-Citation6,Citation8 The data from Active Bacterial Core surveillance (ABCs) in 2007 identified a total of 493 children aged <5 y with IPD after the successful PCV7 implementation in 2000. Among the 427 IPD cases with known serotype, 64% were caused by 6 additional serotypes contained in PCV13. Of these, 42% were caused by serotype 19A.Citation28 We found a similarly increase of serotype 19A in children <5 y of age (5.6% between 2000 and 2009 to 18.3% between 2009 and 2012), which continues to increase (25.0% in 2012) in spite of the low use of PCV among Thai children. PCV7 was introduced in Thailand in 2007, followed by PCV10 and PCV13 in 2011. However, none of these vaccines are included in the Expanded Program on Immunization. Data regarding PCV sales in Bangkok indicate PCV uptake of 14.0% in 2009 and 25.9% in 2012 for infants born in Bangkok. This finding was similar to reports from KoreaCitation12 and ChinaCitation29 that found an increase of 19A before the introduction of PCV7 in the routine childhood immunization program. A study from SingaporeCitation10 also showed an increase of serotype 19A among children aged <5 y with IPD before PCV7 was available, and this became more pronounced following the introduction of PCV7 (26.7% in 2010) These data suggested that other factors were more important than the effect of local use of PCV7 to influence changes in serotypes.Citation30
On the other hand, a study in Thailand of 467 invasive pneumococcal isolates from 60 hospitals countrywide (Thailand Invasive Bacterial Infection Surveillance; Thai IBIS) in 2010 to 2012 demonstrated that the most common pneumococcal serotypes were 3 (10.1%), 6B (9.0%), 23F (7.7%), and 19A (7.1%); and non-vaccine serotype were found in 16.9%.Citation31 The difference in serotype distribution between Bangkok reported in this study and outer provinces reported by Thai IBIS requires further monitoring.
We found that most non-PCV7 serotypes were 19A and 6A, making the PCV13 coverage rate notably higher than PCV10. The 2 additional serotypes of PCV15 (22F and 33F) were rare and resulted in no better coverage of PCV15 over PCV13. However, serotype 22F and 33F were responsible for a significant proportion of IPD in some countries. In England and Wales the increase in non-PCV7 serotypes following the introduction of PCV7 in 2006 were mainly due to serotypes 1, 3, 7F, 19A, and the 2 additional serotypes of PCV15, 22F, and 33F were found in 4.0% and 2.2%, respectively.Citation32 A study of IPD in children <18 y in the US found that serotype 22F accounted for 6.0% of all cases. Almost 22% of 22F isolates were from patients with meningitis compared with 12% of all other isolates (P < 0.001).Citation33 The variations in serotype distribution between geographical areas underscore the need of local epidemiological data to guide prevention strategies, vaccine policy, and new vaccine development.
Due to the emergence of serotype 19A in children younger than 5 y of age, the coverage rate of PCV7 in our network declined from 70% before 2009Citation26,Citation27 to 46% after 2009. The PCV13 serotype coverage rate was highest in the youngest age group, up to the same range of that for PCV7 in the earlier period. These data were similar to a study from Singapore. Thoon et al.Citation10 demonstrated that coverage by PCV7 progressively declined from 78.6% in 2005–2007 to 64.4% in 2008–2010 for children aged <5 y. It is important to follow-up the changes of this vaccine coverage rate over the time. The newer PCVs with broader serotype coverage may be needed in the future.
In 2011, the US and Thai FDA approved the use of PCV13 to prevent IPD in adults aged ≥50 y.Citation34 Data from our collaborative network of the 157 pneumococcal isolates from sterile sites among adults and elderly age ≥50 y during January 2005 to September 2012 demonstrated that the overall serotype coverage by PCV13 was 58%. PCV13 covered 50%, 56%, 59%, and 68% of the invasive isolates from patients aged 50–59, 60–69, 70–79, and ≥80 y, respectively.Citation35
Antimicrobial resistance among S. pneumoniae is a global problem. The ANSORP study of pneumococcal isolates in 11 Asian countries in 2008 and 2009 showed high prevalence of erythromycin resistance (72.7%); with highest rate in China (96.4%), Taiwan (84.9%), and Vietnam (80.7%). Erythromycin resistance of pneumococcal isolates in our study during 2009 to 2012 was 51.3%, a little higher than that reported from Thailand cited in the ANSORP study which was 44.3%. Our findings confirmed that erythromycin resistance among pneumococci is prevalent in the region. Studies from other regions also found high rates of erythromycin resistance from 25–62%.Citation36-Citation38 On the other hand, we found high susceptibilities to penicillin in non-meningeal isolates (91.8%) and fluoroquinolones (97.9–100.0%) but low susceptibility to penicillin in meningeal isolates (50.0%). The similar results was reported by the ANSORP study in 2008–2009 that found low resistance rates to penicillin among pneumococcal isolates caused non-meningeal IPD (3.9%) but high resistance (57.5%) among meningeal isolates.Citation24 Therefore, penicillin should not be used as the empirical treatment in patients who may have meningeal involvement in this region. Resistance to fluoroquinolones was also low, 1.7%, 0.4%, 1.5%, and 13.4% for levofloxacin, moxifloxacin, gatifloxacin, and ciprofloxacin, respectively.Citation24 The high susceptibility rate to penicillin in nonmeningitis cases was also reported in the US, 94.0% and 92.0% in 2008–2009, and 2010–2011, respectively.Citation21 There were variations of susceptibility to cephalosporins among pneumococcal isolates in our study. Cefotaxime and cefditoren (using Spanish MIC breakpoints) provided good in vitro activities (>90.0% susceptibility) while susceptibility to cefdinir was only 50.0%. These results were similar to a report of in vitro activities of cefditoren and 14 other comparator agents against major respiratory tract pathogens from 11 Asian countries. The majority of S. pneumoniae isolates (98.8%) were susceptible to cefditoren, with the MIC50 and MIC90 values ≤0.06 µg/mL and 1 µg/mL, respectively. Ceftriaxone resistance rate was also low at 1.0% while resistance rates of other oral cephalosporins including cefixime, cefprozil, and cedinir were high: 49.3%, 35.7%, and 41.2%, respectively.Citation39 There is no breakpoint for ceditoren defined by Clinical and Laboratory Standards Institute (CLSI). The pharmacokinetic and pharmacodynamics data suggested that the nonsusceptibility breakpoint of cefditoren should be within the range of ≤0.12 to ≤0.5 mg/L.Citation40 The US-FDA approved the breakpoints in the more conservative side. However, considering the favorable pharmacokinetic properties and the lowest MIC90 of cefditoren among β-lactams,Citation41-Citation43 the higher breakpoint was approved by the Spanish Agency. We found a notable discordant susceptibility to cefditoren when applying the US-FDA and Spanish breakpoints. More studies of ceditoren for the treatment of pneumococcal infections are needed to determine the clinical correlation with these breakpoints. Of note, cefotaxime resistance in meningeal isolates was up to 14.3% in our study, vancomycin should be included in the empirical regimen for bacterial meningitis in children in Thailand.
We found serotype 19A was associated with antimicrobial resistance. There were significantly higher rates of resistance to penicillin, cefditoren, and tetracycline among serotype 19A compared with non-19A isolates. The Active Bacterial Core surveillance system in the US reported an increase in invasive pneumococcal disease caused by serotype 19A penicillin-resistant strains from 6.7% in 1998 to 35% in 2005 (P < 0.001).Citation44 Richter et al. also reported an increase in the penicillin-resistance of non-vaccine serotypes, 19A (1.5% to 35.4%) and 35B (1.2% to 12.5%), in US from 1999–2000 to 2004–2005.Citation45 An alarming resistance rate was reported in Taiwan from 2006 to 2010, a study found 70% and 22.5% of serotype 19A isolates resistant to penicillin, ceftriaxone, azithromycin and clindamycin, using meningitis and non-meningitis criteria.Citation46
There were some limitations to our study. As the vaccine uptake rate is low in Bangkok, we were not able to show the real impact of the PCVs. We did not collect the clinical data and we applied meningitis criteria only for isolates from CSF, which may lead to an underestimation of penicillin and cefotaxime resistance in cases of meningitis isolates from blood. Although an increase in the proportion of 19A serotypes was observed, the annual numbers of the isolates in the children ≤5 y were small. Natural fluctuations can appear large if the population studied is relatively small or is limited to one area. Furthermore, there was high proportion of non-PCV15 serotypes that we did not further type so we were unable to detect any non-vaccine emergence serotype. Only one-third of subjects had available clinical data therefore factors associated with serotype changes and mortality could not be assessed. The strengths of this study are that we are able to show the trend of serotype distribution and antimicrobial susceptibilities and up to date information. We also evaluated the coverage rate of the new PCV, PCV15 that might be available in the near future.
In conclusion, we demonstrated changes in serotype distribution of pneumococcal isolates causing IPD in central Thailand. There was an increase in serotype 19A,especially among children ≤5 y, which was significantly associated with higher rates of antimicrobial resistance. PCV13 provided notably higher serotype coverage than PCV10 for both children and the elderly. Susceptibilities to penicillin, cefotaxime, cefditoren (by Spanish breakpoints), linezolid, vancomycin and fluoroquinolones remained high, whereas susceptibilities to macrolides, cefinir, cefditoren (by USFDA breakpoints), and TMP-SMX were low. The results of this study emphasize the need for continued surveillance to monitor the emergence of new serotypes as well as antibiotic susceptibilities in order to provide guidance for future vaccine development, strategies of prevention and treatment.
Materials and Methods
Pneumococci from normally sterile sites from patients followed at hospitals within the “Pneumococcal Laboratory Network” from March 1, 2009, to August 31, 2012, were tested for serotype and antimicrobial susceptibility. This network was established in 2005 in order to share S. pneumoniae isolated from clinical specimens in 4 tertiary care centers in Bangkok, Thailand (Siriraj Hospital, Queen Sirikit National Institute of Child Health, Bhumibol Adulyadej Hospital, and King Chulalongkorn Memorial Hospital). In addition, there were 13 other alliance hospitals (7 private and 6 public hospitals) that regularly submitted clinical pneumococcal isolates to the network. The network also accepted specimens from any government and private hospitals in Bangkok and other provinces. There has not been any change in practices in our surveillance system over time.
The isolates were confirmed to be S. pneumoniae by optochin and bile solubility tests. Only one isolate from each patient was studied. Each isolate was kept at –70 °C in 5% trypticase soy broth plus 20% (v/v) glycerol until use.Citation47
The serotyping was performed using the Quellung test (State Serum Institute, Denmark) for 15 vaccine serotypes (serotypes 1, 3, 4, 5, 7F, 6A, 6B, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F). Isolates that were not 1 of the 15 serotypes were labeled as non-vaccine type (NVT), and were not further serotyped.
Antimicrobial susceptibilities to erythromycin, azithromycin, clarithromycin, clindamycin, tetracycline, trimethoprim- sulfamethoxazole (TMP-SMX), ofloxacin, levofloxacin, linezolid, and vancomycin were performed by disk diffusion method as per CLSI 2013 guidelines.Citation48 The S. pneumoniae ATCC 49619 was used as the control. Minimum inhibitory concentrations (MICs) were determined using standard broth microdilution method following the CLSI guidelines. Cation-adjusted Muleller-Hinton broth supplemented with 3% lysed horse blood was used as medium to determine the MICs of antibiotics against S. pneumoniae. Susceptibility interpretations were based on CLSI clinical breakpointsCitation48 where available. There are 2 recommended breakpoints for cefditoren, one from the US Food and Drug Administration (US FDA) (http://www.fda.gov) and another by the Spanish Regulatory Agency (http://www.aemps.es) for Europe. FDA defines susceptibility for S. pneumoniae as MIC ≤ 0.125mg/L and resistance as ≥0.50 mg/L. The Spanish Agency defines these as ≤0.5 mg/L and ≥2.0 mg/L, respectively for Europe. Data are presented using both breakpoint recommendations. We used meningitis criteria for penicillin and cefotaxime susceptibilities if the specimens were cerebrospinal fluid (CSF) or tissue from central nervous system, which were defined as meningeal isolates. Subjects with both blood and CSF had only CSF included in the analysis and we applied meningitis criteria for antimicrobial susceptibilities of penicillin and cefotaxime. For subjects with non-CSF isolates, either isolate was used and non-meningitis criteria was applied, except if the susceptibility of the 2 isolates were different and then the more resistant isolate was chosen. There was no change in the clinical practice of antibiotic use over the report period.
Serotype coverage calculation was performed by using the serotypes within the vaccine without taking into account of the potential serogroup cross-protection. The current prevalence of S. pneumoniae serotype 19A among those ≤5 y was compared with previously published data from our collaborative network in 2000 to February 2009.Citation26,Citation27 Statistical analysis was performed using χ2 and the Fisher exact test. The criteria for vaccine failure were adopted from a previous published study.Citation49
This study was approved by the Institutional Review Boards of the Committee on Ethics, Faculty of Medicine Siriraj Hospital, Mahidol University.
Disclosure of Potential Conflicts of Interest
All of the authors declare no conflicts of interest associated with the study.
Acknowledgments
This work was partly funded by Pfizer Pharmaceuticals through the investigator initiated research (IIR) grant and in part by the Faculty of Medicine Siriraj Hospital, Mahidol University. We thank the following hospitals for supplying pneumococcal isolates: Bangkok Christian, Bangkok, Bangkok Hua-Hin, Bangkok Phuket, Bangpakok 3, Bhummipol Aduljadej, Bumrungrad International, Chaophya, Charoenkrungpracharak, Hua Chiew, King Chulalongkorn Memorial, Ladprao General, Mahachai 2, Nopparat Rajathanee, Paolo Memorial Samutprakarn, Phayathai, Phayathai 2, Phayathai 3, Pranangklao, Praram 9, Queen Sirikit National Institute of Child Heath, Ramathibodi, Samitivej Srinakarin, Samitivej Sukhumvit, Seriruk, Somdej Prapinklao, Songklanagarind, Synphaet General, Thainakarin, Thammasat Chalermprakiat, Theptarin, Thonburi, Vejthani, and Yanhee Hospitals. We thank Miss Suwande Sapcharoen for her hard work on serotyping and susceptibility testing of pneumococcal isolates. We gratefully thank Professor Philip Alfred Brunell for his critical comments for this manuscript.
References
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al, Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32 - 41; http://dx.doi.org/10.1086/648593; PMID: 19947881
- Bettinger JA, Scheifele DW, Kellner JD, Halperin SA, Vaudry W, Law B, Tyrrell G, Canadian Immunization Monitoring Program, Active (IMPACT). The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000-2007. Vaccine 2010; 28:2130 - 6; http://dx.doi.org/10.1016/j.vaccine.2009.12.026; PMID: 20044050
- van der Linden M, Reinert RR, Kern WV, Imöhl M. Epidemiology of serotype 19A isolates from invasive pneumococcal disease in German children. BMC Infect Dis 2013; 13:70; http://dx.doi.org/10.1186/1471-2334-13-70; PMID: 23384407
- Ingels H, Rasmussen J, Andersen PH, Harboe ZB, Glismann S, Konradsen H, Hoffmann S, Valentiner-Branth P, Lambertsen L, Danish Pneumococcal Surveillance Collaboration Group 2009-2010. Impact of pneumococcal vaccination in Denmark during the first 3 years after PCV introduction in the childhood immunization programme. Vaccine 2012; 30:3944 - 50; http://dx.doi.org/10.1016/j.vaccine.2012.03.060; PMID: 22504662
- de Sevilla MF, García-García JJ, Esteva C, Moraga F, Hernández S, Selva L, Coll F, Ciruela P, Planes AM, Codina G, et al. Clinical presentation of invasive pneumococcal disease in Spain in the era of heptavalent conjugate vaccine. Pediatr Infect Dis J 2012; 31:124 - 8; http://dx.doi.org/10.1097/INF.0b013e318241d09e; PMID: 22173137
- Lepoutre A, Varon E, Georges S, Gutmann L, Lévy-Bruhl D. Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001-2006. Euro Surveill 2008; 13:35 pii: 18962; PMID: 18761883
- Thomas MF, Sheppard CL, Guiver M, Slack MP, George RC, Gorton R, Paton JY, Simmister C, Cliff D, Elemraid MA, et al. Emergence of pneumococcal 19A empyema in UK children. Arch Dis Child 2012; 97:1070 - 2; http://dx.doi.org/10.1136/archdischild-2012-301790; PMID: 23076341
- Williams SR, Mernagh PJ, Lee MH, Tan JT. Changing epidemiology of invasive pneumococcal disease in Australian children after introduction of a 7-valent pneumococcal conjugate vaccine. Med J Aust 2011; 194:116 - 20; PMID: 21299484
- Bautista-Márquez A, Richardson V, Ortiz-Orozco O, Luna-Cruz ME, Carnalla-Barajas MN, Echaniz-Avilés G, Bobadilla-del Valle M, Martínez-Medina L, Montalvo-Vázquez AM, de la Re-Montaño N, et al. Prevalence of pneumococcal disease, serotype distribution, and antimicrobial susceptibility in Mexican children younger than 5 years of age. Arch Med Res 2013; 44:142 - 50; http://dx.doi.org/10.1016/j.arcmed.2012.12.005; PMID: 23291380
- Thoon KC, Chong CY, Tee NW. Early impact of pneumococcal conjugate vaccine on invasive pneumococcal disease in Singapore children, 2005 through 2010. Int J Infect Dis 2012; 16:e209 - 15; http://dx.doi.org/10.1016/j.ijid.2011.11.014; PMID: 22281191
- Liao WH, Lin SH, Lai CC, Tan CK, Liao CH, Huang YT, Wang CY, Hsueh PR. Impact of pneumococcal vaccines on invasive pneumococcal disease in Taiwan. Eur J Clin Microbiol Infect Dis 2010; 29:489 - 92; http://dx.doi.org/10.1007/s10096-010-0873-7; PMID: 20108017
- Choi EH, Kim SH, Eun BW, Kim SJ, Kim NH, Lee J, Lee HJ. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis 2008; 14:275 - 81; http://dx.doi.org/10.3201/eid1402.070807; PMID: 18258121
- Steenhoff AP, Shah SS, Ratner AJ, Patil SM, McGowan KL. Emergence of vaccine-related pneumococcal serotypes as a cause of bacteremia. Clin Infect Dis 2006; 42:907 - 14; http://dx.doi.org/10.1086/500941; PMID: 16511752
- Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, Huang SS, Goldstein R, Hanage WP. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J 2007; 26:468 - 72; http://dx.doi.org/10.1097/INF.0b013e31803df9ca; PMID: 17529860
- Farrell DJ, Klugman KP, Pichichero M. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr Infect Dis J 2007; 26:123 - 8; http://dx.doi.org/10.1097/01.inf.0000253059.84602.c3; PMID: 17259873
- Gertz RE Jr., Li Z, Pimenta FC, Jackson D, Juni BA, Lynfield R, Jorgensen JH, Carvalho MdaG, Beall BW, Active Bacterial Core Surveillance Team. Increased penicillin nonsusceptibility of nonvaccine-serotype invasive pneumococci other than serotypes 19A and 6A in post-7-valent conjugate vaccine era. J Infect Dis 2010; 201:770 - 5; http://dx.doi.org/10.1086/650496; PMID: 20178139
- Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsène JP, Lommel P, Dieussaert I, Schuerman L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009; 28:Suppl S66 - 76; http://dx.doi.org/10.1097/INF.0b013e318199f8ef; PMID: 19325449
- Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines 2009; 8:1479 - 500; http://dx.doi.org/10.1586/erv.09.113; PMID: 19863240
- Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children - Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep 2010; 59:258 - 61; PMID: 20224542
- Skinner JM, Indrawati L, Cannon J, Blue J, Winters M, Macnair J, Pujar N, Manger W, Zhang Y, Antonello J, et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine 2011; 29:8870 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.09.078; PMID: 21964055
- Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011(1.). Emerg Infect Dis 2013; 19:1074 - 83; http://dx.doi.org/10.3201/eid1907.121830; PMID: 23763847
- Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis 2013; 57:952 - 62; http://dx.doi.org/10.1093/cid/cit428; PMID: 23804191
- Jauneikaite E, Jefferies JM, Hibberd ML, Clarke SC. Prevalence of Streptococcus pneumoniae serotypes causing invasive and non-invasive disease in South East Asia: a review. Vaccine 2012; 30:3503 - 14; http://dx.doi.org/10.1016/j.vaccine.2012.03.066; PMID: 22475858
- Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, Lu M, So TM, Hsueh PR, Yasin RM, et al, ANSORP Study Group. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother 2012; 56:1418 - 26; http://dx.doi.org/10.1128/AAC.05658-11; PMID: 22232285
- Dagan R, Givon-Lavi N, Leibovitz E, Greenberg D, Porat N. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J Infect Dis 2009; 199:776 - 85; http://dx.doi.org/10.1086/597044; PMID: 19434927
- Phongsamart W, Srifeungfung S, Dejsirilert S, Chatsuwan T, Nunthapisud P, Treerauthaweeraphong V, Rungnobhakhun P, Chokephaibulkit K. Serotype distribution and antimicrobial susceptibility of S. pneumoniae causing invasive disease in Thai children younger than 5 years old, 2000-2005. Vaccine 2007; 25:1275 - 80; http://dx.doi.org/10.1016/j.vaccine.2006.10.001; PMID: 17092618
- Srifeungfung S, Tribuddharat C, Comerungsee S, Chatsuwan T, Treerauthanaweeraphong V, Rungnobhakhun P, Nunthapisud P, Chokephaibulkit K. Serotype coverage of pneumococcal conjugate vaccine and drug susceptibility of Streptococcus pneumoniae isolated from invasive or non-invasive diseases in central Thailand, 2006-2009. Vaccine 2010; 28:3440 - 4; http://dx.doi.org/10.1016/j.vaccine.2010.02.071; PMID: 20199759
- Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine - United States, 2007. MMWR Morb Mortal Wkly Rep 2010; 59:253 - 7; PMID: 20224541
- Liu Y, Wang H, Chen M, Sun Z, Zhao R, Zhang L, Wang H, Zhang H, Wang L, Chu Y, et al. Serotype distribution and antimicrobial resistance patterns of Streptococcus pneumoniae isolated from children in China younger than 5 years. Diagn Microbiol Infect Dis 2008; 61:256 - 63; http://dx.doi.org/10.1016/j.diagmicrobio.2008.02.004; PMID: 18358662
- Reinert R, Jacobs MR, Kaplan SL. Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine 2010; 28:4249 - 59; http://dx.doi.org/10.1016/j.vaccine.2010.04.020; PMID: 20416266
- Dejsirilert S. Thai IBIS 2010-2012: What did we learn? What’s next? Thai-IBIS Plus Workshop, July 31 – August 1, 2012, Cha-am, Phetchaburi, Thailand. (accessed Jul 25, 2013). Available from http://narst.dmsc.moph.go.th/files_nw/IBISWorkshop31July2012/IBISWorkshop31July2012_ThaiIBIS%202010-2012%20by%20Surang%2030Jul2012%20(1).pdf.
- Pichon B, Ladhani SN, Slack MP, Segonds-Pichon A, Andrews NJ, Waight PA, Miller E, George R. Changes in molecular epidemiology of streptococcus pneumoniae causing meningitis following introduction of pneumococcal conjugate vaccination in England and Wales. J Clin Microbiol 2013; 51:820 - 7; http://dx.doi.org/10.1128/JCM.01917-12; PMID: 23269742
- Ampofo K, Pavia AT, Chris S, Hersh AL, Bender JM, Blaschke AJ, Weng HY, Korgenski KE, Daly J, Mason EO, et al. The changing epidemiology of invasive pneumococcal disease at a tertiary children’s hospital through the 7-valent pneumococcal conjugate vaccine era: a case for continuous surveillance. Pediatr Infect Dis J 2012; 31:228 - 34; http://dx.doi.org/10.1097/INF.0b013e31823dcc72; PMID: 22330164
- Centers for Disease Control and Prevention (CDC). Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep 2012; 61:394 - 5; PMID: 22647745
- Srifuengfung S, Phongsamart W, Tribuddharat C, Chatsuwan T, Rungnobhakhun P, Sapcharoen S, Chokephaibulkit K. Serotype distribution and antibiotic susceptibility of invasive Streptococcus pneumoniae isolates in patients aged 50 years or older in Thailand. Hum Vaccin Immunother 2013; 10; (Forthcoming); PMID: 24030588
- Liu Z, Nachamkin I, Edelstein PH, Lautenbach E, Metlay JP. Serotype emergence and genotype distribution among macrolide-resistant invasive Streptococcus pneumoniae isolates in the postconjugate vaccine (PCV-7) era. Antimicrob Agents Chemother 2012; 56:743 - 50; http://dx.doi.org/10.1128/AAC.05122-11; PMID: 22123697
- Gant CM, Rosingh AW, López-Hontangas JL, van der Heijden M, González-Morán F, Bijlsma JJ, Canton E, RedMiva (Network of Microbiological Vigilance of Comunidad Valenciana). Serotype distribution and antimicrobial resistance of invasive pneumococcal disease strains in the Comunidad Valenciana, Spain, during the winter of 2009-2010: low PCV7 coverage and high levofloxacin resistance. Antimicrob Agents Chemother 2012; 56:4988 - 9; http://dx.doi.org/10.1128/AAC.01201-12; PMID: 22751535
- Shibl AM, Memish ZA, Al-Kattan KM. Antibiotic resistance and serotype distribution of invasive pneumococcal diseases before and after introduction of pneumococcal conjugate vaccine in the Kingdom of Saudi Arabia (KSA). Vaccine 2012; 30:Suppl 6 G32 - 6; http://dx.doi.org/10.1016/j.vaccine.2012.07.030; PMID: 23228355
- Lee MY, Ko KS, Oh WS, Park S, Lee JY, Baek JY, Suh JY, Peck KR, Lee NY, Song JH. In vitro activity of cefditoren: antimicrobial efficacy against major respiratory pathogens from Asian countries. Int J Antimicrob Agents 2006; 28:14 - 8; http://dx.doi.org/10.1016/j.ijantimicag.2006.02.014; PMID: 16777383
- Soriano F, Giménez MJ, Aguilar L. Cefditoren in upper and lower community-acquired respiratory tract infections. Drug Des Devel Ther 2011; 5:85 - 94; http://dx.doi.org/10.2147/DDDT.S9499; PMID: 21340042
- Stefani S, Mezzatesta ML, Fadda G, Mattina R, Palù G, Rossano F, Tufano MA, Schito GC, Nicoletti G. Antibacterial activity of cefditoren against major community-acquired respiratory pathogens recently isolated in Italy. J Chemother 2008; 20:561 - 9; http://dx.doi.org/10.1179/joc.2008.20.5.561; PMID: 19028617
- Fritsche TR, Biedenbach DJ, Jones RN. Update of the activity of cefditoren and comparator oral beta-lactam agents tested against community-acquired Streptococcus pneumoniae isolates (USA, 2004-2006). J Chemother 2008; 20:170 - 4; http://dx.doi.org/10.1179/joc.2008.20.2.170; PMID: 18467241
- Cafini F, Aguilar L, Alou L, Giménez MJ, Sevillano D, Torrico M, González N, Granizo JJ, Martín-Herrero JE, Prieto J. Cidal activity of oral third-generation cephalosporins against Streptococcus pneumoniae in relation to cefotaxime intrinsic activity. Eur J Clin Microbiol Infect Dis 2008; 27:679 - 83; http://dx.doi.org/10.1007/s10096-008-0493-7; PMID: 18299905
- Moore MR, Gertz RE Jr., Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, Gershman K, Reingold A, Farley M, Harrison LH, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis 2008; 197:1016 - 27; http://dx.doi.org/10.1086/528996; PMID: 18419539
- Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004-2005. Clin Infect Dis 2009; 48:e23 - 33; http://dx.doi.org/10.1086/595857; PMID: 19115971
- Tsai HY, Chen YH, Liao CH, Lu PL, Huang CH, Lu CT, Chuang YC, Tsao SM, Chen YS, Liu YC, et al. Trends in the antimicrobial susceptibilities and serotypes of Streptococcus pneumoniae: results from the Tigecycline In Vitro Surveillance in Taiwan (TIST) study, 2006-2010. Int J Antimicrob Agents 2013; 42:312 - 6; http://dx.doi.org/10.1016/j.ijantimicag.2013.05.013; PMID: 23849332
- Spellerberg B, Brandt C. Streptococcus. In: Versalovic J, Carroll KC, Funke G, editors. Manual of Clinical Microbiology, 10th ed. Washington, D.C., USA: ASM Press; 2011. p. 331-49.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial and susceptibility testing: 23rd Informational Supplement (M100-S23). Wayne, PA: Clinical and Laboratory Standards Institute; 2013. Available from: http://xa.yimg.com/kq/groups/17358357/1823053181/name/CLSI%202013%20M100-S23.pdf
- Harboe ZB, Valentiner-Branth P, Ingels H, Rasmussen JN, Andersen PH, Bjerre CC, Goldblatt D, Ashton L, Haston M, Konradsen HB, et al, Danish Pneumococcal Surveillance Collaborating Group. Pediatric invasive pneumococcal disease caused by vaccine serotypes following the introduction of conjugate vaccination in Denmark. PLoS One 2013; 8:e51460; http://dx.doi.org/10.1371/journal.pone.0051460; PMID: 23365635
