Abstract
Despite worldwide vaccination against devastating diseases for decades, millions of children in remote and impoverished regions of the globe die every year from vaccine-preventable infectious diseases. The reasons for incomplete coverage of vaccination programs are based in part on the relatively high costs of conventional vaccinations, including mass production, refrigeration, transportation, and training as well as funding personnel for their administration. Plant-based edible vaccines (PEVs) have been introduced as a revolutionary cost-effective vaccination modality. However, they suffer from major deficiencies that have restricted their application to bench-scale. This article discusses the deficiencies of PEVs and also provides concise overview on the health-promoting, biological and biotechnological features of spirulina (Arthrospira). In short, we envision that spirulina could be considered as a potential alternative biofactory system to the plants toward the production of edible vaccines in high-yield with low-costs that other hosts cannot yet offer.
Challenges in Meeting Global Vaccination and Edible Vaccine Development
Impressive progresses have been achieved in vaccination against infectious diseases; however, the full implementation of global vaccination remains a continuing challenge. According to the World Health Organization (WHO), 1.5 million children under the age of five, living in remote and impoverished parts of the globe, die from diseases that could be prevented by vaccines. This outcome in many developing countries can be exacerbated by economic crises and the high-costs of conventional vaccinations, because of the costs of mass production, purification, refrigeration, and transportation, to providing sterile injection conditions and funding and training personnel for vaccine administration. Therefore, to overcome these problems, further efforts have been performed to seek novel and cost-effective alternate vaccination procedures and technologies. In this regard, PEVs were suggested by Arntzen in 1990s;Citation1 Until now, engineered plants have been advocated for the production of edible vaccines, . but the main question is yet to be addressed: what are the advantages of plants, which have attracted extensive research interests, over other production and vaccination systems?
It could be asserted that (1) Plants are cost-effective in mass-scale production and transportation. (2) Plants surmount storage concerns, while extensive refrigeration facilities are demanded for conventional vaccines. (3) Plant-based edible vaccines are produced at or near the site of use. (4) Extra processes of purification can be eliminated. (5) Such vaccines do not have requirements for sterile processing as well as the training of specialists for their safe delivery.Citation2 (6) Their oral administration can stimulate mucosal as well assystemic immunity.Citation3 (7) The vaccination compliance is high, especially by children, to PEVs. (8) Immunization Reminder Programs (IRPs) are straightforward in these edible vaccines. Despite the perceived advantages of plants as hosts for edible vaccine production, plant-derived vaccines suffer from several shortcomings that have restricted their applicability to the bench-scale until now.
Limitations of Plant Systems for Production of Edible Vaccines
Although there are a large number of investigations on PEVs, they have not yet fulfilled the main aims of vaccination thoroughly. They require time-consuming processing steps from the initial transformation to production of vaccines. Such long period may associate with possible antigen (Ag) changes when most pathogens are ever-changing their Ags unremittingly, hence production of new vaccines in long-term processes may results in vain. It should be highlighted that, because of the abortion of transferred genes from some cells during the regeneration of callus, the consumable organs may accumulate different volumes of Ags.Citation4 Hence, the dosage inconsistency may occur, which is one of the most critical obstacles in clinical rationalization of PEVs applications, since different fruits from the same sprig or fruits from different plants may express various doses of AgCitation5 (). Subsequently, people may be administered with unequal doses of vaccines, resulting in failure of/ambiguity in clinical outcome of vaccination.Citation6
Figure 1. Plant platform limitations of genetic manipulated plant in production of edible vaccines. Plant-based edible vaccines appear to associate with production: vaccine dosage inconsistency, environmental risks and ITIimmune tolerance induction.
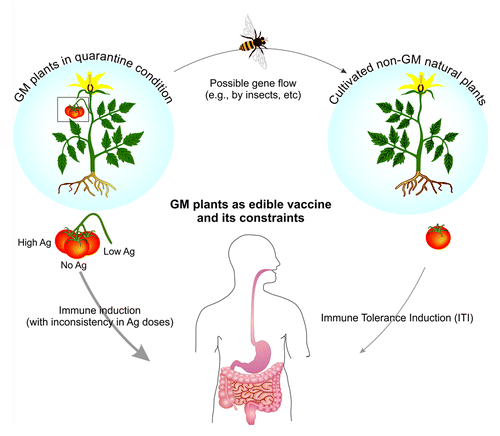
The choice of plant species and target tissue, wherein the protein accumulates, is burdensome (). Thus, the selection of an edible plant that could be used raw (uncooked) is necessary. Nonetheless, some plants products (e.g., potato) need to be cooked prior to its consumption, in which the cooking temperature may result in deformation of Ags structure and even losses in their epitopes. Likewise, climacteric fruits (e.g., banana, tomato) and vegetables (e.g., lettuce), introduced continuously for the production of PEVs, are spoiled during storage or transportation. Moreover, these plants are expensive to be produced considering the quarantine circumstances in greenhouses and great need to water and energy. This clearly means that in comparison with conventional vaccines, this method of vaccines’ development fail to generate low-cost products.
Table 1. Pros and cons of several hosts in edible vaccine production
Of other challenging plant systems, tobacco has been proposed as a robust expression systems even though it is deemed to be an intriguing plant. . In fact, it is not edible because of the presence of toxic compounds such as nicotine. Although this limitation could be transcended with extraction and purification of Ags, this process does not meet the main aims of the cost-effective vaccination strategy. Further, another problem of PEVs is rooted in gene flow.Citation7 According to Cartagena protocols on biosafety, the genetically manipulated (GM) plants must be cultured cautiously in guaranteed fields.Citation8 However, it seems that most developing countries neither do precisely execute the articles of biosafety nor meet the certain rules/regulations in this regard. It should be evoked that inevitable bio-environmental dangers deduced from gene flow and recurrence of unwanted vaccination can result in immune tolerance induction (ITI) against pathogens, and hence already inoculated-individuals may turn vulnerable anew against pathogens ().
Furthermore, most vaccines should be a mixture of several Ags (i.e., multi-component or multivalent vaccines) to prove effectiveness. The expression of several Ags in a single plant and keeping the dose balance appears to be practically improbable. Besides, for vaccination against a pathogen, the culture, transportation, distribution, consumption of tens of transgenic plants and harboring different Ags, deem to be costly and implausible. Therefore, the employment of plants, despite their unique potential, cannot turn our day dreams for the production of easy low-priced vaccines into reality. And possibly the costs of supply and distribution of these plants for effective vaccination exceed the conventional vaccination methods. There is no need to remind that the mucosal immune system (MIS) is not simply stimulated by PEVs and require mucosal adjuvants, while unfortunately the plants do not meet such requirements.
. Limitations of genetic manipulated plant in production of edible vaccines. Plant-based edible vaccines appear to associate with dosage inconsistency, environmental risks and immune tolerance induction.
Edible Germs as Edible Vaccines
Since development and application of PEVs associated with some pivotal limitations,, researchers have recently focused on probiotic bacteria as biofactory for production of bacteria-based edible vaccines.Citation9-Citation11 It should be pointed out that these bacteria, as natural flora of human intestine, have been co-evolved with human body for millions of years.Citation12 Human microbiome project (HMP), which is still running on, will undoubtedly disclose some vital information on the bioimpacts of the commensal microbiome on human health in not far future. Any disruption in balance of these bacteria appears to be seriously associated with human diseases, while the release of live GM-bacteria as edible vaccines within human intestine may inevitably cause inadvertent consequences. It seems the release of such GM-bacteria within human intestine milieu should be with great cautiousness. First, these bacteria are the natural human intestine flora, wherein the release of GM-bacteria may lead to inadvertent colonization. . What will happen then? A high dose of recombinant Ags may be continuously presented to the MIS, which may result in undesired tolerance in the immunosurveillance functionality of the immune system against the target pathogens, or, in a worse scenario, it may lead to a local inflammation because of overreaction of the immune system. . Furthermore, it should not be forgotten that the human gut is an ideal environment for horizontal gene transfer (HGT) within microflora, which may elicit inevitable generation of dangerous pathogens.Citation13 Second, the GM-bacteria are defecated alive and constantly commute between foods and the human body.Citation14 Foods will be infected with the GM-bacteria harboring transgenic Ags. Consequently, global vaccination with these genetically-modified bacteria, which possesses Ags flowed in foods and environment, may lead to a catastrophic situation resulting in contagious outbreaks in part due to ITI ()..
Figure 2. Recombinant Ags flowed by GM- friendly bacteria in intestine (I) and in environment (II). A high dose of recombinant Ag is introduced to the mucosal immune system resulting in immune intolerance induction. And that results in ITI
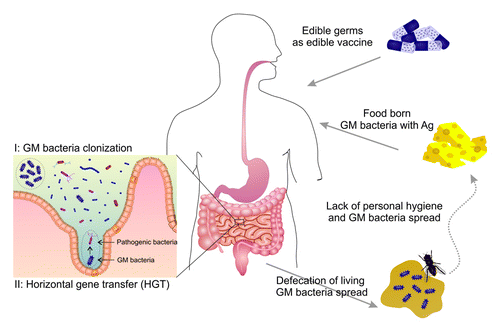
. Recombinant Ags flowed by GM-bacteria in intestine (1) and in environment (2). A high dose of recombinant Ag is introduced to the mucosal immune system resulting in immune tolerance induction.
Spirulina as Tractable Alternate System for Plant in Edible Vaccine Production
Spirulina is a cyanobacterium, whose composition appears to display both prokaryotic and eukaryotic characteristics. Such unique traits make spirulina an excellent candidate for biotechnological applications. Having possessed photosynthesis potential, spirulina can be simply exploited as photosynthesizing bioreactor for efficient production of a wide variety of biotech products, which discriminates it from all other prokaryotes and eukaryotes.
Spirulina, the nature’s richest super nutrient, substitute for mother's milk
Spirulina (Arthrospira) is the nature’s richest super nutrient, with which no plant source may compete. High nutritional value of spirulina and its low cost of production in comparison with plants have put it forward as an attractive candidate to fight against malnutrition. Intergovernmental Institution for the use of Microalgae Spirulina against Malnutrition (IIMSAM) has put forth spirulina to be consumed by children and infants in remote areas under the supervision of the United Nations (UN). It is a crucial source of nutrition which could substitute mother's milk to be used by infants and promote their health.Citation15 In several African and Asian countries, it is used as a major source of protein that is collected from natural water, then dried and consumed. And presently, spirulina has been commercially proposed by human food industry as protein supplement.Citation16 Moreover, the National Aeronautics and Space Administration (NASA) has asserted that the nutritional value of 1000 kg fruits and vegetables equals that of one kg spirulina. This property of spirulina has enabled it to be used in long-term space missions.Citation17,Citation18
Spirulina demonstrates several advantages over other plant nutrients. To mention, but a few: (1) the protein content of spirulina reaches up to 70% of its dry weight in some strains. This property demonstrates that spirulina, if transformed with a strong promoter, could store high doses of spirulina-based edible vaccines.Citation19 Spirulina biomass includes all essential amino acids, large amounts of health promoting lipids, essential fatty acids, ω-3 and ω-6 polyunsaturated fatty acids as γ-linolenic acid (GLA), linoleic acid (LA), stearidonic acid (SDA),eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).Citation20 (2) A collection of vitamins (i.e., B1, B2, B3, B6, B9, B12, vitamin C, D, and E) exist in spirulina.(3) A great deal of potassium and various minerals are present in spirulina. (4) The last, but not least, spirulina is the richest color food with a full spectrum of ten mixed carotenoids. Spirulina’s β carotene is ten times more concentrated than that of carrot.Citation21 It should be also expressed that the malnutritioned children are prone to infectious diseases, hence they may lose their lives not only due to the impacts of pathogens but also because of weakened immune system. All these evidence support the notion of using spirulina as a substitute to colostrums, which can protect children against diseases through combating malnutrition, enhancing immune system and promoting health.
Spirulina as human health promoter
Spirulina possesses great potentials for medical applications. It regulates blood lipid level, lowers plasma triglycerides, and blood pressure,Citation22 hinders neurological damages in aging animals and repairs or avoids stroke-caused damages.Citation23 Spirulina has further applications in curing arthritis, heart disease, diabetes, allergies, obesity, and zinc deficiency.Citation22 They are capable of inhibiting carcinogenesis due to holding anti-oxidant properties in protecting tissues and also reducing toxicity in liver and kidney.
Spirulina suppresses viral infection including herpes virus, cytomegalovirus, influenza virus and human immunodeficiency virus (HIV).Citation24-Citation26 Hundreds of scientific studies have revealed that dietary spirulina can boost the immune system, improve parameters of non-specific defense mechanisms (perhaps through bactericidal and phagocytic functions and lysozyme activity) and increase specific antibody levels (by micro-agglutination).Citation27,Citation28 It has been proven that spirulina represents innate antibacterial activity against pathogens and effectively enhances friendly human bacteria.Citation29
Of the health promoting activities of spirulina is one that is attributed to C-phycocyanin (C-PC). C-PC has been proven to have therapeutic properties including being antioxidant, neuroprotective, anti-inflammatory and anti- cancer.Citation30
Spirulina as a natural immune modulator and mucosal vaccine adjuvant
Induction of intestinal immune system through foreign material is not straightforward biofunction, and in many occasions, the immune system intends to show homeostasis trough tolerance rather than immune induction.Citation31 Therefore, the mere expression of Ags in plants and delivery to intestine could not be responsive as successful vaccination. Sometimes we need an edible adjuvant for effective immune induction that no plant host can offer. Spirulina is a prokaryote with immunostimulatory impacts through CpG-rich oligodeoxynucleotides that could act as adjuvant, leading to innate and acquired immune responses.Citation32,Citation33 It increases phagocytic activity of macrophages, increases accumulation of NK cells into tissue, activates and mobilizes T and B cells and stimulates the production of antibodies (Abs) through enhancing the level of immunoglobulin A (IgA) and M (IgM) and cytokines.Citation34 On the other hand, spirulina consumption appears to restore the balance of intestinal friendly microflora which can in return boost the immune system up against pathogenic bacteria.Citation35
Biological and Biotechnological Potentials of Spirulina vs. Plants
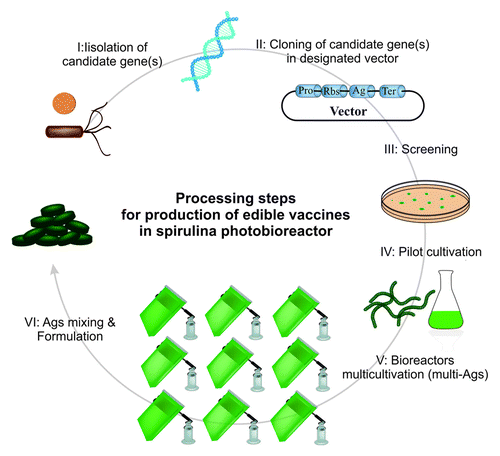
Spirulina is a photosynthetic biosystem that does not need expensive media for cultivation. As a prokaryote, it has rapid growth rate and can simply be genetically manipulated in a short period of time. Since spirulina grows asmulticellular edible microbe, its harvesting is simple and cost-effective without further manipulation. In fact, spirulina has all pros of plants with no cons. It is categorized as Generally Regarded As Safe (GRAS) food source with no contamination of mycotoxins, pesticides and herbicides. The production of spirulina requires low inputs, not yielding limitations of agricultural resources. It is efficient in water and energy usage per mass unit.Citation36 Comparing with green houses, large-scale cultivation of spirulina in photo-bioreactors is more efficient showing high-yielding processes (). Due to adaptation to a wide range of climates, it could be cultured in ordinary pools in high temperature regions of world to modern regulatory photo-bioreactors and outdoor pond cultivations. As a multi-cellular organism, in comparison to other single-celled organisms, it is big enough to be easily collected and harvested. spirulina is an organism which opts for high temperature and survives in a temperature as high as 60 °C,Citation37 hence heat stability of spirulina-based vaccines prove well-promised. Since the whole of multi-cell organism could be consumed as edible vaccine, there is no need to target a specific tissue for the accumulation of Ags and extra processing. Spirulina is a haploid organism that can benefit from the advantages of chloroplast transformation including predictable and targeted DNA integration, possibility of introducing a series of genes, lack of position effect and gene silencing, stable and increased transient expression and reduced environmental dissemination.Citation38 Furthermore, numerous promoters inducible by environmental factors can be used for regulation and enhancement of gene expression. As highlighted previously, the amount of Ag accumulation (low dosage, high dosage and free of Ag) differs in various transgenic plants, tissues and fruits, thus the dosage constancy and reliable vaccination by PEVs seem to be arguable and dubious. However, in the case of spirulina-based vaccines even as multi-valent vaccines, the dose of vaccination could be literally controlled. Millions of cells of transgenic spirulina could be mixed and accessed by all individuals using a single dose of spirulina. Transportation and distribution costs of spirulina-based vaccines are very low. It can be dried to avoid spoilage and then consumed raw. Based upon our preliminary findings (data not shown), the genetic engineering of spirulina is fairly straightforward since transformation to production processes last not more than one month. It should be noted that most pathogens especially viruses experience some forms of “antigenic drift” or “antigenic shift” to escape from the immune system; hence, the previous vaccines will be ineffective against them. With transferring new Ags into spirulina, the vaccines could rapidly be timely updated ( and ).
Figure 3. Transgenic spirulina as a photo-bioreactor fulfills multivalent and multi-edible vaccine. (I) Selection of viral and bacterial pathogens; (II) Cloning candidate genes; (III) Transformation and Screening of transgenic spirulina; (IV) Pilot cultivation; (V) Biomass production in photo-bioreactors; VI. Mixed powder of transgenic spirulina containing different antigens from several pathogens and formulating capsules.
Future Perspective
Considering the innate characteristics of spirulina as a photosynthesized prokaryotic microorganism, we have high hopes for this microorganism to be used as an alternate for plant-based edible vaccines in far more cost-effective manner. We envision that spirulina, in comparison with plant and other organisms, have a robust advantage in reducing the need for sophisticated pieces of work regarding genetic manipulation, biomass production, purification, storage and delivery. Moreover, regarding all properties, we articulate that spirulina could be exploited for production of next generation of vaccines in which we are forced to express several subunits or multi-antigens (e.g., Ags of all flu virusserotypes, malaria vaccine, etc). The transgenic spirulina for each subunit of vaccines could be amplified in separate photo-bioreactors and mixed in only one edible capsule with best dosage consistency that plants cannot offer. The present approach can meet not only the requirement for vaccination in remote areas of the world but also application in suppressing the pandemic diseases and terroristic attacks. This means that millions of vaccine doses could be prepared and stored within a short period of time and be rapidly accessed by all individuals.
Conflict of interests
The authors declare no conflict of interest.
References
- Mason HS, Lam DM, Arntzen CJ. Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci U S A 1992; 89:11745 - 9; http://dx.doi.org/10.1073/pnas.89.24.11745; PMID: 1465391
- Khamsi R. Potatoes pack a punch against hepatitis B. news nature 2005;Available from: http://www.nature.com/news/2005/050214/full/news050214-2.html
- Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol 2012; 12:592 - 605; http://dx.doi.org/10.1038/nri3251; PMID: 22828912
- Sharma M, Sood B. A banana or a syringe: journey to edible vaccines. World J Microbiol Biotechnol 2011; 27:471 - 7; http://dx.doi.org/10.1007/s11274-010-0481-9
- Pelosi A, Shepherd R, Walmsley AM. Delivery of plant-made vaccines and therapeutics. Biotechnol Adv 2012; 30:440 - 8; http://dx.doi.org/10.1016/j.biotechadv.2011.07.018; PMID: 21843627
- Mishra N, Gupta PN, Khatri K, Goyal AK, Vyas SP. Edible vaccines: A new approach to oral immunization. Indian J Biotechnol 2008; 7:283 - 94
- Obembe OO, Popoola JO, Leelavathi S, Reddy SV. Advances in plant molecular farming. Biotechnol Adv 2011; 29:210 - 22; http://dx.doi.org/10.1016/j.biotechadv.2010.11.004; PMID: 21115109
- Lin LL. Cartagena Protocol on Biosafety. Biosafety First 2007:407.
- Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000; 289:1352 - 5; http://dx.doi.org/10.1126/science.289.5483.1352; PMID: 10958782
- Stadler BM, Vogel M, Germond E-J, Fritsche R. Lactic acid bacteria as agents for treating and preventing allergy. Google Patents, 2007.
- Grangette C, Müller-Alouf H, Goudercourt D, Geoffroy M-C, Turneer M, Mercenier A. Mucosal immune responses and protection against tetanus toxin after intranasal immunization with recombinant Lactobacillus plantarum. Infect Immun 2001; 69:1547 - 53; http://dx.doi.org/10.1128/IAI.69.3.1547-1553.2001; PMID: 11179325
- Barzegari A, Saei AA. Designing probiotics with respect to the native microbiome. Future Microbiol 2012; 7:571 - 5; http://dx.doi.org/10.2217/fmb.12.37; PMID: 22568713
- Syvanen M. Evolutionary implications of horizontal gene transfer. Annu Rev Genet 2012; 46:341 - 58; http://dx.doi.org/10.1146/annurev-genet-110711-155529; PMID: 22934638
- Cummins J, Ho M-W. Genetically modified probiotics should be banned. Microb Ecol Health Dis 2005; 17:66 - 8; http://dx.doi.org/10.1080/08910600510044480
- Belay A, Ota Y, Miyakawa K, Shimamatsu H. Current knowledge on potential health benefits of Spirulina. J Appl Phycol 1993; 5:235 - 41; http://dx.doi.org/10.1007/BF00004024
- Koru E. Earth Food Spirulina (Arthrospira): Production and Quality Standarts. In: El-Samragy Y, ed. Food Additive. Turkey: InTech, 2012:191-202.
- Ravi M, De SL, Azharuddin S, Paul SF. The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties. Nutr Diet Suppl 2010; 2:73 - 83
- Saei AA, Omidi AA, Barzegari A. Screening and genetic manipulation of green organisms for establishment of biological life support systems in space. Bioengineered 2013; 4:65 - 71; http://dx.doi.org/10.4161/bioe.22286; PMID: 22992434
- Phang SM, Miah MS, Yeoh BG, Hashim MA. Spirulina cultivation in digested sago starch factory wastewater. J Appl Phycol 2000; 12:395 - 400; http://dx.doi.org/10.1023/A:1008157731731
- Wu LC, Ho JA, Shieh MC, Lu IW. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J Agric Food Chem 2005; 53:4207 - 12; http://dx.doi.org/10.1021/jf0479517; PMID: 15884862
- Tobón-Velasco JC, Palafox-Sánchez V, Mendieta L, García E, Santamaría A, Chamorro-Cevallos G, Limón ID. Antioxidant effect of Spirulina (Arthrospira) maxima in a neurotoxic model caused by 6-OHDA in the rat striatum. J Neural Transm 2013; 120:1179 - 89; http://dx.doi.org/10.1007/s00702-013-0976-2; PMID: 23430275
- Lee EH, Park JE, Choi YJ, Huh KB, Kim WY. A randomized study to establish the effects of spirulina in type 2 diabetes mellitus patients. Nutr Res Pract 2008; 2:295 - 300; http://dx.doi.org/10.4162/nrp.2008.2.4.295; PMID: 20016733
- Wang Y, Chang CF, Chou J, Chen HL, Deng X, Harvey BK, Cadet JL, Bickford PC. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp Neurol 2005; 193:75 - 84; http://dx.doi.org/10.1016/j.expneurol.2004.12.014; PMID: 15817266
- Khan Z, Bhadouria P, Bisen PS. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol 2005; 6:373 - 9; http://dx.doi.org/10.2174/138920105774370607; PMID: 16248810
- Yamani E, Kaba-Mebri J, Mouala C, Gresenguet G, Rey JL. [Use of spirulina supplement for nutritional management of HIV-infected patients: study in Bangui, Central African Republic]. Med Trop (Mars) 2009; 69:66 - 70; PMID: 19499738
- Barrón LB, Torres-Valencia MJ, Chamorro-Cevallos G, Zúñiga-Estrada A. Spirulina in human nutrition and health. In: Gershwin ME, Belay A, eds. Spirulina as an Antiviral Agent. New York: CRC Press,Taylor & Francis, 2008:227-40.
- Watanuki H, Ota K, Tassakka ACMA, Kato T, Sakai M. Immunostimulant effects of dietary< i> Spirulina platensis</i> on carp,< i> Cyprinus carpio</i>. Aquaculture 2006; 258:157 - 63; http://dx.doi.org/10.1016/j.aquaculture.2006.05.003
- Das P, Mishra A, Das M. Spirulina: the superfood and medicine. Algal biotechnology: new vistas 2010:62-76.
- Pradhan J, Das BK, Sahu S, Marhual NP, Swain AK, Mishra BK, et al. Traditional antibacterial activity of freshwater microalga Spirulina platensis to aquatic pathogens. Aquacult Res 2012; 43:1287 - 95; http://dx.doi.org/10.1111/j.1365-2109.2011.02932.x
- Pentón-Rol G, Marín-Prida J, Pardo-Andreu G, Martínez-Sánchez G, Acosta-Medina EF, Valdivia-Acosta A, Lagumersindez-Denis N, Rodríguez-Jiménez E, Llópiz-Arzuaga A, López-Saura PA, et al. C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res Bull 2011; 86:42 - 52; http://dx.doi.org/10.1016/j.brainresbull.2011.05.016; PMID: 21669260
- Chervonsky AV. Intestinal commensals: influence on immune system and tolerance to pathogens. Curr Opin Immunol 2012; 24:255 - 60; http://dx.doi.org/10.1016/j.coi.2012.03.002; PMID: 22445718
- ZHOU Y-c, HU Y-x, MA Z. Animals Study on Spirulina as an Adjuvant Blood Lipid Lowering and Immune Function Enhancing Agent [J]. Practical Preventive Medicine 2006; 2:033.
- Volpi C, Fallarino F, Pallotta MT, Bianchi R, Vacca C, Belladonna ML, Orabona C, De Luca A, Boon L, Romani L, et al. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat Commun 2013; 4:1852; http://dx.doi.org/10.1038/ncomms2874; PMID: 23673637
- Hirahashi T, Matsumoto M, Hazeki K, Saeki Y, Ui M, Seya T. Activation of the human innate immune system by Spirulina: augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int Immunopharmacol 2002; 2:423 - 34; http://dx.doi.org/10.1016/S1567-5769(01)00166-7; PMID: 11962722
- Hironobu W, Kazuki O, Asmi C, Tassakka T, Masahiro S. Immunostimulant effects of dietary Spirulina platensis on carp, Cyprinus carpio. Aquaculture 2006; 258:157 - 63; http://dx.doi.org/10.1016/j.aquaculture.2006.05.003
- Ahsan M, Habib B, Parvin M, Huntington TC, Hasan MR. A review on culture, production and use of spirulina as food for humans and feeds for domestic animals. FAO, 2008.
- Ravi M, De SL, Azharuddin S, Paul SFD. The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties. Nutrition and Dietary Supplements 2010; 2.
- Walker TL, Purton S, Becker DK, Collet C. Microalgae as bioreactors. Plant Cell Rep 2005; 24:629 - 41; http://dx.doi.org/10.1007/s00299-005-0004-6; PMID: 16136314
