Abstract
Food allergies are increasingly common disorders and no therapeutic strategies are yet approved. The unlipidated Omp16 (U-Omp16) is the outer membrane protein of 16 kDa from B. abortus and possesses a mucosal adjuvant property. In this study, we aimed to examine the U-Omp16 capacity to abrogate an allergen-specific Th2 immune response when it is administered as an oral adjuvant in a mouse model of food allergy.
Balb/c mice were sensitized with cholera toxin and cow’s milk proteins (CMP) by gavage and simultaneously treated with U-Omp16 and CMP. Oral challenge with CMP was performed to evaluate the allergic status of mice. Symptoms, local (small bowel cytokine and transcription factor gene expression) and systemic (specific isotypes and spleen cell-secreted cytokines) parameters, and skin tests were done to evaluate the immune response.
We found that the oral administration of U-Omp16 with CMP during sensitization dampened the allergic symptoms, with negativization of immediate skin test and increased skin DTH response. Serum specific IgE and IL-5 were inhibited and a Th1 response was promoted (specific IgG2a antibodies and CMP-induced IFN-γ secretion). We found at the mucosal site an inhibition of the gene expression corresponding to IL-13 and Gata-3, with an induction of IFN-γ and T-bet.
These results indicated that the oral administration of U-Omp16 significantly controlled the allergic response in sensitized mice with a shift of the balance of Th1- and Th2-T cells toward Th1 predominance. These findings suggest that U-Omp16 may be useful as a Th1-directing adjuvant in an oral vaccine.
Introduction
The hygiene hypothesis has been raised for more than 2 decades.Citation1 Although conflicting results have been reported, it is generally accepted that some infections, especially intracellular bacterial and parasitic infections, may have a negative impact on the development of allergic and autoimmune diseases.Citation2-Citation4 The mechanism behind the hygiene hypothesis is believed to be related to immune deviation and/or immune regulation. Indeed, some bacterial infections have been found to alter an allergen-driven T helper 2 (Th2) -cell response to a T helper 1 (Th1)-dominated-cell response. The reduction of allergic reactions and Th2-cell responses was associated with enhanced Th1-cell response.Citation5,Citation6
Cow’s milk allergy (CMA), an immunological mediated reaction to cow’s milk proteins, is one of the most prevalent human food allergies, particularly in infants and young children.Citation7 It is known that allergic disorders are mediated by T lymphocytes secreting Th2 cytokines, resulting in high levels of serum IgE with the risk of life-threatening anaphylaxis after a repeated exposure to the allergen. One of the corrective strategies explored to downregulate the Th2 effector immune response is the induction of a specific Th1 counter-acting immune response that controls the adaptive immunity and the clinical response to the offending allergen.Citation8,Citation9 So, a lower exposition to TLR ligands plus an increase exposition to allergens affect the immunologic homeostasis and influence the development of allergy. Many immunomodulatory strategies that use pro-Th1 adjuvants have been based on this hypothesis.Citation10
The outer membrane protein of 16 kDa from B. abortus (U-Omp16) is a new Brucella pathogen associated molecular pattern (PAMP) that activates dendritic cells (DCs) in vivo and has self-adjuvanting properties when administered by the oral or intraperitoneal route inducing protection against B. abortus challenge. We found that these responses were TLR4 mediated.Citation11 We also demonstrated that the nasal co-administration of U-Omp16 with the model antigen (Ag) ovalbumin (OVA) induced OVA-specific systemic IgG and Th1 immune responses. In addition, the utility of U-Omp16 was also assessed in a mouse model of food allergy. The intranasal administration of U-Omp16 during the sensitization ameliorated the hypersensitivity response of sensitized mice upon oral exposure to cow’s milk proteins (CMP), reduced the clinical signs, decreased anti-CMP IgE serum antibodies and modulated the Th2 response in favor of Th1 immunity.Citation12
Among different mucosal routes, oral delivery is the most easy and acceptable way to administer a formulation, especially in children. Thus, the purpose of this study was to examine the U-Omp16 capacity to downregulate an allergen-specific Th2 immune response when it is administered as an adjuvant through the oral route. These findings may provide a novel therapeutic approach for allergic diseases.
Results
The oral administration of U-Omp16 with CMP controls the induction of allergy
To study the adjuvant capacity of U-Omp16 in an oral formulation, mice were intragastrically (i.g.) administered with U-Omp16 during the sensitization phase and the induction of an allergic reaction was studied. As control, a group of mice received CpG (Th1 adjuvant) with CMP by gavage, another group of mice received only CMP (no sensitization) and OVA was used as a non-related antigen ( shows a schematic representation of the experimental protocol). An oral challenge following the sensitization phase was performed to evidence the induction of hypersensitivity reactions immediately after the exposure to the allergen. The clinical signs were scored () and we evidenced that treated animals (Sens/Omp16 and Sens/CpG) showed significant lower clinical scores compared with sensitized animals exposed to CMP (Sens/PBS) (average score 0.6 for Sens/CpG, 1.0 for Sens/Omp16 and 3.0 for Sens/PBS; P < 0.001), which suggests that the allergic sensitization was ameliorated with the use of these adjuvants. No symptoms were observed in control animals that received only CMP or in animals that were sensitized to CMP and then challenged with OVA (score 0).
Figure 1. Experimental design and in vivo assays. (A) Schematic overview of the experimental design for the food allergy mouse model in BALB/c mice. (B) Hypersensitivity scores of sensitized mice 30 min after last challenge with CMP. Each point represents an individual mouse. Mice were treated with PBS+CMP (Control) or CpG+CMP (Sens/CpG) as controls, or U-Omp16+CMP (Sens/Omp16), and intragastric challenge was performed with CMP or OVA, as control. Mean and standard deviation are indicated. Data are representative of 2 independent experiments (n/group = 5). (C) Cutaneous test performed before the oral challenge showing the blue color corresponding to extravasation of blue Evans in a positive reaction. CMP, OVA, or PBS were inoculated in mice’s ears and blue Evans, in the tail vein. (D) Delayed-type hypersensitivity (DTH) response to CMP was assayed 3 wk after the last boost to evaluate the cellular immune response in vivo. Twenty μg of CMP were injected into one footpad, and saline was injected into the contra lateral footpad, as a negative control. The thickness of both footpads was measured 48 h later. Results are shown as mean increment in the hind footpad between right and left foot ± SEM at 48 h for each group (n/group = 5). Significant difference from CMP alone treated group is indicated (*P < 0.05). Results are representative of 2 independent experiments.
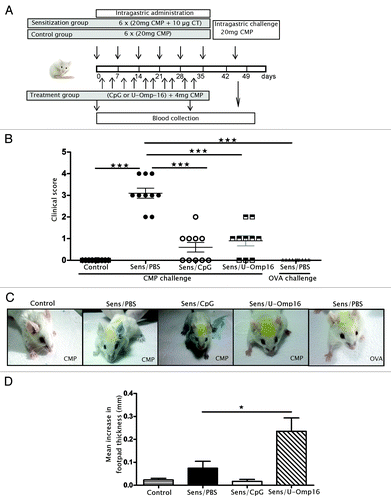
We indirectly demonstrated that this suppressed reaction could be linked to a lower presence of membrane-bound IgE to mast cells through the cutaneous test. shows that an immediate extravasation of the blue dye was only observed in sensitized mice that were subcutaneously injected with CMP in vehicle. No increase in vascular permeability was observed in mice treated with U-Omp16 or CpG plus CMP or in control animals that received only CMP and were injected with CMP. Milk-sensitized mice that were injected with OVA showed no extravasation of the dye. These findings indirectly indicate that IgE-sensitization of skin mast cells is lower in U-Omp16- or CpG-treated animals compared with cells from sensitized mice. This situation may be extended to tissue mast cells and circulating basophils and reflects the absence of immediate reaction following the oral challenge with the allergen. To further investigate if U-Omp16 promoted a specific Th1-mediated immune response, a DTH response was examined. We found a statistically significant increased DTH reaction in mice that received U-Omp16 as adjuvant plus the allergen (average footpath thickness = 0.24), compared with control or sensitized animals (average footpath thickness = 0.03 or 0.07, respectively) ().
The intragastric administration of U-Omp16 promotes a Th1-immune response
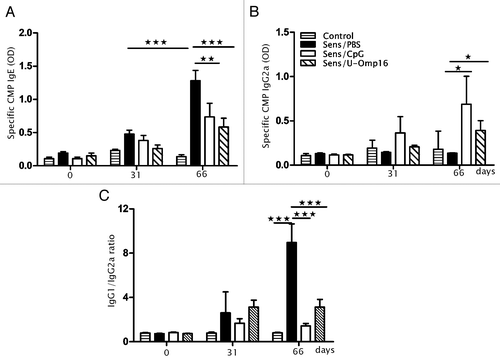
To analyze the specific immune response induced by the adjuvants, the production of the different CMP-specific immunoglobulins was monitored during the protocol. Specific IgE antibodies were significantly increased in the sensitized animals after the oral challenge, whereas low levels of specific IgE was detected in the animals that received only CMP. Besides, we observed that the oral administration of U-Omp16 plus CMP reduced the CMP-specific IgE levels as much as 55%, similar to 43% achieved with the CpG treatment (), whereas levels of CMP-specific IgG2a increased as much as 68% with Omp16/CMP treatment, and 80% with CpG/CMP treatment (). This isotype remained unchanged in control mice, which suggests that it was promoted with the intragastric administration of U-Omp16 or CpG.
Figure 2. U-Omp16 when administered as an oral adjuvant for treatment induces a modulation of specific isotypes. Determination of CMP-specific serum (A) IgE, (B) IgG2a after the oral CMP sensitization and treatment; C) IgG1/IgG2a ratio. Data represents the mean ± SEM from each group of 10 mice (***P < 0.001, **P < 0.01, *P < 0.05 vs CMP treated group). These results are representative of 2 independent experiments.
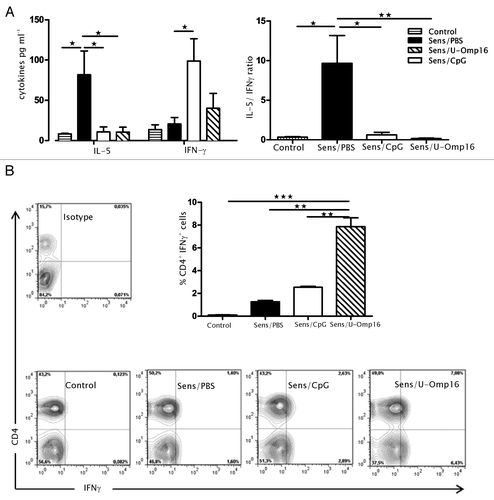
To confirm the pro-Th1 oral adjuvant properties of U-Omp16, the cytokine production of Ag-stimulated spleen cells was studied. As depicted in , we found that IL-5 was highly secreted by cells from sensitized animals (85 ± 20 pg/mL), while IFN-γ was very low (15 ± 4.1 pg/mL). The co-administration of CMP with U-Omp16 or CpG induced the production of IFN-γ in splenocytes (45 ± 4.8 pg/mL for U-Omp16/CMP and 100 ± 21 pg/mL for CpG/CMP) with a reduced secretion of IL-5 (10 ± 3.2 pg/mL for U-Omp16/CMP) (). Importantly, CD4+ T cells were found as being a relevant source of IFN-γ in U-Omp16 treated mice compared with CMP treated mice (7.08 ± 1.10% sensitized/U-Omp16 vs 1.26 ± 1.12% sensitized/PBS, P < 0.05) ().
Figure 3. Treatment with U-Omp16 stimulates the induction of CD4+ CMP-specific T cells that produce IFN-γ. (A) Spleen cells were collected 24 h after the oral challenge and stimulated in vitro with 350 µg/mL of CMP for 72 h. Levels of IL-5 and IFN-γ in culture supernatants of spleen cells from sensitized and treated mice were determined by ELISA. Supernatants were analyzed in triplicate and IL-5/IFN-γ is also depicted. (B) Spleen cells from sensitized and treated mice were incubated with brefeldin A for the last 4 h of culture, and then were stained with specific anti-CD4 (PE) monoclonal antibody. Subsequently, cells were fixed, permeabilized, and stained with an anti-IFN-γ (FITC) or isotype control (FITC) monoclonal antibodies. Samples were analyzed by flow cytometry and data are expressed as mean values ± SEM (*P < 0.05 vs CMP treated group). Results shown are representative of 2 independent experiments.
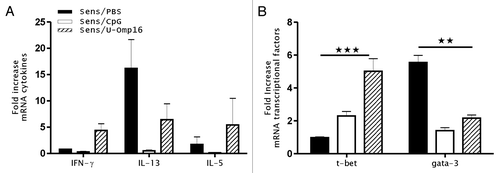
To further evaluate the intestinal induction of a Th1-immune response, the whole-tissue gene expression of cytokines and transcriptional factors was analyzed as an indirect marker of the local immune response induction. The data output showed in is expressed as a fold-difference expressed as mean ± SEM of individual gene expression ratio of treated to control animals. We found increased transcript levels corresponding to IL-13, IL-5, and Gata-3 genes in the jejunum of sensitized mice (fold increase of 16.2 ± 5.1 for IL-13, 2.1 ± 1.1 for IL-5, and 5.8 ± 0.3 for Gata-3, compared with treated animals which showed upregulated gene expression of IFN-γ and T-bet (4.7 ± 0.5 and 5.1 ± 0.8 of fold increase, respectively) (). Remarkably, U-Omp16-treated animals showed the highest transcript levels of these Th1 T-cell differentiation markers.
Figure 4. U-Omp16 induces a modulation of cytokine and transcription factor gene expression at the mucosal site. (A) mRNA expression for cytokines (IL-5, IL-13, and IFN-γ) and (B) transcription factors (T-bet and Gata-3) was quantified 24 h after oral challenge in jejunum segments. β-actin was assessed as a housekeeping gene for standardization in each sample and fold increased was calculated by comparing sensitized and control mice gene expression. Data represents the mean ± SEM from each group and are representative of 2 independent experiments (***P < 0.001, **P < 0.01, *P < 0.05 vs CMP treated group).
Overall these findings provide a strong evidence of the pro-Th1 mucosal adjuvant properties of U-Omp16 that could control the IgE-mediated allergic reaction in sensitized mice.
Discussion
Mucosal vaccines have several advantages compared with systemic vaccines in terms of practicity in administration, safety, regulatory aspects, and production and purity. However, the development of mucosal adjuvants has been the most challenging point to overcome and is a crucial step when defining vaccine candidates. Although it has been demonstrated that mucosal vaccines contribute to stimulate protective immune responses against mucosal and non-mucosal infectious, and to control the aberrant immune response in inflammatory (allergy, autoimmunity)Citation13,Citation14 and non-inflammatory disorders (cancer),Citation15 a few commercially available vaccines have been approved.Citation16,Citation17
Regarding food allergy there is an unmet clinical need for an effective therapy; thus development of therapeutic interventions is a research priority. Allergen immunotherapy is associated with a risk of anaphylaxis, and in the last decades, significant progress has been made for the management of allergic diseases. Studies are concentrated on long-term efficacy, safety due to local and systemic side effects, and the use of safe and effective adjuvants in human vaccines. Therefore promising antigen-specific therapies are based on the development of novel adjuvants and innovative methods for delivery, in combination with new routes of administration, that skew the immune response away from a Th2-mediated allergic response.
Novel immunologic adjuvants based on pathogen-associated molecular patterns, such as TLR agonists, constitute relevant mucosal adjuvants, which have been successfully used in experimental animal models and in clinical trials.Citation18-Citation21 In particular CpG-oligodeoxynucleotide (ODN)-containing formulations (TLR9 agonists), monophosporyl lipid A (TLR4 agonist), and flagellin (TLR5 agonist) have been widely investigated.Citation18,Citation19,Citation22 In this work we have studied the properties of U-Omp16 as an oral adjuvant using an IgE-mediated mouse model of food allergy. Our findings indicate that U-Omp16 delivered by the oral route drives a Th1 immune response to co-delivered CMP, and abolished the Th2-mediated IgE secretion that induced the allergic immune response in mice. This Brucella PAMP promoted the induction of CD4-producing IFN-γ cells that mediated the mucosal immunomodulatory effect observed. It has been previously demonstrated that this newly described PAMP activates dendritic cells through binding to TLR4.Citation11 These results, in accordance with our previous works,Citation11,Citation12 prompt us to propose the use U-Omp16 as a mucosal adjuvant to enhance or control an Ag-specific immune response. Of note, the immunomodulatory properties of U-Omp16 were comparable to CpG administration. Several reports have described that the administration of the allergen with oligodeoxynucleotides containing unmethylated CpG motifs by subcutaneous, intraperitoneal, nasal, or oral administration shift the balance between Th1- and Th2- T cells toward a Th1 predominance, with the reduction of the allergic reaction in mice.Citation23-Citation27 Beeh et al. have conducted a randomized, double-blind, placebo-controlled clinical trial in patients with persistent allergic asthma requiring long-term treatment with inhaled corticosteroids (ICS). Patients received weekly subcutaneous injections of QbG10 (bacteriophage Qbeta-derived virus-like particle with CpG-motif G10 inside), while ICS treatment was steeply withdrawn. In treated patients asthma improved and a considerable reduction of ICS was achieved. On the other hand, patients receiving placebo, the withdrawal of ICS led to a worsening.Citation20 The clinical efficacy of immunomodulatory agents containing a CpG motif has also demonstrated by Klimek et al.Citation28 in other atopic conditions.
In the food allergy mouse model here employed the cholera toxin was used as a pro-Th2 mucosal adjuvant. The immunomodulatory properties of this bacterial enterotoxin are not based on TLR binding. It binds via the B subunit to gangliosides present in the cell membrane of nucleated cells (GM1) and promotes the synthesis of cytosol cyclic AMP. Due to severe unwanted side effects developed in vaccinated subjectsCitation29-Citation32 this mucosal adjuvant has not been approved for human vaccines.
The aim of the most advanced novel allergen-specific immunotherapy is to interfere with the Th2 pro-allergic mechanisms, re-directing the immune response to a mixed Th1-Th2 response by the administration of small doses of allergens with or without adjuvants. Such an immunological response may be achieved by mimicking infections with natural pathogens. Therefore stimulating TLRs that promote a Th1-T cell based immune response may be an attractive way to achieve this goal. To this end, we used the Brucella PAMP U-Omp16 that has proved to be effective as a pro-Th1 auto-adjuvant and intranasal adjuvant to control food allergy. In this work we succeeded in inducing the production of IFN-γ and a Th1-mediated immune response that halted the development of the allergic Th2-based reaction, using the recombinant U-Omp16 as an oral adjuvant. Of note, the use of this novel TLR agonist showed a significant impact in the inhibition of symptoms immediately following the exposure to the allergen in sensitized and treated mice. Concomitantly, the production of IgE was systemically suppressed, which was also reflected at the cellular level (negative skin test). In addition, the DTH test was higher in treated mice compared with sensitized and placebo treated animals indicating the presence of specific Th1 cells. In concordance, IFN-γ and IgG2a were raised indicating a Th1-mediated immune response induced with the oral administration of U-Omp16 and CpG. We could also evidence that Th2 cells were controlled at the mucosal site with a reduction of Gata-3 gene expression and induction of T-bet. Overall these findings clearly indicate that U-Omp16 significantly contributes to the control of the allergic immune response in an IgE-mediated mouse model of food allergy. Further research with IFN-γ neutralizing antibodies or in IFN-γ null mice is required to confirm the central role of this Th1 cytokine in the suppression of the allergic reaction using oral U-Omp16.
In conclusion, we have studied the anti-allergic properties of the bacterial adjuvant U-Omp16 through the oral route, and we demonstrated that the intestinal IgE-mediated allergic sensitization was dampened. Although further research is mandatory to confirm the IFN-γ-based immunomodulatory effect of U-Omp16, this approach may show promise for safe oral use of this novel mucosal adjuvant for the treatment of food allergy.
Materials and Methods
Mice
Male 8-wk-old specific pathogen-free BALB/c mice were purchased from the School of Animal Science at the University of La Plata. Mice were housed in appropriate conventional animal care facilities and handled according to international guidelines required for animal experiments. Animals were grouped in 5 mice per condition and experiments were repeated at least twice.
All the experimental protocols of this study were conducted in strict agreement with the international ethical standards for animal experimentation (Helsinki Declaration and its amendments, Amsterdam Protocol of welfare and animal protection and National Institutes of Health, USA NIH, guidelines: Guide for the Care and Use of Laboratory Animals). All experimental procedures were reviewed and approved by the local Institutional Animal Care and Use Committee at the School of Animal Science (University of La Plata).
Antigens and adjuvants
CMP extract and the recombinant U-Omp16 were obtained as previously described.Citation33,Citation12 Briefly, recombinant U-Omp16 was isolated from bacterial cytoplasm and then purified by affinity chromatography with a Ni-NTA resin (Qiagen). Expression and purification of the recombinant protein was checked by SDS-PAGE followed by Coomasie Blue staining and to confirm the identity of the U-Omp16, western blot was performed and developed with anti–Omp16-specific mAb (data not shown). Protein concentration was determined by the bicinchoninic acid [2-(4-carboxyquinolin-2-yl)quinoline-4-carboxylic acid] assay with bovine seroalbumin as a standard (Pierce). Depletion of lipopolysaccharide (LPS) was performed with a Sepharose-polymyxin B resine (Sigma-Aldrich). Endotoxin determination was performed with Limulus amoebocyte chromogenic assay (LONZA). Protein preparation contained less than 0.10 endotoxin U/mg proteins. The CpG-ODNs contained 2 CpG motifs (ODN 1826) and were purchased from Invivogen. The complete sequence for CPG-ODNs is 5′-TCCATGACGT TCCTGACGTT-3′.
Sensitization and treatment of mice
Mice were sensitized as previously described.Citation33 Briefly, mice received 6 weekly intragastric (i.g) doses of 20 mg of CMP administered as homogenized commercial non-fat dry milk, plus 10 μg of cholera toxin (CT) (Sigma Aldrich) in a final volume of 200 μL of bicarbonate buffer (sensitized mice). As control group naïve mice received 6 weekly i.g doses of 20 mg CMP with PBS. To modulate the allergic sensitization, mice received by gavage during the sensitization 4 µg CMP plus 100 µg U-Omp16 (Sens/Omp16), 4 µg CMP plus 100 µg of CpG (Sens/CpG) as a positive treatment control, or 4 µg CMP in PBS as negative treatment control (Sens/PBS) twice a week. Ten days after the final boost mice were i.g challenged with 20 mg CMP and symptoms were immediately evaluated in a blinded fashion by 2 other researchers. Individual mice only received one treatment. Blood samples were collected during the whole protocol and sera were stored at –20 °C until use. The experimental design is shown in .
In vivo evaluation of the allergic reaction
Assessment of clinical signs
Symptoms were observed between 30 and 60 min after the oral challenge in a blinded fashion by 2 independent researchers. Clinical scores were assigned according to the following range: 0 = no symptoms; 1 = scratching and rubbing around the nose and head; 2 = puffiness around the eyes and mouth, diarrhea, pilo- erection, reduced activity, and/or decreased activity with increase respiratory rate; 3 = wheezing, labored respiration, cyanosis around the mouth and the tail; 4 = no activity after prodding, or tremor and convulsion; and 5 = death.
Cutaneous test
Mice were injected intradermically with 20 μg of CMP in 20μL of sterile saline in one ear and saline alone in the other ear as negative control. Mice were also injected intravenously (tail vein) with 100 µL of 0.1% Evans blue dye (Anedra). The presence of blue color in the ear minutes after the injection was considered positive.
DTH test
Twenty-one days after the last boost the delayed-type hypersensitivity (DTH) response was measured by determining the footpad swelling after a subcutaneous injection of 20 μg of CMP in 20 μL PBS into one hind footpad. As a negative control saline was similarly injected into the contra-lateral footpad. Footpad swelling was measured 48 h post injection with a digital micrometer with a minimum increment of 0.01 mm.
In vitro evaluation of the allergic reaction
Serum specific IgE detection
For the evaluation of specific IgE antibodies against CMP serum samples were tested by EAST as described before.Citation33 Briefly, cyanogen bromide-activated cellulose paper discs were coupled with CMP extracts containing 1.75 mg/mL of protein. Discs were blocked with ethanolamine, and incubated overnight at 4 °C with 50 µL of serum samples. IgE isotype was revealed using a biotinylated anti-mouse IgE monoclonal antibody (BD Pharmigen) at 1:1000 dilution during 5 h at 4 °C, followed with an alkaline phosphatase-streptavidin conjugate (Sigma-Aldrich) at 1:3000 dilution during 30 min at 37 °C. The enzymatic activity was revealed with p-nitrophenyl phosphate (Biochemika, Fluka) and stopped with 0.1 M EDTA. Optical density (OD) was measured at 405 nm.
Serum specific IgG1 and IgG2a detection
CMP-specific IgG1 and IgG2a antibodies were measured by ELISA as previously described.Citation33 Briefly, Maxi Sorp ELISA plates (NUNC) were coated overnight at 4 °C with 1 μg/100 μL CMP in carbonate-bicarbonate buffer (pH 9.6). Coated plates were blocked with 5% equine serum in saline for 2 h at 37 °C and then incubated with sera (1/200) for 1 h at 37 °C. Bound antigen-specific immunoglobulins were detected using a sheep anti-mouse isotype-specific antibody (The Binding Site) (1/1000, 1 h at 37 °C), followed by incubation with a horseradish peroxidase-conjugated goat anti-sheep antibody (Jackson Immunoresearch) (1/10 000, 1 h at 37 °C). The reaction was developed with o-phenylenediamine (Sigma-Aldrich), and stopped with 2 M H2SO4. OD was measured at 492 nm.
Cytokine response of stimulated splenocytes analysis
Twenty-four hours after the oral challenge mice were killed and spleens were aseptically removed. Single cell suspensions were prepared in complete medium. Splenocytes (4 × 106 cells/well) were cultured for 72 h at 37 °C in a humidified atmosphere containing 5% CO2 with complete medium alone (RPMI supplemented with 10% FBS, 10U/mL penicillin, and 10mg/mL streptomycin) as control or in complete medium containing CMP (0.35 mg/mL) for CMP-specific cell stimulation. Supernatants were harvested and assayed for IL-5 and IFN-γ by ELISA using commercially available kits (Invitrogen) according to manufacturer’s instructions. For cytokine staining by flow cytometry, spleen cells from sensitized and treated mice were incubated with brefeldin A (BD Bioscience) for the last 4 h of culture, and then stained with specific anti-CD4 (PE) monoclonal Ab (BD Bioscience). Subsequently, cells were fixed, permeabilized, and stained with an anti-IFN-γ (FITC) or isotype control (FITC) monoclonal Abs (BD Bioscience). Sample acquisition was performed with a FACScalibur cytometer using QuestProCell software. The data were analyzed with the FlowJo software.
Mucosal gene expression analysis
The jejunum was aseptically removed from mice killed by cervical dislocation 24 h after oral challenge and mRNA was isolated using illustra RNAspin mini isolation kit according the manufacturer’s specifications (GE Healthcare). Peyer’s patches were discarded prior to tissue processing. Briefly, samples were homogenized in buffer lysis with β-mercaptoethanol, then lysates were filtrated to eliminate clumps or debris and the RNA was precipitated with ethanol. Suspensions were centrifuged for RNA binding to silica. Finally, DNase was added and after washing, RNA was eluted in water. The amount of the extracted RNA was determined by UV absorption with a spectrophotometer and the optical density ratio of OD280nm/OD260nm was used as a purity measure. Complementary DNA (cDNA) was obtained from 1 µg of RNA using M-MLV reverse transcriptase and random primers (Invitrogen) and mRNA expression was determined by real-time quantitative PCR. The experimental procedure was performed on ABI prims sequence detection system using SYBRGreen fluorescence (BioRad). The thermal cycling conditions were: 10 min at 95 °C, followed by 40 amplification cycles with 1 min annealing/extension at 60 °C, and 15 s of denaturation at 95 °C. The sequences of primers were designed with the Perlprimer software using nucleotide sequences present in a GeneBank database. β-actin was used to standardize the total amount of cDNA and the fold change in mRNA expression was defined as the ratio of the normalized values corresponding to the sensitized mouse to that of control mouse. Genes of interest were IFN-γ (sense-TGGCATAGAT GTGGAAGAAA AGAG, antisens-TGCAGGATTT TCATGTCACC AT), IL-5 (sense-TACTCATAAA AATCACCAGC, antisense-TTATTAATGA CAGGTTTTGG), IL13 (sense-ACATCACACA AGACCAGACT, antisense-TTTGTTATAA AGTGGGCTAC), Gata-3 (sense-CTACATGCTC TGTGAATCAG, antisense-CATCTTTTCT TATTTTGGTG), and T-bet (sense-TGTCCAGTCA GTAACTTTCA, antisense-CAGTCACCTG AGTCTTCTCT).
Statistical analysis
All statistical analysis and plotting were performed using GraphPad Prism 5 software. Data were displayed as mean ± SEM. T test was conducted if 2 experimental groups were performed, whereas when more than 2 groups were conducted, the significance of the difference was determined using ANOVA test followed by the Bonferroni post test. When data did not fit a Gaussian distribution, a logarithmical transformation was done to archive a normal distribution. A P value < 0.05 was considered as statistically significant.
| Abbreviations: | ||
| Ag | = | Antigen |
| CMA | = | Cow’s milk allergy |
| CMP | = | Cow’s milk protein |
| CT | = | cholera toxin |
| DTH | = | delayed-type hypersensitivity |
| i.g | = | intragastric |
| LPS | = | lipopolysaccharide |
| OD | = | optical density |
| OVA | = | ovalbumin |
| U-Omp16 | = | unlipidated outer membrane protein of 16 kDa from B. abortus |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants of the University of La Plata (X557) and Agencia Nacional de Promoción Científica (PICT 2202). Authors have contributed as follows: conceived and designed experiments: P.L.S., A.E.I., J.C., and G.H.D.; performed the experiments: P.L.S. and A.E.I.; analyzed the data: P.L.S., A.E.I., J.C., and G.H.D.; contributed with reagents, materials, analysis tools: J.C., C.A.F., and G.H.D., wrote the manuscript: P.L.S. and G.H.D.
References
- Strachan DP. Hay fever, hygiene, and household size. BMJ (Clinical Research Ed); 299:1259–60.
- Hertzen LC. The hygiene hypothesis in the development of atopy and asthma--still a matter of controversy?. QJM 1998; 91:767 - 71; http://dx.doi.org/10.1093/qjmed/91.11.767; PMID: 10024940
- Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 2000; 55:Suppl 1 S2 - 10; http://dx.doi.org/10.1136/thorax.55.suppl_1.S2; PMID: 10943631
- Okada H, Kuhn C, Feillet H, Bach J-F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol 2010; 160:1 - 9; http://dx.doi.org/10.1111/j.1365-2249.2010.04139.x; PMID: 20415844
- Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both?. Immunology 2004; 112:352 - 63; http://dx.doi.org/10.1111/j.1365-2567.2004.01925.x; PMID: 15196202
- Bilenki L, Gao X, Wang S, Yang J, Fan Y, Han X, Qiu H, Yang X. Dendritic cells from mycobacteria-infected mice inhibits established allergic airway inflammatory responses to ragweed via IL-10- and IL-12-secreting mechanisms. J Immunol 2010; 184:7288 - 96; http://dx.doi.org/10.4049/jimmunol.0902829; PMID: 20483754
- Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, Sigurdardottir ST. The prevalence of food allergy : a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638-46
- Daser A, Meissner N, Herz U, Renz H. Role and modulation of T-cell cytokines in allergy. Curr Opin Immunol 1995; 7:762 - 70; http://dx.doi.org/10.1016/0952-7915(95)80045-X; PMID: 8679117
- Gieni RS, Yang X, HayGlass KT. Allergen-specific modulation of cytokine synthesis patterns and IgE responses in vivo with chemically modified allergen. J Immunol 1993; 150:302 - 10; PMID: 7678032
- Kitagaki K, Businga TR, Kline JN. Oral administration of CpG-ODNs suppresses antigen-induced asthma in mice. Clin Exp Immunol 2006; 143:249 - 59; http://dx.doi.org/10.1111/j.1365-2249.2005.03003.x; PMID: 16412048
- Pasquevich KA, García Samartino C, Coria LM, Estein SM, Zwerdling A, Ibañez AE, Barrionuevo P, Oliveira FS, Carvalho NB, Borkowski J, et al. The protein moiety of Brucella abortus outer membrane protein 16 is a new bacterial pathogen-associated molecular pattern that activates dendritic cells in vivo, induces a Th1 immune response, and is a promising self-adjuvanting vaccine against systemic and oral acquired brucellosis. J Immunol 2010; 184:5200 - 12; http://dx.doi.org/10.4049/jimmunol.0902209; PMID: 20351187
- Ibañez AE, Smaldini P, Coria LM, Delpino MV, Pacífico LGG, Oliveira SC, Risso GS, Pasquevich KA, Fossati CA, Giambartolomei GH, et al. Unlipidated outer membrane protein Omp16 (U-Omp16) from Brucella spp. as nasal adjuvant induces a Th1 immune response and modulates the Th2 allergic response to cow’s milk proteins. PLoS One 2013; 8:e69438; http://dx.doi.org/10.1371/journal.pone.0069438; PMID: 23861971
- Boutajangout A, Goni F, Knudsen E, Schreiber F, Quartermain D, Frangione B, Chabalgoity A, Sigurdsson EM. Diminished amyloid-beta burden in Tg2576 Mice Following a Prophylactic Oral Immunization with a Salmonella-based Aβ Derivative Vaccine. J Alzheimers Dis 2009; 18:961 - 72; PMID: 19749432
- Moingeon P, Lombardi V, Saint-Lu N, Tourdot S, Bodo V, Mascarell L. Adjuvants and vector systems for allergy vaccines. Immunol Allergy Clin North Am 2011; 31:407 - 19, xii; http://dx.doi.org/10.1016/j.iac.2011.03.001; PMID: 21530828
- Nguyen CT, Hong SH, Ung TT, Verma V, Kim SY, Rhee JH, Lee SE. Intranasal immunization with a flagellin-adjuvanted peptide anticancer vaccine prevents tumor development by enhancing specific cytotoxic T lymphocyte response in a mouse model. Clin Exp Vaccine Res 2013; 2:128 - 34; http://dx.doi.org/10.7774/cevr.2013.2.2.128; PMID: 23858404
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6:148 - 58; http://dx.doi.org/10.1038/nri1777; PMID: 16491139
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11:Suppl S45 - 53; http://dx.doi.org/10.1038/nm1213; PMID: 15812489
- Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol 2009; 21:23 - 9; http://dx.doi.org/10.1016/j.coi.2009.01.004; PMID: 19246182
- Blaas SH, Stieber-Gunckel M, Falk W, Obermeier F, Rogler G. CpG-oligodeoxynucleotides stimulate immunoglobulin A secretion in intestinal mucosal B cells. Clin Exp Immunol 2009; 155:534 - 40; http://dx.doi.org/10.1111/j.1365-2249.2008.03855.x; PMID: 19220839
- Beeh K-M, Kanniess F, Wagner F, Schilder C, Naudts I, Hammann-Haenni A, Willers J, Stocker H, Mueller P, Bachmann MF, et al. The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J Allergy Clin Immunol 2013; 131:866 - 74; http://dx.doi.org/10.1016/j.jaci.2012.12.1561; PMID: 23384679
- Malyala P, Chesko J, Ugozzoli M, Goodsell A, Zhou F, Vajdy M, O’Hagan DT, Singh M. The potency of the adjuvant, CpG oligos, is enhanced by encapsulation in PLG microparticles. J Pharm Sci 2008; 97:1155 - 64; http://dx.doi.org/10.1002/jps.21065; PMID: 17683059
- Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol 2008; 9:769 - 76; http://dx.doi.org/10.1038/ni.1622; PMID: 18516037
- Broide DH, Stachnick G, Castaneda D, Nayar J, Miller M, Cho JY, Roman M, Zubeldia J, Hayashi T, Raz E. Systemic administration of immunostimulatory DNA sequences mediates reversible inhibition of Th2 responses in a mouse model of asthma. J Clin Immunol 2001; 21:175 - 82; http://dx.doi.org/10.1023/A:1011078930363; PMID: 11403224
- Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, Eiden JJ, Kagey-Sobotka A, Creticos PS, Lichtenstein LM, Spiegelberg HL, et al. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J Allergy Clin Immunol 2000; 106:124 - 34; http://dx.doi.org/10.1067/mai.2000.107927; PMID: 10887315
- Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J Immunol 2000; 164:5575 - 82; http://dx.doi.org/10.4049/jimmunol.164.11.5575; PMID: 10820231
- Teshima R, Okunuki H, Sato Y, Akiyama H, Maitani T, Sawada J. Effect of oral administration of CpG ODN-OVA on WBB6F1-W/Wv mice. Allergol Int. 2006; 55:43–8
- Weiss R, Scheiblhofer S, Thalhamer J. Allergens are not pathogens: Why immunization against allergy differs from vaccination against infectious diseases. Hum Vaccin Immunother 2013; 10:10; PMID: 24280693
- Klimek L, Willers J, Hammann-Haenni A, Pfaar O, Stocker H, Mueller P, Renner WA, Bachmann MF. Assessment of clinical efficacy of CYT003-QbG10 in patients with allergic rhinoconjunctivitis: a phase IIb study. Clin Exp Allergy 2011; 41:1305 - 12; http://dx.doi.org/10.1111/j.1365-2222.2011.03783.x; PMID: 21672053
- Fujihashi K, Koga T, van Ginkel FW, Hagiwara Y, McGhee JR. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 2002; 20:2431 - 8; http://dx.doi.org/10.1016/S0264-410X(02)00155-X; PMID: 12057597
- Glueck R. Pre-clinical and clinical investigation of the safety of a novel adjuvant for intranasal immunization. Vaccine 2001; 20:Suppl 1 S42 - 4; http://dx.doi.org/10.1016/S0264-410X(01)00292-4; PMID: 11587809
- Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med 2004; 350:896 - 903; http://dx.doi.org/10.1056/NEJMoa030595; PMID: 14985487
- Levine MM, Black RE, Clements ML, Lanata C, Sears S, Honda T, Young CR, Finkelstein RA. Evaluation in humans of attenuated Vibrio cholerae El Tor Ogawa strain Texas Star-SR as a live oral vaccine. Infect Immun 1984; 43:515 - 22; PMID: 6693169
- Smaldini P, Curciarello R, Candreva A, Rey MA, Fossati CA, Petruccelli S, Docena GH. In vivo evidence of cross-reactivity between cow’s milk and soybean proteins in a mouse model of food allergy. Int Arch Allergy Immunol 2012; 158:335 - 46; http://dx.doi.org/10.1159/000333562; PMID: 22472742
