Abstract
Introduction of Haemophilus influenzae type b (Hib) vaccine in low- and middle-income countries has been limited by cost and availability of Hib conjugate vaccines for a long time. It was previously recognized by the Institute for Translational Vaccinology (Intravacc, originating from the former Vaccinology Unit of the National Institute of Public Health [RIVM] and the Netherlands Vaccine Institute [NVI]) that local production of a Hib conjugate vaccine would increase the affordability and sustainability of the vaccine and thereby help to speed up Hib introduction in these countries. A new affordable and a non-infringing production process for a Hib conjugate vaccine was developed, including relevant quality control tests, and the technology was transferred to a number of vaccine manufacturers in India, Indonesia, and China. As part of the Hib technology transfer project managed by Intravacc, a preclinical toxicity study was conducted in the Netherlands to test the safety and immunogenicity of this new Hib conjugate vaccine. The data generated by this study were used by the technology transfer partners to accelerate the clinical development of the new Hib conjugate vaccine.
A repeated dose toxicity and local tolerance study in rats was performed to assess the reactogenicity and immunogenicity of a new Hib conjugate vaccine compared to a licensed vaccine. The results showed that the vaccine was well tolerated and immunogenic in rats, no major differences in both safety and immunogenicity in rats were found between the vaccine produced according to the production process developed by Intravacc and the licensed one. Rats may be useful to verify the immunogenicity of Hib conjugate vaccines and for preclinical evaluation.
In general, nonclinical evaluation of the new Hib conjugate vaccine, including this proof of concept (safety and immunogenicity study in rats), made it possible for technology transfer partners, having implemented the original process with no changes in the manufacturing process and vaccine formulation, to start directly with phase 1 clinical trials.
Abbreviations:
- Hib, Haemophilus influenzae type b
- PRP, poly-ribosylribitol phosphate (Hib capsular polysaccharide)
- PRP-T, Hib capsular polysaccharide conjugated to Tetanus Toxoid
- IgG, Immunoglobulin G
- QC, quality control
- Hib, Haemophilus influenzae type b
- SPF, specific pathogen free
- ELISA, enzyme-linked immunosorbent assay
- RIVM, The National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu)
- NVI, Netherlands Vaccine Institute
- Intravacc, Institute for Translational Vaccinology
- LCB, Laboratory of Control of Biological Products
- WHO, World Health Organization
- EP, European Pharmacopeia
- NIBSC, National Institute for Biological Standards and Control (UK)
- OECD, Organization for Economic Cooperation and Development
Introduction
Haemophilus influenzae type b (Hib) conjugate vaccines have been highly efficacious in reducing Hib-related disease incidence in developed countries, mainly meningitis and pneumonia. Although safe and effective Hib conjugate vaccines have been available since the late 1980s, the introduction of these vaccines in low- and middle-income countries did not start until 20 y later. The availability of Hib conjugate vaccines could be improved by assuring a sustainable local production in low-and middle-income countries; this was only possible by enabling access to (Hib) conjugate technology. The Institute for Translational Vaccinology (Intravacc, originating from the former Vaccinology Unit of the National Institute of Public Health [RIVM] and the Netherlands Vaccine Institute [NVI]) developed an up-scalable, non-infringing, and affordable production process of Hib conjugate vaccine plus the related quality control (QC) tests and transferred the technology to several vaccine manufacturers in India, Indonesia, and ChinaCitation1-5
Hib vaccine developed by Intravacc consists of purified polyribosylribitol phosphate (Hib capsular polysaccharide, PRP) conjugated to a carrier protein (tetanus toxoid, routinely prepared by Bio Farma). Tetanus toxoid, when covalently bound to PRP (PRP-T), is capable of inducing a T-cell-dependent B-cell immune response to the polysaccharide. The Hib technology developed resulted in stable intermediate and final products and allowed both freeze-dried and liquid formulations of several Hib vaccines.
Based on this technology, the Indian partners have succeeded in producing, marketing, and prequalifying both standalone and combined Hib conjugate vaccines.Citation6-8 Prequalification is a service provided by WHO to United Nations Children's Fund (UNICEF) and other United Nations agencies to assure that vaccines considered for purchase by such agencies meet global standards of quality, safety, and efficaccy.Citation9 Local production by emerging manufacturers as a result of the technology transfer has contributed to an effective and sustainable way in increasing the Hib conjugate vaccine supply.Citation10,11
Nonclinical evaluation, including a safety and immunogenicity study in rats, was part of the Hib technology transfer project at Intravacc. The first clinical lot was produced in collaboration between Intravacc and Bio Farma: the production of polysaccharide, production of tetanus toxoid, freeze-drying and filling of the final lot took place at Bio Farma, and the conjugation of the Hib-polysaccharide to tetanus toxoid took place at Intravacc. The safety and immunogenicity of this clinical lot, produced under Good Manufacturing Practices, was tested in a repeated dose toxicity and local tolerance study in the Netherlands according to WHO guidelines on nonclinical evaluation of vaccines.Citation12 Based on the preclinical data from this study, phase 1 clinical trials could be initiated in Indonesia using the same lot.Citation2
Although Intravacc as the owner/transferee of the technology had no intention of marketing the Hib conjugate vaccine, it chose to evaluate the new Hib conjugate vaccine and thereby the developed process nonclinically and to provide the data to all technology transfer partners in order to achieve an efficient and cost-effective technology transfer. The nonclinical data provided by Intravacc could be used by all technology transfer partners to build their registration dossier, to obtain approval from independent ethics committees and regulatory authorities to perform clinical trials with the product produced in their own facilities and to obtain marketing authorization. In this way, it was not necessary for each individual partner to generate similar data, an approach supported by WHO.Citation12 This approach can be set as an example for other technology transfer projects, assuring that the local requirements are taken into account.
Since relatively few publications are available on preclinical evaluation of Hib conjugate vaccines, it was chosen to publish the design and results of the preclinical safety study performed within the framework of the Hib technology transfer project. The primary objective of this preclinical study was to assess the systemic and local toxicity/reactogenicity of this new Haemophilus influenzae type b conjugate vaccine after repeated intramuscular vaccinations in male and female rats in comparison to a commercial Hib conjugate vaccine. In addition, the antibody response was measured in rat sera to confirm that the Hib conjugate, produced by the production process developed by Intravacc was immunogenic in rats.
Results
Toxicity study
No major abnormalities were observed in the appearance, general condition, growth, food consumption, hematological and clinical chemistry values, organ weights and gross necropsy, and histopathological findings in any of the groups. Temporary signs of local irritation, including erythema and hematomas, were observed at the injection sites among animals of all groups indicating an effect induced by the injection procedure. Twenty-one days after the last injection, no signs of local reactions were apparent at the injection site, except for localized myodegeneration in a single animal treated with the licensed vaccine.
In both Hib conjugate vaccine–treated groups, the mean body weights at the end of the study were lower in comparison with that of the placebo group, but the differences were only statistically significant for female rats (5%). Body weights at day 63 and mean food conversion efficiencies at day 77 are summarized in . Other effects were observed alternatively in rats treated with the test and licensed vaccine: increase in neutrophils and lymphocytes, decrease in the albumin/globulin ratio, weight increase of the popliteal lymph nodes. These effects may be indicative for a normal immune response following vaccination. The degree and incidence of these effects were comparable between both groups and were not statistically significant.
Table 1. Summary of injection site reactions and body weights at day 63 (sacrifice of main group) and mean food conversion efficiencies at day 77 (total study period)
In addition, a slight increase was observed in the weight of the draining popliteal lymph nodes only in animals treated with the test vaccine. This effect was no longer observed at day 21 after the last vaccination and was likely caused by the activated immune response following vaccination. summarizes the most important Haematology and Clinical chemistry data.
Table 2. Summary of main haematology and clinical chemistry data
Immunogenicity
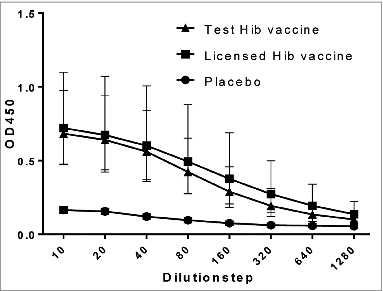
Blood was collected at necropsy from all animals and used to evaluate the immune response after vaccination by determining the level of serum IgG specific for Hib-polysaccharide (PRP). PRP-specific IgG was detected in both sera from the test Hib group and the licensed vaccine group, but not in sera from the placebo group (). Some difference was seen between male and female rats, especially for the test vaccine. This difference was not further investigated since the primary objective of the assay was to prove that the Hib-vaccines were immunogenic in rats.
Figure 1. PRP- specific IgG antibodies in serum after vaccination. Rats were vaccinated with 3 doses of a licensed Hib vaccine (square) and the test Hib vaccine (triangle) on day 0, 28, and 56. As a negative control a group of rats was vaccinated using a placebo (circle). The level of PRP- specific IgG antibodies were measured in a rat-anti-PRP ELISA at OD450
Discussion/Conclusions
Technology Transfer has been proven to be one of the fastest routes in getting access to know-how. Intravacc has a longstanding history in supporting manufacturers to establish production capacity by providing access to the required technologies. Transferring the technology of Hib conjugate vaccine has helped a number of emerging manufacturers to establish local Hib conjugate vaccine manufacturing capacity, to get access to innovative conjugation technology and to acquire more regulatory expertise on (Hib) conjugate vaccines. The strategy followed by the technology transferee (Intravacc) in the Hib technology transfer project was to develop a pilot-scale process as soon as possible, to generate all necessary supportive data and to provide the data to all technology transfer partners. This included immunogenicity and safety data, generating thereby a proof of concept for the Hib technology to be transferred independent of the site were the product is produced, an approach that can be followed for other technology transfer programs. In this case, it was not needed to generate preclinical data by each individual partner, a time and money saving approach. Knowing that some local authorities may not accept this WHO approach, intensive communication with the Indonesian regulatory authorities was needed before being able to start with the preclinical study in the Netherlands. Ultimately, the Indonesian partner was able to start with the clinical trials without repeating the preclinical study in Indonesia.Citation2 Further, both the Indian partners and the Chinese partner were able to rely on this preclinical data when submitting their clinical plans and registration dossiers to the local authorities.
Rats were chosen because of their proven suitability as an animal model for toxicological studies and for Hib conjugate vaccine immunogenicity studies.Citation13,14 Beside rats, several other animal models have been described in the literature to be used successfully to study the immunogenicity of Hib vaccines, including mice, guinea pigs, and rabbits.Citation15,16 Despite that, there are a limited number of publications describing the preclinical evaluation of Hib conjugate vaccines.Citation17-21
Because of the absence of major abnormalities in the appearance, general condition, growth, food consumption, hematological and clinical chemistry values, organ weights and gross necropsy, and histopathological findings, it can be concluded that the investigational Hib conjugate vaccine was equally well-tolerated by the animals as the licensed vaccine. In addition, both the new Hib conjugate vaccine and the licensed vaccine induce PRP-specific IgG antibodies. The animal model used during this study is thus considered to be suitable for preclinical evaluation of Hib conjugate vaccines.
The Hib conjugate vaccine produced using Intravacc's technology is already licensed and prequalified as part of several combination-vaccines, containing other childhood vaccines such as DTP.Citation7,8,22 Therefore, it can be stated now only with near certainty that the approach followed in this Hib technology transfer project can be followed for other technology transfer projects, provided that the local regulatory authorities are involved from the very beginning of the project, including in the design of the preclinical study.
Materials and Methods
The repeated-dose toxicity and local tolerance study was performed by an external and independent research laboratory: TNO Nutrition and Food Research in Zeist, the Netherlands, and conducted in accordance with the Organization for Economic Cooperation and Development (OECD) Principles for Good Laboratory Practice.Citation23 summarizes the study design including the most important observations, analyses, and measurements performed during the study.
Table 3. Study design of the repeated dose toxicity study with Haemophilus influenza b vaccine
Vaccines
Both the investigational Hib conjugate vaccine (lot number FPH012) and the licensed Hib conjugate vaccine (lot number U1059, RIVM) were stand-alone freeze-dried vaccines consisting of Hib capsular polysaccharide conjugated to Tetanus Toxoid (PRP-T). Both Hib conjugate vaccines had the same composition: an equivalent of 10 μg polysaccharide and approximately 20 μg tetanus toxoid per human dose in the presence of sucrose. The test Hib vaccine used was a clinical lot produced by Intravacc and Bio Farma; based on the process developed by Intravacc and transferred to the technology transfer partnersCitation24,25 meeting all WHO and EP requirements.Citation26-28 The Hib polysaccharide and tetanus toxoid were produced and purified at Bio Farma, the polysaccharide was conjugated to tetanus toxoid at Intravacc. Tetanus toxoid was routinely produced by Bio Farma and was meeting all WHO Hib requirementsCitation26 for a carrier protein. The polysaccharide was conjugated to tetanus toxoid at Intravacc using the conjugation method originally described by John Robbins and collaborators (National Institutes of Health, Bethesda, USA).Citation29 The purified concentrated conjugate bulk was sent to Indonesia for formulation, freeze-drying, and filling.Citation2 Part of this (pre)clinical lot was sent to the Netherlands to be used for the preclinical study and part was used at a later stage for a clinical study in Indonesia under the auspices of Bio Farma.Citation2 Each vial of the freeze-dried Hib vaccines, containing the equivalent of one human dose, was reconstituted in 0.25 mL phosphate-buffered saline (PBS, Gibco BRL, Life Technologies Ltd). Reconstituted vaccines were used within 4 h after reconstitution. PBS was used for the control animals (placebo).
Test animals
Fifty-four animals (27 males and 27 females) young adult Wistar outbred rats (Charles River) were obtained from a colony maintained under Specific Pathogen Free (SPF) conditions. The age of the animals at the time of commencement of the study was 8 to 10 wk. The animals were randomly assigned to one of the following groups: test Hib conjugate vaccine, placebo, and licensed vaccine. To correct for differences in the mean weight of animals between groups after computer randomization, 2 animals from the test group had to be exchanged with 2 animals from the group to be used for the licensed vaccine.
None of the animals showed any signs of illness and/or anomalies. A serological investigation of the microbiological status was also conducted. All animals examined were assigned to the study. The animals were acclimatized to the laboratory conditions for 10 d before the start of the study.
Injections
Rats received a total of 3 injections with the test Hib conjugate vaccine, the placebo, or the licensed Hib conjugate vaccine. The vaccines and the placebo were administered on day 0, 28, and 56 by intramuscular route alternatingly in the gastrocnemius or thigh muscle in the hind legs. At each immunization, 0.125 mL of the Hib conjugate vaccine, the placebo, or the reference vaccine was injected in both the left and right limb, which in total was equal to one human dose. The following parameters were assessed during the study: clinical signs, body weight, food intake, hematology and clinical chemistry of the blood, examination at necropsy for gross macroscopic changes, organ weights, and histopathology of various tissues and organs (including the injection site). Of each group a subgroup of animals was sacrificed on day 63, consisting of 12 animals (6 males and 6 females) per dose group. The remaining animals (3 males and 3 females/group) were sacrificed on day 77 (recovery group).
Collection of blood
Blood samples were collected at necropsy from all animals and transferred to the Laboratory of Control of biological products (LCB, at that time part of NVI), for evaluating the immunogenicity of the vaccines by determining antibody production against Hib-polysaccharide. Serum was prepared by allowing blood to coagulate for approximately 2 h at 37 °C and overnight at 4 °C. After coagulation the serum was separated by centrifugation. The serum samples were stored at −30 °C after inactivation for 30 min at 56 °C.
Serology
An enzyme-linked immunosorbent assay (ELISA) was performed to measure antibodies specific for Hib-polysaccharide. For this ELISA, Immulon II plates were coated with Human Serum Albumin conjugated to Hib-polysaccharide (HbO-HA). 2-fold serial dilution ranges were made of the rat sera, starting at 1/10 till 1/1280. Following overnight incubation at room temperature, 100 μL of a peroxidase-labeled sheep-anti-rat IgG solution, in BSA (1% w/v) and Tween 20 (0.05% v/v), was added to each well and the plates were incubated for one hour at 37 °C. After adding 100 μL Tetramethyl-benzidine (TMB) substrate (in sodium acetate) to each well, the plates were incubated for 10 min at room temperature; sulphuric acid was used to stop the reaction. The Optical Density (OD) was measured at 450 nm using an ELISA reader. On each ELISA plate, a positive control was tested. As positive control a pool of rat sera was used, these rats were immunized using 2 injections at day 0 and day 7 of the licensed Hib vaccine.
Reagents and chemicals
The following reagents and chemicals were used for the ELISA:
HbO-HA: Lederle- Praxis Biologicals, lyophilized, 1 mg/vial, obtained from NIBSC.
BSA: Sigma A-4503.
Tween 20: Merck 822184.
TMB: Sigma T2885.
Sheep anti rat IgG solution: Amersham.
Sulphuric acid, concentrated: H2SO4, MW 98.07, 95–97%, (Merck nr. 100731).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge the contribution of all RIVM/NVI personal to the Hib-project, especially Annemarie Bouwman-Kelder for generating valuable data and Coenraad Hendriksen for reviewing the final version of the manuscript. The contribution of Babs O Fabriek, TNO Nutrition and Food Research, in reviewing the manuscript is also very much appreciated.
Funding
The Hib technology transfer project was funded by the various partners (Bio Farma, Biological E., Serum Institute of India, and Glovax/Shanghai Institute of Biological Products), through bilateral license agreements. In the beginning it was needed to use seed capital from Intravacc (originating from RIVM/NVI) to study the feasibility of the project and generate Proof of Concept data.
References
- Jadhav S, Datla M, Kreeftenberg H, Hendriks J. The Developing Countries Vaccine Manufacturers’ Network (DCVMN) is a critical constituency to ensure access to vaccines in developing countries. Vaccine 2008; 26:1611-5; PMID:18294742; http://dx.doi.org/10.1016/j.vaccine.2008.01.034
- Beurret M, Hamidi A, Kreeftenberg H. Development and technology transfer of Haemophilus influenzae type b conjugate vaccines for developing countries. Vaccine 2012; 30:4897-906; PMID:22683521; http://dx.doi.org/10.1016/j.vaccine.2012.05.058
- Sharma HJ, Multani AS, Dutta AK, Joshi SM, Malik S, Bhardwaj S, Chakravarty A, Namjoshi GS, Parekh S, Verma V. Safety and immunogenicity of an indigenously developed Haemophilus influenzae type b conjugate vaccine through various phases of clinical trials. Hum Vaccin 2009; 5:483-7; PMID:19395868
- Li H, Li MG, Tang J, Liang L, Li YN, He L, Ye Q. Development of a quantitative ELISA method for total antibody of Haemophilus influenzae type b. Chinese Journal of Biologicals 2011; 24:974-6.
- Kreeftenberg JG, Hamidi A. Annex 5: Lessons learned in the transfer of Hib conjugate vaccine technology and the consequences for access to this vaccine in developing countries. World Health Organization (WHO); 2004. Report No.: WHO/IVB/04.21. Available from: 627 http://whqlibdoc.who.int/hq/2004/WHOIVB04.21(302KB).pdf
- Sharma HJ, Yadav S, Lalwani SK, Kapre SV, Jadhav SS, Chakravarty A, Parekh SS, Palkar S, Bhardwaj SH, Namjoshi GS, et al. Immunogenicity and safety of an indigenously manufactured reconstituted pentavalent (DTwP-HBV+Hib) vaccine in comparison with a foreign competitor following primary and booster immunization in Indian children. Hum Vaccin 2011; 7:451-7; PMID:21403463; http://dx.doi.org/10.4161/hv.7.4.14208
- Sharma HJ, Patil VD, Lalwani SK, Manglani MV, Ravichandran L, Kapre SV, Jadhav SS, Parekh SS, Ashtagi G, Malshe N, et al. Assessment of safety and immunogenicity of two different lots of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae type b vaccine manufactured using small and large scale manufacturing process. Vaccine 2012; 30:510-6; PMID:22119927; http://dx.doi.org/10.1016/j.vaccine.2011.11.067
- Sharma H, Yadav S, Lalwani S, Gupta V, Kapre S, Jadhav S, Chakravarty A, Parekh S, Palkar S. A phase III randomized, controlled study to assess the immunogenicity and tolerability of DTPw-HBV-Hib, a liquid pentavalent vaccine in Indian infants. Vaccine 2011; 29:2359-64; PMID:21288803; http://dx.doi.org/10.1016/j.vaccine.2011.01.054
- WHO Expert Committee on Biological Standardization. WHO TRS No. 978- Sixty-first Report, 2010. Annex 6: Procedure for assessing the acceptability, in principle, of vaccines for purchase by United Nations agencies.
- WHO report on Increasing access to vaccines through technology transfer and local production, 2011: http://www.who.int/phi/publications/Increasing_Access_to_Vaccines_Through_Technology_Transfer.pdf
- WHO report on Trends in Local Production of Medicines and Related Technology Transfer, 2011: http://apps.who.int/medicinedocs/documents/s19063en/s19063en.pdf
- World Health Organization. Annex1: WHO guidelines on nonclinical evaluation of vaccines. WHO TRS No. 927; 2005.
- Fernández-Santana V, Cardoso F, Rodriguez A, Carmenate T, Peña L, Valdés Y, Hardy E, Mawas F, Heynngnezz L, Rodríguez MC, et al. Antigenicity and immunogenicity of a synthetic oligosaccharide-protein conjugate vaccine against Haemophilus influenzae type b. Infect Immun 2004; 72:7115-23; PMID:15557635; http://dx.doi.org/10.1128/IAI.72.12.7115-7123.2004
- Mawas F, Newman G, Burns S, Corbel MJ. Suppression and modulation of cellular and humoral immune responses to Haemophilus influenzae type B (Hib) conjugate vaccine in hib-diphtheria-tetanus toxoids-acellular pertussis combination vaccines: a study in a rat model. J Infect Dis 2005; 191:58-64; PMID:15593004; http://dx.doi.org/10.1086/426396
- Fusco PC, Michon F, Laude-Sharp M, Minetti CA, Huang CH, Heron I, Blake MS. Preclinical studies on a recombinant group B meningococcal porin as a carrier for a novel Haemophilus influenzae type b conjugate vaccine. Vaccine 1998; 16:1842-9; PMID:9795390; http://dx.doi.org/10.1016/S0264-410X(98)00174-1
- Gupta RK, Anderson R, Cecchini D, Rost B, Xu J, Gendreau K, Saroff DL, Marchant C, Siber GR. Evaluation of a guinea pig model to assess interference in the immunogenicity of different components of a combination vaccine comprising diphtheria, tetanus and acellular pertussis (DTaP) vaccine and haemophilus influenzae type b capsular polysaccharide conjugate vaccine. Biologicals 1999; 27:167-76; PMID:10600208; http://dx.doi.org/10.1006/biol.1999.0204
- Siber GR, Anderson R, Habafy M, Gupta RK. Development of a guinea pig model to assess immunogenicity of Haemophilus influenzae type b capsular polysaccharide conjugate vaccines. Vaccine 1995; 13:525-31; PMID:7483772; http://dx.doi.org/10.1016/0264-410X(94)00042-L
- Fernandez S, Cisney ED, Ulrich RG. Enhancement of serum and mucosal immune responses to a Haemophilus influenzae Type B vaccine by intranasal delivery. Clin Vaccine Immunol 2013; 20:1690-6; PMID:23986319; http://dx.doi.org/10.1128/CVI.00215-13
- Bacardí D, Cosme K, Aldana L, Merino N, Suárez J, Mosqueda O, Urquiza D, Romero J, Madrigal R, Amaya R, et al. Preclinical safety testing of the Quimi-Hib® vaccine adjuvanted with aluminum phosphate during product development. Biotecnol Apl 2013; 30:118-24
- Caulfield MJ, Smith JG, Wang S, Capen RC, Blondeau C, Lentsch S, Arminjon F, Sabouraud A. Immunogenicity of a hexavalent combination vaccine in rhesus monkeys. Vaccine 2000; 19:902-7; PMID:11115714; http://dx.doi.org/10.1016/S0264-410X(00)00299-1
- Fusco PC, Michon F, Laude-Sharp M, Minetti CASA, Huang CH, Heron I, Blake MS. Preclinical studies on a recombinant group B meningococcal porin as a carrier for a novel Haemophilus influenzae type b conjugate vaccine. Vaccine 1998; 16:1842-9; PMID:9795390; http://dx.doi.org/10.1016/S0264-410X(98)00174-1
- Alliance GAVI. Indian manufacturer cuts price of childhood vaccine by 30 percent: http://www.gavialliance.org/library/news/press-releases/2013/pentavalent-vaccine-30-percent-price-drop/
- OECD series on principles of good laboratory practice and compliance monitoring. Ann Ist Super Sanita. 1997;33(1):1-172.
- Hamidi A, Beurret M, inventors;2009(Sept. 6). Process for producing a capsular polysaccharide for use in conjugate vaccines. De Staat der Nederlanden, vert. door de minister van VWS, The Hague, NL, assignee. US Pat 7,582,459 B2.
- Hamidi A, Beurret M, inventors;2010(Mar. 3). Process for producing a capsular polysaccharide for use in conjugate vaccines. De Staat der Nederlanden, vert. door de minister van VWS, The Hague, NL, assignee. Eur Pat Spec 1 664 319 B1.
- WHO Expert Committee on Biological Standardization. Forty-ninth report. Annex 1. Recommendations for the production and control of Haemophilus influenzae type b conjugate vaccines. WHO Technical Report Series 2000;897:27-60.
- WHO Expert Committee on Biological Standardization. Forty-first report. WHO TRS No. 814 1991 Annex 1. Requirements for Haemophilus type b conjugate vaccines.
- European Pharmacopoeia 5.0. Haemophilus type b conjugate vaccine. 01/2005: 1219.
- Chu C, Schneerson R, Robbins JB, Rastogi SC. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun 1983; 40:245-56; PMID:6601061
