Abstract
Economic evaluation of vaccination programs can be challenging and does not always fully capture the benefits provided. Reasons for this include the difficulties incurred in accurately capturing the health and economic impact of infectious diseases and how different diseases may interact with each other. Rotavirus infection, for example, peaks at a similar time than other infectious diseases, such as RSV and influenza, which can cause hospital overcrowding and disruption, and may pose a risk to more vulnerable children due to limited availability of isolation facilities. Another challenge, specific to evaluating childhood vaccination, is that QoL cannot be accurately measured in children due to a lack of validated instruments. Childhood diseases also incur a care giver burden, due to the need for parents to take time off work, and this is important to consider. Finally, for diseases such as RVGE, cost-effectiveness analyses in which longer time horizons are considered may not reflect the short-term benefits of vaccination. Further quantification of the economic impact of childhood diseases is thus required to fully highlight the true benefits of childhood vaccination that may be realized. Herein we explore the limitations of existing economic evaluations for childhood vaccination, and how economic analyses could be better adapted in future.
Introduction
Today’s healthcare policy makers operate in a world of cost containment and value-driven decision making. In this context, health economics evaluation is often an essential component of Health Technology Assessment (HTA) and constitutes a key decision tool in determining whether investment is worthwhile. However, existing economic evaluations usually focus on a narrow set of benefits which may lead to substantial underestimates of the true value of vaccination. In particular, evaluations of vaccination programs that focus on cost-effectiveness analyses, which consider only direct medical costs from a payer perspective, can be too restrictive and may not always fully reflect the economic value of vaccination.Citation1
The economic evaluation of rotavirus (RV) vaccination, which has recently been recommended in several European countries including France, Germany, and the United Kingdom (UK), is a case in point. RV is a frequent cause of RV gastroenteritis (RVGE) in children under the age of 5 y, and 2 vaccines, Rotarix® and RotaTeq®, are currently licensed for the prevention of RVGE. Although a number of cost-effectiveness analyses have been conducted for RV vaccination worldwide, uncertainty remains regarding the cost-effectiveness of RV vaccination in developed countries. This is due in part to the heterogeneity of models used and to a lack of broader health economic considerations. Indeed, most studies have found RV vaccination to be cost-effective in certain scenarios only, for example when the effects of herd immunity and a societal perspective are considered.Citation2
Although the benefits of childhood vaccination are widely acknowledged, current economic evaluations of pediatric vaccines can underestimate the real value provided for a number of reasons. First, overlap in the peaks of seasonal pediatric infections, and consequent nosocomial infections, results in considerable pressure on and disruption to healthcare systems (including GP and emergency consultations, and hospitalizations), which is often not captured by cost-effectiveness models. Second, when conducting cost-effectiveness analysis of childhood diseases, it is extremely difficult to accurately value the quality of life (QoL) in children.Citation2 For this reason, it is important to consider the related burden to parents and care givers, in terms of both QoL and productivity losses. Third, for diseases such as RVGE, where most of the vaccination benefits are realized in the short-term, the value of vaccination may not be fully demonstrated in cost-effectiveness analyses which focus on long-term outcomes.
Herein we examine the limitations of current cost-effectiveness analyses for assessing the full economic value of childhood vaccination, and we provide recommendations for further work required to overcome these limitations. We focus our attention on RV vaccination, given that it combines particularities related to infectious disease seasonality, childhood interventions, and short-term benefits.
Challenges in Evaluating Seasonal Infectious Diseases
Seasonality of infections, and its impact on resource utilization, is not often considered in economic evaluations, with one of the key issues being the scarcity of evidence to quantify this. Indeed, while pressure imposed on hospital resources as a result of overlap with seasonal peaks of other infectious diseases is recognized, this is difficult to quantify in monetary terms. In the case of RV vaccination, for example, it can be difficult to accurately predict the impact of overlap between the peak season of RV infection, from January to April, with the peak seasons of influenza and respiratory syncytial virus (RSV) infections. The main route of transmission of RV is by contact, either directly, or indirectly via the fecal-oral route, and the virus can be excreted both prior to onset of symptoms and during diarrhea. Since RV is highly resistant in the environment, and can retain infectivity for at least 4 h on human hands, and for several days in the air and on inanimate surfaces,Citation3 nosocomial RV can result in a high burden of disease even when infection-control measures are in place. Seasonal overlap of RV with other diseases can pose an additional burden on already busy health services, including not only hospitals but potentially also GP surgeries and emergency departments, and can lead to increases in transmission of infections, use of hospital resources, and hospital disruption, which may be associated with further direct costs.Citation4 Indeed, in Europe, RV accounts for more than 87 000 hospitalizations, of which 11% to 25% are nosocomial,Citation5,Citation6 and almost 700 000 outpatient visits annually.Citation7 A prospective cohort study conducted in Italy estimated costs of €8 019 155 per year resulting from nosocomial RV infection.Citation8
Additional costs resulting from overlap of RV with other infections are likely to occur in a number of areas. Increased burden on hospital capacity can put pressure on staff, affecting their ability to deliver high-quality care to children or delaying planned elective surgeries for other children. Moreover, nosocomial RV infection may lead to hospital re-admissions or increased resource use that may not be fully captured in cost-effectiveness analyses. For example, RV may increase the severity of the initial disease for which the child was hospitalized and increase the duration of hospitalization (with studies reporting increases of between 1.7 d and 5.9 d), putting further pressure on staff and resulting in additional costs to treat the primary infection.Citation9 This may be particularly problematic when RV occurs in already vulnerable children who are specifically at risk of nosocomial infections due to repeated or prolonged hospital stays, such as children born prematurely, or children with chronic respiratory, gastroenterologic, or neurologic diseases. The effect of nosocomial RV infection on vulnerable children was demonstrated by a study in a Canadian pediatric hospital, in which 59% of children who had contracted nosocomial RVGE had a chronic underlying medical condition, and in which 29% of patients were premature or dysmature at birth.Citation10 Concomitant peaks of infections also limit the availability of isolation facilities, especially in the emergency-room setting, potentially endangering vulnerable children and increasing the risk of further infection.
Outbreaks of nosocomial RV infection can also cause considerable disruption to hospital organization, as illustrated by a retrospective investigation of an RVGE outbreak in a neonatal medium care unit in the Netherlands.Citation11 During this nosocomial outbreak of RVGE, a number of procedures had to be put in place to reduce the spread of the infection, including hand washing after changing diapers, cohorting infants with diarrhea, having separate medical equipment and clothes for infected children, restricting contact with infants to only parents and specific caregivers, and even, when cases persisted, a 3-d ward closure and chlorine disinfection of all surfaces. Additionally, in a study investigating levels of RV infection over a 5-y period, no correlation was found between improvement in hand-washing compliance and stringent infection-control practices and decrease in the transmission rate of RV.Citation12 Vaccination to prevent RV may therefore provide a considerable benefit in preventing similar outbreaks, which can have severe consequences for the quality of healthcare and incur significant additional healthcare costs. Indeed, in a retrospective study investigating change in bed-day occupancy before and after introduction of the RotaTeq® vaccine in Finland, there was a reduction in bed days for RVGE of between 79% and 92% after introduction of the vaccination program.Citation13
The challenge of quantifying the cost burden associated with seasonal overlap of RV infection with other childhood diseases therefore represents a key limitation of cost-effectiveness analyses. Although the current evidence available does suggest that seasonality of RV infection may have an important economic impact, further research to generate better data are needed in order to quantify this adequately. In particular, real-world data on hospital capacity constraints during peak RVGE season and research into the additional risk of nosocomial infection as a result of hospital overcrowding, are important in order to demonstrate more accurately the full value of RV vaccination. For example, a pilot study in Belgium reported a significant reduction in average hospital pattern and personnel measurement scores (calculated from analysis of a number of factors, including bed-day occupancy, bed turnover, and nosocomial infection) with the introduction of RV vaccine (P < 0.05), supporting the notion that vaccination may relieve the seasonal burden of infections on hospitals.Citation14
One area worthy of investigation is the impact of capacity constraints on the quality and cost of hospital care. Opportunity costs incurred by the hospital could be quantified by investigating the percentage of beds used during the peak season of RV infection, the extent of delays to childhood elective surgeries, the number and duration of ward closures, or the additional costs for complication of a pediatric disease due to RV nosocomial infection, which can be reflected by a diagnosis-related group (DRG) change or a higher DRG weight. Comparisons between pre-vaccination and post-vaccination data in countries that have implemented RV vaccination would provide additional evidence, enabling better assessment of the economic impact of the seasonal burden of RV vaccination.
Care Giver Burden Associated with Childhood Diseases
Another significant limitation of cost-effectiveness analysis for childhood diseases is that it is extremely difficult to accurately value the utility in children, because the instruments used to quantify this in adults are not appropriate and difficult to administer in children,Citation2 leading to uncertainty in cost-effectiveness results. For this reason, it is important to consider the related burden to parents and care givers, which manifests itself in terms of QoL burden (increased parental stress), economic impact on the family (time taken off work to care for the child, increased childcare costs), and societal burden (loss of working days, reduced productivity). Vaccination against childhood diseases therefore provides an additional economic benefit that is not captured in cost-effectiveness analysis adopting the third-party-payer perspective.
In a prospective study (REVEAL) in 1102 children with RVGE, conducted across 7 European countries, at least one parent/care giver had to take time off work to nurse a sick child in 39% to 91% of cases in the hospital setting and in 44% to 64% of cases in the emergency-department setting.Citation15 The mean number of working days lost ranged from 2.3 d (France, hospital setting) to 7.5 d (UK, primary-care setting), which constituted a considerable economic burden from a societal perspective.Citation15 Additional childcare arrangements were required in up to 21% of episodes in this study, representing an economic burden on families affected. However, while there is some evidence demonstrating a high burden to care givers, there is a need for further research data to more accurately quantify the economic impact of this, as this can have a considerable impact on the findings of cost-effectiveness analyses.
In 2014, the UK will move to value-based pricing (VBP), whereby the economic impacts of treatments, including vaccines, will be considered more broadly than previously, and not just in terms of direct benefits (such as reduction in hospitalization costs) but also in terms of indirect and societal benefits (such as reduction in working days lost). This underlines an important trend in how decision-makers are assessing the economic value of therapeutic interventions. A VBP approach will require consideration of the economic and QoL burden of RV to care givers in economic evaluations of RV vaccination programs.
Short-Term Benefits of Rotavirus Vaccination
Cost-utility evaluations (which are commonly included in HTA submissions to support reimbursement) may not adequately demonstrate the economic benefits of vaccines that yield relatively short-term benefits, since such evaluations usually assess vaccines' benefits over a long-term horizon. Since RVGE is an acute disease with a short duration, the clinical, social, and health-economic benefits offered by RV vaccination are realized over a short time period, with most of the benefits occurring within 5 y of vaccination. Already, a clear clinical benefit of vaccination has been reported in countries where vaccination programs for RV have been implemented for a few years. For example, in Belgium, a 65% to 83% reduction in RV hospitalizations was observed, and in Finland hospitalizations and outpatient clinic visits decreased by 76% and 81%, respectively.Citation16,Citation17 It is therefore important that these considerable short-term benefits are fully captured.
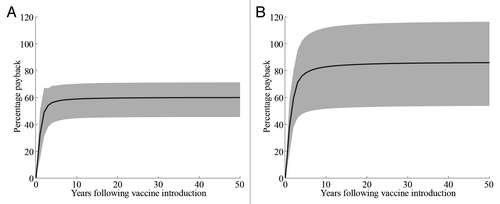
Percentage payback measures the probability that costs expended on an intervention will be recouped, and may effectively demonstrate the short-term benefits of vaccination. A dynamic model by Atkins and colleagues in England and Wales demonstrated a high and rapid percentage payback for RV vaccination. In the model, all costs of RV vaccination were recouped within 10 y at a price of £45 per vaccine course, and over 80% of costs were recouped at the higher price of £60 per course over the same time frame ().Citation18
Figure 1. Budget impact analysis showing the cumulative mean annual percentage payback predicted for introduction of RV vaccination in England and Wales. The 2 graphs show different vaccine waning and cost scenarios: (A) immediate vaccine immunity waning after vaccination, concomitant vaccine administration; (B) delayed vaccine immunity waning after vaccination, concomitant vaccine administration. The black line is the median, and the gray area represents the 95% predictive range. Price for a full-course regimen is taken to be £60. Discount rate is fixed at 3.5%. SOURCE: 18; Reprinted from Vaccine, Vol 30, Atkins K E, Shim E, Carroll S, Quilici, S, Galvani, A P, The cost-effectiveness of pentavalent rotavirus vaccination in England and Wales, Pages 6766–6776, Copyright (2012), with permission from Elsevier.
A Markov model by Vitale and colleagues, which estimated the cost and benefits of vaccinating newborns in an Italian population, in comparison with no vaccination and considering both the national health service and societal perspectives, produced similar findings.Citation19 The budget impact analysis in this model showed that, as early as the second year after introduction, the costs of the vaccine would be more than offset by savings from prevented cases of RVGE and reduced hospitalization, and this saving would increase in subsequent years. Savings amounted to €34 440 over 5 y, equivalent to a saving of €4.64 per child from the national health service perspective. An even stronger economic impact of introducing the vaccine would have been observed if absenteeism and loss of productivity had been considered. There is, therefore, a strong case for consideration of payback and budget impact analyses, which better reflect the short-term benefits of RV vaccination, as an addition to cost-effectiveness analyses which focus on a longer time horizon. The use of payback analyses may also help to overcome the issue of QoL evaluation in children and to inform policy makers on the economic feasibility of implementing a RV vaccination program.
Conclusion
Economic evaluation of vaccines can be challenging and does not always reflect the full benefits provided due to several features specific to infectious diseases and childhood interventions. Difficulty in accurately predicting the real burden on hospitals due to overlap of seasonal infections and the risk this might pose to more vulnerable children can lead to an underestimation of the true value of vaccination. Indeed, in addition to reducing healthcare costs, vaccination has the potential to increase the efficiency of healthcare systems. Furthermore, cost-effectiveness analyses are inherently limited when evaluating childhood diseases, because there are no validated instruments to quantify QoL in children. The QoL impact and economic burden to the care giver is therefore particularly important, and should be considered when evaluating the economic value of childhood disease. Lastly, the considerable short-term benefits of RV vaccination (as demonstrated by a rapid predicted payback) are not fully captured by cost-effectiveness analyses, which tend to focus on longer-term impact of interventions. Payback analysis may be a complementary way to reflect the economic value of RV vaccination.
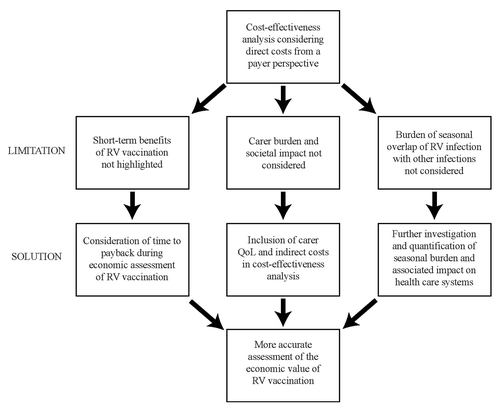
In the future, a number of actions could be taken to better consider the full value of implementing childhood vaccination programs such as RV vaccination as summarized in . First, investigation of the seasonal burden of infections (and its impact on hospital resources, efficiency, and outcomes for more vulnerable children) needs to be performed, and should be incorporated into cost-effectiveness analysis. Second, indirect costs and societal burden, including QoL impact and economic burden to care givers, should be considered within cost-effectiveness analyses. This is particularly important given that the QoL impact on children cannot be easily quantified. Lastly, using payback analysis as an additional methodology during economic evaluation would be valuable, and would ensure that the benefits provided by vaccines offering short-term benefits are considered. While further work is needed to robustly quantify the impact of the alternative economic evaluations discussed, it is clear that a broader range of economic benefits should be considered to ensure that payers fully understand the value of childhood vaccines such as the RV vaccine, and make decisions accordingly.
| Abbreviations: | ||
| DRG | = | diagnosis-related group |
| HTA | = | Health Technology Assessment |
| QoL | = | quality of life |
| RSV | = | respiratory syncytial virus |
| RV | = | rotavirus |
| RVGE | = | rotavirus gastroenteritis |
| UK | = | United Kingdom |
Disclosure of Potential Conflicts of Interest
The preparation of this paper was funded by Sanofi Pasteur MSD, the manufacturer of the RotaTeq® vaccine for the prevention of rotavirus gastroenteritis. B.B. and M.L. received honoraria from Sanofi Pasteur MSD for their contributions to the paper. Medical writing services from HERON™ Commercialization, a PAREXEL company, were funded by Sanofi Pasteur MSD, and F.R.S. is an employee at HERON. V.R. is employed by Sanofi Pasteur MSD. We hereby confirm that all financial or other relationships that may be construed as a conflict of interest have been disclosed.
References
- Postma MJ, Standaert BA. Economics of vaccines revisited. Hum Vaccin Immunother 2013; 9:1139 - 41; http://dx.doi.org/10.4161/hv.23447; PMID: 23364247
- Aballéa S, Millier A, Quilici S, Caroll S, Petrou S, Toumi M. A critical literature review of health economic evaluations of rotavirus vaccination. Hum Vaccin Immunother 2013; 9:1272 - 88; http://dx.doi.org/10.4161/hv.24253; PMID: 23571226
- Ansari SA, Springthorpe VS, Sattar SA. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis 1991; 13:448 - 61; http://dx.doi.org/10.1093/clinids/13.3.448; PMID: 1866549
- Morgan C, Adlard N, Carroll S, Parvataneni L. Burden on UK secondary care of rotavirus disease and seasonal infections in children. Curr Med Res Opin 2010; 26:2449 - 55; http://dx.doi.org/10.1185/03007995.2010.518135; PMID: 20818925
- Wildi-Runge S, Allemann S, Schaad UB, Heininger U. A 4-year study on clinical characteristics of children hospitalized with rotavirus gastroenteritis. Eur J Pediatr 2009; 168:1343 - 8; http://dx.doi.org/10.1007/s00431-009-0934-z; PMID: 19205732
- Fischer TK, Bresee JS, Glass RI. Rotavirus vaccines and the prevention of hospital-acquired diarrhea in children. Vaccine 2004; 22:Suppl 1 S49 - 54; http://dx.doi.org/10.1016/j.vaccine.2004.08.017; PMID: 15576202
- Soriano-Gabarró M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J 2006; 25:Suppl S7 - 11; http://dx.doi.org/10.1097/01.inf.0000197622.98559.01; PMID: 16397431
- Festini F, Cocchi P, Mambretti D, Tagliabue B, Carotti M, Ciofi D, Biermann KP, Schiatti R, Ruggeri FM, De Benedictis FM, et al. Nosocomial Rotavirus Gastroenteritis in pediatric patients: a multi-center prospective cohort study. BMC Infect Dis 2010; 10:235; http://dx.doi.org/10.1186/1471-2334-10-235; PMID: 20696065
- Gleizes O, Desselberger U, Tatochenko V, Rodrigo C, Salman N, Mezner Z, Giaquinto C, Grimprel E. Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J 2006; 25:Suppl S12 - 21; http://dx.doi.org/10.1097/01.inf.0000197563.03895.91; PMID: 16397425
- Verhagen P, Moore D, Manges A, Quach C. Nosocomial rotavirus gastroenteritis in a Canadian paediatric hospital: incidence, disease burden and patients affected. J Hosp Infect 2011; 79:59 - 63; http://dx.doi.org/10.1016/j.jhin.2011.04.020; PMID: 21723643
- Widdowson MA, van Doornum GJ, van der Poel WH, de Boer AS, van de Heide R, Mahdi U, Haanen P, Kool JL, Koopmans M. An outbreak of diarrhea in a neonatal medium care unit caused by a novel strain of rotavirus: investigation using both epidemiologic and microbiological methods. Infect Control Hosp Epidemiol 2002; 23:665 - 70; http://dx.doi.org/10.1086/501991; PMID: 12452294
- Anderson EJ, Rupp A, Shulman ST, Wang D, Zheng X, Noskin GA. Impact of rotavirus vaccination on hospital-acquired rotavirus gastroenteritis in children. Pediatrics 2011; 127:e264 - 70; http://dx.doi.org/10.1542/peds.2010-1830; PMID: 21262887
- Hartwig S, Uhari M, Renko M, Hemming M, Vesikari T. Bed occupancy for rotavirus gastroenteritis in two Finnish hospitals before and after the implementation of the vaccination program with Rotateq. European Society for Paediatric Infectious Diseases. 2014. Ref Type: Conference Proceeding.
- Alwan A, Strens D, Raes M, Standaert B. Improvement in hospital quality of care after the introduction of rotavirus vaccination: a pilot study in Belgium. 2013. Ref Type: Conference Proceeding.
- Van der Wielen M, Giaquinto C, Gothefors L, Huelsse C, Huet F, Littmann M, Maxwell M, Talayero JM, Todd P, Vila MT, et al, REVEAL Study Group. Impact of community-acquired paediatric rotavirus gastroenteritis on family life: data from the REVEAL study. BMC Fam Pract 2010; 11:22; http://dx.doi.org/10.1186/1471-2296-11-22; PMID: 20230601
- Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J 2011; 30:Suppl S1 - 5; http://dx.doi.org/10.1097/INF.0b013e3181fefa1f; PMID: 21183833
- Hemming M, Räsänen S, Huhti L, Paloniemi M, Salminen M, Vesikari T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr 2013; 172:739 - 46; http://dx.doi.org/10.1007/s00431-013-1945-3; PMID: 23361964
- Atkins KE, Shim E, Carroll S, Quilici S, Galvani AP. The cost-effectiveness of pentavalent rotavirus vaccination in England and Wales. Vaccine 2012; 30:6766 - 76; http://dx.doi.org/10.1016/j.vaccine.2012.09.025; PMID: 23000223
- Vitale F, Barbieri M, Dirodi B, Vitali Rosati G, Franco E. [A full economic evaluation of extensive vaccination against rotavirus with RIX4414 vaccine at National and Regional level in Italy]. Ann Ig 2013; 25:43 - 56; PMID: 23435779