Abstract
Bacille Calmette-Guérin (BCG), the only licensed vaccine for the prevention of tuberculosis (TB), provides only limited protection against certain forms of Mycobacterium tuberculosis (Mtb) infection. While infection with Mtb can be treated with antibiotics, the therapy is expensive, toxic, and requires several months for treatment. In addition, the emergence of drug resistant strains limits the impact of antibiotics and underlines the importance of developing a more effective vaccine to control this disease. Given that pulmonary TB is the most common form of the disease, a vaccine capable of inducing lung-resident immunity may be advantageous for combating this infection. New advances in pulmonary delivery make this route of vaccination feasible and affordable. Here, we evaluate the safety and immunogenicity of an aerosolized Ad35-based vaccine, AERAS-402, delivered to the lungs in nonhuman primates as part of a GLP acute and chronic toxicology and safety study. In this study, animals received three high doses (1 x 1011 vp) of AERAS-402 by inhalation via a nebulizer at 1-week intervals. Aerosol delivery of AERAS-402 resulted in an increase in relative lung weights as well as microscopic findings in the lungs, mediastinal lymph nodes, bronchus-associated lymphatic tissue, and the naso-oropharynx that were consistent with the induction of an immune response during the acute phase. These findings resolved by the chronic phase and were considered to be non-adverse. Furthermore, we observed transient vaccine-specific immune responses in the peripheral blood as well as sustained high-level polyfunctional CD4+ and CD8+ T cell responses in the bronchoalveolar lavage fluid of vaccinated nonhuman primates. The data suggest that pulmonary delivery of Ad35-based vaccines can be safe and can induce potent lung-resident immunity.
Introduction
Created nearly a century ago, Bacille Calmette-Guérin (BCG) remains the only licensed vaccine for the prevention of tuberculosis (TB). While the vaccine is effective at limiting certain forms of childhood TB,Citation1-Citation5 adult pulmonary disease continues to be a tremendous burden. In 2011, the WHO reports there were 8.7 million new Mycobacterium tuberculosis (Mtb) infections and more than 1.4 million deaths from TB, despite widespread vaccination with BCG.Citation6 While drug therapies are available, they require months of treatment that is both expensive and toxic. Furthermore, drug-resistant strains of Mtb, MDR (multidrug-resistant) and XDR (extensively drug-resistant), as well as totally drug-resistant TB (TDR), make it clear that the impact of drug therapy is limited.Citation7-Citation12 A new safe and effective vaccine for TB is desperately needed.
The majority of people exposed to Mtb fail to develop active disease.Citation13 However, data suggest that at least some people are latently infected and disease can become active when the immune system is suppressed and yet others progress to disease rapidly.Citation13 There is mounting evidence that cellular immunity is particularly important for controlling Mtb. For example, in humans HIV infection greatly increases the chance for active Mtb infection,Citation14,Citation15 particularly as CD4+ T cells diminish,Citation16 suggesting those cells play a critical role in controlling/preventing TB. Nonhuman primate (NHP) models, which most closely model the immune responses of humans, have implicated CD8+ T cells in protection against TB. In these studies, depletion of CD8+ T cells results in the loss of control of TB infection.Citation17 The application of modern vaccine technology offers potential improvements compared with the current BCG vaccine, including recombinant BCG, adjuvanted BCG, the use of other vaccine platforms (i.e., protein/adjuvant formulations, viral vectors, recombinant bacteria), and prime/boost vaccination strategies, which are effective at inducing cellular immunity. Given that pulmonary TB is the most common form of TB in adults, it may be beneficial to establish lung-resident immunity for protecting against Mtb. Challenge with extremely low numbers of Mtb may result in infection and acute disease or latent infection in cynomolgus macaques,Citation18 but quickly leads to disease in rhesus macaques (unpublished data). While the exact numbers of bacteria involved in human infection are not entirely known, bacterial numbers are expected to be very low. The presence of Mtb-specific T cells in the lungs at the time of infection may be advantageous to the host for controlling or eliminating Mtb, particularly before the infectious organism is allowed to replicate to uncontrollable numbers. Several studies have demonstrated that tissue-resident antigen presenting cells (APCs), primarily dendritic cells (DCs), are able to “imprint” homing capabilities on antigen-specific T cells, directing the T cells back to the tissue where the APC originated.Citation19-Citation22 Therefore, it may be possible to exploit immunological imprinting by targeting lung-resident DCs by aerosol vaccination to elicit lung-resident Mtb-specific T cells.
Aerosol vaccination presents a unique set of challenges and opportunities for combating infection with Mtb.Citation23,Citation24 There have been numerous advances toward the delivery of vaccines to the lungs, including stable dry powder formulations.Citation23,Citation25-Citation27 However, the greatest difficulty with aerosol vaccination is the susceptibility and sensitivity of the lungs to collateral damage from immune responses and inflammation. Inflammatory responses in the lung can do as much, if not more, damage to the lungs than infectious organisms.Citation23,Citation28,Citation29 It may be for this reason that activation of some lung-resident cells, such as alveolar macrophages, appear to limit the inflammatory processes.Citation30 However, despite the challenges and risks associated with aerosol vaccination, the potential benefit of eliciting lung-resident immunity is substantial. Several studies have shown that intranasal (i.n.) immunization using a recombinant adenovirus, either alone or following a BCG prime, provides protection from Mtb over that observed with BCG in miceCitation31-Citation33 and guinea pigs.Citation34 Furthermore, this protection is superior to that observed following parenteral immunization with the same vectors.Citation31,Citation33,Citation34 Interestingly, the inoculum volume given i.n. to mice impacts the location of the immune response, with larger volumes being more likely to deliver to the lungs rather than just the upper respiratory tract.Citation35 Protection in mice appears to be dependent upon the i.n. delivery of larger volumes, suggesting that lung delivery is critical for protection in this model.Citation32
The primary concern with any aerosol vaccination is safety. Delivering foreign substances, particularly vaccines which are designed to elicit immune responses, to the lungs carries the risk of eliciting potentially harmful reactions that could result in significant damage to the airways and, in the case of TB, potentially exacerbate disease as a result of immunopathology. In order to address this concern, we examined lung immunization in a NHP toxicology study using a vaccine currently undergoing clinical testing, AERAS-402/Ad35 (hereafter referred to as AERAS-402). AERAS-402 is a recombinant, replication-deficient Adenovirus serotype 35 vaccine encoding a fusion protein of the Mtb antigens 85A, 85B, and TB10.4.Citation36 Intramuscular injection of AERAS-402 has been shown to be safe and immunogenic in animal models and humans.Citation31,Citation36,Citation37 A prior study in nonhuman primates by Song et al. evaluating aerosol delivery of AERAS-402 examined immune responses following 2 immunizations, 8 wk apart, at dose levels of 109, 1010, and 1011 virus particles per immunization.Citation38 This study demonstrated potent T cell responses in the lungs of vaccinated animals. Here we evaluate the toxicology and immunogenicity of three high doses of AERAS-402 (1011 virus particles per immunization) delivered to the lungs of rhesus monkeys using a nebulizer and given at one week intervals, which is potentially more toxic to the animals. In addition, the inherent nature of aerosol immunization also increases the risk that the eyes may be exposed to the vaccine. Therefore, a single high dose of the vaccine was also administered, dropwise, to the eyes of some animals. The data show that three aerosol vaccinations elicited a transient low-level T cell response in the blood followed by much higher numbers of vaccine-specific T cells in the lungs. Antigen-specific T cells were highly polyfunctional, making IFN-γ, IL-2, and TNF. Despite the high dose, rapid dosing regimen, and high pulmonary levels of T cells, aerosol administration of AERAS-402 did not elicit adverse effects in the lungs of vaccinated animals. Furthermore, ocular administration did not elicit any adverse effects in the eyes. Together these data suggest that lung vaccination with AERAS-402 was able to safely induce lung-resident T cell responses in NHPs.
Results
Toxicology
The primary purpose of this study was to assess the toxicity of aerosol vaccination with AERAS-402 following three doses (n + 1) in male and female rhesus monkeys. The study also evaluated the toxicity following a single ocular topical administration in anticipation of accidental ocular exposure during immunization.
There were no vaccine-related effects for either route of administration on mortality, clinical observations, cageside and post-dose observations, body weights, body weight changes, body temperatures, food consumption, ophthalmology, radiography, clinical pathology, or gross pathology. Vaccine-related organ weight alterations were limited to apparent increased absolute and relative lung weights in Group 1 males and females (aerosol inhalation) at SD 18. This finding correlated with test article-related microscopic findings at SD 18 that included: in the lung, minimal to mild lymphocytic infiltrates, minimal lymphoid hyperplasia, and minimal alveolar macrophages (); in mediastinal lymph nodes, minimal to moderate lymphoid hyperplasia and minimal to mild sinus histiocytosis; in bronchus-associated lymphatic tissue, minimal lymphoid hyperplasia and minimal to mild sinus histiocytosis (); and in naso-oropharynx, minimal to mild lymphoid hyperplasia. At SD 43, there were no test article-related organ weight alterations or microscopic findings ( and ). There were no vaccine-related organ weight or microscopic pathology findings in Group 3 males or females (ocular topical application) with the exception of mild lymphoid hyperplasia that was observed in the male animal. This hyperplasia was considered unlikely to be associated with the vaccine because the female did not exhibit this finding. The toxicology findings are summarized in .
Figure 1. Lung histology. Lungs from control (A and B) or vaccinated (C and D) animals were collected on Study Day 18 (A and C) and 43 (B and D). Tissues were sectioned, H&E stained, and examined by microscopy. Arrows with stems indicate perivascular infiltrates. Arrows without stems indicate alveolar macrophages. Bars indicate 100 µm scale.
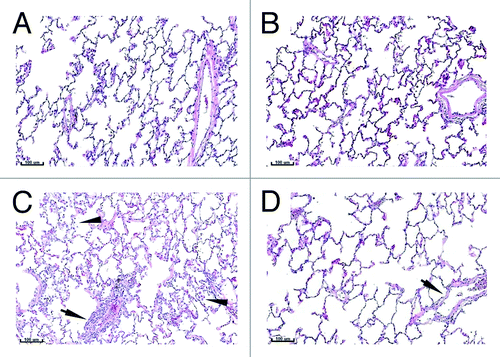
Figure 2. BALT histology. Lungs from control (A and B) or vaccinated (C and D) animals were collected on Study Day 18 (A and C) and 43 (B and D). Tissues were sectioned, H&E stained and examined by microscopy. Arrows indicate bronchus-associated lymphoid tissue (BALT). The asterisk indicates the presence of a germinal center. Bars indicate 200 µm scale.
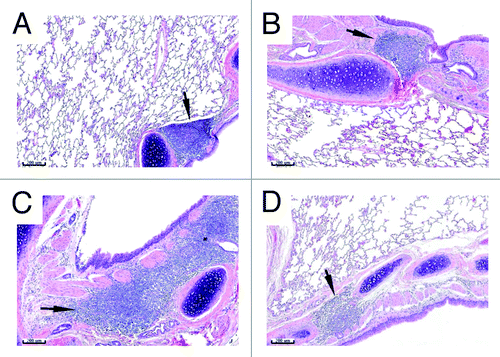
Table 1. Toxicology results
Neutralizing antibodies
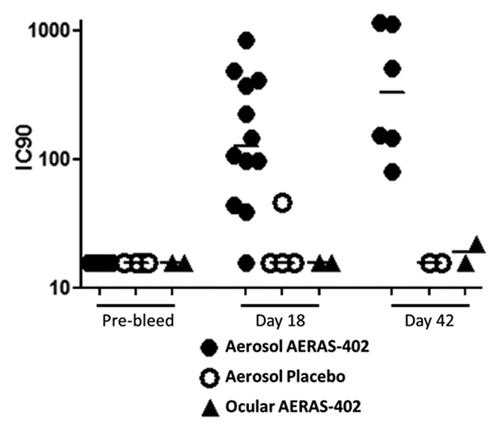
Animals were screened prior to inclusion in this study and at each necropsy time point to determine neutralizing antibody (nAb) responses to Ad35. Only animals lacking a nAb response were included in the study to ensure maximal vaccine effect (for safety measurements). The results show that 11/12 animals (91.7%) that received three aerosol immunizations with 1 x 1011 viral particles (vp) on study days 1, 8, and 15 had detectable neutralizing antibodies three days following the last immunization ( and ). The single animal that failed to mount a detectable nAb response at study day 18 was positive for neutralizing antibodies by study day 43, where 100% of the animals receiving the aerosol immunizations were positive for nAb. Only 1 out of 4 control animals mounted a detectable Ad35 nAb response, which was very low (IC90 = 46) on study day 18. One animal receiving AERAS-402 by ocular administration had a very low nAb level (IC90 = 22) on study day 43. Together the data suggest that three doses of the AERAS-402 vaccine given by aerosol are able to elicit serum nAb to the Ad35 vector, consistent with prior observations for two doses.Citation38 Ocular administration is not effective at driving vector-specific antibody responses. Given that the eye is an immunologically privileged siteCitation39 these results were not unexpected.
Table 2. Serum neutralizing antibodies
Figure 3. Serum neutralizing antibodies. Serum was collected from animals prior to study inclusion (pre-bleed) and at each necropsy time point (SD 18 and 42). The Ad35 neutralizing antibody assay was performed and the 90% inhibitory concentration (IC90) calculated for animals receiving three aerosol immunizations on SD 1, 8, and 15 with AERAS-402 (closed circles) or placebo (open circles), or following a single ocular dose of AERAS-402 on study day 1 (triangles). Responses below the limit of detection are reported as an IC90 = 16. Bars represent group median IC90 results.
T cell responses in the blood
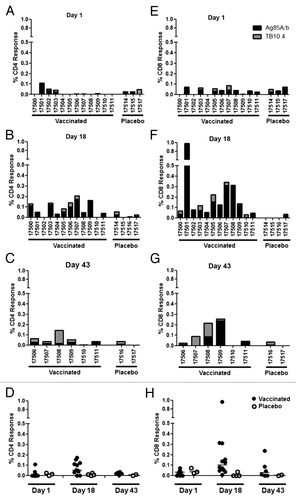
PBMC were collected from animals prior to immunization and on study days 18 and 43 in order to assess the ability of aerosol administration of AERAS-402 to elicit cellular immune responses 3 d or 28 d following the last immunization, respectively. Intracellular cytokine staining (ICS) revealed that the immune response consisted primarily of CD8+ T cells directed against the Ag85A/b peptide pool, which is typically the dominant response following intramuscular immunization with this viral vector ( and previously describedCitation36,Citation37). This CD8+ T cell response was higher on day 18 and diminished by day 43 (). Responses (total response; IFN-γ, IL-2, or TNF alone or in any combination) in the peripheral blood on day 18 ranged from 0.01% to 0.96% with an average response of 0.19%. CD4+ T cell responses were also observed, although not to the magnitude observed with the CD8+ T cell compartment. Again, these responses were primarily against Ag85A/b and peaked on day 18, ranging from 0% to 0.18% with an average response of 0.07% ( and Fig. S1). Interestingly, greater responses were observed in the CD4+ compartment to the subdominant antigen, TB10.4, on day 43. However, these responses remained below those observed against Ag85A/b for most animals ().
Figure 4. PBMC T cell responses. Animals were immunized with aerosol AERAS-402 or placebo on study days 1, 8, and 15. Blood was collected prior to the first immunization (day 1; A and E) and following the second and third immunizations (days 18 and 43; B, F and C, G, respectively). PBMCs were isolated for the measurement of CD4+ (A-D) and CD8+ (E-H) DMSO-subtracted T cell responses against Ag85A/b and TB10.4 (black and gray bars, respectively) using intracellular cytokine staining for IFN-γ, IL-2 or TNF alone or in any combination. The time course of Ag85A/b-specific T cell responses are shown in plots D and H, for both vaccinated (closed circles) and placebo (open circles) groups. Numbers below bar charts represent unique animal numbers. Circles on dot plots represent individual animal responses. Bars on dot plots represent the median response.
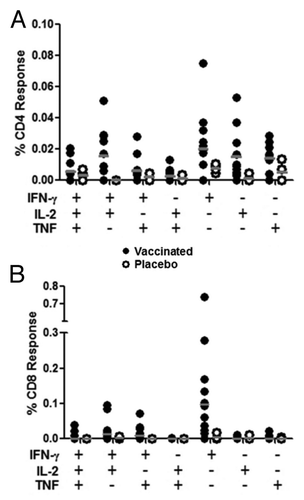
Boolean gates were generated to examine the functionality of the PBMC immune response to Ag85A/b following aerosol vaccination on day 18 for both CD4+ and CD8+ T cells. As expected from the total response, polyfunctional analysis revealed very low CD4+ responses, typically less than 0.04% for any given cytokine combination. The response primarily consisted of cells secreting IFN-γ or IL-2 either alone or in combination (). Interestingly, the CD8+ T cell response was largely composed of cells secreting IFN-γ alone. However, there were some bifunctional (IFN-γ + IL-2 and IFN-γ + TNF) cells observed (). Together the data suggest that the dominant response in the peripheral blood following aerosol immunization with AERAS-402/Ad35 is a transient monofunctional (IFN-γ) CD8+ T cell response against the dominant Ag85A/b peptide pool.
Figure 5. Polyfunctional analysis of PBMC T cell responses on day 18. Animals were immunized with aerosol AERAS-402 (closed circles) or placebo (open circles) on study days 1, 8, and 15. Blood was collected on study day 18 and evaluated by intracellular cytokine staining for production of IFN-γ, IL-2, or TNF for both CD4+ (A) and CD8+ (B) T cells. Circles represent individual responses for each possible cytokine combination. Grey bars represent the group median response.
T cell responses in the lung
The primary purpose for delivering a vaccine to the lung is to generate lung-resident immunity. Therefore, at each necropsy time point (study days 18 and 43), bronchoalveolar samples were collected from two animals immunized with aerosol AERAS-402 and two animals that received aerosol placebo. Cells were isolated for stimulation and intracellular cytokine staining (ICS). Total ICS responses (IFN-γ, IL-2, or TNF alone or in combination) showed much higher percentages of Ag85A/b-specific responses in the lungs of immunized animals than in the periphery. Responses in the lungs were very similar between CD4+ and CD8+ T cells at day 18 with averages of 2.74% and 3.62%, respectively. However, by day 43, we observed a dramatic increase in lung-resident Ag85A/b-specific T cells in the CD8+ T cell compartment, consistent with prior studies using aerosol delivery of AERAS-402.Citation38 The T cell responses increased to an average of 6.74% and 29.03% for CD4+ and CD8+ T cells, respectively. This pattern of CD8+ T cell dominant responses was also observed in the peripheral blood. However, in contrast, lung T cell responses continued to increase at day 43 (–).
Figure 6. Lung T cell responses following aerosol immunization. Animals were immunized with aerosol AERAS-402 (black bars) or placebo (white bars) on study days 1, 8, and 15. Two animals each from vaccinated and placebo control animals were pre-selected for BAL collection at the time of necropsy at each necropsy time point (days 18 and 43). Cells were isolated from BAL and CD4+ and CD8+ responses (production of IFN-γ, IL-2, or TNF alone or in combination) evaluated by intracellular cytokine staining following stimulation with Ag85A/b. Bars represent the background-subtracted Ag85A/b response for each animal. Numbers are unique animal identifiers.
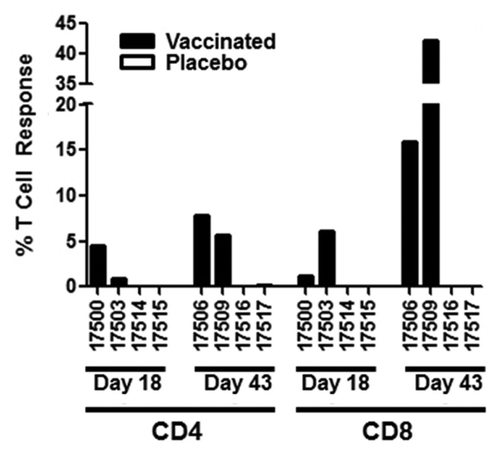
Figure 7. Polyfunctional analysis of lung T cell responses on study days 18 and 43. Animals were immunized with aerosol AERAS-402 (black bars) or placebo (white bars) on study days 1, 8, and 15. Two animals each from vaccinated and placebo groups were pre-selected for BAL collection at each necropsy time point (days 18 and 43). Cells were isolated and evaluated by intracellular cytokine staining to measure IFN-γ, IL-2, and TNF production by CD4+ (A-B) and CD8+ (C-D) T cells on study days 18 (A,C) and 43 (B,D). Bars represent background-subtracted Ab85A/b responses for individual animals for each of the possible cytokine combinations. Grey bars below plots refer to the functionality shown in the pie charts. Pie charts represent the functionality (3 functions, 2 functions, or one function) of the T cell response for each animal.
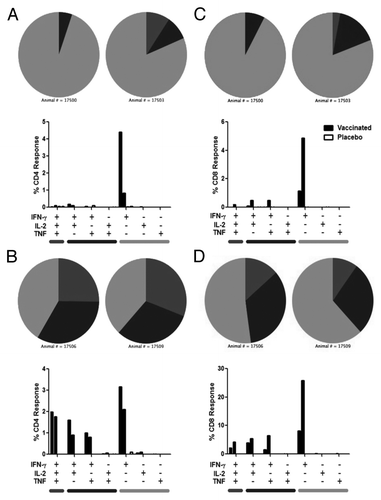
We then examined the functionality of the responding cells in the lungs at days 18 and 43. The results show a similar pattern of functionality on day 18, with the response being primarily driven by cells making IFN-γ alone. However, by day 43, we observed an increase in the polyfunctionality of both CD4+ and CD8+ T cells. CD4+ and CD8+ T cells exhibited a large increase in polyfunctional (IFN-γ, IL-2, and TNF) and bifunctional T cells (IFN-γ with either IL-2 or TNF; ).
Discussion
Aerosol vaccines are designed to elicit antigen-specific T cells in the lungs, with the goal of providing a more rapid and robust response to infection.Citation23 Challenge studies in mice and guinea pigs suggest that mucosal (intranasal) vaccination with recombinant adenoviral vaccines is more effective at inducing protective responses than parenteral vaccination. (Wang et al.Citation33 and Xing et al.Citation34). While intranasal delivery in mice accesses both the nasal and lung compartments, studies have shown that larger volumes of vaccine, which are more likely to result in lung delivery, are required for the induction of protective responses. (Minne et al.Citation35 and Ronan et al.Citation32). Together, these studies suggest lung delivery of AERAS-402 may enhance the efficacy of this vaccine. Song et al. previously reported enhanced CD4+ and CD8+ T cell responses in the lungs of nonhuman primates following aerosol delivery of AERAS-402, but not following delivery of this vaccine by intramuscular administration. (Song et al.Citation38). Whether the generation of T cell responses in the lungs of vaccinated animals is required for protection from Mtb challenge remains to be determined.
The primary concern with aerosol immunization is safety. The induction of an immune response in the lungs has the potential for inducing immunopathology.Citation23,Citation28,Citation29 Early studies with adenoviral vectors, in particular adenovirus serotype 5 (Ad5) vectors, delivered to the lungs of patients with cystic fibrosis (CF) revealed dose-limiting toxicity in humans.Citation40 Toxicity included fever of greater than 101 °F as well as pleuritic chest pain and focal radiographic infiltrates following administration of a high dose (2.1 x 1011 vp). Similar to what was observed in this and other primate studies,Citation41,Citation42 symptoms were transient and largely resolved by day 10.Citation40 However, there are several differences in the CF human trial compared with the toxicology study presented here. The dose given in the human trial is more than twice the dose given in this toxicology study. Further, a large portion of the human population has pre-existing immunity to Ad5 compared with Ad35, possibly adding to the inflammatory responses observed when Ad5 was delivered to the lungs. Finally, vaccination is typically given to healthy individuals with the intent on eliciting immune responses. With the CF trial, the vector was administered to patients with diseased lungs, which can impact deposition of the viral particles and may have contributed to the increased inflammatory responses.Citation43,Citation44
This study was performed to evaluate the toxicity of a recombinant adenovirus serotype 35 vector (Ad35) delivered by the aerosol route in NHPs, specifically addressing concerns regarding the potential for damage to the lungs following the delivery of a recombinant viral vaccine. NHPs are particularly valuable for assessing safety and toxicology because they are genetically and anatomically close to humans. NHPs have proven useful for studying infections of the lung, including tuberculosis, where they exhibit a similar spectrum of disease as seen in humans.Citation45 Vaccine-related organ weight alterations in vaccinated animals were limited to apparent increased absolute and relative lung weights. This was associated with microscopic findings in the lung (), mediastinal lymph nodes, bronchus-associated lymphatic tissue (), and the naso-oropharynx, which completely resolved following the recovery interval (day 43; and ). In addition, aerosol vaccination induced the formation of germinal centers in the BALT (). While these effects are expected responses to a vaccine administered via the aerosol route and were considered non-adverse, it is unknown what impact an unrelated infection would have during this transient period of lung inflammation. Based on the toxicology results, the delivery of the Ad35 vector by aerosol to the lungs was safe in NHPs at a dose of 1 x 1011 vp. Furthermore, accidental exposure to the eyes is a concern with aerosol vaccines. However, no adverse effects were reported following administration of a full dose (1 x 1011 vp) of the Ad35 vaccine by topical administration to the eyes, further supporting the safety of this vaccine.
AERAS-402 administration via repeated aerosol inhalation resulted in ‘vaccine take’ (i.e., a combined immunogenicity endpoint based on neutralizing vector antibody generation and induction of T cell immunity, both in lung tissue and peripheral blood). All animals receiving the aerosol vaccine generated neutralizing antibodies to Ad35, suggesting that the vaccine is effective at eliciting vector-specific humoral immunity in nonhuman primates.
While the number of animals is limited, evaluation of cellular immunity following aerosol immunization suggests a transient CD4+ and CD8+ T cell-mediated immune response in the blood of immunized animals followed by a sustained T cell response in the lungs ( and ). While the CD8+ response was reliably of a greater magnitude than observed with CD4+ T cells, the CD4+ response was substantial, particularly in the lungs. Prior studies examining gut immunity show tissue-resident APCs confer homing properties to the T cells they stimulate.Citation19-Citation22 More recently this has also been shown for the lungs, which showed CCR4 was expressed on lung-homing T cells in mice.Citation45 We examined the expression of CCR4 on vaccine-specific T cells on a limited number of samples following aerosol delivery of AERAS-402 and found no expression of CCR4 on these cells (data not shown). Further studies will be necessary to determine whether lung immunization in NHPs and humans is able to confer similar immune imprinting as was reported in mice. Interestingly, there are reports of respiratory infections priming T cell responses in the spleen.Citation46 While we see no direct evidence for splenic priming in this study, including no changes in splenic weight or histopathology, the possibility remains that aerosol immunization may have primed splenic responses that were too low in magnitude or duration to observe at later time points. Despite the lack of evidence of immune imprinting, the resulting proportion of lung resident CD8+ T cells that were specific for the vaccine antigens was very high in the animals examined (42.17% and 15.89%).
The quality of the immune response following aerosol AERAS-402 delivery appeared to change over time in the immunized animals. At day 18, the immune response was largely monofunctional in the animals tested, with T cells making IFN-γ in response to ex vivo stimulation, and was consistent in both the blood and the lungs. However, by day 43 the response appeared to have matured and was largely polyfunctional in nature ( and ). The apparent change in the functional capacity of these cells may be due to the number of vaccinations and/or the timing of the immune evaluation. We cannot rule out that the observed responses were not representative of the larger group given the limited number of animals tested. The third immunization was given on study day 15, only three days before the day 18 time point. This proximity to the third vaccination may not have allowed enough time for the response to expand functionality following the third vaccination. However, it may also be that the monofunctional T cells are not as long-lived and self-sustaining as their polyfunctional counterparts. The secretion of IL-2 is believed to be important for T cell survival and proliferation.Citation47 Therefore, those cells that make IL-2 may be better able to persist and proliferate compared with cells that are secreting only IFN-γ. Regardless of the cause, the end result was the accumulation of highly functional cells in the lungs of immunized animals for at least one month following the final immunization (day 43).
As mentioned before, there were no adverse vaccine-related findings in the lungs or other organs of immunized animals at study day 43 despite the presence of large numbers of highly functional T cells. While the vaccine was shown to be safe, we do not yet know whether the presence of polyfunctional T cells in the lungs at the time of exposure to Mtb will be beneficial or harmful. Current challenge models are not able to accurately reflect the low levels of bacterial exposure in human infection. Animal models of Mtb infection typically rely on the deposition of large numbers of bacteria into the lung either by instillation using a bronchoscope or by aerosol infection. While models have been examined using low numbers of bacteria, these numbers do not yet approach the theorized numbers of bacteria that are predicted to result in natural infection. Large numbers of bacteria may be beyond the ability of the host immune system to control, making the interpretation of these models difficult at best. The only model to date that evaluates natural transmission involves the use of guinea pigs placed in a ventilation shaft that carries air from Mtb-infected patients.Citation48,Citation49 While this model may more accurately reflect natural infection in humans, it is biologically distant from humans compared with the NHP model. Models will need to be further developed to deliver biologically relevant numbers of bacteria in order to fully understand whether Mtb-specific T cells present in the lungs at the time of infection can impact bacterial burden in a beneficial manner and do so in a way that is safe for the host.
Here we have shown the results of a GLP acute and chronic toxicology and safety study in NHPs for aerosol delivery of a recombinant adenoviral vaccine, AERAS-402. The data suggest that aerosol immunization with this recombinant Ad35 vaccine is safe and immunogenic in NHPs. However, there are several strengths and limitations to this study. A major strength was that the toxicology portion of this study was performed under GLP conditions to ensure the quality, reliability, consistency, and reproducibility of the results. GLP studies are typically performed in small animal models, such as mice, guinea pigs, or rabbits due to the difficulty and expense of performing these studies in larger animals. This GLP study was performed in nonhuman primates, which is incredibly beneficial to the field of aerosol vaccines due to the genetic and anatomic similarity to humans. However, animals were unable to be challenged due to restrictions of GLP requirements in conducting safety/toxicology studies. Therefore, this GLP study and a separate challenge study (Darrah et al., manuscript in preparation) were conducted at nearly the same time, but at different locations, in anticipation of future clinical trials. The evaluation of immunogenicity was limited to the number of animals and number of time points needed to measure “vaccine take” as is required in safety and toxicology studies. Despite the limited number of animals, the immune responses observed are consistent to what has been reported previously (Song et al.), suggesting that the delivery of the vaccine was successful and appropriate for measuring the safety of this vaccine by aerosol delivery. It is important to note that this safety and toxicology assessment does not examine potential issues that may arise following exposure to Mtb. Further testing will be required to specifically address the impact of having large numbers of highly functional vaccine-specific T cells in the lungs at the time of Mtb exposure/challenge for both safety and efficacy.
Materials and Methods
Animals and vaccination
Nine male and nine female Chinese rhesus macaques were housed at Bridge Laboratories in Gaithersburg, Maryland and were identifiable by tattoo. Animals ranged from 2.8 to 6 kg in weight and 44 to 59 mo of age at the start of the study. Prior to arrival, animals were screened for Ad35 neutralizing antibodies. All animals selected for use in this study had titers of ≤ 16, a negative result for this assay. Animals were acclimated for 48 d prior to vaccination and randomized by age, weight, and sex to receive vaccine or control by lung or ocular administration (below). This study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Bridge Laboratories and was compliant with provisions of the USDA Animal Welfare Act, the PHS Policy on Humane Care and Use of Laboratory Animals, and the US. Interagency Research Animal Committee Principles for the Utilization and Care of Research Animals. This study, with the exception of immunological analyses, was conducted in compliance with GLP regulations (21 CFR Part 58).
Vaccination
The AERAS-402 vaccine is a live recombinant replication-deficient adenovirus encoding a fusion protein of three mycobacterial proteins (Ag85A, Ag85B, and TB10.4). Frozen viral stocks were manufactured by Crucell (Leiden, The Netherlands) and supplied to Bridge Laboratories by Aeras (Rockville, MD) and were accompanied by a Certificate of Analysis. The viral stocks were kept frozen at -75 ± 15 °C and diluted just prior to vaccination to deliver a dose of 1 x 1011 viral particles. The vaccine or control was delivered in a biosafety cabinet using a PARI eFlow SCF aerosolization device (generates droplets of ~3–4 μm, which can reach small airways and alveoliCitation50) for lung delivery (1 mL) or topical administration to both eyes at a dose volume of 0.5 mL per eye by drop instillation. Twelve monkeys (3/group/sex) were assigned to Groups 1 and 2 to receive the AERAS-402 vaccine via aerosol inhalation on Study Day (SD) 1, 8, and 15, with a full gross necropsy on SD 18 and 43, respectively. Two animals (1/sex) were assigned to Group 3 to receive AERAS-402 via ocular topical application on SD 1, with a full gross necropsy on SD 43. Four animals (1/group/sex) were assigned to Groups 4 and 5 to receive placebo (10 mM Tris pH7.4, 75 mM NaCl, 5% (w/v) sucrose, 1 mM MgCl2, 100 µM EDTA, 0.5% (v/v) ethanol, 10 mM l-histidine, 0.02% (w/v) polysorbate 80, pH7.4) via aerosol inhalation and ocular topical administration on SD 1, 8, and 15, with a full gross necropsy on SD 18 and 43, respectively ().
Table 3. Study design
Toxicology testing
Parameters evaluated during the study included mortality, clinical, cageside, and post-dose observations, body weights, body weight changes, body temperatures, food consumption, ophthalmology (ocular route only), radiography (inhalation route only), immunology, clinical pathology (clinical chemistry, hematology, coagulation, and urinalysis), gross pathology, absolute and relative organ weights (inhalation route only), and histopathology.
Cageside observations included observation for mortality, moribundity, general health and signs of toxicity. Clinical observations included evaluation of respiratory rate and breathing pattern, skin and fur characteristics, eye and mucous membranes, respiratory, circulatory, autonomic and central nervous systems, and somatomotor and behavior patterns. Daily qualitative food consumption was evaluated using the following scale: poor = 1−4 biscuits eaten, fair = 5−8 biscuits eaten, good = 9−12 biscuits eaten. Body temperatures were collected by implanted microchip and/or rectal probe.
Chest X-rays were conducted using a MinXray HF8015+ Ultralight. The images were captured digitally by IDEXX Digital Radiography, using control box Model #FCB-810. The images were displayed/recorded on a Dell Latitude ATC/D360 Model #PP18L, using IDEXX Equiview software. All images were taken at 56 kV and either 0.04 or 0.06 msec depending on the size of the subject. Animals were anesthetized prior to examination.
Ophthalmologic observations were conducted using indirect ophthalmoscopy and slit-lamp biomicroscopy (as needed) following administration of a mydriatic solution. Animals were anesthetized prior to examination.
Blood and urine specimens were collected prior to vaccination and just prior to necropsy for evaluation of hematology, serum chemistry, and urinalysis.
Necropsy
Animals were euthanized by injection of sodium pentobarbital equivalent (Fatal-Plus™) followed by exsanguination, and subjected to necropsy. Animals were necropsied as soon as possible after the time of death. A gross necropsy, which included examination of the external surface of the body, all orifices, the cranial, thoracic, and abdominal cavities, and their contents, was performed. Bronchoalveolar lavage samples were taken during necropsy for applicable animals (one per sex per aerosol-vaccinated group) as outlined below. Total lung weight was obtained prior to lavage. Organ weights were collected for animals in Groups 1, 2, 4, and 5 only. Organs (lungs, spleen, brain, kidneys, and liver) were weighed as soon as possible after dissection at scheduled necropsies. Paired organs were weighed together. Tissues were preserved in 10% neutral buffered formalin (NBF) with the exception of eyes (with optic nerve) and testes (with epididymides), which were preserved in modified Davidson’s fixative and subsequently transferred to 10% NBF. All preserved tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin by Bridge. Slides were subsequently shipped to Vet Path Services, Inc. (Mason, OH) and examined by a board-certified veterinary pathologist.
Histopathology
All tissues were processed using a Leica ASP300 processor (Leica Microsystems, Buffalo Grove, IL), embedded in paraffin using a Leica EG1160 Paraffin Embedding Center (Leica Microsystems, Buffalo Grove, IL), sectioned using a Leica RM 2125 Microtome, (Leica Microsystems, Buffalo Grove, IL), and stained with hematoxylin and eosin using a Hacker Linear Stainer (Hacker, Fairfield, NJ). The slides were read by a board-certified veterinary pathologist. Photomicroscopy of selected sections of the lung was done using an Olympus s CX41 (Olympus, Center Valley, PA) and a Motic VM600 digital slide scanner (Motic Instruments, Richmond, BC, Canada). Images were processed using Motic Digital Slide Assistant Lite V1 (Motic Instruments, Richmond, BC, Canada).
Immunology
Prior to study inclusion and at necropsy, approximately 1 mL of whole blood was collected from the femoral vein of all animals into serum separator tubes. Tubes were inverted several times and maintained at room temperature prior to centrifugation. Samples were centrifuged at 1500 rcf for 15 min at 25 °C. The resultant serum was transferred to microcentrifuge tubes and shipped to Aeras (on the day of collection) on dry ice. Samples were then shipped to Dr. Dan Barouch’s laboratory at Harvard for evaluation of neutralizing antibodies.
Prior to dosing on SD 1 and at necropsy, blood samples (up to 8 mL) were collected from the femoral vein of all animals in Groups 1, 2, 4, and 5. Blood samples were collected in green-top CPT tubes (cell preparation tubes with sodium citrate) and shipped at ambient temperature (on the day of collection) to Aeras, where peripheral blood mononuclear cells (PBMCs) were prepared for intracellular cytokine staining (ICS; below).
On SD 18 and 43, one animal/sex in Groups 1 and 4 as well as Groups 2 and 5, respectively, was euthanized by injection of sodium pentobarbital equivalent (Fatal-Plus™) followed by exsanguination. The thoracic cavity was opened, and the lung was rapidly removed and weighed. A small incision was made in the main bronchus leading to the left diaphragmatic lobe and the bronchi to the other lobes were ligated. Care was taken to avoid bleeding. The diaphragmatic lobe was lavaged by administration of five consecutive 10-mL aliquots of Sterile Saline for Injection, USP. The infused saline was gently aspirated into a syringe. The lavage fluid from the syringe was ejected carefully into labeled 50-mL conical polypropylene tubes, the volume was recorded, and then diluted 1:1 (volume:volume) with RPMI cell culture media. The tubes were placed on cold packs or wet ice and shipped (on the day of collection) to the Vaccine Research Center (VRC) for evaluation by flow cytometry (below).
Neutralizing antibodies
Ad35-specific NAb titers were assessed by luciferase-based virus neutralization assays as described previously.Citation51,Citation52 A549 human lung carcinoma cells were plated at a density of 1 × 104 cells per well in 96-well plates and infected with E1/E3-deleted, replication-incompetent Ad35 expressing luciferase at a multiplicity of infection (MOI) of 500 with 2-fold serial dilutions of serum in 200 μL reaction volumes. Following a 24-h incubation, luciferase activity in the cells was measured using the Steady-Glo Luciferase Reagent System (Promega, Madison, Wisconsin) with a Victor 1420 Multilabel Counter (Perkin Elmer, Wellesley, Massachusetts). Neutralization titers were defined as the maximum serum dilution that neutralized 90% of luciferase activity.
Intracellular cytokine staining
Intracellular cytokine staining was performed on freshly isolated PBMCs. Briefly, CPT tubes were centrifuged and the PBMCs collected (NOTE: PBMCs from one animal were not obtained due to the lack of a PBMC layer following centrifugation). Cells were then counted and stimulated for 5–6 h with peptide pools (15-mer peptides overlapping by 11 amino acids) for Ag85A/b (peptide pool spanning the entirety of Ag85A and parts of Ag85B not found in Ag85A) and TB10.4 (both peptide pools from JPT, Berlin, Germany). Positive and negative controls were cells stimulated with SEA/SEB and DMSO (peptide carrier), respectively. Stimulations were performed in the presence of Brefeldin A and the costimulatory antibodies α-CD28 and α-CD49d (all from BD). Following stimulation, cells were washed and stained for viability (LIVE/DEAD violet dye; Invitrogen, Carlsbad, California). Cells were then washed and stained for phenotypic markers CD14 (Pacific Blue), CD16 (Pacific Blue) and CD4 (PerCP-Cy5.5) (All BD). Cells were then washed and then fixed and permeabilized with BD Cytofix/Cytoperm (BD) and then stained for CD3 (APC-Cy7), CD8 (APC), IFN-γ (FITC), IL-2 (PE), and TNF (PE-Cy7) (All BD). Cells were then washed, fixed, and analyzed by multiparameter flow cytometry on a modified Becton Dickinson LSR II. Standard data analysis using FlowJo (TreeStar, Ashland, Oregon) and Pestle and Spice software (kindly provided by Dr. Mario Roederer) was performed to identify the proportion of CD4+ or CD8+ T cells that responded by synthesizing cytokines following peptide stimulation. Summary data are presented in the form of the fraction of CD4+ or CD8+ cells that produced one or more of the cytokines IFN-γ, IL-2, or TNF, following background subtraction.
BAL
Prior to vaccination, two animals from vaccinated groups and two animals from placebo groups were selected for BAL collection at each future necropsy time point for evaluation of lung immune responses. The thoracic cavity was opened, and the lung was rapidly removed and weighed. A small incision was made in the main bronchus leading to the left diaphragmatic lobe and the bronchi to the other lobes were ligated. The diaphragmatic lobe was lavaged by administration of five consecutive 10-mL aliquots of sterile physiological saline, and then gently aspirated into a syringe. The lavage fluid was placed into conical polypropylene tubes, the volume was recorded, and then diluted 1:1 (volume:volume) with RPMI cell culture media. Cells were isolated from the BAL by centrifugation and washing followed by incubating with R10 (RPMI supplemented with 10% fetal bovine serum, L-glutamine, and Pen/Strep) containing DNase I (Roche, Indianapolis, Indiana). Cells were than strained, counted, and stimulated with a pool of overlapping 15-mer peptides with 11 amino acid overlap (Ag85A/b, covers the entire Ag85A protein and parts of Ag85B protein not found in Ag85A; JPT), overnight in the presence of Brefeldin A to inhibit the release of newly generated cytokines from the cells and the costimulatory antibodies α-CD28 and α-CD49d. Unstimulated control wells were treated identically except for the addition of peptides, to estimate the “background” (level of cytokine-producing cells which are not specific to the desired antigen). After overnight culture, cells were stained for viability (LIVE/Dead aqua dye; Invitrogen), CD4 (QDot 605; BD), and CD8 (QDot 655; conjugated by the VRC). Cells were then washed, fixed and permeabilized using BD Cytofix/Cytoperm (BD), and then stained for CD3 (APC-Cy7), IL-2 (APC), IFN-γ (FITC), and TNF (PE-Cy7) (All from BD). Cells were analyzed by multiparameter flow cytometry on a modified Becton Dickinson LSR II. Standard data analysis using FlowJo (TreeStar) and Pestle and Spice software (provided by Dr. Mario Roederer) was performed to identify the proportion of CD4+ or CD8+ T cells that responded by synthesizing cytokines following peptide stimulation. Summary data are presented in the form of the fraction of CD4+ or CD8+ cells that produced one or more of the cytokines IFN-γ, IL-2, or TNF, following background subtraction.
Additional material
Download Zip (88.3 KB)Acknowledgments
This work was funded by the Bill and Melinda Gates Foundation and the NIH (grant numbers AI095985, AI078526, and AI096040).
Conflicts of Interest
D.H., R.W., K.H., and V.D. are employed by Aeras. M.D., M.P., and J.S. are employed by Crucell. C.S.G. is employed by Smithers Avanza, who was contracted to perform the study.
References
- Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol 2005; 3:656 - 62; http://dx.doi.org/10.1038/nrmicro1211; PMID: 16012514
- Barker LF, Brennan MJ, Rosenstein PK, Sadoff JC. Tuberculosis vaccine research: the impact of immunology. Curr Opin Immunol 2009; 21:331 - 8; http://dx.doi.org/10.1016/j.coi.2009.05.017; PMID: 19505813
- Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993; 22:1154 - 8; http://dx.doi.org/10.1093/ije/22.6.1154; PMID: 8144299
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006; 367:1173 - 80; http://dx.doi.org/10.1016/S0140-6736(06)68507-3; PMID: 16616560
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014; 58:470 - 80; http://dx.doi.org/10.1093/cid/cit790; PMID: 24336911
- Global Tuberculosis Report 2012. Geneva, Switzerland: World Health Organization, 2012.
- Cohn DL, Bustreo F, Raviglione MC, International Union Against Tuberculosis and Lung Disease. Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD Global Surveillance Project. Clin Infect Dis 1997; 24:Suppl 1 S121 - 30; http://dx.doi.org/10.1093/clinids/24.Supplement_1.S121; PMID: 8994791
- Falzon D, Jaramillo E, Schünemann HJ, Arentz M, Bauer M, Bayona J, Blanc L, Caminero JA, Daley CL, Duncombe C, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011; 38:516 - 28; http://dx.doi.org/10.1183/09031936.00073611; PMID: 21828024
- Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. Totally drug-resistant tuberculosis in India. Clin Infect Dis 2012; 54:579 - 81; http://dx.doi.org/10.1093/cid/cir889; PMID: 22190562
- Masjedi MR, Tabarsi P, Baghaei P, Jalali S, Farnia P, Chitsaz E, Amiri M, Mansouri D, Velayati AA. Extensively drug-resistant tuberculosis treatment outcome in Iran: a case series of seven patients. Int J Infect Dis 2010; 14:e399 - 402; http://dx.doi.org/10.1016/j.ijid.2009.07.002; PMID: 19818664
- Velayati AA, Farnia P, Masjedi MR, Ibrahim TA, Tabarsi P, Haroun RZ, Kuan HO, Ghanavi J, Farnia P, Varahram M. Totally drug-resistant tuberculosis strains: evidence of adaptation at the cellular level. Eur Respir J 2009; 34:1202 - 3; http://dx.doi.org/10.1183/09031936.00081909; PMID: 19880622
- Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest 2009; 136:420 - 5; http://dx.doi.org/10.1378/chest.08-2427; PMID: 19349380
- Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis 2003; 3:578 - 90; http://dx.doi.org/10.1016/S1473-3099(03)00741-2; PMID: 12954564
- Chaisson RE, Martinson NA. Tuberculosis in Africa--combating an HIV-driven crisis. N Engl J Med 2008; 358:1089 - 92; http://dx.doi.org/10.1056/NEJMp0800809; PMID: 18337598
- Pacheco AG, Durovni B, Cavalcante SC, Lauria LM, Moore RD, Moulton LH, Chaisson RE, Golub JE. AIDS-related tuberculosis in Rio de Janeiro, Brazil. PLoS One 2008; 3:e3132; http://dx.doi.org/10.1371/journal.pone.0003132; PMID: 18781195
- Feller L, Wood NH, Chikte UM, Khammissa RA, Meyerov R, Lemmer J. Tuberculosis part 3: HIV-tuberculosis co-infection. SADJ 2009; 64:352 - 4; PMID: 20034289
- Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y, Halliday L, Fortman J, McAllister M, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog 2009; 5:e1000392; http://dx.doi.org/10.1371/journal.ppat.1000392; PMID: 19381260
- Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, Bigbee C, Chiosea I, Capuano SV, Fuhrman C, Klein E, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun 2009; 77:4631 - 42; http://dx.doi.org/10.1128/IAI.00592-09; PMID: 19620341
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004; 21:527 - 38; http://dx.doi.org/10.1016/j.immuni.2004.08.011; PMID: 15485630
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 2003; 424:88 - 93; http://dx.doi.org/10.1038/nature01726; PMID: 12840763
- Picker LJ. Control of lymphocyte homing. Curr Opin Immunol 1994; 6:394 - 406; http://dx.doi.org/10.1016/0952-7915(94)90118-X; PMID: 7917107
- Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur J Immunol 2002; 32:1445 - 54; http://dx.doi.org/10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E; PMID: 11981833
- Hokey DA, Misra A. Aerosol vaccines for tuberculosis: a fine line between protection and pathology. Tuberculosis (Edinb) 2011; 91:82 - 5; http://dx.doi.org/10.1016/j.tube.2010.09.007; PMID: 21067975
- Schwander S, Dheda K. Human lung immunity against Mycobacterium tuberculosis: insights into pathogenesis and protection. Am J Respir Crit Care Med 2011; 183:696 - 707; http://dx.doi.org/10.1164/rccm.201006-0963PP; PMID: 21075901
- Jin TH, Tsao E, Goudsmit J, Dheenadhayalan V, Sadoff J. Stabilizing formulations for inhalable powders of an adenovirus 35-vectored tuberculosis (TB) vaccine (AERAS-402). Vaccine 2010; 28:4369 - 75; http://dx.doi.org/10.1016/j.vaccine.2010.04.059; PMID: 20444437
- Lu D, Hickey AJ. Pulmonary vaccine delivery. Expert Rev Vaccines 2007; 6:213 - 26; http://dx.doi.org/10.1586/14760584.6.2.213; PMID: 17408371
- Sené C, Bout A, Imler JL, Schultz H, Willemot JM, Hennebel V, Zurcher C, Valerio D, Lamy D, Pavirani A. Aerosol-mediated delivery of recombinant adenovirus to the airways of nonhuman primates. Hum Gene Ther 1995; 6:1587 - 93; http://dx.doi.org/10.1089/hum.1995.6.12-1587; PMID: 8664383
- Hodge LM, Marinaro M, Jones HP, McGhee JR, Kiyono H, Simecka JW, Immunoglobulin A. Immunoglobulin A (IgA) responses and IgE-associated inflammation along the respiratory tract after mucosal but not systemic immunization. Infect Immun 2001; 69:2328 - 38; http://dx.doi.org/10.1128/IAI.69.4.2328-2338.2001; PMID: 11254590
- Simecka JW, Jackson RJ, Kiyono H, McGhee JR. Mucosally induced immunoglobulin E-associated inflammation in the respiratory tract. Infect Immun 2000; 68:672 - 9; http://dx.doi.org/10.1128/IAI.68.2.672-679.2000; PMID: 10639432
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3:23 - 35; http://dx.doi.org/10.1038/nri978; PMID: 12511873
- Radosevic K, Wieland CW, Rodriguez A, Weverling GJ, Mintardjo R, Gillissen G, Vogels R, Skeiky YA, Hone DM, Sadoff JC, et al. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun 2007; 75:4105 - 15; http://dx.doi.org/10.1128/IAI.00004-07; PMID: 17526747
- Ronan EO, Lee LN, Tchilian EZ, Beverley PC. Nasal associated lymphoid tissue (NALT) contributes little to protection against aerosol challenge with Mycobacterium tuberculosis after immunisation with a recombinant adenoviral vaccine. Vaccine 2010; 28:5179 - 84; http://dx.doi.org/10.1016/j.vaccine.2010.05.075; PMID: 20558252
- Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, Hitt M, Xing Z. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol 2004; 173:6357 - 65; http://dx.doi.org/10.4049/jimmunol.173.10.6357; PMID: 15528375
- Xing Z, McFarland CT, Sallenave JM, Izzo A, Wang J, McMurray DN. Intranasal mucosal boosting with an adenovirus-vectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis. PLoS One 2009; 4:e5856; http://dx.doi.org/10.1371/journal.pone.0005856; PMID: 19516906
- Minne A, Louahed J, Mehauden S, Baras B, Renauld JC, Vanbever R. The delivery site of a monovalent influenza vaccine within the respiratory tract impacts on the immune response. Immunology 2007; 122:316 - 25; http://dx.doi.org/10.1111/j.1365-2567.2007.02641.x; PMID: 17521369
- Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med 2010; 181:1407 - 17; http://dx.doi.org/10.1164/rccm.200910-1484OC; PMID: 20167847
- Hoft DF, Blazevic A, Stanley J, Landry B, Sizemore D, Kpamegan E, Gearhart J, Scott A, Kik S, Pau MG, et al. A recombinant adenovirus expressing immunodominant TB antigens can significantly enhance BCG-induced human immunity. Vaccine 2012; 30:2098 - 108; http://dx.doi.org/10.1016/j.vaccine.2012.01.048; PMID: 22296955
- Song K, Bolton DL, Wei CJ, Wilson RL, Camp JV, Bao S, Mattapallil JJ, Herzenberg LA, Herzenberg LA, Andrews CA, et al. Genetic immunization in the lung induces potent local and systemic immune responses. Proc Natl Acad Sci U S A 2010; 107:22213 - 8; http://dx.doi.org/10.1073/pnas.1015536108; PMID: 21135247
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol 2003; 74:179 - 85; http://dx.doi.org/10.1189/jlb.1102574; PMID: 12885934
- Zuckerman JB, Robinson CB, McCoy KS, Shell R, Sferra TJ, Chirmule N, Magosin SA, Propert KJ, Brown-Parr EC, Hughes JV, et al. A phase I study of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis. Hum Gene Ther 1999; 10:2973 - 85; http://dx.doi.org/10.1089/10430349950016384; PMID: 10609658
- McDonald RJ, Lukason MJ, Raabe OG, Canfield DR, Burr EA, Kaplan JM, Wadsworth SC, St George JA. Safety of airway gene transfer with Ad2/CFTR2: aerosol administration in the nonhuman primate. Hum Gene Ther 1997; 8:411 - 22; http://dx.doi.org/10.1089/hum.1997.8.4-411; PMID: 9054516
- Lerondel S, Vecellio None L, Faure L, Sizaret PY, Sene C, Pavirani A, Diot P, Le Pape A. Gene therapy for cystic fibrosis with aerosolized adenovirus-CFTR: characterization of the aerosol and scintigraphic determination of lung deposition in baboons. J Aerosol Med 2001; 14:95 - 105; http://dx.doi.org/10.1089/08942680152007945; PMID: 11495490
- Meyer KC, Sharma A. Regional variability of lung inflammation in cystic fibrosis. Am J Respir Crit Care Med 1997; 156:1536 - 40; http://dx.doi.org/10.1164/ajrccm.156.5.9701098; PMID: 9372672
- Garcia-Contreras L, Hickey AJ. Aerosol treatment of cystic fibrosis. Crit Rev Ther Drug Carrier Syst 2003; 20:317 - 56; http://dx.doi.org/10.1615/CritRevTherDrugCarrierSyst.v20.i5.10; PMID: 14959788
- Mikhak Z, Strassner JP, Luster AD. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J Exp Med 2013; 210:1855 - 69; http://dx.doi.org/10.1084/jem.20130091; PMID: 23960189
- Turner DL, Bickham KL, Farber DL, Lefrançois L. Splenic priming of virus-specific CD8 T cells following influenza virus infection. J Virol 2013; 87:4496 - 506; http://dx.doi.org/10.1128/JVI.03413-12; PMID: 23388712
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006; 6:595 - 601; http://dx.doi.org/10.1038/nri1901; PMID: 16868550
- Dharmadhikari AS, Basaraba RJ, Van Der Walt ML, Weyer K, Mphahlele M, Venter K, Jensen PA, First MW, Parsons S, McMurray DN, et al. Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb) 2011; 91:329 - 38; http://dx.doi.org/10.1016/j.tube.2011.03.002; PMID: 21478054
- Mills CC, O’Grady F, Riley RL. Tuberculin conversion in the “naturally infected” guinea pig. Bull Johns Hopkins Hosp 1960; 106:36 - 45; PMID: 14422642
- Swift D, Asgharian B, Kimbell J. Use of mathematical aerosol deposition models in predicting the distribution of inhaled therapeutic aerosols. In: Hickey A, ed. Inhaled Aerosols: Physical and Biological Basis for Therapy. New York, NY: Marcel Dekker, Inc., 2006.
- Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng’ang’a D, Brandariz KL, Abbink P, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 2011; 29:5203 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.05.025; PMID: 21619905
- Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, Goudsmit J, Havenga MJ, Kostense S. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol 2003; 41:5046 - 52; http://dx.doi.org/10.1128/JCM.41.11.5046-5052.2003; PMID: 14605137
