Abstract
Rotavirus is the leading cause of severe diarrhea among children <5 years worldwide. Currently licensed rotavirus vaccines have been efficacious and effective, with many countries reporting substantial declines in diarrheal and rotavirus-specific morbidity and mortality. However, the full public health impact of these vaccines has not been realized. Most countries, including those with the highest disease burden, have not yet introduced rotavirus vaccines into their national immunization programs. Research activities that may help inform vaccine introduction decisions include (1) establishing effectiveness, impact, and safety for rotavirus vaccines in low-income settings; (2) identifying potential strategies to improve performance of oral rotavirus vaccines in developing countries, such as zinc supplementation; and (3) pursuing alternate approaches to oral vaccines, such as parenteral immunization. Policy- and program-level barriers, such as financial implications of new vaccine introductions, should be addressed to ensure that countries are able to make informed decisions regarding rotavirus vaccine introduction.
Background
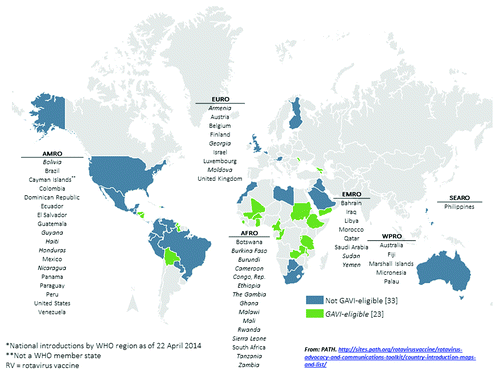
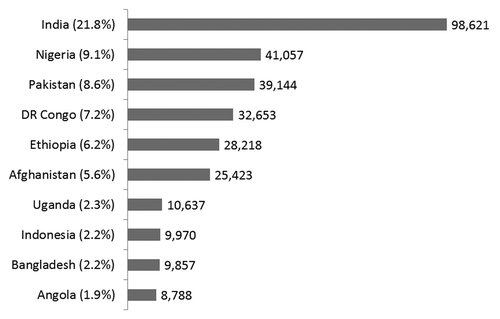
Diarrhea is a major cause of death among children <5 y of age globally.Citation1 Rotavirus is the leading cause of severe diarrhea, resulting in an estimated 453 000 deaths in 2008, most of which occurred in developing countries of sub-Saharan Africa and South-East Asia ().Citation2 Rotavirus also causes considerable morbidity, with global estimates of 2.3 million hospitalizations and 24 million outpatient visits annually among children aged <5 y.Citation2,Citation3 Data from the Global Rotavirus Surveillance Network of the World Health Organization (WHO), a network of sentinel surveillance sites in over 50 countries, indicate that rotavirus is responsible for ~40% of acute gastroenteritis hospitalizations among children <5 y of age in regions without widespread rotavirus vaccine use.Citation4 Since improvements in water and sanitation do not prevent the majority of rotavirus disease, rotavirus vaccines are an essential part of an integrated approach to the control of diarrhea that also includes interventions, such as access to safe drinking water, sanitation, and handwashing facilities, breast feeding, vitamin A and zinc supplementation, and appropriate case management.Citation5 Since 2009, WHO, with support from its Strategic Advisory Group of Experts (SAGE) on Immunization, has recommended that rotavirus vaccines be included in all national immunization programs and considered a priority, particularly in countries with high diarrhea-related mortality.Citation6 By April 2014, 56 countries had introduced rotavirus vaccines into their national immunization programs, and ~50 more likely will follow within the next several years.Citation7,8
Figure 1. 10 countries with the greatest number of rotavirus deaths in 2008. Adapted from: Tate et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 y before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12(2):136–141.
This review will discuss the 2 globally licensed rotavirus vaccines and other vaccines locally available or in development, the current status of vaccine introduction, information on the impact of vaccine introduction on immunization systems and disease burden, vaccine safety issues, and future considerations for vaccine introduction.
Rotavirus Vaccines
Since 2006, 2 rotavirus vaccines have been licensed and used globally—Rotarix (GlaxoSmithKline) and RotaTeq (Merck and Co., Inc.). Rotarix is a live, attenuated vaccine containing a single G1P[8] human rotavirus strain. RotaTeq is a live, attenuated vaccine containing 5 human-bovine reassortant rotavirus strains—G1P7[5], G2P7[5], G3P7[5], G4P7[5], and G6P1A[8] (). Both vaccines are administered orally to infants starting at a minimum age of 6 wk, with a minimum 4 wk interval between doses (2 doses per Rotarix course, 3 doses per RotaTeq course).Citation6 Previous WHO administration recommendations for upper age limits of 15 wk for the first dose of vaccine and 32 wk for the last dose of vaccine were removed in 2013.Citation6 For both vaccines, clinical trials conducted in high and upper-middle-income countries in the Americas, Asia, and Europe demonstrated vaccine efficacy of 72–100% in preventing severe rotavirus disease during 1- to 3-y follow-up periods, while trials conducted in lower income countries in Africa and Asia demonstrated vaccine efficacy of 49–72% ().Citation9-Citation17 Although definite reasons for this lower efficacy are unknown, reasons proposed include factors that can result in interference of uptake of a live, oral vaccine, such as breast milk, stomach acid, maternal antibodies, and co-administration of oral poliovirus vaccine (OPV), and factors that may cause an impaired immune response to vaccine, such as malnutrition, and other infections (e.g., human immunodeficiency virus, malaria, and tuberculosis).Citation18,Citation19
Table 1. Currently licensed and globally recommended rotavirus vaccines
China and Vietnam have locally manufactured oral vaccines that are licensed for use only within these countries (). Available in China, the Lanzhou lamb rotavirus vaccine (LLR; Lanzhou Institute of Biological Products) is a live, attenuated vaccine containing a single G10P[12] lamb rotavirus strain. The recommended schedule of administration is one dose annually for children 2 mo to 3 y of age and one dose at age 3–5 y.Citation20 Since 2000, over 30 million doses have been sold, but little is known about vaccine efficacy, safety, and vaccination coverage since LLR is available only through the private market.Citation20 Available in Vietnam, Rotavin-M1 (POLYVAC) is a live, attenuated vaccine containing a single G1P[8] human rotavirus strain similar to Rotarix. The recommended schedule of administration is 2 doses, starting at a minimum age of 6 wk, given at least 30 d apart.Citation21 Phase I and II studies demonstrated immunogenicity and safety profiles similar to Rotarix;Citation21 efficacy data currently are unavailable. Recently, India licensed its own locally manufactured oral vaccine, ROTAVAC (Bharat Biotech International, Ltd) (). ROTAVAC is a live, attenuated vaccine containing a single neonatal rotavirus G9P[11] strain, 116E. The recommended schedule of administration is 3 doses at 6, 10, and 14 wk. The phase III trial demonstrated vaccine efficacy of ~56% in preventing severe rotavirus diarrhea.Citation22 Bharat Biotech is pursuing WHO pre-qualification of the vaccine so that it may be available globally.Citation23
Table 2. Rotavirus vaccines that are regionally used, recently licensed, or in development
Additional live oral and parenteral rotavirus vaccines are in development or clinical trial stages, but not yet licensed (). Five live, single to multi-strain vaccines, including 2 lamb-human reassortant vaccines from China (Lanzhou and NF-R7), the previously licensed rhesus-human reassortant vaccine RotaShield, a bovine-human reassortant vaccine (UK), and a neonatal human strain vaccine from Australia (RV3), currently are undergoing clinical trials. One subunit rotavirus vaccine currently is undergoing a phase I trial, while several other candidates, including an inactivated rotavirus vaccine (IRV), are in pre-clinical stages of development. Alternative schedules, such as administration of a neonatal dose (RotaShield, RV3) are also being explored.
Current Status of Rotavirus Vaccine Introduction
As of April 2014, 56 countries have introduced rotavirus vaccines into their national immunization programs; an additional 4 have introduced vaccines regionally, and 3 have widespread coverage through the private market ().Citation7 Although Australia and countries in the Americas and Europe were the earliest to introduce after vaccine licensure, countries in other regions have followed, many with support from the GAVI Alliance, a public-private global health partnership with a mission to save children’s lives and protect people’s health by increasing access to immunization in poor countries. As of March 2014, 16 countries have GAVI Alliance support to introduce rotavirus vaccines nationally, 4 countries have GAVI applications under review, and 11 countries are planning to submit GAVI applications.Citation7,8 In addition, 16 non-GAVI eligible countries are planning introductions, making a total of 47 more countries planning to introduce rotavirus vaccines within the next several years. However, despite this progress and a universal recommendation for inclusion of rotavirus vaccines in national immunization programs, 84 countries have no reported plans to introduce rotavirus vaccines. The majority (70%) of these countries are located in Europe, South-East Asia, and the Western Pacific.
Vaccine Impact
While data from clinical trials provide important information on vaccine efficacy and safety in controlled settings, program and field conditions may differ with regards to vaccine management and administration and thus impact both vaccine effectiveness and the immunization system. Evaluation of the impact of vaccine introduction on immunization systems and disease burden is crucial for improving and sustaining immunization programs and stimulating introduction of additional vaccines.
To help evaluate the programmatic impact of new vaccine introduction on immunization systems, WHO recommends post introduction evaluations (PIEs) 6–12 mo after any new vaccine introduction.Citation24 Findings from these evaluations may provide countries with information regarding the impact of rotavirus vaccine introduction on various program topics (e.g., vaccination coverage recording and reporting, cold chain and vaccine management, monitoring and supervision, training and knowledge of health-care workers, waste management, adverse events following immunization, and advocacy and communication planning), which may be used to improve local immunization systems and inform subsequent vaccine introductions. From 2009–2013, rotavirus vaccine PIEs were conducted in 13 countries in Africa, Latin America, the Eastern Mediterranean region, and Europe.Citation25
Monitoring of disease trends and vaccine effectiveness studies can provide timely information necessary to evaluate the impact of vaccine introduction on disease burden. Findings generated from Rotarix and RotaTeq impact assessments, mostly in Australia and countries in Europe and the Americas, have demonstrated substantial declines of 22–50% in diarrhea-related mortality,Citation26-Citation29 17–55% in diarrhea-related hospitalizations,Citation27,Citation30-Citation45 and 49–91% in rotavirus-specific hospitalizations among children <5 y of age ().Citation30-Citation34,Citation36,Citation37,Citation42-Citation59 Many of these studies also have reported potential indirect benefits for unvaccinated older children and young adults, with reductions of 6–51% in diarrhea-related hospitalizationsCitation30-Citation32,Citation35,Citation37,Citation45,Citation60-Citation62 and 20–92% in rotavirus-specific hospitalizations().Citation31,Citation32,Citation34,Citation37,Citation42-Citation45,Citation49,Citation50,Citation54,Citation56,Citation5,Citation61-Citation63 Additional studies have demonstrated vaccine effectiveness in preventing rotavirus hospitalizations similar to vaccine efficacy observed in clinical trials. High and upper-middle-income countries including Australia (certain regions), Taiwan, Austria, Belgium, France, Germany, Northern Israel, Spain, Mexico, Brazil (certain regions), and the US have reported vaccine effectiveness estimates of 79–100%,Citation37,Citation38,Citation53,Citation60,Citation64-Citation83 while lower income countries including Bolivia, El Salvador, and Nicaragua have reported vaccine effectiveness estimates of 43–92% ().Citation84-Citation89
Table 3. Summary of rotavirus vaccine impact studies among children <5 y – disease trendsa
Table 4. Summary of rotavirus vaccine impact studies – indirect benefits for unvaccinated individualsa
Table 5. Vaccine effectiveness (VE) against rotavirus hospitalizations
To date, both Rotarix and RotaTeq have provided protection against a range of rotavirus strains, as demonstrated by the Rotarix clinical trials conducted in Africa, for which vaccine efficacy was ~60–64% for both G1 (contained in Rotarix) and non-G1 (not contained in Rotarix) rotavirus types,Citation90 and by various vaccine effectiveness studies conducted in Australia, Europe, and the Americas, for which vaccine effectiveness estimates were 71–95% against rotavirus strains not contained in RotaTeq and/or Rotarix.Citation38,Citation66,Citation70,Citation78,Citation80,Citation83,Citation89,Citation91 Monitoring of rotavirus strains continues in order to detect any global changes in strain prevalence and any emergence of unusual strains, and allow for strain-specific measures of vaccine effectiveness in the event that there is concern about vaccine effectiveness against an emergent or novel strain.
Vaccine Safety
In 1999, the first licensed rotavirus vaccine, RotaShield (Wyeth), was withdrawn from US market within a year after licensure due to an association with intussusception, an obstruction of the small intestine that can require radiological or surgical intervention. It was estimated that ~1 excess case of intussusception occurred per 10 000 infants vaccinated with RotaShield.Citation92 Pre-licensure clinical trials for Rotarix and RotaTeq that included 60 000–70 000 infants each did not demonstrate an increased risk for intussusception,Citation14,Citation16 However, post-licensure monitoring studies were recommended to detect a possible low risk that may not have been identified in the clinical trials. In 2011, findings from studies conducted in Mexico (Rotarix only) and Australia (Rotarix and RotaTeq) reported a low level risk of intussusception with both Rotarix and RotaTeq on the order of ~1–2 excess cases per 100 000 vaccinated infants, mostly within the first week after the first dose of vaccine.Citation93-Citation9 These risks were lower than the risk associated with RotaShield, and a subsequent review of available data and a risk-benefit analysis of rotavirus vaccination conducted by the WHO Global Advisory Committee on Vaccine Safety (GACVS) determined that the benefits of rotavirus vaccination of all infants greatly exceeded the risks of intussusception associated with vaccination ().Citation96 Additional analyses to examine the risk-benefit of rotavirus vaccination without upper age restrictions estimated that universal rotavirus vaccination in low- and low-middle-income countries could prevent an additional 47 200 (range: 18 000–63 700) rotavirus deaths while potentially causing an additional 294 (range: 161–471) intussusception deaths among a cohort of children <5 y of age.Citation98 These analyses contributed to the 2013 WHO recommendation that upper age restrictions for rotavirus vaccination be removed to allow for greater vaccination coverage and potentially greater reductions in the number of rotavirus deaths as the benefits of vaccination continue to outweigh the risks of intussusception.Citation6
Table 6. Risk of intussusception and benefits of rotavirus vaccination in Mexico, Brazil, Australia, and the USa
Recently, new data from the US also have demonstrated a low risk of intussusception with both vaccines. In 2 studies conducted among separate managed care populations, an approximate risk of 1–5 excess cases of intussusception per 100 000 infants vaccinated with rotavirus vaccine was reported.Citation98,Citation99 While the risk-benefit of rotavirus vaccination with these 2 vaccines remains approximately the same as that seen when using the earlier risk estimates from Mexico and Australia, questions remain as to whether this risk may be higher in any particular subgroup of infants and whether the level of risk seen in high- and middle-income countries will occur in low-income countries where vaccine efficacy is lower.Citation100 Additional special studies and continued post-introduction intussusception monitoring, especially in low-income countries, may help answer these questions.
Future Considerations
Countries worldwide have established estimates of the considerable diarrheal disease burden of rotavirus.Citation4,Citation6 Existing licensed rotavirus vaccines have proven to be efficacious in clinical trials and effective in post-introduction evaluations, with many countries demonstrating adequate capacity to introduce these vaccines into routine immunization programs and substantial declines in diarrheal and rotavirus-specific morbidity and mortality (–). Despite this, the full public health impact of these vaccines on rotavirus disease and child mortality has not been realized as most countries, including some with the highest disease burden, have not yet introduced rotavirus vaccines into their national immunization programs. Why is this the case? What are the remaining questions to be answered? What are the potential barriers to introduction?
Several key research activities may help to address remaining questions about rotavirus vaccine use under field conditions and inform vaccine introduction decisions, especially in low-income countries. These include: (1) establishing effectiveness, impact, and post-licensure safety of the current WHO-prequalified rotavirus vaccines in low-income settings; (2) identifying potential strategies to improve performance of oral rotavirus vaccines in developing countries, such as zinc supplementation to potentially strengthen the immune response to vaccination, withholding breast feeding just before and after vaccine administration to prevent interference of vaccine uptake by maternal antibodies, or a neonatal dosing schedule to help increase the amount of rotavirus disease prevented in settings where children may acquire disease at an earlier age; and (3) pursuing alternate approaches to oral vaccines, such as parenteral vaccines to bypass possible interference of vaccine uptake by gastric acid and breast milk to improve vaccine efficacy in developing countries.Citation18
To identify potential barriers to introduction at a policy level, one may consider key issues in the decision-making process to introduce a new vaccine.Citation101 Once barriers are identified, solutions may be implemented (). While each country should have a mechanism for an evidence-based, informed-decision making process, which may include expert groups that provide technical advice to national immunization programs, such as National Immunization Technical Advisory Groups (NITAGs) (http://www.healthinternetwork.com/immunization/sage/national_advisory_committees/en/) or other advisory committees on immunization,Citation101 barriers and concerns may differ by country or even region. For example, one country may be concerned with vaccine introduction costs while another may be concerned with the programmatic impact of vaccine introduction or vaccine efficacy and safety. Given the potential diversity of opinion, it will be crucial to understand issues at the local policy making level to best inform decision makers.
Table 7. Key issues and potential barriers and related questions in the decision to introduce rotavirus vaccine
During the past several years, significant progress has been made in the prevention and control of rotavirus diarrhea. The introduction of rotavirus vaccines into the national immunization programs of over 50 countries has resulted in substantial declines in rotavirus-related morbidity and mortality. However, questions remain that will need to be answered by additional research, and policy- and program-level barriers should be removed to ensure that countries are able to make informed decisions regarding rotavirus vaccine introduction and to help realize the full potential impact of these vaccines.
| Abbreviations: | ||
| GACVS | = | Global Advisory Committee on Vaccine Safety |
| LLR | = | Lanzhou lamb rotavirus vaccine |
| SAGE | = | Strategic Advisory Group of Experts on Immunization |
| WHO | = | World Health Organization |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Financial Support
The authors have indicated that they have no financial relationships relevant to this article to disclose.
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al, Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379:2151 - 61; http://dx.doi.org/10.1016/S0140-6736(12)60560-1; PMID: 22579125
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136 - 41; http://dx.doi.org/10.1016/S1473-3099(11)70253-5; PMID: 22030330
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565 - 72; http://dx.doi.org/10.3201/eid0905.020562; PMID: 12737740
- World Health Organization. Global rotavirus information and surveillance bulletin.2013; 7:1-11.
- World Health Organization/United Nations Children's Fund. Ending preventable child deaths from pneumonia and diarrhoea by 2025: the integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). France: WHO Press; 2013.
- World Health Organization. Rotavirus vaccines. WHO position paper – January 2013. Wkly Epidemiol Rec 2013; 88:49 - 64; PMID: 23424730
- International Vaccine Access Center. VIMS report: global vaccine introduction. Johns Hopkins Bloomberg School of Public Health; March 2014. Available from: http://www.jhsph.edu/research/centers-and-institutes/ivac/vims/IVAC-VIMS-Report-2014-Mar.pdf
- 8. PATH. Rotavirus vaccine introductions: worldwide. PATH; April 2014. Available from: http://sites.path.org/rotavirusvaccine/rotavirus-advocacy-and-communications-toolkit/country-introduction-maps-and-list/
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606 - 14; http://dx.doi.org/10.1016/S0140-6736(10)60889-6; PMID: 20692030
- Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, Muto H, Smolenov I, Suryakiran PV, Han HH. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011; 29:6335 - 41; http://dx.doi.org/10.1016/j.vaccine.2011.05.017; PMID: 21640780
- Linhares AC, Velázquez FR, Pérez-Schael I, Sáez-Llorens X, Abate H, Espinoza F, López P, Macías-Parra M, Ortega-Barría E, Rivera-Medina DM, et al, Human Rotavirus Vaccine Study Group. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181 - 9; http://dx.doi.org/10.1016/S0140-6736(08)60524-3; PMID: 18395579
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289 - 98; http://dx.doi.org/10.1056/NEJMoa0904797; PMID: 20107214
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, Teoh YL, Tang H, Boudville I, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 2009; 27:5936 - 41; http://dx.doi.org/10.1016/j.vaccine.2009.07.098; PMID: 19679216
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al, Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11 - 22; http://dx.doi.org/10.1056/NEJMoa052434; PMID: 16394298
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757 - 63; http://dx.doi.org/10.1016/S0140-6736(07)61744-9; PMID: 18037080
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al, Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23 - 33; http://dx.doi.org/10.1056/NEJMoa052664; PMID: 16394299
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615 - 23; http://dx.doi.org/10.1016/S0140-6736(10)60755-6; PMID: 20692031
- Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most?. J Infect Dis 2009; 200:Suppl 1 S39 - 48; http://dx.doi.org/10.1086/605035; PMID: 19817613
- Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine 2012; 30:Suppl 1 A30 - 5; http://dx.doi.org/10.1016/j.vaccine.2011.11.093; PMID: 22520134
- Fu C, He Q, Xu J, Xie H, Ding P, Hu W, Dong Z, Liu X, Wang M. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine 2012; 31:154 - 8; http://dx.doi.org/10.1016/j.vaccine.2012.10.078; PMID: 23127516
- Dang DA, Nguyen VT, Vu DT, Nguyen TH, Nguyen DM, Yuhuan W, Baoming J, Nguyen DH, Le TL, Rotavin-M1 Vaccine Trial Group. A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine 2012; 30:Suppl 1 A114 - 21; http://dx.doi.org/10.1016/j.vaccine.2011.07.118; PMID: 22520120
- Department of Biotechnology GoI. The need for rotavirus vaccines in India: understanding efficacy and impact. 2013; http://www.defeatdd.org/sites/default/files/node-images/ROTAVAC%20efficacy%20fact%20sheet_FINAL.pdf. Accessed February 3, 2014.
- Department of Biotechnology GoI. Internationl social innovation partnership leads to lifesaving vaccine. 2013; http://www.defeatdd.org/sites/default/files/node-images/ROTAVAC%20history_FINAL_0.pdf. Accessed February 3, 2014.
- World Health Organization. New vaccine post-introduction evaluation (PIE) tool. Geneva, Switzerland: WHO Document Services; 2010.
- Personal communication with World Health Organization. Expanded Programme on Immunization.
- Bayard V, DeAntonio R, Contreras R, Tinajero O, Castrejon MM, Ortega-Barría E, Colindres RE. Impact of rotavirus vaccination on childhood gastroenteritis-related mortality and hospital discharges in Panama. Int J Infect Dis 2012; 16:e94 - 8; http://dx.doi.org/10.1016/j.ijid.2011.09.003; PMID: 22154592
- do Carmo GM, Yen C, Cortes J, Siqueira AA, de Oliveira WK, Cortez-Escalante JJ, Lopman B, Flannery B, de Oliveira LH, Carmo EH, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med 2011; 8:e1001024; http://dx.doi.org/10.1371/journal.pmed.1001024; PMID: 21526228
- Lanzieri TM, Linhares AC, Costa I, Kolhe DA, Cunha MH, Ortega-Barria E, Colindres RE. Impact of rotavirus vaccination on childhood deaths from diarrhea in Brazil. Int J Infect Dis 2011; 15:e206 - 10; http://dx.doi.org/10.1016/j.ijid.2010.11.007; PMID: 21193339
- Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, Parashar U, Patel M. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med 2010; 362:299 - 305; http://dx.doi.org/10.1056/NEJMoa0905211; PMID: 20107215
- Bégué RE, Perrin K. Reduction in gastroenteritis with the use of pentavalent rotavirus vaccine in a primary practice. Pediatrics 2010; 126:e40 - 5; http://dx.doi.org/10.1542/peds.2009-2069; PMID: 20587671
- Chang HG, Smith PF, Tserenpuntsag B, Markey K, Parashar U, Morse DL. Reduction in hospitalizations for diarrhea and rotavirus infections in New York state following introduction of rotavirus vaccine. Vaccine 2010; 28:754 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.10.075; PMID: 19896451
- Clarke MF, Davidson GP, Gold MS, Marshall HS. Direct and indirect impact on rotavirus positive and all-cause gastroenteritis hospitalisations in South Australian children following the introduction of rotavirus vaccination. Vaccine 2011; 29:4663 - 7; http://dx.doi.org/10.1016/j.vaccine.2011.04.109; PMID: 21575665
- Cortes JE, Curns AT, Tate JE, Cortese MM, Patel MM, Zhou F, Parashar UD. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med 2011; 365:1108 - 17; http://dx.doi.org/10.1056/NEJMoa1000446; PMID: 21992123
- Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J 2010; 29:489 - 94; PMID: 20354464
- Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis 2010; 201:1617 - 24; http://dx.doi.org/10.1086/652403; PMID: 20402596
- Dey A, Wang H, Menzies R, Macartney K. Changes in hospitalisations for acute gastroenteritis in Australia after the national rotavirus vaccination program. Med J Aust 2012; 197:453 - 7; http://dx.doi.org/10.5694/mja12.10062; PMID: 23072242
- Field EJ, Vally H, Grimwood K, Lambert SB. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics 2010; 126:e506 - 12; http://dx.doi.org/10.1542/peds.2010-0443; PMID: 20732946
- Gurgel RG, Bohland AK, Vieira SC, Oliveira DM, Fontes PB, Barros VF, Ramos MF, Dove W, Nakagomi T, Nakagomi O, et al. Incidence of rotavirus and all-cause diarrhea in northeast Brazil following the introduction of a national vaccination program. Gastroenterology 2009; 137:1970 - 5; http://dx.doi.org/10.1053/j.gastro.2009.07.046; PMID: 19632228
- Lanzieri TM, Costa I, Shafi FA, Cunha MH, Ortega-Barria E, Linhares AC, Colindres RE. Trends in hospitalizations from all-cause gastroenteritis in children younger than 5 years of age in Brazil before and after human rotavirus vaccine introduction, 1998-2007. Pediatr Infect Dis J 2010; 29:673 - 5; http://dx.doi.org/10.1097/INF.0b013e3181da8f23; PMID: 20300045
- Molto Y, Cortes JE, De Oliveira LH, Mike A, Solis I, Suman O, Coronado L, Patel MM, Parashar UD, Cortese MM. Reduction of diarrhea-associated hospitalizations among children aged < 5 Years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J 2011; 30:Suppl S16 - 20; http://dx.doi.org/10.1097/INF.0b013e3181fefc68; PMID: 21183835
- Quintanar-Solares M, Yen C, Richardson V, Esparza-Aguilar M, Parashar UD, Patel MM. Impact of rotavirus vaccination on diarrhea-related hospitalizations among children < 5 years of age in Mexico. Pediatr Infect Dis J 2011; 30:Suppl S11 - 5; http://dx.doi.org/10.1097/INF.0b013e3181fefb32; PMID: 21183834
- Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J 2011; 30:e120 - 5; http://dx.doi.org/10.1097/INF.0b013e318214b811; PMID: 21436757
- Sáfadi MA, Berezin EN, Munford V, Almeida FJ, de Moraes JC, Pinheiro CF, Racz ML. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in São Paulo, Brazil. Pediatr Infect Dis J 2010; 29:1019 - 22; PMID: 20543761
- Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, Nolasco JB, De Oliveira LH, Pastor D, Tate JE, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J 2011; 30:Suppl S6 - 10; http://dx.doi.org/10.1097/INF.0b013e3181fefa05; PMID: 21048524
- Yen C, Tate JE, Wenk JD, Harris JM 2nd, Parashar UD. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics 2011; 127:e9 - 15; http://dx.doi.org/10.1542/peds.2010-1393; PMID: 21172995
- Anderson EJ, Rupp A, Shulman ST, Wang D, Zheng X, Noskin GA. Impact of rotavirus vaccination on hospital-acquired rotavirus gastroenteritis in children. Pediatrics 2011; 127:e264 - 70; http://dx.doi.org/10.1542/peds.2010-1830; PMID: 21262887
- Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, Akikusa JD, Kelly JJ, Kirkwood CD. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:Suppl S25 - 9; http://dx.doi.org/10.1097/INF.0b013e3181fefdee; PMID: 21183837
- Custodio H, Masnita-Iusan C, Wludyka P, Rathore MH. Change in rotavirus epidemiology in northeast Florida after the introduction of rotavirus vaccine. Pediatr Infect Dis J 2010; 29:766 - 7; http://dx.doi.org/10.1097/INF.0b013e3181dbf256; PMID: 20661105
- Eberly MD, Gorman GH, Eide MB, Olsen CH, Rajnik M. The effect of rotavirus immunization on rotavirus gastroenteritis hospitalization rates in military dependents. Vaccine 2011; 29:650 - 9; http://dx.doi.org/10.1016/j.vaccine.2010.11.041; PMID: 21129394
- Hemming M, Räsänen S, Huhti L, Paloniemi M, Salminen M, Vesikari T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr 2013; 172:739 - 46; http://dx.doi.org/10.1007/s00431-013-1945-3; PMID: 23361964
- Lambert SB, Faux CE, Hall L, Birrell FA, Peterson KV, Selvey CE, Sloots TP, Nissen MD, Grimwood K. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med J Aust 2009; 191:157 - 60; PMID: 19645646
- Msimang VM, Page N, Groome MJ, Moyes J, Cortese M, Seheri M, Kahn K, Chagan M, Madhi SA, Cohen C. Impact of Rotavirus Vaccine on Childhood Diarrheal Hospitalization Following Introduction into the South African Public Immunization Program. Pediatr Infect Dis J 2013; 32:1359 - 64; http://dx.doi.org/10.1097/INF.0b013e3182a72fc0; PMID: 23934208
- Paulke-Korinek M, Kollaritsch H, Aberle SW, Zwazl I, Schmidle-Loss B, Vécsei A, Kundi M. Sustained low hospitalization rates after four years of rotavirus mass vaccination in Austria. Vaccine 2013; 31:2686 - 91; http://dx.doi.org/10.1016/j.vaccine.2013.04.001; PMID: 23597718
- Paulke-Korinek M, Kundi M, Rendi-Wagner P, de Martin A, Eder G, Schmidle-Loss B, Vecsei A, Kollaritsch H. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 2011; 29:2791 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.01.104; PMID: 21320539
- Paulke-Korinek M, Rendi-Wagner P, Kundi M, Kronik R, Kollaritsch H. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in austrian children. Pediatr Infect Dis J 2010; 29:319 - 23; PMID: 19935446
- Payne DC, Staat MA, Edwards KM, Szilagyi PG, Weinberg GA, Hall CB, Chappell J, Curns AT, Wikswo M, Tate JE, et al, New Vaccine Surveillance Network (NVSN). Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US Counties, 2006-2009. Clin Infect Dis 2011; 53:245 - 53; http://dx.doi.org/10.1093/cid/cir307; PMID: 21705316
- Tate JE, Panozzo CA, Payne DC, Patel MM, Cortese MM, Fowlkes AL, Parashar UD. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics 2009; 124:465 - 71; http://dx.doi.org/10.1542/peds.2008-3528; PMID: 19581260
- Zeller M, Rahman M, Heylen E, De Coster S, De Vos S, Arijs I, Novo L, Verstappen N, Van Ranst M, Matthijnssens J. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine 2010; 28:7507 - 13; http://dx.doi.org/10.1016/j.vaccine.2010.09.004; PMID: 20851085
- Zlamy M, Kofler S, Orth D, Würzner R, Heinz-Erian P, Streng A, Prelog M. The impact of Rotavirus mass vaccination on hospitalization rates, nosocomial Rotavirus gastroenteritis and secondary blood stream infections. BMC Infect Dis 2013; 13:112; http://dx.doi.org/10.1186/1471-2334-13-112; PMID: 23452879
- Cortese MM, Leblanc J, White KE, Jerris RC, Stinchfield P, Preston KL, Meek J, Odofin L, Khizer S, Miller CA, et al. Leveraging state immunization information systems to measure the effectiveness of rotavirus vaccine. Pediatrics 2011; 128:e1474 - 81; http://dx.doi.org/10.1542/peds.2011-1006; PMID: 22084328
- Gastañaduy PA, Curns AT, Parashar UD, Lopman BA. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA 2013; 310:851 - 3; http://dx.doi.org/10.1001/jama.2013.170800; PMID: 23982372
- Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204:980 - 6; http://dx.doi.org/10.1093/infdis/jir492; PMID: 21878425
- Anderson EJ, Shippee DB, Weinrobe MH, Davila MD, Katz BZ, Reddy S, Cuyugan MG, Lee SY, Simons YM, Yogev R, et al. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin Infect Dis 2013; 56:755 - 60; http://dx.doi.org/10.1093/cid/cis1010; PMID: 23349228
- Adlhoch C, Hoehne M, Littmann M, Marques AM, Lerche A, Dehnert M, Eckmanns T, Wichmann O, Koch J. Rotavirus vaccine effectiveness and case-control study on risk factors for breakthrough infections in Germany, 2010-2011. Pediatr Infect Dis J 2013; 32:e82 - 9; http://dx.doi.org/10.1097/INF.0b013e3182720b71; PMID: 23334342
- Boom JA, Tate JE, Sahni LC, Rench MA, Hull JJ, Gentsch JR, Patel MM, Baker CJ, Parashar UD. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 2010; 125:e199 - 207; http://dx.doi.org/10.1542/peds.2009-1021; PMID: 20083525
- Boom JA, Tate JE, Sahni LC, Rench MA, Quaye O, Mijatovic-Rustempasic S, Patel MM, Baker CJ, Parashar UD. Sustained protection from pentavalent rotavirus vaccination during the second year of life at a large, urban United States pediatric hospital. Pediatr Infect Dis J 2010; 29:1133 - 5; http://dx.doi.org/10.1097/INF.0b013e3181ed18ab; PMID: 20634776
- Braeckman T, Van Herck K, Meyer N, Pirçon JY, Soriano-Gabarró M, Heylen E, Zeller M, Azou M, Capiau H, De Koster J, et al, RotaBel Study Group. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ 2012; 345:e4752; http://dx.doi.org/10.1136/bmj.e4752; PMID: 22875947
- Castilla J, Beristain X, Martínez-Artola V, Navascués A, García Cenoz M, Alvarez N, Polo I, Mazón A, Gil-Setas A, Barricarte A. Effectiveness of rotavirus vaccines in preventing cases and hospitalizations due to rotavirus gastroenteritis in Navarre, Spain. Vaccine 2012; 30:539 - 43; http://dx.doi.org/10.1016/j.vaccine.2011.11.071; PMID: 22122860
- Chang WC, Yen C, Wu FT, Huang YC, Lin JS, Huang FC, Yu HT, Chi CL, Lin HY, Tate JE, et al. Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Pediatr Infect Dis J 2014; 33:e81 - 6; http://dx.doi.org/10.1097/INF.0000000000000105; PMID: 24569388
- Correia JB, Patel MM, Nakagomi O, Montenegro FM, Germano EM, Correia NB, Cuevas LE, Parashar UD, Cunliffe NA, Nakagomi T. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis 2010; 201:363 - 9; http://dx.doi.org/10.1086/649843; PMID: 20047501
- Cortese MM, Immergluck LC, Held M, Jain S, Chan T, Grizas AP, Khizer S, Barrett C, Quaye O, Mijatovic-Rustempasic S, et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics 2013; 132:e25 - 33; http://dx.doi.org/10.1542/peds.2012-3804; PMID: 23776114
- Desai SN, Esposito DB, Shapiro ED, Dennehy PH, Vázquez M. Effectiveness of rotavirus vaccine in preventing hospitalization due to rotavirus gastroenteritis in young children in Connecticut, USA. Vaccine 2010; 28:7501 - 6; http://dx.doi.org/10.1016/j.vaccine.2010.09.013; PMID: 20851087
- Donauer S, Payne DC, Edwards KM, Szilagyi PG, Hornung RW, Weinberg GA, Chappell J, Hall CB, Parashar UD, Staat MA. Determining the effectiveness of the pentavalent rotavirus vaccine against rotavirus hospitalizations and emergency department visits using two study designs. Vaccine 2013; 31:2692 - 7; http://dx.doi.org/10.1016/j.vaccine.2013.03.072; PMID: 23583814
- Gagneur A, Nowak E, Lemaitre T, Segura JF, Delaperrière N, Abalea L, Poulhazan E, Jossens A, Auzanneau L, Tran A, et al, IVANHOE investigators. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine 2011; 29:3753 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.03.035; PMID: 21443962
- Justino MC, Linhares AC, Lanzieri TM, Miranda Y, Mascarenhas JD, Abreu E, Guerra SF, Oliveira AS, da Silva VB, Sanchez N, et al. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belém, Brazil. Pediatr Infect Dis J 2011; 30:396 - 401; http://dx.doi.org/10.1097/INF.0b013e3182055cc2; PMID: 21150692
- Martinón-Torres F, Bouzón Alejandro M, Redondo Collazo L, Sánchez Lastres JM, Pértega Díaz S, Seoane Pillado MT, Martinón Sánchez JM, ROTACOST research team. Effectiveness of rotavirus vaccination in Spain. Hum Vaccin 2011; 7:757 - 61; http://dx.doi.org/10.4161/hv.7.7.15576; PMID: 21521947
- Muhsen K, Shulman L, Kasem E, Rubinstein U, Shachter J, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, et al. Effectiveness of rotavirus vaccines for prevention of rotavirus gastroenteritis-associated hospitalizations in Israel: a case-control study. Hum Vaccin 2010; 6:450 - 4; http://dx.doi.org/10.4161/hv.6.6.11759; PMID: 20448471
- Payne DC, Boom JA, Staat MA, Edwards KM, Szilagyi PG, Klein EJ, Selvarangan R, Azimi PH, Harrison C, Moffatt M, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009-2011. Clin Infect Dis 2013; 57:13 - 20; http://dx.doi.org/10.1093/cid/cit164; PMID: 23487388
- Snelling TL, Andrews RM, Kirkwood CD, Culvenor S, Carapetis JR. Case-control evaluation of the effectiveness of the G1P[8] human rotavirus vaccine during an outbreak of rotavirus G2P[4] infection in central Australia. Clin Infect Dis 2011; 52:191 - 9; http://dx.doi.org/10.1093/cid/ciq101; PMID: 21288843
- Snelling TL, Schultz R, Graham J, Roseby R, Barnes GL, Andrews RM, Carapetis JR. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis 2009; 49:428 - 31; http://dx.doi.org/10.1086/600395; PMID: 19566443
- Staat MA, Payne DC, Donauer S, Weinberg GA, Edwards KM, Szilagyi PG, Griffin MR, Hall CB, Curns AT, Gentsch JR, et al, New Vaccine Surveillance Network (NVSN). Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics 2011; 128:e267 - 75; http://dx.doi.org/10.1542/peds.2010-3722; PMID: 21768317
- Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics 2010; 125:e208 - 13; http://dx.doi.org/10.1542/peds.2009-1246; PMID: 20100757
- Yen C, Figueroa JR, Uribe ES, Carmen-Hernández LD, Tate JE, Parashar UD, Patel MM, Richardson López-Collado V. Monovalent rotavirus vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. J Infect Dis 2011; 204:783 - 6; http://dx.doi.org/10.1093/infdis/jir390; PMID: 21810915
- Cardellino A, Khawaja S, Sánchez Cruz E, Mast TC. Effectiveness of vaccination with the pentavalent rotavirus vaccine in Nicaragua as determined using the screening method. Hum Vaccin Immunother 2013; 9:1449 - 53; http://dx.doi.org/10.4161/hv.24338; PMID: 23571175
- de Palma O, Cruz L, Ramos H, de Baires A, Villatoro N, Pastor D, de Oliveira LH, Kerin T, Bowen M, Gentsch J, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 2010; 340:c2825; http://dx.doi.org/10.1136/bmj.c2825; PMID: 20551120
- Mast TC, Khawaja S, Espinoza F, Paniagua M, Del Carmen LP, Cardellino A, Sánchez E. Case-control study of the effectiveness of vaccination with pentavalent rotavirus vaccine in Nicaragua. Pediatr Infect Dis J 2011; 30:e209 - 15; http://dx.doi.org/10.1097/INF.0b013e31822a8527; PMID: 21768920
- Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, Gonzalez A, Malespin O, Amador JJ, Umaña J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009; 301:2243 - 51; http://dx.doi.org/10.1001/jama.2009.756; PMID: 19491186
- Patel M, Pedreira C, De Oliveira LH, Umaña J, Tate J, Lopman B, Sanchez E, Reyes M, Mercado J, Gonzalez A, et al. Duration of protection of pentavalent rotavirus vaccination in Nicaragua. Pediatrics 2012; 130:e365 - 72; http://dx.doi.org/10.1542/peds.2011-3478; PMID: 22753550
- Patel MM, Patzi M, Pastor D, Nina A, Roca Y, Alvarez L, Iniguez V, Rivera R, Tam KI, Quaye O, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 2013; 346:f3726; http://dx.doi.org/10.1136/bmj.f3726; PMID: 23783434
- Steele AD, Neuzil KM, Cunliffe NA, Madhi SA, Bos P, Ngwira B, Witte D, Todd S, Louw C, Kirsten M, et al. Human rotavirus vaccine Rotarix™ provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infect Dis 2012; 12:213; http://dx.doi.org/10.1186/1471-2334-12-213; PMID: 22974466
- Patel MM, Glass R, Desai R, Tate JE, Parashar UD. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure?. Lancet Infect Dis 2012; 12:561 - 70; http://dx.doi.org/10.1016/S1473-3099(12)70029-4; PMID: 22742639
- Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, Zanardi LR, Setia S, Fair E, LeBaron CW, et al, Rotavirus Intussusception Investigation Team. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001; 344:564 - 72; http://dx.doi.org/10.1056/NEJM200102223440804; PMID: 11207352
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, Booy R, Bines JE, PAEDS/APSU Study Group. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine 2011; 29:3061 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.01.088; PMID: 21316503
- Patel MM, López-Collada VR, Bulhões MM, De Oliveira LH, Bautista Márquez A, Flannery B, Esparza-Aguilar M, Montenegro Renoiner EI, Luna-Cruz ME, Sato HK, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med 2011; 364:2283 - 92; http://dx.doi.org/10.1056/NEJMoa1012952; PMID: 21675888
- Velázquez FR, Colindres RE, Grajales C, Hernández MT, Mercadillo MG, Torres FJ, Cervantes-Apolinar M, DeAntonio-Suarez R, Ortega-Barria E, Blum M, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J 2012; 31:736 - 44; http://dx.doi.org/10.1097/INF.0b013e318253add3; PMID: 22695189
- World Health Organization. Rotavirus vaccine and intussusception:report from an expert consultation. Wkly Epidemiol Rec 2011; 86:317 - 21; PMID: 21800466
- Patel MM, Clark AD, Sanderson CF, Tate J, Parashar UD. Removing the age restrictions for rotavirus vaccination: a benefit-risk modeling analysis. PLoS Med 2012; 9:e1001330; http://dx.doi.org/10.1371/journal.pmed.1001330; PMID: 23109915
- Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, Klein NP, Glanz JM, Jacobsen SJ, Naleway A, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med 2014; 370:513 - 9; http://dx.doi.org/10.1056/NEJMoa1311738; PMID: 24422678
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, Selvam N, Selvan M, Lee GM, Nguyen M. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med 2014; 370:503 - 12; http://dx.doi.org/10.1056/NEJMoa1303164; PMID: 24422676
- Glass RI, Parashar UD. Rotavirus vaccines--balancing intussusception risks and health benefits. N Engl J Med 2014; 370:568 - 70; http://dx.doi.org/10.1056/NEJMe1315836; PMID: 24422677
- World Health Organization. Vaccine introduction guidelines - adding a vaccine to a national immunization programme: decision and implementation. Geneva, Switzerland: WHO Document Services; 2003.
- Australian Government Therapeutic Goods Administration. Rotavirus vaccination and risk of intussusception. 2011; http://www.tga.gov.au/safety/alerts-medicine-rotavirus-110225.htm. Accessed August 24, 2011.
- Cortese MM. Summary of intussusception risk and benefits of rotavirus vaccination in the United States. Adivsory Committee on Immunization Practices Meeting; June 20, 2013, 2013; Atlanta, GA.