Abstract
Similar features between the immune system of healthy elderly people and of younger individuals subjected to conditions of chronic immune activation are progressively being observed. This is raising the hypothesis that chronic immune activation may cause the premature aging of the immune system. Here we dissect this theory by comparing changes occurring to B-cells during healthy aging to the ones occurring during chronic immune activation in younger individuals. Moreover, we discuss how these changes may affect or predict response to vaccination in immune compromised individuals.
Introduction
Life expectancy has consistently increased during the last decades with a greater number of healthy aged individuals. In parallel, improved treatment of primary and secondary immune diseases has been achieved over the last years resulting in increased survival of individuals with chronic disease. A similar feature of these 2 populations of somewhat healthy individuals is the decreased ability of the immune system to undergo de novo or recall immune responses and to respond to vaccination. Interestingly, additional common features between the immune system of healthy elderly people and of younger individuals subjected to conditions of chronic immune activation are progressively being observed thus raising the hypothesis that chronic immune activation may cause the premature aging of the immune system. In particular, several defects of the B-cell compartment have been described.
Overview on B-Cell Development and Function
B-cells arise in the bone marrow (BM) from hematopoietic stem cells (HSC). Pro- and pre- B-cell precursors are characterized by the expression of CD10 and interleukin (IL) -7 receptor α. In the BM and in the presence of IL-7, B-cells rearrange the variable (V), diversity (D), and joining (J) regions of the immunoglobulin (Ig), or antibody (Ab), heavy (H) (VDJ), and light (L) (VJ) chain genes respectively, through a process involving RAG-1 and RAG-2 gene products.Citation1 After the Ig gene rearrangement is complete, RAG-1 and 2 gene expression is downregulated and immature B-cells in the BM express CD5, lose the expression of IL-7 receptor α and begin to express IgM as part of a not yet functional B-cell receptor (BCR).Citation1,Citation2 Immature-transitional B-cells are found in peripheral blood. They still express CD10, co-express IgD and IgM and present an activated phenotype with high expression of CD24 and CD38.Citation3-Citation5 These cells home mainly to the spleen where they differentiate into CD27+IgM+ memory B-cells.Citation3 Immature-transitional B-cell frequencies are high in healthy children and slowly decrease with age until stable range levels are reached around 13 y of age.Citation6 Their role, especially in the spleen marginal zone (MZ) is to provide a first line defense against quickly replicating pathogens, such as encapsulated bacteria. Mature B-cells can be found in the periphery as well as in secondary lymphoid organs. They are negative for CD10 and are able to recognize antigens (Ags). In particular, mature naïve B-cells brightly express surface IgD and poorly IgM while mature memory B-cells express CD27 and surface IgG, IgA, or IgE. The pool of resting memory B-cells increases over time upon encounter with Ags through vaccination or natural infection.Citation7,Citation8 Resting mature B-cells, both naïve and memory, are characterized by high expression of CD21 while they downregulate this molecule after activation.Citation9 In the absence of CD21 or Ig measurements, the frequency of CD27- naïve B-cells is reciprocal to the one of CD27+ memory B-cells.Citation10 A summary of the phenotypic, molecular, and functional changes taking place during B-cell development is shown in .
Table 1. Phenotypic, molecular, and functional changes during B-cell development
The ability of mature B-cells to produce highly specific Abs in the germinal center (GC) is dependent on Ig affinity maturation.Citation11 This is a highly regulated process, controlled by the interaction between T-cell membrane CD40 ligand with the CD40 molecule on the surface of activated B-cells and by soluble T-cell cytokines in the GCs. Ligation of these cytokines, in particular of IL-4, IL-10, and IL-21, to cognate receptors on B-cells causes direct downstream activation of STAT6 and NFkB and particularly of activation-induced deaminase (AID) transcription.Citation11 The action of AID introduces double strand breaks in the Ig germline leading to the introduction of point mutations called somatic hyper-mutations (SHMs) in the V region of both naïve and memory B-cells, and to class switch recombination (CSR) in the C region of naïve B-cells. SHMs are meant to modulate the Ab-Ag affinity while CSR to allow the transcription of the selected V region in association with IgG, IgA, or IgE having improved effector functions than IgM.Citation11
Switching from IgM to IgG production and secretion is normally a phenomenon restricted to Ag triggered B-cells. Nonetheless, bacterial CpG and viral double stranded RNA can directly activate B-cells through TLR9 and TLR3 ligation respectively, inducing IgG and IgA CSR.Citation12,Citation13 Most TLRs are localized in intracellular compartments and ligate viral nucleic acid in endosomes; this localization ensures that TLRs will not bind to the host nucleic acid which is not normally accessible in these compartments and it does not trigger TLRs.Citation14 Human B-cells express TLR1, TLR3 and TLR6 through TLR10 and their levels increase upon B-cell activation, particularly those of TLR9 and 10.Citation13,Citation15 Maintenance of serological memory has been shown to be performed by long-lived PCs recirculating between the periphery and the BM and by memory B-cells being continuously restimulated by bystander T-cell cytokines and microbial products triggering TLRs in the absence of specific Ags.Citation16
B-Cells during Healthy Aging
The pure effect of aging on the immune system is not an easy task to assess in humans, rather than in aged mice models, as decline of immune functions may cause several age associated inflammatory pathologies having an impact on the different parameters analyzed.Citation17 In view of this, below we will discuss the immune features of healthy aged individuals i.e., centenarians and those individuals over 65 y of age having a general healthy status.Citation18
The number or total B-cells in peripheral blood declines with age but whether this is due to decreased B-cell generation or extravasation from the BM is not clear. Despite the frequencies of B-cell precursors and immature B-cells seem to be maintained throughout life in the BM,Citation19,Citation20 the observation that the proportion of HSC relatively to BM stromal cells is lower with old age may point out to reduced B-cell generation in aged individuals.Citation21,Citation22 Nonetheless, while no data are available for humans, stromal cell-derived IL-7 levels seem to be reduced in old mice thus suggesting that the aged BM may not provide adequate conditions for proper B-cell development.Citation23 As previously stated, the frequency of immature-transitional B-cells in peripheral blood is physiologically low in aged individuals,Citation6 therefore their role during healthy aging is not easy to assess. Though, one study related increased systemic autoimmunity to impaired IL-10 production by immature-transitional B-cells (identified as CD19+CD24highCD38high) in aged individuals.Citation24 Nonetheless, decreased numbers but not frequencies of spleen IgM+ memory B-cells are observed in elderly as compared with young individuals.Citation25 Decreased frequencies of peripheral IgM+ memory B-cells have instead been observed in the elderly.Citation26 This may relate to weak responses to pneumococcal challenge in elderly subjects.Citation27 Peripheral mature B-cells seem to be the most affected by the aging process both in terms of altered composition and function.Citation28 Discordant data on the distribution of naïve/memory B-cells in the elderly have been reported. A dramatic decrease of CD27+ memory B-cells in parallel to an increase of CD27- naïve B-cells has been described.Citation29 Nonetheless, naïve B-cells did not differ significantly between young and elderly individuals in terms of absolute numbers.Citation29 On the contrary, a reduction of CD27- naïve B-cells, with no reciprocal increase of CD27+ memory B-cells, had previously been reported in older individuals and centenarians.Citation30 A possible explanation for these conflicting data has later been suggested to be the substantial age difference between the cohorts analyzed.Citation31 Different levels of inflammation and immune activation may be observed between elderly individuals and centenarians.Citation32 With this respect, discrimination of naïve and memory B-cells by surface CD27 alone does not in fact take into account different ratios between resting and activated cells (being CD21high or CD21low respectively) as well as different ratios between switched and IgM+ memory B-cells. Moreover, the measurement of resting memory B-cells in the elderly may be biased by another factor, i. e. the accumulation of a CD27-IgD- double negative (DN) B-cell population.Citation31 These expanded B-cells in the elderly have in fact been suggested to be late or exhausted memory B-cells that have lost the expression of CD27.Citation31 Interestingly, B-cell immunophenotyping based on the activation markers CD24 and CD38 also revealed another population of CD24-CD38- B-cells to be increased in elderly individuals.Citation5 These cells produce the pro-inflammatory cytokine TNF-α which might contribute to the inflammatory status often observed in the elderly.Citation5 A summary of the effect of healthy aging on the different B-cell subpopulations has been incorporated in .
As previously stated, the ability of B-cells to produce Ag-specific Abs relies on the upregulation of AID leading to SHM and CSR in the GC. Physiological age-related thymic involution and reduced T-cell generation may affect both the required number of interactions between T- and B-cells and the yield of soluble T-cell cytokines in the GCs.Citation33 As a result of this and of intrinsic B-cell defects, the ability of B-cells to upregulate AID expression and to undergo CSR decreases with age.Citation29 In addition, the Ig repertoire of different B-cell subtypes including DN B-cells, from elderly individuals presents a reduced number of SHMs.Citation34
Signs of Premature B-Cell Senescence are Observed during Human Immune Deficiency Virus (HIV)-1 Infection and in Other Diseases Characterized by Chronic Immune Activation
Among all immune diseases, human immune deficiency virus (HIV)-1 infection has been extensively studied. Intrinsic defects in B-cells have to date been described in chronically HIV-1 infected individuals.Citation35 These include hyperactivation and increased susceptibility to exhaustion and apoptosis in vitro.Citation36,Citation37 Moreover, the B-cell repertoire of infected patients is affected by an increase of dysfunctional immature-transitional B-cells, in conditions of high viremia,Citation38,Citation39 and by memory B-cell depletion which occurs during the first stages of primary infection resulting in loss of serologic memory throughout the later stages of chronic infection.Citation40,Citation41 Initiation of antiretroviral therapy (ART) early during primary infection in adults or within the first year of life in HIV-1 vertically infected children is able to preserve high numbers and function of antigen-specific memory B-cells gained during vaccination.Citation40,Citation42 Nonetheless, B-cell defects may only partially be reversed even upon early ART and suppression of viral replication as patients still experience impaired vaccination responses to influenza and other vaccines.Citation43 Residual chronic immune activation related to microbial products translocation between damaged tissue in the gut and other body compartments has been reported even in treated patients.Citation44
A number of studies describe similar B-cell impairments for HIV-1 infected individuals and healthy elderly people.Citation31,Citation45-Citation47 In particular, altered B-cell subpopulations resembling DN B-cells in the elderlyCitation31 have also been described in HIV-1 infected individuals.Citation47 Moreover, AID expression is also impaired during HIV-1 infection.Citation47 This may be due to suboptimal T-cell cytokine concentration and T-cell help, to B-cell hyperactivation or to a direct effect of HIV-1 viral components on AID transcription.Citation47-Citation49 However, this last hypothesis is less likely to be correct as different viral components were shown to have opposite effects on AID.Citation48,Citation49 Recently, high frequencies of DN B-cells, in parallel to high frequencies of mature activated (MA) B-cells (CD19+CD10-CD21-),Citation9 were confirmed in HIV-1 infected pediatric patients.Citation50 Interestingly, a longer time on ART related with lower DN while an earlier start of ART related with lower frequencies of MA in this population.Citation51 This suggests that different levels of chronic immune activation during HIV-1 infection may actually relate with the appearance of these senescent and activated B-cells. The relation between high frequencies of both DN and MA with immune senescence during vertical HIV-1 infection was confirmed by direct correlation with the frequencies of terminal effector CD4+ T-cells.Citation50 Moreover, shorter lymphocyte telomere length was reported for viremic HIV-1 infected children as compared with healthy controls.Citation52 Interestingly, increased frequencies of both DN and MA were also observed in the blood of kidney transplant pediatric patients.Citation53 In view of this, it is interesting to consider HIV-1 infected and kidney transplant patients as different models of secondary immune deficiency characterized by chronic immune activation. As during HIV-1 infection in fact, patients with solid organ transplantation may also experience chronic immune activation due to the persistence of a foreign antigen (the transplanted organ) and are immune deficient because of the immune suppressive therapies administered after transplantation. Previously, premature senescence of T-cells was observed in long-term survivors of kidney transplantation.Citation54 Increased DN-like B-cells associated with a high disease activity were also observed in the blood of patients affected by systemic lupus erythematous (SLE) but not in patients with chronic hepatitis C virus (HCV) infection.Citation55 During HCV infection, immune activation with high frequencies of MA B-cells has also been observedCitation56 whereas high frequencies of dysfunctional immature transitional B-cells have been observed during both SLECitation4 and HCV infection.Citation56 This may relate with different degrees of inflammation and immune activation in these pathologies. During HIV-1 infection in fact, dysfunctional immature transitional B-cells increase only during the acute phases of the infection and tail off during the chronic phase.Citation4 In view of this, it can be hypothesized that chronic rather than acute inflammation and immune activation, may lead to premature immune senescence with high frequencies of DN B-cells. A summary of the effect of chronic immune activation on the different B-cell subpopulations has also been incorporated in .
Implications of B-Cell Senescence for Vaccination
Loss of protective immunity and impaired vaccination responses are common features between healthy elderly individuals and immune compromised patients. Due to high risk of complications in case of influenza infection, yearly immunization against seasonal influenza is highly recommended for both elderly and immune compromised individuals.Citation57 Therefore, data on vaccination response in these 2 populations is mostly available for influenza. The role of impaired AID expression on the success of such vaccination has previously been pointed out in both healthy elderly individuals and HIV-1 infected patients.Citation46,Citation58 In both these studies, the ability of B-cells to upregulate AID upon in vitro polyclonal stimulation was assessed prior to A(H1N1)pdm09 vaccination and in both cases the distribution of the different levels of AID fold increase, measured in a mixed cohort of elderly and younger individuals in one case and of HIV-1 infected and age-matched healthy individuals in the other, related to the magnitude of antibody response measured 1 mo after vaccination.Citation46,Citation58 Recently and in a similar manner, the frequencies of both DN and MA B-cells measured in a mixed cohort of vertically HIV-1 infected patients and age-matched healthy subjects prior to 2012–2013 seasonal influenza vaccination could predict the strength of humoral response to the single strains contained in the vaccine.Citation50 These data suggest that measuring the frequencies of DN and MA B-cells could be a useful tool for vaccination design, in terms of number of vaccine doses, in both elderly and immune compromised individuals. Concerns exist on whether vaccination against seasonal influenza should be performed with one or multiple vaccine doses in populations at high risk of complications due to influenza infection. A significant increase of polyclonal antibodies with low affinity and increased reactivity to lymphocytes (named anti-lymphocyte antibodies or ALA) was reported after 2012–2013 seasonal influenza vaccination in both HIV-1 and kidney transplant pediatric patients but not in healthy controls and this directly correlated with the frequencies of both DN and MA.Citation53 These additional data suggest that the quality of an immune response triggered by possibly any vaccination in immune compromised individuals may depend on the degree of B-cell senescence and on their activation status.Citation53 Another recent report also related impaired antibody response to influenza vaccination in both HIV-1 infected or uninfected aging women to immune activation and inflammation measured by high levels of plasma IL-21 and of TNFα respectively.Citation59
Conclusions and Future Perspectives
Several evidences indicate that chronic immune activation may cause the premature aging of the immune system and that this can negatively impact and even predict responses to influenza vaccination. However, whether upregulation of AID expression in vitro or the frequencies of MA and DN may be confirmed as predictive biomarkers of vaccination response in immune compromised individuals needs to be tested in large studies including different vaccine types and different models of immune disorders starting from SLE where high DN B-cells have previously been reported.Citation55 The need of such biomarkers is particularly critical for vaccination of severe immune compromised individuals as they may need multiple vaccine doses in order to achieve immune-protection.Citation60 Other populations experiencing suboptimal antibody production during vaccination and chronic immune activation include the large number of patients affected by inflammatory bowel diseases (IBD). Several B-cell disturbances, including B-cell hyperactivation and dysfunctional responses to TLR agonists, have in fact been described in patients affected by IBD.Citation61-Citation63 Whether signs of premature aging of the immune system possibly related to suboptimal antibody production during vaccination exist in these patients has never been investigated.
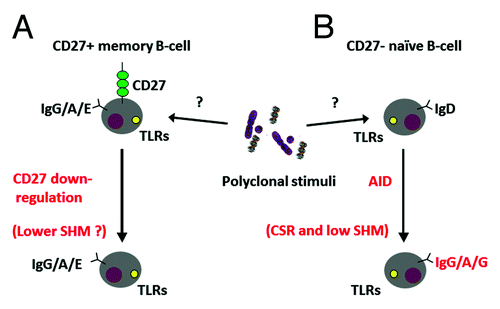
In addition, the nature of DN B-cells must be further investigated. In the elderly, these cells have recently been characterized as having a tissue trafficking phenotype in terms of expression of different chemokine receptors and as being able to produce granzyme B.Citation64 Whether the pro-inflammatory environment observed during chronic immune activation in younger individuals could similarly influence the trafficking phenotype and function of DN B-cells also in this population is not known. Moreover, as previously stated, DN B-cells have been defined as late or exhausted memory B-cells.Citation31 In view of this, a low number of SHM should not be expected in this population as SHMs would accumulate at each cell division in pre-established memory B-cells during specific recall or bystander activation. The possibility of increased circulation of microbial products between body compartments due to damaged and inflamed tissue cannot be excluded nor in immune compromised or in healthy aged individuals.Citation44,Citation65 Increased microbial products would trigger non-specific B-cell activation and potentially cause CD27-IgD+ naïve B-cells to switch into CD27-IgD- B-cells.Citation47 With this respect, it would be interesting to assess the antigenic specificity of DN B-cells in order to verify whether they belong to the memory B-cell pool. These theories on the origin of DN B-cells have been summarized in .
Figure 1. (A) DN B-cells may be late or exhausted memory B-cells which have lost the expression of CD27 carrying a decreased number of SHMs in the Ig V region. However, a low number of SHM should not be expected in this population as SHMs would accumulate at each cell division in pre-established memory B-cells during specific recall or bystander activation. (B) DN B-cells may be naïve B-cells being triggered by polyclonal stimuli due to increased circulation of microbial products between body compartments due to damaged and inflamed tissue. In this scenario, CD27-IgD+ naïve B-cells would switch to CD27-IgD- B-cells.
References
- Sagaert X, De Wolf-Peeters C. Classification of B-cells according to their differentiation status, their micro-anatomical localisation and their developmental lineage. Immunol Lett 2003; 90:179 - 86; http://dx.doi.org/10.1016/j.imlet.2003.09.007; PMID: 14687723
- Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood 2002; 99:3892 - 904; http://dx.doi.org/10.1182/blood.V99.11.3892; PMID: 12010786
- Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev 2004; 197:179 - 91; http://dx.doi.org/10.1111/j.0105-2896.2004.0109.x; PMID: 14962195
- Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010; 32:129 - 40; http://dx.doi.org/10.1016/j.immuni.2009.11.009; PMID: 20079667
- Buffa S, Pellicano M, Bulati M, Martorana A, Goldeck D, Caruso C, Pawelec G, Colonna-Romano G.. A novel B cell population revealed by a CD38/CD24 gating strategy: CD38(-)CD24 (-) B cells in centenarian offspring and elderly people. Age (Dordr). 2013; 35:5 2009 - 24
- Kyriakides TC, Guarino P. Timing of antiretroviral treatment initiation. JAMA 2001; 285:1702 - 3; http://dx.doi.org/10.1001/jama.285.13.1702; PMID: 11277817
- Piątosa B, Wolska-Kuśnierz B, Pac M, Siewiera K, Gałkowska E, Bernatowska E. B cell subsets in healthy children: reference values for evaluation of B cell maturation process in peripheral blood. Cytometry B Clin Cytom 2010; 78(:6 ):372 - 81; http://dx.doi.org/10.1002/cyto.b.20536; PMID: 20533385
- Luning Prak ET, Ross J, Sutter J, Sullivan KE. Age-related trends in pediatric B-cell subsets. Pediatr Dev Pathol 2011; 14:45 - 52; http://dx.doi.org/10.2350/10-01-0785-OA.1; PMID: 20658930
- Moir S, Malaspina A, Ho J, Wang W, Dipoto AC, O’Shea MA, Roby G, Mican JM, Kottilil S, Chun TW, et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis 2008; 197:572 - 9; http://dx.doi.org/10.1086/526789; PMID: 18240953
- Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today 2000; 21:204 - 6; http://dx.doi.org/10.1016/S0167-5699(00)01605-4; PMID: 10782048
- Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol 2008; 26:261 - 92; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090248; PMID: 18370922
- He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol 2004; 173:4479 - 91; PMID: 15383579
- Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, He B, Chen K, Cerutti A. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol 2008; 181:276 - 87; PMID: 18566393
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004; 5:987 - 95; http://dx.doi.org/10.1038/ni1112; PMID: 15454922
- Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood 2003; 102:956 - 63; http://dx.doi.org/10.1182/blood-2002-11-3355; PMID: 12689944
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002; 298:5601 2199 - 202
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured?. Nutr Rev 2007; 65:S173 - 6; http://dx.doi.org/10.1301/nr.2007.dec.S173-S176; PMID: 18240544
- Caruso C, Buffa S, Candore G, Colonna-Romano G, Dunn-Walters D, Kipling D, Pawelec G. Mechanisms of immunosenescence. Immun Ageing 2009; 6:10; http://dx.doi.org/10.1186/1742-4933-6-10; PMID: 19624841
- Rossi MI, Yokota T, Medina KL, Garrett KP, Comp PC, Schipul AH Jr., Kincade PW. B lymphopoiesis is active throughout human life, but there are developmental age-related changes. Blood 2003; 101:576 - 84; http://dx.doi.org/10.1182/blood-2002-03-0896; PMID: 12393702
- Nuñez C, Nishimoto N, Gartland GL, Billips LG, Burrows PD, Kubagawa H, Cooper MD. B cells are generated throughout life in humans. J Immunol 1996; 156:866 - 72; PMID: 8543844
- Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev 2000; 117:57 - 68; http://dx.doi.org/10.1016/S0047-6374(00)00137-8; PMID: 10958923
- Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res 2001; 19:117 - 25; http://dx.doi.org/10.1016/S0736-0266(00)00010-3; PMID: 11332607
- Stephan RP, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood 1998; 91:75 - 88; PMID: 9414271
- Duggal NA, Upton J, Phillips AC, Sapey E, Lord JM. An age-related numerical and functional deficit in CD19(+) CD24(hi) CD38(hi) B cells is associated with an increase in systemic autoimmunity. Aging Cell 2013; 12:873 - 81; http://dx.doi.org/10.1111/acel.12114; PMID: 23755918
- Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev 2011; 10:330 - 5; http://dx.doi.org/10.1016/j.arr.2010.08.004; PMID: 20728581
- Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol 2005; 175:3262 - 7; PMID: 16116217
- Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med 2003; 197:939 - 45; http://dx.doi.org/10.1084/jem.20022020; PMID: 12682112
- Ademokun A, Wu YC, Dunn-Walters D. The ageing B cell population: composition and function. Biogerontology 2010; 11:125 - 37; http://dx.doi.org/10.1007/s10522-009-9256-9; PMID: 19937382
- Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, Blomberg BB. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol 2008; 180:5283 - 90; PMID: 18390709
- Colonna-Romano G, Bulati M, Aquino A, Scialabba G, Candore G, Lio D, Motta M, Malaguarnera M, Caruso C. B cells in the aged: CD27, CD5, and CD40 expression. Mech Ageing Dev 2003; 124:389 - 93; http://dx.doi.org/10.1016/S0047-6374(03)00013-7; PMID: 12714244
- Colonna-Romano G, Bulati M, Aquino A, Pellicanò M, Vitello S, Lio D, Candore G, Caruso C. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev 2009; 130:681 - 90; http://dx.doi.org/10.1016/j.mad.2009.08.003; PMID: 19698733
- Candore G, Caruso C, Colonna-Romano G. Inflammation, genetic background and longevity. Biogerontology 2010; 11:565 - 73; http://dx.doi.org/10.1007/s10522-010-9286-3; PMID: 20549353
- Ginaldi L, Loreto MF, Corsi MP, Modesti M, De Martinis M. Immunosenescence and infectious diseases. Microbes Infect 2001; 3:851 - 7; http://dx.doi.org/10.1016/S1286-4579(01)01443-5; PMID: 11580980
- Buffa S, Bulati M, Pellicanò M, Dunn-Walters DK, Wu YC, Candore G, Vitello S, Caruso C, Colonna-Romano G. B cell immunosenescence: different features of naive and memory B cells in elderly. Biogerontology 2011; 12:473 - 83; http://dx.doi.org/10.1007/s10522-011-9353-4; PMID: 21879287
- Cagigi A, Nilsson A, De Milito A, Chiodi F. B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design. Vaccine 2008; 26:3016 - 25; http://dx.doi.org/10.1016/j.vaccine.2007.11.063; PMID: 18164520
- Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, Liu S, Adelsberger J, Lapointe R, Hwu P, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci U S A 2001; 98:10362 - 7; http://dx.doi.org/10.1073/pnas.181347898; PMID: 11504927
- Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, Krishnan SR, Planta MA, Turney JF, Justement JS, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med 2004; 200:587 - 99; http://dx.doi.org/10.1084/jem.20032236
- Malaspina A, Moir S, Ho J, Wang W, Howell ML, O’Shea MA, Roby GA, Rehm CA, Mican JM, Chun TW, et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci U S A 2006; 103:2262 - 7; http://dx.doi.org/10.1073/pnas.0511094103; PMID: 16461915
- Cagigi A, Palma P, Nilsson A, Di Cesare S, Pensieroso S, Kakoulidou M, Bernardi S, Rossi P, Chiodi F. The impact of active HIV-1 replication on the physiological age-related decline of immature-transitional B-cells in HIV-1 infected children. AIDS 2010; 24:2075 - 80; http://dx.doi.org/10.1097/QAD.0b013e32833c3298; PMID: 20588160
- Titanji K, Chiodi F, Bellocco R, Schepis D, Osorio L, Tassandin C, Tambussi G, Grutzmeier S, Lopalco L, De Milito A. Primary HIV-1 infection sets the stage for important B lymphocyte dysfunctions. AIDS 2005; 19:1947 - 55; http://dx.doi.org/10.1097/01.aids.0000191231.54170.89; PMID: 16260900
- Titanji K, De Milito A, Cagigi A, Thorstensson R, Grützmeier S, Atlas A, Hejdeman B, Kroon FP, Lopalco L, Nilsson A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 2006; 108:1580 - 7; http://dx.doi.org/10.1182/blood-2005-11-013383; PMID: 16645169
- Pensieroso S, Cagigi A, Palma P, Nilsson A, Capponi C, Freda E, Bernardi S, Thorstensson R, Chiodi F, Rossi P. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci U S A 2009; 106:7939 - 44; http://dx.doi.org/10.1073/pnas.0901702106; PMID: 19416836
- Cagigi A, Nilsson A, Pensieroso S, Chiodi F. Dysfunctional B-cell responses during HIV-1 infection: implication for influenza vaccination and highly active antiretroviral therapy. Lancet Infect Dis 2010; 10:499 - 503; http://dx.doi.org/10.1016/S1473-3099(10)70117-1; PMID: 20610332
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365 - 71; http://dx.doi.org/10.1038/nm1511; PMID: 17115046
- Khurana S, Frasca D, Blomberg B, Golding H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PLoS Pathog 2012; 8:e1002920; http://dx.doi.org/10.1371/journal.ppat.1002920; PMID: 23028320
- Cagigi A, Pensieroso S, Ruffin N, Sammicheli S, Thorstensson R, Pan-Hammarström Q, Hejdeman B, Nilsson A, Chiodi F. Relation of activation-induced deaminase (AID) expression with antibody response to A(H1N1)pdm09 vaccination in HIV-1 infected patients. Vaccine 2013; 31:2231 - 7; http://dx.doi.org/10.1016/j.vaccine.2013.03.002; PMID: 23499520
- Cagigi A, Du L, Dang LV, Grutzmeier S, Atlas A, Chiodi F, Pan-Hammarström Q, Nilsson A. CD27(-) B-cells produce class switched and somatically hyper-mutated antibodies during chronic HIV-1 infection. PLoS One 2009; 4:e5427; http://dx.doi.org/10.1371/journal.pone.0005427; PMID: 19412542
- Qiao X, He B, Chiu A, Knowles DM, Chadburn A, Cerutti A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol 2006; 7:302 - 10; http://dx.doi.org/10.1038/ni1302; PMID: 16429138
- He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol 2006; 176:3931 - 41; PMID: 16547227
- Cagigi A, Rinaldi S, Di Martino A, Manno EC, Zangari P, Aquilani A, Cotugno N, Nicolosi L, Villani A, Bernardi S, et al. Premature immune senescence during HIV-1 vertical infection relates with response to influenza vaccination. J Allergy Clin Immunol 2013; PMID: 24290278
- Cagigi A, Rinaldi S, Cotugno N, Manno EC, Santilli V, Mora N, Zangari P, Aquilani A, Kuekou HT, Giaquinto C, et al. Early Highly-Active Antiretroviral Therapy Enhances B-Cell Longevity: A 5 Year Follow-Up. Pediatr Infect Dis J 2013; In press PMID: 24378939
- Côté HC, Soudeyns H, Thorne A, Alimenti A, Lamarre V, Maan EJ, Sattha B, Singer J, Lapointe N, Money DM, et al, CIHR Emerging Team in HIV therapy, aging (CARMA). Leukocyte telomere length in HIV-infected and HIV-exposed uninfected children: shorter telomeres with uncontrolled HIV viremia. PLoS One 2012; 7:e39266; http://dx.doi.org/10.1371/journal.pone.0039266; PMID: 22815702
- Cagigi A, Rinaldi S, Santilli V, Mora N, C Manno E, Cotugno N, Zangari P, Aquilani A, Guzzo I, Dello Strologo L, et al. Premature ageing of the immune system relates to increased anti-lymphocyte antibodies (ALA) after an immunization in HIV-1-infected and kidney-transplanted patients. Clin Exp Immunol 2013; 174:274 - 80; PMID: 23841754
- Li P, Tian C, Ge N, Wang H, Liu L, Lou F, Björkholm M, Xu D. Premature senescence of T cells in long-term survivors of renal transplantation. Biochem Biophys Res Commun 2011; 407:599 - 604; http://dx.doi.org/10.1016/j.bbrc.2011.03.079; PMID: 21426898
- Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner EC, Sanz I. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol 2007; 178:6624 - 33; PMID: 17475894
- Sugalski JM, Rodriguez B, Moir S, Anthony DD. Peripheral blood B cell subset skewing is associated with altered cell cycling and intrinsic resistance to apoptosis and reflects a state of immune activation in chronic hepatitis C virus infection. J Immunol 2010; 185:3019 - 27; http://dx.doi.org/10.4049/jimmunol.1000879; PMID: 20656924
- Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices--United States, 2013-2014. MMWR Recomm Rep 2013; 62:RR-07 1 - 43; PMID: 24048214
- Frasca D, Diaz A, Romero M, Landin AM, Phillips M, Lechner SC, Ryan JG, Blomberg BB. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine 2010; 28:8077 - 84; http://dx.doi.org/10.1016/j.vaccine.2010.10.023; PMID: 20974306
- Parmigiani A, Alcaide ML, Freguja R, Pallikkuth S, Frasca D, Fischl MA, Pahwa S. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS One 2013; 8:e79816; http://dx.doi.org/10.1371/journal.pone.0079816; PMID: 24236161
- Cagigi A, Cotugno N, Giaquinto C, Nicolosi L, Bernardi S, Rossi P, Douagi I, Palma P. Immune reconstitution and vaccination outcome in HIV-1 infected children: present knowledge and future directions. Hum Vaccin Immunother 2012; 8(:12 ):1784 - 94; http://dx.doi.org/10.4161/hv.21827; PMID: 22906931
- Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, Zhang Y, Nikolajczyk BS, Farraye FA, Ganley-Leal LM. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol 2009; 86:1007 - 16; http://dx.doi.org/10.1189/jlb.0309203; PMID: 19589946
- Berkowitz D, Peri R, Lavy A, Kessel A. Increased Toll-like receptor 9 expression by B cells from inflammatory bowel disease patients. Hum Immunol 2013; 74:1519 - 23; http://dx.doi.org/10.1016/j.humimm.2013.08.285; PMID: 24007656
- Frasca D, Andrisani G, Diaz A, Felice C, Guidi L, Blomberg BB. AID in aging and autoimmune diseases. Autoimmunity 2013; 46:168 - 75; http://dx.doi.org/10.3109/08916934.2012.750300; PMID: 23190037
- Bulati M, Buffa S, Martorana A, Candore G, Lio D, Caruso C, Colonna-Romano G. Trafficking phenotype and production of granzyme B by double negative B cells (IgG(+)IgD(-)CD27(-)) in the elderly. Exp Gerontol 2014; 54:123 - 129; http://dx.doi.org/10.1016/j.exger.2013.12.011; PMID: 24389059
- Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013; 13:875 - 87
