Abstract
While the NGcGM3/VSSP vaccine, a preparation consisting in very small sized proteoliposomes (VSSP) obtained by the incorporation of the NGcGM3 ganglioside into the outer membrane protein (OMP) complex of Neisseria meningitides, is currently studied in late stage clinical trials in breast cancer and melanoma patients, mechanisms involved in the vaccine’s antitumor effect are insufficiently understood. Here we have addressed the role of adaptive and innate immune cells in mediating the protective effect of the vaccine. To this aim we selected the 3LL-D122 Lewis lung spontaneous metastasis model. Unexpectedly, inoculation of the vaccine in tumor bearing C57BL/6 mice, either by subcutaneous (sc) or intraperitoneal (ip) routes, induced similar anti-metastatic effect. Regardless the T-independent nature of NGcGM3 ganglioside as antigen, the antimetastatic effect of NGcGM3/VSSP is dependent on CD4+ T cells. In a further step we found that the vaccine was able to promote the increase, maturation, and cytokine secretion of conventional DCs and the maturation of Bone Marrow-derived plasmacytoid DCs. In line with this result the in vivo IFNα serum level in ip vaccinated mice increased as soon as 2h after treatment. On the other hand the infiltration of NK1.1+CD3- and NK1.1+CD3+ cells in lungs of vaccinated mice was significantly increased, compared with the presence of these cells in control animal lungs. In the same way NGcGM3/VSSP mobilized acquired immunity effector cells into the lungs of vaccinated tumor bearing mice. Finally and not less noteworthy, leukocyte infiltration in lungs of tumor bearing mice correlates with vaccine induced inhibition of lung metastization.
Introduction
Gangliosides have been considered attractive targets for cancer immunotherapy based on their higher abundance in tumors when compared with the corresponding normal tissues.Citation1,Citation2 They have an important role in tumor progression and metastization events.Citation3-Citation5 Gangliosides are also powerful stimulators of in vivo tumor growthCitation6 and they impair multiple events of the immune response, operating as soluble factors in the immunosuppression induced by tumors.Citation7,Citation8 N-glycolyl (NGc) gangliosides, and particularly NGcGM3, have received attention as a privileged target for cancer therapy.Citation9 NGcGM3 contributes to cancer progression, not only by influencing dendritic cell differentiation and maturation, but also down-modulating the CD4 expression in T cellsCitation10 and impairing CD4+CD25- T lymphocyte functions.Citation11
Many clinical trials have been performed with a NGcGM3-containing vaccine for the immunotherapy of melanomaCitation12-Citation15 and breast cancer.Citation16,Citation17 The vaccine consisted in very small sized proteoliposomes (VSSP) obtained by the incorporation of the NGcGM3 ganglioside into the outer membrane protein (OMP) complex of Neisseria meningitides.Citation18 All NGcGM3/VSSP treated patients developed anti-NGcGM3 antibody titers and the hyper-immune sera recognize both NGcGM3+ murine tumor cell lines L1210 and P3X63.Citation16,Citation17
In a previous paper we showed that tumor cells express the NGcGM3 ganglioside in the murine model of spontaneous lung metastasis induced by the 3LL-D122 Lewis lung carcinoma tumor cells.Citation19 This murine model is much related to the disease occurring in cancer patients, who in many cases develop metastatic lesions after surgical treatment. The 3LL-D122 Lewis lung carcinoma spontaneous metastasis murine model was consistent with an increased expression of NGcGM3 from primary tumors to metastatic lesions, as observed in human breast cancer samples.Citation20 Additionally, 2 therapeutic subcutaneous (sc) injections of the vaccine, in the neoadjuvant setting, inhibited spontaneous metastasis on the lungs of treated mice.
Active immunization with different ganglioside-based approaches has given rise to the generation of high titers of IgM and IgG antibodies,Citation21-Citation23 but in all cases only generation of humoral response have been reported.Citation24-Citation26 Interestingly, in a Phase Ib/IIa clinical trial in patients with advanced cutaneous melanoma, the NGcGM3/VSSP vaccine induced vitiligo in 3 out of 12 patients, although the nominal antigen NGcGM3 is not present in melanocytes.Citation12 Experimental evidence has suggested a role for CD8+ cytotoxic T lymphocytes (CTLs) in the pathophysiology of vitiligo, a pigmentation disorder with focal loss of melanocytes in the skin.Citation27 The involvement of CTLs has not been associated with anti-ganglioside immune response, due to their T-independent nature as antigens. Considering this finding, in the present paper, we focused in searching cellular immune effectors responsible of the antimetastatic effect observed in mice treated with NGcGM3/VSSP, using the 3LL-D122 murine spontaneous metastasis model.
Results
Similar anti-metastatic effect of the NGcGM3/VSSP vaccine is observed, regardless of the route of administration
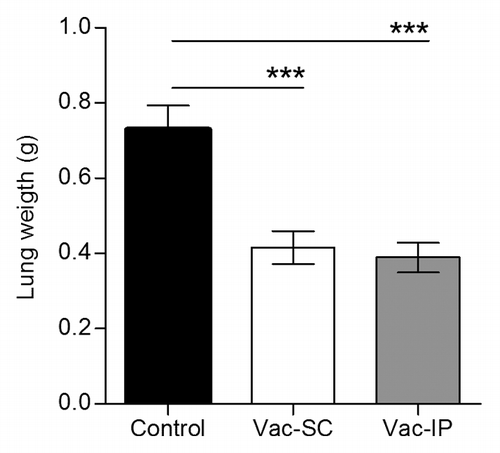
In experimental animal models numerous factors influence the immune response generated with a vaccine, including the route of administration, which targets distinct populations of dendritic cells (DCs). Previously, a therapeutic benefit after sc NGcGM3/VSSP treatment of mice challenged with the 3LL-D122 tumor (spontaneous lung metastasis model) was documented.Citation19 So we wonder if, in the same experimental model, the intraperitoneal (ip) route of administration of the vaccine also produces an anti-metastatic effect. The NGcGM3/VSSP vaccine was injected ip or sc in different groups of mice, 7 and 14 d following the intra-footpad tumor cells implantation. After removing primary tumors by surgery, the animals were sacrificed and the metastatic spread assessed through lungs weights. Mice injected with the vaccine showed reduced amount of metastasis in the lungs, compared with control animals just receiving PBS (P < 0.001, One-way ANOVA, ), despite the route of administration. Noteworthy, lungs weights in sc vaccinated animals were similar to those coming from ip vaccinated mice (P > 0.05, One-way ANOVA). This unexpected result may indicate a similar capability of the vaccine to activate different site resident DCs.
Figure 1. Irrespective of the route of administration the anti-metastatic effect of the NGcGM3/VSSP vaccine (in a spontaneous lung metastases murine model) is similar. C57BL/6 mice were inoculated with 3LL-D122 cells (2 x 105/mouse) into the right hind footpad, and treated twice with the NGcGM3/VSSP vaccine (Vac), either subcutaneous (Vac-SC) or intraperitoneal (Vac-IP), 7 and 21 d after tumor inoculation. Control mice were injected with PBS. Vac treated animals had significantly fewer pulmonary metastases measured as lung weight, relative to control group. Differences were not observed between the 2 routes (P > 0.05). Column bars represent mean values and error bars correspond to standard errors. The P value was calculated with ANOVA and the Tukey multiple comparison tests (***P < 0.001).
NGcGM3/VSSP anti-metastatic effect depends on CD4+ T cells
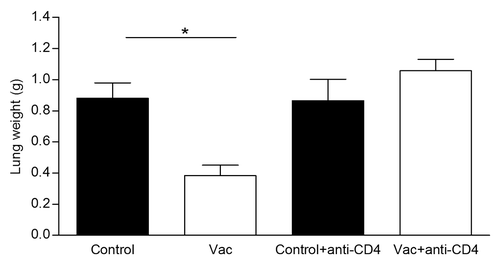
Despite the T-independent nature of NGcGM3 ganglioside as antigen, the role of CD4+ T cells in the NGcGM3/VSSP vaccine capacity to avoid metastization of 3LL-D122, was investigated in vivo by a cell-type specific depletion procedure. After injecting twice the vaccine in tumor-bearing mice as previously described, depletion of CD4+ T cells was achieved by anti-CD4+ monoclonal antibody (mAb) administration 24 h after each vaccine injection. Control groups received PBS or PBS plus the anti-CD4+ mAb. shows the abolishment of the anti-metastatic effect of the NGcGM3/VSSP vaccine in mice deficient in CD4+ T cells, suggesting that this population is involved in the anti-metastatic effect of the vaccine.
Figure 2. CD4+ T cells are involved in the NGcGM3/VSSP vaccine anti-metastatic effect. Depletion of T cells was achieved injecting anti CD4 mAb, 1 d after each vaccine immunization. Column bars represent mean values and error bars correspond to the standard error. The P value was calculated with ANOVA and the Tukey multiple comparison tests (*P < 0.05).
The NGcGM3/VSSP vaccine promotes the increase, maturation, and cytokine secretion of conventional Dendritic cells
To explore the possible direct connection of the NGcGM3/VSSP vaccine immune response with professional Antigen Presenting Cells (APC), bone marrow (BM) precursors from C57BL/6 mice were differentiated in GM-CSF-enriched medium. BM-derived conventional DC (BM-cDC) were cultured overnight with additional stimuli (LPS or NGcGM3/VSSP) or just with medium (immature cDC) and then the maturation profile of these cells was measured by CD40 and CD86 expression by flow cytometry. As shown in , incubation with NGcGM3/VSSP induced 50% of CD11c+CD40+ cells, similarly to LPS (45%), a well-known inducer DCs maturation. Induction of the costimulatory molecule CD86 by the vaccine was even higher (43%) than that induced by LPS (28%).
Figure 3. The NGcGM3/VSSP vaccine promotes the increase, maturation, and cytokine secretion of Bone Marrow-derived conventional-DC in vitro and in vivo. BM cells of C57BL/6 mice were differentiated in GM-CSF-enriched medium. Cells were cultured overnight in the presence of LPS-1 µg/mL (C+), NGcGM3/VSSP (VAC-1 µg/mL), or medium (im-cDC). (A) Frequency of mature CD11c+ cells. (B) IL-6 and IL-12p40 secretion by NGcGM3/VSSP treated cDC in culture. Data are representative of 2 independent experiments. (C) C57BL/6 mice were injected sc with the NGcGM3/VSSP vaccine. The bar diagram shows the percentage of mature CD11c+ cells in draining lymph nodes, 3 d after Vac or PBS (control) treatments.
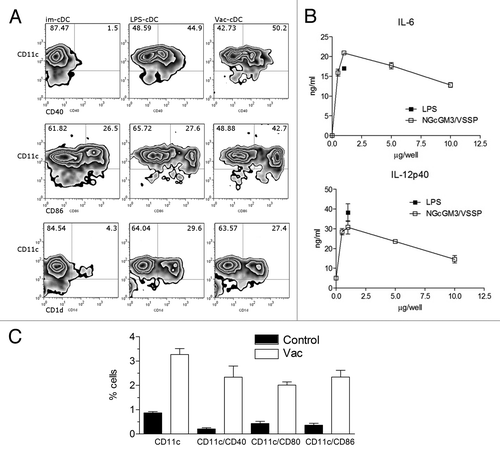
CD1d expression was also examined in the BM-cDC cultures due to the role of this molecular complex in glycolipid presentation to NKT cells.Citation28,Citation29 While only 4.5% of the im-cDC were CD1d+, 27% of vaccine treated DCs upregulated their surface expression of CD1d ().
Simultaneously, we tested the capacity of this mature-BM-cDC to secrete inflammatory cytokines. BM-cDC exposed to the vaccine produced high levels of IL-6 and IL-12p40 after overnight incubation, in a dose dependent manner (), demonstrating their functional activation. To confirm these evidences in an in vivo setting, mice were injected sc with NGcGM3/VSSP and lymph nodes were collected 3 d later to evaluate DCs recruitment to lymph nodes. A 3-fold increase in the frequency of CD11c+ cells was observed in the draining lymph node of vaccinated mice as compared with controls. Mobilized Dendritic cells were also phenotypically more mature, according to the expression of surface maturation markers CD40, CD80, and CD86 ().
NGcGM3/VSSP vaccine increases the maturation of Bone Marrow-derived plasmacytoid Dendritic cells
Plasmacytoid Dendritic cells (pDCs) are 1 of the 2 main populations of dendritic cells and they are blood resident APC. After confirming the anti-metastatic effect of the ip administered NGcGM3/VSSP vaccine, we hypothesized that pDCs could be activated by the vaccine. To this purpose, BM of C57BL/6 mice were differentiated in FLT3L-enriched medium and pDCs were isolated as B220+ cells. Cells were cultured overnight in the presence of LPS, CpG-A, NGcGM3/VSSP, or without stimuli. The ganglioside-based preparation induced the maturation of BM-pDC, measured by the upregulation of the CD86 marker on B220+ cells (). The IFNα production by stimuli maturated BM-pDC in culture was also assayed. Only CpG-A was able to induce detectable levels of this cytokine ().
Figure 4. The NGcGM3/VSSP vaccine increases the maturation of Bone Marrow-derived plasmacytoid Dendritic cells. BM of C57BL/6 mice were differentiated in FLT3L-enriched medium. B220+ purified cells were cultured overnight in the presence of LPS (0.5 µg/mL), CpG-A (5 µg/mL), NGcGM3/VSSP (Vac 0.1 µg/mL), or medium (im-pDC). (A) Frequency of CD86+B220+ cells. (B) IFNα secretion by NGcGM3/VSSP treated pDC in culture. Data are representative of 2 independent experiments. (C) C57BL/6 mice were injected ip with the NGcGM3/VSSP vaccine and serum levels of IFNα, IL6 and IL12p40 were determined by ELISA at 2-h intervals.
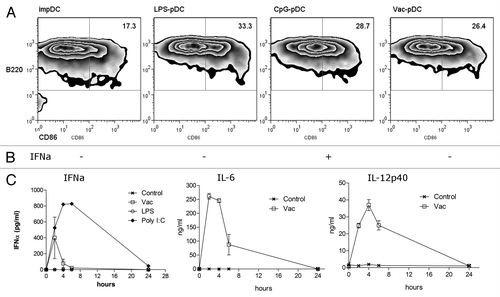
Next mice were challenged with the vaccine and the levels of IL-6, IL-12, and type I interferons, in sera of ip vaccinated mice were quantified by ELISA at early time points. Interestingly, IFNα level was rapidly increased as soon as 2 h after treatment, suggesting the in vivo activation of pDCs. The levels of IL-6 and IL-12p40 were also rapidly increased, showing a peak 2 and 4 h after vaccine injection, respectively (). IFNγ remained undetected in vaccinated mice sera (data not shown). All together, the data show that the vaccine induce a strong cytokine response in vivo and the maturation of both conventional and plasmacytoid DCs in vitro.
NGcGM3/VSSP directed innate immunity effector cells toward metastatic lungs
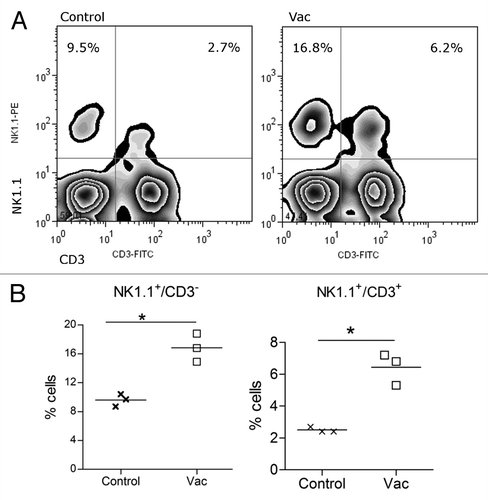
We noted previously that depleting NK1.1+ cells aborted the anti-metastatic effect of the NGcGM3 based vaccine, injected in 3LL-D122 tumor bearing syngeneic mice.Citation19 Then we wondered if vaccination could increase the frequency of NK1.1+ cells in the lungs of these animals. Immunized or controls mice were sacrificed 3 d after the second vaccine administration and the frequency of NK and NKT cells measured by Flow Cytometry. The infiltration of NK1.1+CD3- and NK1.1+CD3+ cells in lungs of vaccinated mice was significantly increased, compared with the presence of these cells in control animal lungs ().
Figure 5. Natural killer cells infiltrate the lungs of vaccinated tumor bearing animals. 3LL tumor bearing C57BL/6 mice, treated with Vac or PBS (Control), were sacrificed 3 d after the second vaccine administration and the frequency of NK and NKT cells measured in lungs by Flow Cytometry. (A) Density dot plot of a representative animal per group. (B) Individual numbers of NK or NKT cells in total lung infiltrating cells (percent) in vaccinated and control mice. Data are representative of 2 independent experiments (*P < 0.05).
NGcGM3/VSSP mobilized acquired immunity effector cells into lungs of vaccinated tumor bearing mice
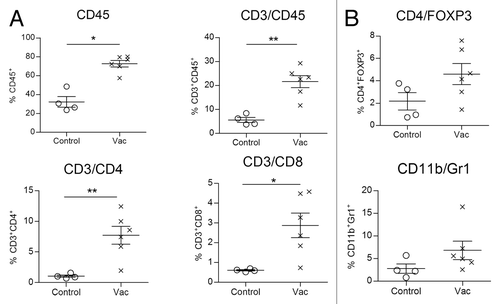
Based on the observation that the anti-metastatic effect of the NGcGM3/VSSP vaccine in the Lewis lung cancer model depends on both CD4+ and CD8+ T cells, their frequencies in lungs of vaccinated or control tumor bearing mice were assessed. Twenty-one days after primary tumor removal by surgery, lungs from sacrificed mice were processed and lymphocyte frequency determined. The percentage of infiltrating CD45+ cells was significantly higher in lungs of vaccinated animals, as well as was the percentage of T cells, identified as CD45+CD3+, CD3+CD4+, and CD3+CD8+ cells (). The infiltration of regulatory populations, such as regulatory T cells (Tregs: CD4+Foxp3+) and myeloid derived suppressor cells (MDSC: CD11b+Gr1+) was also measured. The frequency of these regulatory cells were similar in lungs of vaccinated and control tumor recipients ().
Figure 6. Vaccination of tumor bearing animals induces lung infiltration by adaptive immunity effector cells. Vac or PBS (control) treated C57BL/6 mice, inoculated with 3LL-D122 cells, were sacrificed 21 d after primary tumor removal. The frequency of effector and regulatory cells was measured in animal lungs by Flow Cytometry. (A) Individual numbers of CD45+, CD3+ CD45+, CD3+ CD4+, or CD3+ CD8+ cells in total lung infiltrating cells (percent) in vaccinated and control mice. (B) Frequency of regulatory populations, top: regulatory T cells (CD4/Foxp3) and bottom: myeloid derived suppressor cells (CD11b/Gr1).
Leukocyte infiltration in lungs of tumor bearing mice correlates with vaccine induced inhibition of lung metastization
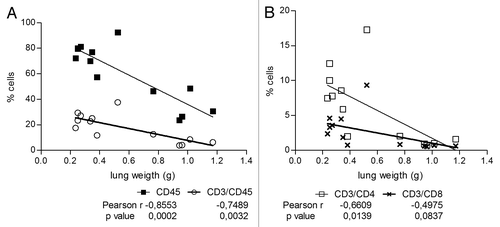
To verify a possible link between the vaccine induced immune infiltration and the anti-metastatic effect, percentages of CD45+, CD45+CD3+, CD3+CD4+, and CD3+CD8+ cells and the corresponding individual mice lungs weights (as a surrogate of metastization) were correlated. Clearly, while tumor bearing non vaccinated mice showed a higher lung weights with lower percentage of infiltrating leukocyte and T cells frequencies pattern, in vaccinated mice lower lung weights were associated to an increased presence of these cell populations. Statistical analysis of these 2 variables revealed negative Pearson r coefficients ranging between 0.855–0.498 (), establishing an inverse relationship between vaccine induced cell infiltration and tumor metastization. The significant correlation (P < 0.05) obtained for infiltrating CD45+, CD3+, and CD4+ cells support the idea that these cells, properly mobilized to the vaccinated tumor bearing mice lungs, were responsible for controlling metastization. No significant correlation was achieved for CD3+CD8+ cells.
Figure 7. Leukocyte and CD4+ T cells infiltration correlate with vaccine induced inhibition of lung metastization. Vac treated or Control C57BL/6 mice, inoculated with 3LL-D122 cells, were sacrificed 21 d after primary tumor removal and the frequency of (A) CD45 and CD3, or (B) CD4 and CD8 cells in animal lungs was correlated with lung metastases quantified by weighing the lungs. The Pearson r coefficients and P values were calculated using GraphPad Prism 5 software.
Discussion
Tumor elimination by vaccine-mediated therapy requires the generation of immune response. It is generally accepted that the route of administration is a critical factor influencing the vaccine induced immune response. Previously, we have demonstrated the therapeutic benefit after sc NGcGM3/VSSP treatment in the spontaneous lung metastasis model with the 3LL-D122 tumor. Herein we described that this NGcGM3 containing vaccine induced similar anti-metastatic effect on the Lewis spontaneous lung metastasis model regardless the route of administration. At the cellular immunity level different routes means targeting distinct populations of DC. The sc route typically involves cDC and the IP, targets also pDC. Our results showed that NGcGM3/VSSP vaccine promotes the increase, maturation, and secretion IL-12/IL-6 by cDC. IL-12 produced by DC cells is crucial for the development of Th1 responses.Citation30,Citation31 On the other hand, it has been reported that production of IL-6 by DC is critical for T cell activation and it renders antigen-specific T cells, refractory to the suppressive activity of Tregs cells.Citation32 In the spontaneous tumor model that we used, Katz et al.Citation33 reported a complete protection against spontaneous metastases after combining human recombinant IL-6 and irradiated 3LL-D122 tumor cells. Our results are in accordance also with the ones published by Mesa et al.,Citation34 who used the acetylated variant of GM3 ganglioside integrated into VSSP evidencing a potent activation and cytokine secretion of cDC, suggesting that the bacterial component of the vaccine is relevant for APC activation and function.
In contrast to cDC, pDC are characterized as type I IFN-producing cells that engage endosomal TLRs and provide an initial line of host defense against viral infection.Citation35,Citation36 Various studies indicate that the presence of activated tumor-associated pDCs results in tumor regression in mice.Citation37 In this direction, a significant result was the maturation of Bone Marrow-derived plasmacytoid DC by the NGcGM3 based vaccine. However, vaccine maturated BM-pDC does not produce detectable levels of IFNα in culture supernatant. Curiously enough, IFNα level in animal sera was rapidly increased after vaccine IP treatment, suggesting the in vivo activation of pDC.
Another important result is the upregulation of CD1d molecule on BM-DC after NGcGM3/VSSP treatment, considering the glycolipid nature of NGcGM3. The expression of CD1d molecules is essential for the selection and activation of NKT cells.Citation29,Citation38 In mice, CD1d has been detected on professional APC including monocytes, macrophages, and some subsets of DC.Citation32,Citation39 It has been reported that the levels of CD1d expression can be modulated during infection. Skold et al.Citation40 revealed that cell surface CD1d expression on APC is regulated and affects T cell activation under physiological conditions. CD1d upregulation requires 2 signals, 1 provided by IFNγ and a second mediated by microbial products or by the pro-inflammatory cytokine TNF. Additionally, Raghuraman et al.Citation41 showed that infection of APC with the intracellular pathogen Listeria monocytogenes leads to upregulation of CD1d, and that IFNβ was required to mediate this upregulation. The changes in surface CD1d expression on DC by our vaccine support the idea of NKT cell activation during NGcGM3/VSSP immunization.
The nature of the vaccine is an additional element influencing the type of immune effectors elicited and the protection efficacy. Numerous cancer vaccine approaches have induced antibodies, capable of binding specifically to tumor antigens. However, these have generally not been enough to produce a real tumor rejection. Active immunization with different ganglioside-based approaches has given rise to the generation of high titers of IgM and IgG antibodies.Citation21-Citation23 In mice, these Abs have shown to mediate protection in models of challenge with ganglioside expressing tumors.Citation42 In humans, anti-ganglioside induced antibodies were capable of mediating complement dependent cytotoxicity of gangliosides expressing melanoma cells in the majority of patients and were associated with a significantly improved disease-free and overall survival responses, but in all cases only generation of humoral response have been described.Citation24-Citation26 We previously reported the immunogenicity of the NGcGM3/VSSP vaccine in chicken and mice models, and confirmed the capability of the sera of vaccinated animals to identify the ganglioside on positive NGcGM3 myeloma cells.Citation19 Furthermore, in the phase I clinical trial in advanced breast cancer patients, performed with NGcGM3/VSSP vaccine, all treated patients developed anti-NGcGM3 antibody titers (IgM and IgG) and the hyper-immune sera increased complement-mediated cytotoxicity vs. NGcGM3 positive P3X63 myeloma cells.Citation16 In the controlled Phase II clinical trial conducted in patients with metastatic breast cancer with NGcGM3/VSSP, the vaccine was immunogenic and showed a sustained increase of both IgG and IgM antibody titters against NGcGM3. Antibodies were able to recognize both NGcGM3+ murine tumor cell lines L1210 and P3X63. Hyperimmune sera from vaccinated patients were able to prevent the NGcGM3 mediated CD4 down-modulationCitation10 on T lymphocytes. Herein, regardless of the T independent nature of NGcGM3 ganglioside as antigen, we focused in searching cellular immune effectors. We tested in vivo, using a cell-type specific depletion procedure, the role of CD4+ T cells in the NGcGM3/VSSP vaccine capacity to avoid metastization of 3LL-D122 primary tumors in mice. Interestingly, the anti-metastatic effect of the NGcGM3/VSSP vaccine was abolished in mice deficient in CD4+ T cells, suggesting the involvement of this population from the adaptive immune system in the anti-metastatic effect of the vaccine. In our first reportCitation19 similar in vivo experiments, using cell-type specific depletion trials, demonstrated a clear involvement of CD8+ T cells and NK1.1+ cells in the anti-metastatic effect elicited by the vaccine. Then here we measured the frequency of NK and NKT cells in lungs of 3LL-D122 tumor bearing syngeneic mice and we found a significantly increased infiltration of NK+CD3- and NK1.1+CD3+ cells 3 d after vaccine treatment in lungs of vaccinated mice compared with control animals. Even more, 21 d after primary tumor removal by surgery, lungs from vaccinated mice were obtained and examined the frequency of acquired immunity effector cells by facs. The percentage of infiltrating CD45+ cells was significantly higher in lungs of vaccinated animals, as well as was the percentage of CD4+ and CD8+ T cells. Mazorra et al.Citation43 using in vivo cell-type specific depletion experiment in the melanoma tumor model and preventive administration of a NAcGM3-based vaccine to C57BL/6 mice, reported the involvement of CD8+ T cells, but not NK1.1+ cells, neither CD4+ T, in the effector phase of the antitumor immune response. Nevertheless, our results demonstrated the recruitment of innate and adaptative immune effector cells toward the metastatic site of tumor bearing mice, by a ganglioside-based vaccine. As well, leukocyte infiltration in the lungs of tumor bearing mice correlated with the vaccine induced inhibition of lung metastization. A strong lymphocytic infiltration has been reported to be associated with major improvements in clinical outcome for many different tumor types, including lung cancer. Recently, studies conducted by Galon et al.,Citation44,Citation45 on tumors from colorectal cancer patients, demonstrated that total high densities of CD3+ T cells, CD8+ cytotoxic T cells and CD45RO+ memory T cells were clearly associated with a longer disease-free survival and/or overall survival. On the other hand the existence of several different subpopulations of CD4+ T cells could explain the apparently contradictory results about their infiltration pattern in tumors and clinical outcomes. Future experiments to define the subpopulation and functionality of CD4+ T cells involved in the NGcGM3/VSSP vaccine anti-metastatic effect are warranted.
Materials And Methods
Animals and cells
C57BL/6 female mice, 8- to 12-wk-old, purchased from the Center for Laboratory Animal Production (CENPALAB), were treated according to the Cuban National Laboratory Animal Use Guidelines. The murine 3LL-D122 cell lineCitation46 of the Lewis lung carcinoma, of C57BL/6 origin was grown in DMEM-F12 (Gibco) supplemented with 10% fetal calf serum (FCS) (Hyclone), 2 mM L-glutamine, 1 mM sodium pyruvate, penicillin 100 U/mL, and streptomycin 100 µg/mL (Life Technologies). BM-cDC were differentiated in vitro from BM of C57BL/6 mice using GM-CSF. BM-pDC were purified from in vitro culture of BM of C57BL/6 mice on FLT3-L enriched medium, by positive selection, using B220+ microbeads (Miltenyi Biotec). For isolation of leukocytes from lungs, cell suspension were obtained by digestion with Collagenase D (1 mg/mL; Sigma) and DNase I (1 mg/mL; Sigma) for 30 min at 37 °C.
NGcGM3/VSSP vaccination in the spontaneous murine tumor model
NGcGM3/VSSP vaccine was prepared as described by Estevez.Citation18 C57BL/6 mice challenged with 3LL-D122 clone (2 × 105/mouse) into the right hind footpad, were treated twice with NGcGM3/VSSP (200 µg per mouse), 7 and 21 d after tumor implantation, as described previously,Citation19 using the sc or the ip routes. Primary tumors were surgically removed and 21 d after, animals were sacrificed. Spontaneous lung metastases were quantified through lung weights, established as a surrogate of the number and size of metastasis.Citation47,Citation48 Control groups received PBS.
In vivo CD4-depletion study
MAbs to CD4 were purified from culture supernatants of the YTS 191 (anti-CD4, ECACC) rat hybridoma by ammonium sulfate precipitation. In vivo depletion was done by intra-peritoneal injection with 1mg of anti-CD4 mAb, 1 d after each vaccine immunization. This dose has been previously shown to deplete more than 98% of the cell subset. Rat IgG (Sigma) at 500 µg/injection was used as isotype control in previous experiments with the same design, without any change in the current results (unpublished results).
Flow cytometric analysis
Total freshly isolated cells from lungs were stained for 20 min at 4 °C with the following antibodies purchased from eBioscience: anti-CD45-FITC, anti-CD4-FITC, anti-CD8-PE, anti-CD3-PE Cy5, anti-FoxP3-PE, anti-CD25-PE Cy5, anti-Gr1-PE, anti-CD11b-PE Cy5, anti-NK1.1-PE, anti-CD3-FITC. For DC analysis, using freshly isolated cells from lymph nodes or BM-DCs, cells were stained with antibodies to CD11c, CD86, CD40, CD80, and CD1d, all from eBioscience. Stained cells were acquired with a Partec flow cytometer and analyzed with FLOWJO software (version 4.5.4; Tree Star).
Cytokine production
In vitro assay: BM-cDC or BM-pDC were cultured in 96-well culture plates at 37 °C in the presence of LPS (Sigma), NGcGM3/VSSP, CpG-A (InVivoGen), or medium (IMDM, Gibco). Culture supernatants were collected 18 h later and analyzed by ELISA.
In vivo assay: Mice were injected ip with NGcGM3/VSSP, sera were collected 2, 4, 6, and 24 h after vaccine injection and analyzed for the content of IFN-α, IL-6 and IL-12p40.
Cytokine ELISA kits were purchased from PBL Biomedical Laboratories (IFN-α) and Thermo Fischer Scientific (IL-12p40 and IL-6).
Statistical analyses
Mann–Whitney U test for paired comparison of values or One-way ANOVA, combined with the Tukey test, for multiple comparisons were employed. Differences were considered significant if P < 0.05. The Pearson r coefficients and P values were calculated using GraphPad Prism 5 software.
| Abbreviations: | ||
| VSSP | = | very small sized proteoliposomes |
| OMP | = | outer membrane protein |
| sc | = | subcutaneous |
| ip | = | intraperitoneal |
| cDCs | = | conventional dendritic cells |
| pDCs | = | plasmacytoid dendritic cells |
| BM | = | bone marrow |
| NGc | = | N-glycolyl |
| CTLs | = | cytotoxic T lymphocytes |
| PBS | = | phosphate buffer saline |
| mAb | = | monoclonal antibody |
| APC | = | Antigen Presenting Cells |
| LPS | = | lipopolysaccharide |
| IFN | = | Interferon |
| Tregs | = | regulatory T cells |
| MDSC | = | myeloid derived suppressor cells |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Judith Raymond for technical assistance with animal handling. This work was supported by the CIM´s budget for Research and Development.
References
- Hakomori S, Young WW Jr., Patt LM, Yoshino T, Halfpap L, Lingwood CA. Cell biological and immunological significance of ganglioside changes associated with transformation. Adv Exp Med Biol 1980; 125:247 - 61; http://dx.doi.org/10.1007/978-1-4684-7844-0_24; PMID: 6244722
- Livingston PO, Hood C, Krug LM, Warren N, Kris MG, Brezicka T, Ragupathi G. Selection of GM2, fucosyl GM1, globo H and polysialic acid as targets on small cell lung cancers for antibody mediated immunotherapy. Cancer Immunol Immunother 2005; 54:1018 - 25; http://dx.doi.org/10.1007/s00262-005-0663-8; PMID: 15926079
- Birklé S, Zeng G, Gao L, Yu RK, Aubry J. Role of tumor-associated gangliosides in cancer progression. Biochimie 2003; 85:455 - 63; http://dx.doi.org/10.1016/S0300-9084(03)00006-3; PMID: 12770784
- Choi HJ, Chung TW, Kang SK, Lee YC, Ko JH, Kim JG, Kim CH. Ganglioside GM3 modulates tumor suppressor PTEN-mediated cell cycle progression--transcriptional induction of p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway. Glycobiology 2006; 16:573 - 83; http://dx.doi.org/10.1093/glycob/cwj105; PMID: 16574813
- Ferrone S. Changes in serum ganglioside and antibody levels in soft tissue sarcoma: are they the cause or the effect of tumor progression?. Cancer J 2002; 8:369 - 70; http://dx.doi.org/10.1097/00130404-200209000-00006; PMID: 12416893
- Alessandri G, Filippeschi S, Sinibaldi P, Mornet F, Passera P, Spreafico F, Cappa PM, Gullino PM. Influence of gangliosides on primary and metastatic neoplastic growth in human and murine cells. Cancer Res 1987; 47:4243 - 7; PMID: 2440560
- Ladisch S, Kitada S, Hays EF. Gangliosides shed by tumor cells enhance tumor formation in mice. J Clin Invest 1987; 79:1879 - 82; http://dx.doi.org/10.1172/JCI113031; PMID: 3584474
- Ladisch S. Tumor cell gangliosides. Adv Pediatr 1987; 34:45 - 58; PMID: 3318303
- Fernandez LE, Gabri MR, Guthmann MD, Gomez RE, Gold S, Fainboim L, Gomez DE, Alonso DF. NGcGM3 ganglioside: a privileged target for cancer vaccines. Clin Dev Immunol 2010; 2010:814397; http://dx.doi.org/10.1155/2010/814397; PMID: 21048926
- de Leòn J, Fernández A, Mesa C, Clavel M, Fernández LE. Role of tumour-associated N-glycolylated variant of GM3 ganglioside in cancer progression: effect over CD4 expression on T cells. Cancer Immunol Immunother 2006; 55:443 - 50; http://dx.doi.org/10.1007/s00262-005-0041-6; PMID: 16208470
- de León J, Fernández A, Clavell M, Labrada M, Bebelagua Y, Mesa C, Fernández LE. Differential influence of the tumour-specific non-human sialic acid containing GM3 ganglioside on CD4+CD25- effector and naturally occurring CD4+CD25+ regulatory T cells function. Int Immunol 2008; 20:591 - 600; http://dx.doi.org/10.1093/intimm/dxn018; PMID: 18310617
- Osorio M, Gracia E, Rodríguez E, Saurez G, Arango MdelC, Noris E, Torriella A, Joan A, Gómez E, Anasagasti L, et al. Heterophilic NeuGcGM3 ganglioside cancer vaccine in advanced melanoma patients: results of a Phase Ib/IIa study. Cancer Biol Ther 2008; 7:488 - 95; http://dx.doi.org/10.4161/cbt.7.4.5476; PMID: 18285705
- Fernández LE, Alonso DF, Gomez DE, Vázquez AM. Ganglioside-based vaccines and anti-idiotype antibodies for active immunotherapy against cancer. Expert Rev Vaccines 2003; 2:817 - 23; http://dx.doi.org/10.1586/14760584.2.6.817; PMID: 14711364
- Osorio M, Gracia E, Reigosa E, Hernandez J, de la Torre A, Saurez G, Perez K, Viada C, Cepeda M, Carr A, et al. Effect of vaccination with N-glycolyl GM3/VSSP vaccine by subcutaneous injection in patients with advanced cutaneous melanoma. Cancer Manag Res 2012; 4:341 - 5; http://dx.doi.org/10.2147/CMAR.S22617; PMID: 23055778
- Pérez K, Osorio M, Hernández J, Carr A, Fernández LE. NGcGM3/VSSP vaccine as treatment for melanoma patients. Hum Vaccin Immunother 2013; 9:1237 - 40; http://dx.doi.org/10.4161/hv.24115; PMID: 23442598
- Carr A, Rodríguez E, Arango MdelC, Camacho R, Osorio M, Gabri M, Carrillo G, Valdés Z, Bebelagua Y, Pérez R, et al. Immunotherapy of advanced breast cancer with a heterophilic ganglioside (NeuGcGM3) cancer vaccine. J Clin Oncol 2003; 21:1015 - 21; http://dx.doi.org/10.1200/JCO.2003.02.124; PMID: 12637465
- Mulens V, de la Torre A, Marinello P, Rodríguez R, Cardoso J, Díaz R, O’Farrill M, Macias A, Viada C, Saurez G, et al. Immunogenicity and safety of a NeuGcGM3 based cancer vaccine: Results from a controlled study in metastatic breast cancer patients. Hum Vaccin 2010; 6:6; http://dx.doi.org/10.4161/hv.6.9.12571; PMID: 20855939
- Estevez F, Carr A, Solorzano L, Valiente O, Mesa C, Barroso O, Sierra GV, Fernandez LE. Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP). Vaccine 1999; 18:190 - 7; http://dx.doi.org/10.1016/S0264-410X(99)00219-4; PMID: 10501249
- Labrada M, Clavell M, Bebelagua Y, León Jd, Alonso DF, Gabri MR, Veloso RC, Vérez V, Fernández LE. Direct validation of NGcGM3 ganglioside as a new target for cancer immunotherapy. Expert Opin Biol Ther 2010; 10:153 - 62; http://dx.doi.org/10.1517/14712590903443084; PMID: 20088712
- Carr A, Mullet A, Mazorra Z, Vázquez AM, Alfonso M, Mesa C, Rengifo E, Pérez R, Fernández LE. A mouse IgG1 monoclonal antibody specific for N-glycolyl GM3 ganglioside recognized breast and melanoma tumors. Hybridoma 2000; 19:241 - 7; http://dx.doi.org/10.1089/02724570050109639; PMID: 10952412
- Helling F, Livingston PO. Ganglioside conjugate vaccines. Immunotherapy against tumors of neuroectodermal origin. Mol Chem Neuropathol 1994; 21:299 - 309; http://dx.doi.org/10.1007/BF02815357; PMID: 8086040
- Livingston PO. Approaches to augmenting the immunogenicity of melanoma gangliosides: from whole melanoma cells to ganglioside-KLH conjugate vaccines. Immunol Rev 1995; 145:147 - 66; http://dx.doi.org/10.1111/j.1600-065X.1995.tb00080.x; PMID: 7590824
- Ritter G, Boosfeld E, Adluri R, Calves M, Oettgen HF, Old LJ, Livingston P. Antibody response to immunization with ganglioside GD3 and GD3 congeners (lactones, amide and gangliosidol) in patients with malignant melanoma. Int J Cancer 1991; 48:379 - 85; http://dx.doi.org/10.1002/ijc.2910480312; PMID: 2040532
- Chapman PB, Morrisey D, Panageas KS, Williams L, Lewis JJ, Israel RJ, Hamilton WB, Livingston PO. Vaccination with a bivalent G(M2) and G(D2) ganglioside conjugate vaccine: a trial comparing doses of G(D2)-keyhole limpet hemocyanin. Clin Cancer Res 2000; 6:4658 - 62; PMID: 11156217
- Chapman PB, Morrissey DM, Panageas KS, Hamilton WB, Zhan C, Destro AN, Williams L, Israel RJ, Livingston PO. Induction of antibodies against GM2 ganglioside by immunizing melanoma patients using GM2-keyhole limpet hemocyanin + QS21 vaccine: a dose-response study. Clin Cancer Res 2000; 6:874 - 9; PMID: 10741710
- Ragupathi G, Livingston PO, Hood C, Gathuru J, Krown SE, Chapman PB, Wolchok JD, Williams LJ, Oldfield RC, Hwu WJ. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin Cancer Res 2003; 9:5214 - 20; PMID: 14614001
- Steitz J, Brück J, Lenz J, Büchs S, Tüting T. Peripheral CD8+ T cell tolerance against melanocytic self-antigens in the skin is regulated in two steps by CD4+ T cells and local inflammation: implications for the pathophysiology of vitiligo. J Invest Dermatol 2005; 124:144 - 50; http://dx.doi.org/10.1111/j.0022-202X.2004.23538.x; PMID: 15654968
- Speak AO, Cerundolo V, Platt FM. CD1d presentation of glycolipids. Immunol Cell Biol 2008; 86:588 - 97; http://dx.doi.org/10.1038/icb.2008.42; PMID: 18542099
- MacDonald HR. Development and selection of NKT cells. Curr Opin Immunol 2002; 14:250 - 4; http://dx.doi.org/10.1016/S0952-7915(02)00329-1; PMID: 11869900
- Chamilos G, Lewis RE, Hu J, Xiao L, Zal T, Gilliet M, Halder G, Kontoyiannis DP. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci U S A 2008; 105:9367 - 72; http://dx.doi.org/10.1073/pnas.0709578105; PMID: 18583479
- Gilliet M, Lande R. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr Opin Immunol 2008; 20:401 - 7; http://dx.doi.org/10.1016/j.coi.2008.06.008; PMID: 18611439
- Kmieciak M, Basu D, Payne KK, Toor A, Yacoub A, Wang XY, Smith L, Bear HD, Manjili MH. Activated NKT cells and NK cells render T cells resistant to myeloid-derived suppressor cells and result in an effective adoptive cellular therapy against breast cancer in the FVBN202 transgenic mouse. J Immunol 2011; 187:708 - 17; http://dx.doi.org/10.4049/jimmunol.1100502; PMID: 21670315
- Katz A, Shulman LM, Revel M, Feldman M, Eisenbach L. Combined therapy with IL-6 and inactivated tumor cells suppresses metastasis in mice bearing 3LL lung carcinomas. Int J Cancer 1993; 53:812 - 8; http://dx.doi.org/10.1002/ijc.2910530518; PMID: 8449606
- Mesa C, de León J, Rigley K, Fernández LE. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for dendritic cell activation. Vaccine 2006; 24:Suppl 2 S2 - 42, 3; http://dx.doi.org/10.1016/j.vaccine.2005.08.111; PMID: 16823920
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol 2008; 8:594 - 606; http://dx.doi.org/10.1038/nri2358; PMID: 18641647
- Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev 2010; 234:142 - 62; http://dx.doi.org/10.1111/j.0105-2896.2009.00881.x; PMID: 20193017
- Vermi W, Soncini M, Melocchi L, Sozzani S, Facchetti F. Plasmacytoid dendritic cells and cancer. J Leukoc Biol 2011; 90:681 - 90; http://dx.doi.org/10.1189/jlb.0411190; PMID: 21730085
- Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol 2000; 1:521 - 5; http://dx.doi.org/10.1038/82782; PMID: 11101875
- Spada FM, Borriello F, Sugita M, Watts GF, Koezuka Y, Porcelli SA. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur J Immunol 2000; 30:3468 - 77; http://dx.doi.org/10.1002/1521-4141(2000012)30:12<3468::AID-IMMU3468>3.0.CO;2-C; PMID: 11093166
- Sköld M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J Immunol 2005; 175:3584 - 93; http://dx.doi.org/10.4049/jimmunol.175.6.3584; PMID: 16148102
- Raghuraman G, Geng Y, Wang CR. IFN-beta-mediated up-regulation of CD1d in bacteria-infected APCs. J Immunol 2006; 177:7841 - 8; http://dx.doi.org/10.4049/jimmunol.177.11.7841; PMID: 17114455
- Zhang H, Zhang S, Cheung NK, Ragupathi G, Livingston PO. Antibodies against GD2 ganglioside can eradicate syngeneic cancer micrometastases. Cancer Res 1998; 58:2844 - 9; PMID: 9661900
- Mazorra Z, Mesa C, Fernández A, Fernández LE. Immunization with a GM3 ganglioside nanoparticulated vaccine confers an effector CD8(+) T cells-mediated protection against melanoma B16 challenge. Cancer Immunol Immunother 2008; 57:1771 - 80; http://dx.doi.org/10.1007/s00262-008-0503-8; PMID: 18351335
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960 - 4; http://dx.doi.org/10.1126/science.1129139; PMID: 17008531
- Galon J, Fridman WH, Pagès F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res 2007; 67:1883 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-06-4806; PMID: 17332313
- Eisenbach L, Hollander N, Greenfeld L, Yakor H, Segal S, Feldman M. The differential expression of H-2K versus H-2D antigens, distinguishing high-metastatic from low-metastatic clones, is correlated with the immunogenic properties of the tumor cells. Int J Cancer 1984; 34:567 - 73; http://dx.doi.org/10.1002/ijc.2910340421; PMID: 6490207
- Eisenbach L, Segal S, Feldman M. MHC imbalance and metastatic spread in Lewis lung carcinoma clones. Int J Cancer 1983; 32:113 - 20; http://dx.doi.org/10.1002/ijc.2910320118; PMID: 6862690
- Gorelik E, Segal S, Feldman M. Control of lung metastasis progression in mice: role of growth kinetics of 3LL Lewis lung carcinoma and host immune reactivity. J Natl Cancer Inst 1980; 65:1257 - 64; PMID: 6933271
