Abstract
When oral vaccines are administered to children in lower- and middle-income countries, they do not induce the same immune responses as they do in developed countries. Although not completely understood, reasons for this finding include maternal antibody interference, mucosal pathology secondary to infection, malnutrition, enteropathy, and previous exposure to the organism (or related organisms). Young children experience a high burden of cholera infection, which can lead to severe acute dehydrating diarrhea and substantial mortality and morbidity. Oral cholera vaccines show variations in their duration of protection and efficacy between children and adults. Evaluating innate and memory immune response is necessary to understand V. cholerae immunity and to improve current cholera vaccine candidates, especially in young children. Further research on the benefits of supplementary interventions and delivery schedules may also improve immunization strategies.
Introduction
Vibrio cholerae is a highly transmissible organism that is found in aquatic reservoirs.Citation1 Cholera is a rapidly spreading, severely dehydrating diarrheal disease that represents a global challenge, with over 50 countries considered to be endemic for the disease.Citation2 Cholera outbreaks have become more frequent and prolonged in recent years in endemic areas with recurrent seasonal patterns, as well as non-endemic areas that are initiated by exogenous introduction of V. cholerae following complex emergencies, such as refugee crises or natural disasters ().Citation3 Recent crises in Pakistan, sub-Saharan Africa and Haiti provide contextual experience to support this idea, in which a sudden disruption results in the collapse of already tenuous water and sanitary facilities.Citation4-Citation6 Estimations project that 1.4 billion people are at risk for cholera worldwide, with half of the resulting deaths being found in children under five years of age.Citation7 Oral cholera vaccines (OCV) offer protection to those vaccinated through direct immunity and indirect protection to unvaccinated individuals via herd immunity.Citation8,Citation9 Other intervention strategies are also required alongside vaccination in order to increase protection against cholera even further. Such measures include timely access to rehydration, antibiotics, hand washing, and improved water/sanitation initiatives. Most developing country populations live in conditions that perpetuate disease transmission and improvements in standards of living can take a long time to achieve. V. cholerae has the potential to cause large, rapidly spreading severe outbreaks that often cripple those public health systems that have limited clinical and financial resources.
Figure 1. Cholera world map, a disease of poverty.Citation3
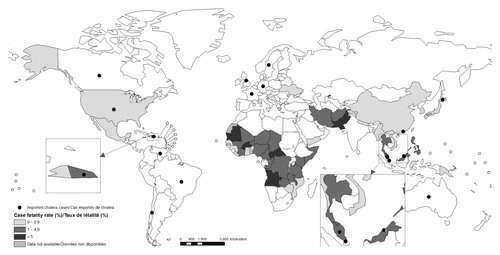
Lower reported efficacy of oral vaccines in children from resource poor countries have led to concerns regarding the public health impact of OCVs.Citation10 While not completely understood, the most likely explanations for this reduced efficacy in poorer countries are linked to malnutrition, maternal antibody interference, parasitic infection, enteropathy, and the presence of pre-exisiting antibodies, all of which have the potential to compromise the mucosal immune response pathway. The following review aims to discuss key findings and measures that are important in optimizing both immunogenicity and efficacy of oral cholera vaccines in high-risk areas.
Pathogenesis and Immunological Basis of Cholera Vaccination
Infection requires small-intestine colonization by V. cholerae, with its pathogenicity mediated by a two-subunit cholera toxin. The pentameric B subunit binds the bacteria to the epithelial surface and is not associated with any toxicity, while the A subunit is responsible for stimulating a cascade causing severe secretory diarrhea.Citation11 Since vibrio pathogens have a mucosal port of entry, vaccination eliciting locally produced intestinal antibodies and stimulating immunological memory makes oral vaccines an attractive option for immunizing populations in resource-limited settings.Citation12
Many currently available cholera vaccines work through inducing serum and mucosal antibodies to interfere with microbial invasion of the bloodstream. Humans can mount a systemic and local mucosal immune response in cholera.Citation13 Antibody mediated protection is primarily directed toward the lipopolysaccharide (LPS) O antigen of cholera organisms and to a lesser degree to cholera toxin. While both O1 and O139 serogroups can elicit serum antitoxin responses, cross protection has not been shown to occur.Citation14 Since the pathogen is thought to be non-invasive, it is widely believed that secretory IgA (sIgA) released from the intestinal lumen may play a substantial role in conferring protection. Serum vibriocidal antibody titers increase with age and studies have shown these to be inversely related to the risk of acquiring a cholera infection.Citation15 Many surrogates of protection following vaccination are relative, meaning that protection is difficult to objectively quantify. In cholera, seroconversion is defined by a 4-fold increase in serum vibriocidal antibody titers against baseline and has served as an indicator that immune responses has been elicited in vaccinnees. While antibody levels in sufficient quantities are thought to prevent infection and severe disease, it should be noted that an adequate memory B cell and cell-mediated immune response are valuable for the control of established infection.Citation16 Other parameters showing association with protection against cholera disease include LPS IgA and CTB IgA antibodies,Citation17 and LPS IgG memory B-cell response.Citation18
Certain intrinsic and genetic host factors are well recognized in influencing the outcome of V. cholerae exposure.Citation19 Cholera infected subjects, who are blood group O suffer more severe symptoms and fatal outcomes,Citation20 but have also responded with higher antibody responses following the administration of a live OCV (CVD 103-HgR).Citation21 Other significant factors in determining outcome of disease include repeated infections and poor nutritional status, which are both linked to low socioeconomic status and poor environmental conditions.Citation22
Immune Response to Oral Vaccination in Developing Countries
Immunization is a powerful and cost effective health intervention, preventing approximately three million deaths and protecting over 100 million lives from illness and disability every year.Citation23 Over two thirds of the world’s population live in developing countries, where infectious diseases cause most of the mortality among children under five years of age.Citation24 In general, oral mucosal/enteric vaccines, whether live, killed, viral, or bacterial, have been less immunogenic and efficacious when given to those living in LDCs, especially in young children.Citation25,Citation26 Under performance has been observed in vaccines for both diarrheal and non-diarrheal diseases alike (). With specific regards to the new modified bivalent oral cholera vaccine, efficacy was much lower in children under 5 y (42%). Nevertheless, more cases seemed to be prevented by vaccination (10.5/1000) for children aged 1–5 y, compared with older age groups (5.5/1000 in 5–15 y and 3.1/1000 in ≥ 15 y). Though the reasons for this are not completely understood, issues relating to the intestinal environment appear to be important in vaccine hypo-responsiveness. Key factors associated with poor vaccine performance in developing countries include protein energy and micronutrient malnutrition, interference by maternal placental and breast milk antibodies, parasitic infections, and intestinal mucosal damage following environmental enteropathy. Another reason could be exposure to related organisms that elicit antibodies that cross react with either non-living or live attenuated vaccines, thus diminishing potency in LDCs. Infants and toddlers have limited exposure to antigens and therefore, mount inadequate anamnestic immune responses.Citation25 This is likely seen in all age groups from non-endemic areas due to a lack of any initial exposure.Citation27 For three years following El Tor V. cholerae disease, both young children and older individuals mount similar vibriocidal and Ab specific responses after natural infection.Citation28 Despite these similarities between the two age groups, currently available oral vaccines are less efficacious and offer a shorter duration of protection in young children than that found in older individuals. Memory B cells (MBC) are thought to play a crucial role in mediating long-term protective immunity by facilitating a rapid anamnestic response upon re-exposure to the antigen.Citation29 A recent investigation from an endemic region has demonstrated a significantly larger and longer lasting MBC response in adults who had natural cholera infection compared with those receiving vaccination with a killed oral cholera vaccine.Citation30 Meanwhile, helper T cells play an important role in the development of B-cell immunity, warranting further research of both MBC and cell-mediated immunity responses in children.Citation31,Citation32
Table 1. Under-performing oral vaccines in the developing world
Although this phenomenon of diminished immunogenicity has been observed in all age groups, there are certain, specific confounding factors attributed to young infants. A level of immunity is transferred to the neonate in the form of maternal serum IgG via the placenta and sIgA and nonspecific protective immune cells through breast milk.Citation33 Specifically, breast milk from mothers in cholera endemic countries contain important antibodies and human milk glycans vital for the immune response.Citation34 While these serve as a valuable protective mechanism against disease, they can also modulate vaccine response. However, frequent intake of breast milk may inhibit the natural protection offered by antibodies and glycans found in maternal breast milk by preventing the vaccine strains from binding to the gut lumen.Citation35 Researchers in Bangladesh demonstrated higher vibriocidal immune responses in children for whom breastfeeding was withheld for 3 h before administration of a killed OCV compared with that of children who were breastfed 1 h before vaccination.Citation36 Though breastfeeding has been documented to reduce the risk of severe cholera in infants,Citation37,Citation38 numerous examples of severe disease in neonates and young children have been increasingly reported.Citation39,Citation40
Immune response relies on overall nutrition, and in the case of infants, maternal macro and micro nutrition. Malnutrition in resource-limited settings is commonly related to socio-economic status, which is linked to poor living conditions and a higher exposure to microbes. These concomitant factors commonly lead to repeated infection. Low anthropometric Z scores (weight for age) have been associated with a 9.5 odds ratio (OR) increase for overall mortality from diarrhea, in addition to increased and prolonged diarrhea in patients hospitalized with V. cholerae diarrhea.Citation41,Citation42 Micronutrients, such as Vitamin ACitation43 and vitamin DCitation44 have been shown to improve vaccine immune response in infants and young children. Zinc supplementation has been shown to significantly lower diarrheal and respiratory disease, as well as improve morbidity from infections in infants and children.Citation45,Citation46 Conversely, zinc deficiency can play a role in decreased response to respiratory vaccines.Citation27,Citation47,Citation48 Although the above studies provide encouraging results with the addition of zinc supplementation, challenges remain in implementing feasible schedules to ensure adequate levels of zinc are attained in the highest risk infants. Other micronutrients, such as vitamins B6, B12, C, E, folate, selenium, copper, magnesium, and iron need to be researched further, but have already been shown to offer great potential in improving immune function.Citation49
In contrast to those living in developed countries with relatively higher levels of sanitation, the mucosal gut lining of individuals living in impoverished settings of developing countries often reflect their continual exposure to fecal-contaminated environments. This leads to the intestinal microflora exhibiting a different composition, which is characterized by villous atrophy and crypt hyperplasia leading to inflammation of and malabsorption by the gut. This altered pathology of the gut’s function is known as environmental or tropical enteropathy. Frequent exposure to bacteria, viruses and parasites may serve as a valuable defense mechanism since such pathogens would have to face this primed, hostile immune environment. Instead of activating the innate immune system and enhancing the adaptive response, oral vaccines may be ultimately attenuated by a highly-activated immune system.Citation50 Furthermore, increased exposure to related organisms sharing common epitopes of the cholera vaccine may likely induce antibodies that can reduce OCV potency. While deworming has demonstrated significant effects on vibriocidal antibody responses to the live OCV, CVD 103-HgR in Ecuadorian children,Citation51 there was no beneficial effect observed in the immune response of Bangladeshi children treated with antihelminthics, who received Dukoral®.Citation27 Maintenance of normal gut microflora and architecture could be critical for normal intestinal function and mucosal immune response.
Overview of Immune Response to Potential OCV Vaccination
Currently, six oral cholera vaccines are in active use or in active clinical evaluation. This includes 2 killed and 4 live OCVs ().
Table 2. Oral cholera vaccines
Killed oral cholera vaccines- capitalizing on dosing schedule flexibility
Dukoral® is the first internationally licensed oral cholera vaccine. Though pre-qualified by the World Health Organization (WHO), it has mainly been used as a travelers’ vaccine, primarily due to its high price and formulation process. The vaccine is co-administered with a buffer (mixed with clean water) to protect the B subunit from being altered by gastric acid. A large randomized placebo-controlled trial in Bangladesh of predecessors to Dukoral® (with and without the recombinant cholera toxin B subunit) in approximately 90 000 adults and children of 2 y and older revealed an 85% efficacy after six months and maintained 50% protection at 3 y of follow up for older children and adults.Citation52
Considering the need to provide a less expensive cholera vaccine for resource-poor countries with endemic cholera, a transfer of the Dukoral® good manufacturing process (GMP) occurred, culminating in the development of a whole-cell, killed oral cholera vaccine in Vietnam. The Vietnamese vaccine differs from Dukoral® in several important aspects. Since it does not contain cholera toxin B subunit, it does not elicit antitoxic immunity, nor does it require co-administration with buffer. It is also less expensive to manufacture. Over 20 million doses of this vaccine have been administered in public health programs in Vietnam. This vaccine has since been reformulated to an oral WC-only vaccine called mORCVAX®.
In order to comply with WHO standards, transfer of manufacturing technologies for this Vietnamese vaccine has resulted in the development and licensure of Shanchol®, a low cost alternative OCV produced in India. Several double-blind, randomized controlled trials (RCT) have evaluated a 2-dose regimen of this modified whole-cell vaccine. Shanchol® consists of five strains of V. cholerae killed whole cells, including O1 classical and El Tor biotypes, plus O139 at 10E11 cfu. The vaccine is safe and highly immunogenic against V. cholerae O1, with 91% seroconversion rates of vibriocidal antibodies among Vietnamese adults.Citation53 A similar trial conducted in Kolkata, India, revealed high background immunity, yielding 53% seroconversion in adults and 80% in children.Citation54 Another highly endemic area in Bangladesh demonstrated an overall 73% immune response in infants (1–2 y), toddlers (2–5 y), and adults (18 y+) with no increase in any adverse events in vaccinees when compared with the placebo group.Citation55 While previous studies measured immune response 14 d after the second dose, the Bangladesh study found similarly high responses 7 d after the second dose. When comparing responses between one and two vaccine doses, investigators from Kolkata found that a single dose of Shanchol® elicited a 4-fold increase in serum vibriocidal antibodies at a higher percentage when compared with two doses in both adults (65% → 46%) and children (87% →82%).Citation56 No increase in seroconversion following a second dose has been observed as a mechanism of the immune response generated.Citation57,Citation58 It may also suggest that the intestinal mucosal response elicited by the first dose could be blunting the vibriocidal response of the second dose. Higher levels of pre-existing cross reactive OCV antibodies and presumably higher immunity in endemic areas also correlate with lower vaccine response.Citation52 Still, vibriocidal antibody response does not truly reflect protection and at best is an indirect correlate of protection.
Shanchol® has conferred 67% protection for 5 y in a double-blind, randomized, controlled trial among more than 67 000 children and adults in Kolkata, India.Citation59 Children aged 1–5 y, who are at greatest risk for severe disease, were significantly less protected with a collective protective efficacy of 42% over 5 y. A large OCV trial in Bangladesh has enrolled 267 000 individuals aged one year and above to measure the vaccine’s feasibility and effectiveness.Citation60 This was a three-armed study aimed to compare the vaccine against the vaccine plus interventions to promote standard washing and point-of-use water treatment in the community, and a control arm that promotes standard sanitary practices only. This study aims to evaluate the feasibility and impact of a vaccine together with important community interventions in a high-risk cholera endemic area. Several characteristics make the vaccine advantageous for use in the developing world. Shanchol® does not contain the B subunit toxin and therefore does not require a buffer, making it simpler to administer and cheaper to manufacture. It is licensed for individuals from one year of age and above, and is administered in two doses. In September 2011, WHO included Shanchol® on its list of prequalified vaccines, which has enabled purchase by United Nations (UN) agencies and widened its potential for implementation in affected areas. Recommendations by WHO for use of OCV as part of a total control package for endemic and epidemic area was first published in 2010.Citation61
Investigations evaluating innovative delivery packages and schedules targeting high-risk populations (children under 5 y, HIV-positive individuals, pregnant women) are needed to improve current efficacy levels and optimize immunization program effectiveness. Data regarding the safety and immunogenicity of the modified, killed, whole-cell vaccine among infants need to be gathered in order to pave the way for the possible integration of this vaccine into cholera-endemic areas, where infants and children are most at risk.Citation62 Furthermore, since no data exist regarding the concomitant use of this vaccine with other routine infant vaccines, it is important to determine if any interference exists between Shanchol® and other antigens included in the Expanded Program on Immunization (EPI). Providing the killed, whole-cell vaccine as part of the EPI could make the introduction of cholera vaccines in endemic areas easier. A clinical trial evaluating safety and immunogenicity in the infant population (< 1 y) is also being planned. Considering the increased immune response following the first dose in a 14-d schedule, a recently completed study measuring responses after a prolonged dosing interval of 28 d suggests no difference in seroconversion rates between the 2 and 4 wk schedule.Citation63 With no rise in seroconversion rates after a second dose, efforts to evaluate a single dose regimen in a clinical field trial is being planned in order to evaluate its potential use in an epidemic setting. Also, similar vibriocidal responses reported for the 14 and 28-d schedule may endorse the inclusion of OCV into national immunization schemes, such as the EPI schedule for infants, which could assist in producing higher vaccination rates in endemic areas. Furthermore, flexibility with the administration of 2 doses over a month could ease logistical requirements in an epidemic setting following a natural disaster, allowing for stabilization of community infrastructure, as well as aiding in the delivery of OCV.
Live oral cholera vaccines- potential for extended protection with a single dose
There are currently four live attenuated oral cholera vaccines in active clinical programs. As well as improving administration schedules by presenting the possibility of a single dose regimen, these vaccine candidates potentially offer greater efficacy, rapid onset of protection, and longer duration, which would be valuable in conferring the highest level of containment in outbreak settings.Citation64 All four vaccines are administered as a single dose, require buffer co-administration, and are in the planning stages for phase 3 protective efficacy studies.
The vaccine candidate with the longest clinical history is CVD 103-HgR (University of Maryland, USA), which has been shown to be safe and immunogenic in North American volunteers and generated antibodies within one week of dosing.Citation65 Despite a large phase 3 efficacy trial in Indonesian children and adults failing to show protection during any of the four years of follow up,Citation66 the vaccine was associated with a 79% risk reduction of cholera when given via mass vaccination following an epidemic in Micronesia.Citation67 Due to these conflicting results, commercial production was suspended in 2004. However, its favorable safety and immunogenicity profiles in adults, children and HIV positive individuals has led to efforts to re-commercialize this vaccine (PXVX0200, PAXVAX, USA).Citation68-Citation71 Phase 3 trials are expected to be completed in mid-2014. The lyophylized formulation is the only live vaccine candidate that does not need to be stored frozen (-20 C), but still requires cold chain and co-administration with a buffer. Plans for subsequent generation formulations aim to be more suited to being used in endemic setting use by negating the need for cold chain or a buffer.
Other live vaccines actively being tested and in advanced stages of clinical trials include Peru-15 (CholeraGarde®, Harvard Medical School, USA), V. cholerae 638 (Finlay Institute, Cuba), and VA 1.4 (Government of India). Peru-15 has gone through successful safety/immunogenicity trials in US volunteers, as well as in Bangladeshi children aged 9 mo to 5 y of age, where infants were noted to have the lowest antibody titer response.Citation72 Early results from Bangladesh indicate no interference between Peru-15 and the measles vaccine in infants less than one year of age.Citation73 A recently completed trial with this vaccine in Thailand also demonstrated safety and immunogenicity in HIV positive adults.Citation74 Investigators in Cuba have developed a new oral vaccine containing the 638 live, attenuated cholera strain. Phase 1 and 2 trials in adult populations from Cuba and Mozambique have demonstrated favorable safety and immunogenicity.Citation75,Citation76 The Indian Department of Biotechnology is moving forward with a new live OCV (VA1.3) that has been shown to be safe and has produced an excellent immune response when tested in adult males from Kolkata, India. This vaccine, derived from a non-toxigenic strain of Vibrio cholerae El Tor, Inaba did not result in any increased adverse events compared with placebo and elicited a 57% seroconversion rate in adult males from the endemic setting of Kolkata.Citation77 It has been reformulated and since renamed as VA 1.4.
Considerations in OCV Implementation and Delivery
Contrary to conventional belief, cholera vaccine protective efficacy does not need to be very high in order to be effective as a public health intervention. Vaccine herd protection is the ability to reduce person-person transmission through mass vaccination programs, which adds to a vaccine’s protective efficacy thereby increasing the public health impact of OCVs. For highly transmissible diseases, protective efficacy does not necessarily serve as an absolute measure of vaccine protection, as is the case in outbreak settings with poor access to clean water (urban slums or refugee camps), where incidence can rise to as high as 50 cases/1000. Even with a lower efficacy, a vaccine demonstrating herd protective effects that is used in high incidence areas, could prevent a large number of severe cholera cases. Currently available whole-cell, killed OCVs have been shown to provide significant herd protection in both vaccinated and non-vaccinated populations, including children too young to be vaccinated, even in areas of modest vaccine coverage. This provides convincing evidence to public health decision makers when deciding on including a vaccine into their EPI programs or not. Simulation models suggest that when vaccine coverage rates over 50% are achieved with the modified, killed, whole-cell vaccine, outbreaks in endemic regions could be reduced by 93%.Citation8,Citation9 Furthermore, it has been deemed very cost effective in high incidence areas such as India, Bangladesh, and Mozambique.Citation78,Citation79 A recent investment case estimating vaccine cost effectiveness for over 30 countries projected to be early OCV adopters found the vaccine to be very cost effective, especially for programs targeting children.Citation80,Citation81 When considering vaccine delivery, isolating specific age groups may prove to add extra operational and ethical challenges. The price per dose of Shanchol® currently stands at US$1.85, though this may gradually decline as more producers enter the market. Proven effectiveness with herd protection in all age groups, a highly tolerated safety profile, simple administration, and low cost are vital characteristics that make a vaccine useful in endemic and post-disaster settings.
The updated WHO position recommends the use of OCVs as a tool to help control endemic cholera and should be considered in epidemic situations.Citation82 Important areas that still require research to be performed include updating disease burden rates and trends, as well as an improvement in rapid, reliable, and inexpensive surveillance methods. As with any vaccine, improvements in the logistical use of OCVs (heat stability, multi-dose vials, single-dose regimen) would greatly aid in facilitating its introduction into high risk field settings in resource-scarce countries. Short-term modeling analysis of the Haitian epidemic suggests that expedient use of a limited supply of OCVs could have had substantial impact if targeted to high-risk populations, especially when complementing improvements in water and sanitation.Citation83 Discussions for the possible use of a cholera vaccine stockpile following the disasters in Pakistan and Haiti offer an attractive mechanism for OCV introduction, with the hopes of yielding both public health and humanitarian benefits.Citation84 A comprehensive cholera response, that links prevention with care will require a concerted international effort to reach those who are most affected and in greatest need.
Public immunization campaigns with the modified, killed, whole-cell OCV have successfully been performed in high risk areas, including India, Bangladesh, Haiti, and Guinea.Citation60,Citation85-Citation87 Though most trials have been conducted in Asia, the vaccine is not expected to act any differently in non-Asian populations. Even so, clinical investigations have recently been completed in Zanzibar (Dukoral®), while a trial in Ethiopia (Shanchol®), demonstrated similar safety and immunogenicity in healthy African adults and children to those found in Asian trials.Citation88,Citation89 These trials can help pave the way for the possible use of this vaccine in any endemic and outbreak setting throughout resource-scarce areas. A double blind, phase 3, randomized, controlled trial is underway to generate evidence for the potential use of a single dose OCV regimen. The critical issue is the question of efficacy and the duration of protection of a single dose, which would be an important step toward beginning the dossier for single-dose vaccination in outbreak settings. Another ongoing study evaluating the effect of booster doses suggests that a single booster dose of killed OCV can elicit vibriocidal titers similar to those levels produced by a primary series in adults and children residing in endemic areas, which could help to ease logistical challenges faced in maintaining protection in cholera endemic areas.Citation90 Investigations on memory B cell and cell-mediated immune responses are lacking in children, and such studies would be interesting to offer important insights into differences in protection offered by natural infection vs. current vaccine options.
Conclusion
Both killed and live oral cholera vaccines will face several key challenges, which will be crucial in improving the protective impact of vaccines in areas and communities at high risk for endemic and epidemic cholera. Complementary behavioral modification efforts to improve water sanitation and hygiene in high risk communities will also provide great benefit in preventing other high disease burden enteric water-borne diseases, such as enterotoxigenic E. coli, Shigella, and Rotavirus.
Even though reduced immunogenicity in the LDC vaccinee presents numerous challenges in achieving effective protection against cholera, many innovative approaches are being investigated. Safe water supplies combined with adequate sanitation and improved hygiene are essential to reduce cholera and other enteric diseases in the future.Citation91 Unfortunately, such progress can take decades to achieve and will provide little relief to unstable and mobile populations. In this context, a safe and affordable vaccine to protect against cholera would be a welcome public health tool. Continued efforts to evaluate and optimize immunization strategies will be vital in achieving maximum benefit to those most at risk.
| Abbreviations: | ||
| EPI | = | Expanded Programme on Immunization |
| HIV | = | human immunodeficiency virus |
| LDC | = | least developing countries |
| OCV | = | oral cholera vaccine |
| RCT | = | randomized controlled trial |
| UN | = | United Nations |
| WHO | = | World Health Organization |
Acknowledgments
We are grateful to Dr Daniel T. Leung (icddr,b and Massachusetts General Hospital, Harvard Medical School) for his helpful review of this manuscript.
Potential conflicts of interest: SND and AC are staff of the International Vaccine Institute, which has supported the reformulation and use of the bivalent inactivated oral cholera vaccine, Shanchol® globally.
References
- Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, Kariuki S, Croucher NJ, Choi SY, Harris SR, Lebens M, et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 2011; 477:462 - 5; http://dx.doi.org/10.1038/nature10392; PMID: 21866102
- World Health Organization. Cholera, 2012. Wkly Epidemiol Rec 2013; 88:321 - 34; PMID: 23980290
- World Health Organization. Countries reporting cholera cases 2011. Global Health Observatory Map Gallery. September 28, 2012 [cited September 30, 2013].
- Enserink M. Public health. No vaccines in the time of cholera. Science 2010; 329:1462 - 3; http://dx.doi.org/10.1126/science.329.5998.1462; PMID: 20847246
- Nossiter A. Cholera epidemic envelops coastal slums in West Africa, in New York TImes: Dakar, Senegal.
- Ministere de la Sante Publique et de la Population, Republique d'Haiti (MSPP). 2013 [cited; Available from: http://www.mspp.gouv.ht/site/index.php?option=com_content&view=article&id=120&Itemid=1.
- Ali M, Lopez AL, You YA, Kim YE, Sah B, Maskery B, Clemens J. The global burden of cholera. Bull World Health Organ 2012; 90:209 - 218A; http://dx.doi.org/10.2471/BLT.11.093427; PMID: 22461716
- Ali M, Emch M, von Seidlein L, Yunus M, Sack DA, Rao M, Holmgren J, Clemens JD. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet 2005; 366:44 - 9; http://dx.doi.org/10.1016/S0140-6736(05)66550-6; PMID: 15993232
- Longini, I.M., Jr., et al., Controlling endemic cholera with oral vaccines. PLoS Medicine / Public Library of Science, 2007. 4(11): p. e336.
- Pastor M, Pedraz JL, Esquisabel A. The state-of-the-art of approved and under-development cholera vaccines. Vaccine 2013; 31:4069 - 78; http://dx.doi.org/10.1016/j.vaccine.2013.06.096; PMID: 23845813
- Sánchez J, Holmgren J. Virulence factors, pathogenesis and vaccine protection in cholera and ETEC diarrhea. Curr Opin Immunol 2005; 17:388 - 98; http://dx.doi.org/10.1016/j.coi.2005.06.007; PMID: 15963708
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11:Suppl S45 - 53; http://dx.doi.org/10.1038/nm1213; PMID: 15812489
- Cash RA, Music SI, Libonati JP, Craig JP, Pierce NF, Hornick RB. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis 1974; 130:325 - 33; http://dx.doi.org/10.1093/infdis/130.4.325; PMID: 4443613
- Albert MJ, Alam K, Rahman AS, Huda S, Sack RB. Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J Infect Dis 1994; 169:709 - 10; http://dx.doi.org/10.1093/infdis/169.3.709; PMID: 8158063
- Mosley WH, Ahmad S, Benenson AS, Ahmed A. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bull World Health Organ 1968; 38:777 - 85; PMID: 5303331
- Harris AM, Bhuiyan MS, Chowdhury F, Khan AI, Hossain A, Kendall EA, Rahman A, LaRocque RC, Wrammert J, Ryan ET, et al. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect Immun 2009; 77:3850 - 6; http://dx.doi.org/10.1128/IAI.00369-09; PMID: 19528207
- Clemens JD, Stanton BF, Chakraborty J, Sack DA, Khan MR, Huda S, Ahmed F, Harris JR, Yunus M, Khan MU, et al. B subunit-whole cell and whole cell-only oral vaccines against cholera: studies on reactogenicity and immunogenicity. J Infect Dis 1987; 155:79 - 85; http://dx.doi.org/10.1093/infdis/155.1.79; PMID: 3540139
- Patel SM, Rahman MA, Mohasin M, Riyadh MA, Leung DT, Alam MM, Chowdhury F, Khan AI, Weil AA, Aktar A, et al. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin Vaccine Immunol 2012; 19:842 - 8; http://dx.doi.org/10.1128/CVI.00037-12; PMID: 22518009
- O’Connor D, Pollard AJ. Characterizing vaccine responses using host genomic and transcriptomic analysis. Clin Infect Dis 2013; 57:860 - 9; http://dx.doi.org/10.1093/cid/cit373; PMID: 23728145
- Faruque AS, Mahalanabis D, Hoque SS, Albert MJ. The relationship between ABO blood groups and susceptibility to diarrhea due to Vibrio cholerae 0139. Clin Infect Dis 1994; 18:827 - 8; http://dx.doi.org/10.1093/clinids/18.5.827; PMID: 8075282
- Lagos R, Avendaño A, Prado V, Horwitz I, Wasserman S, Losonsky G, Cryz S Jr., Kaper JB, Levine MM. Attenuated live cholera vaccine strain CVD 103-HgR elicits significantly higher serum vibriocidal antibody titers in persons of blood group O. Infect Immun 1995; 63:707 - 9; PMID: 7822046
- Chowdhury F, Khan AI, Harris JB, LaRocque RC, Chowdhury MI, Ryan ET, Faruque AS, Calderwood SB, Qadri F. A comparison of clinical and immunologic features in children and older patients hospitalized with severe cholera in Bangladesh. Pediatr Infect Dis J 2008; 27:986 - 92; http://dx.doi.org/10.1097/INF.0b013e3181783adf; PMID: 18833030
- O’Hagan DT, Rappuoli R. Novel approaches to pediatric vaccine delivery. Adv Drug Deliv Rev 2006; 58:29 - 51; http://dx.doi.org/10.1016/j.addr.2005.12.002; PMID: 16480788
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al, Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379:2151 - 61; http://dx.doi.org/10.1016/S0140-6736(12)60560-1; PMID: 22579125
- Pasetti MF, Simon JK, Sztein MB, Levine MM. Immunology of gut mucosal vaccines. Immunol Rev 2011; 239:125 - 48; http://dx.doi.org/10.1111/j.1600-065X.2010.00970.x; PMID: 21198669
- Sack DA, Svennerholm AM. Determinants of Responses to Oral Vaccines in Developing Countries.. Ann Nestle 2008; 66:71 - 9; http://dx.doi.org/10.1159/000159366
- Qadri F, Bhuiyan TR, Sack DA, Svennerholm AM. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine 2013; 31:452 - 60; http://dx.doi.org/10.1016/j.vaccine.2012.11.012; PMID: 23153448
- Ali M, Emch M, Park JK, Yunus M, Clemens J. Natural cholera infection-derived immunity in an endemic setting. J Infect Dis 2011; 204:912 - 8; http://dx.doi.org/10.1093/infdis/jir416; PMID: 21849288
- Leung DT, Rahman MA, Mohasin M, Riyadh MA, Patel SM, Alam MM, Chowdhury F, Khan AI, Kalivoda EJ, Aktar A, et al. Comparison of memory B cell, antibody-secreting cell, and plasma antibody responses in young children, older children, and adults with infection caused by Vibrio cholerae O1 El Tor Ogawa in Bangladesh. Clin Vaccine Immunol 2011; 18:1317 - 25; http://dx.doi.org/10.1128/CVI.05124-11; PMID: 21697337
- Uddin T, Aktar A, Xu P, Johnson RA, Rahman MA, Leung DT, Afrin S, Akter A, Alam MM, Rahman A, et al. Immune Responses to O-Specific Polysaccharide and Lipopolysaccharide of Vibrio cholerae O1 Ogawa in Adult Bangladeshi Recipients of an Oral Killed Cholera Vaccine and Comparison to Responses in Patients with Cholera. Am J Trop Med Hyg 2014; 90:873 - 81; http://dx.doi.org/10.4269/ajtmh.13-0498; PMID: 24686738
- Leung DT, Rahman MA, Mohasin M, Patel SM, Aktar A, Khanam F, Uddin T, Riyadh MA, Saha A, Alam MM, et al. Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin Vaccine Immunol 2012; 19:690 - 8; http://dx.doi.org/10.1128/CVI.05615-11; PMID: 22441386
- Arifuzzaman M, Rashu R, Leung DT, Hosen MI, Bhuiyan TR, Bhuiyan MS, Rahman MA, Khanam F, Saha A, Charles RC, et al. Antigen-specific memory T cell responses after vaccination with an oral killed cholera vaccine in Bangladeshi children and comparison to responses in patients with naturally acquired cholera. Clin Vaccine Immunol 2012; 19:1304 - 11; http://dx.doi.org/10.1128/CVI.00196-12; PMID: 22739692
- Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr 2010; 156:Suppl S3 - 7; http://dx.doi.org/10.1016/j.jpeds.2009.11.021; PMID: 20105662
- Glass RI, Svennerholm AM, Stoll BJ, Khan MR, Hossain KM, Huq MI, Holmgren J. Protection against cholera in breast-fed children by antibodies in breast milk. N Engl J Med 1983; 308:1389 - 92; http://dx.doi.org/10.1056/NEJM198306093082304; PMID: 6843632
- Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 2003; 21:3406 - 12; http://dx.doi.org/10.1016/S0264-410X(03)00342-6; PMID: 12850349
- Ahmed T, Svennerholm AM, Al Tarique A, Sultana GN, Qadri F. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine 2009; 27:1433 - 9; http://dx.doi.org/10.1016/j.vaccine.2008.12.036; PMID: 19146904
- Glass RI, Stoll BJ. The protective effect of human milk against diarrhea. A review of studies from Bangladesh. Acta Paediatr Scand Suppl 1989; 351:131 - 6; http://dx.doi.org/10.1111/j.1651-2227.1989.tb11225.x; PMID: 2692384
- Clemens JD, Sack DA, Harris JR, Khan MR, Chakraborty J, Chowdhury S, Rao MR, van Loon FP, Stanton BF, Yunus M, et al. Breast feeding and the risk of severe cholera in rural Bangladeshi children. Am J Epidemiol 1990; 131:400 - 11; PMID: 2301350
- Khan AM, Hossain MS, Khan AI, Chisti MJ, Chowdhury F, Faruque AS, Salam MA. Bacterial enteropathogens of neonates admitted to an urban diarrhoeal hospital in Bangladesh. J Trop Pediatr 2009; 55:122 - 4; http://dx.doi.org/10.1093/tropej/fmn090; PMID: 18840632
- Siddique AK, Ahmed S, Iqbal A, Sobhan A, Poddar G, Azim T, Sack DA, Rahman M, Sack RB. Epidemiology of rotavirus and cholera in children aged less than five years in rural Bangladesh. J Health Popul Nutr 2011; 29:1 - 8; http://dx.doi.org/10.3329/jhpn.v29i1.7560; PMID: 21528784
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J, Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008; 371:243 - 60; http://dx.doi.org/10.1016/S0140-6736(07)61690-0; PMID: 18207566
- Palmer DL, Koster FT, Alam AK, Islam MR. Nutritional status: a determinant of severity of diarrhea in patients with cholera. J Infect Dis 1976; 134:8 - 14; http://dx.doi.org/10.1093/infdis/134.1.8; PMID: 820813
- Cassani B, Villablanca EJ, De Calisto J, Wang S, Mora JR. Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol Aspects Med 2012; 33:63 - 76; http://dx.doi.org/10.1016/j.mam.2011.11.001; PMID: 22120429
- Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect 2006; 134:1129 - 40; http://dx.doi.org/10.1017/S0950268806007175; PMID: 16959053
- Larson CP, Roy SK, Khan AI, Rahman AS, Qadri F. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr 2008; 26:356 - 65; PMID: 18831230
- Larson CP, Nasrin D, Saha A, Chowdhury MI, Qadri F. The added benefit of zinc supplementation after zinc treatment of acute childhood diarrhoea: a randomized, double-blind field trial. Trop Med Int Health 2010; 15:754 - 61; http://dx.doi.org/10.1111/j.1365-3156.2010.02525.x; PMID: 20374562
- Brooks WA, Yunus M, Santosham M, Wahed MA, Nahar K, Yeasmin S, Black RE. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet 2004; 363:1683 - 8; http://dx.doi.org/10.1016/S0140-6736(04)16252-1; PMID: 15158629
- Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr 2004; 24:255 - 75; http://dx.doi.org/10.1146/annurev.nutr.23.011702.073054; PMID: 15189121
- Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr 2007; 98:Suppl 1 S29 - 35; http://dx.doi.org/10.1017/S0007114507832971; PMID: 17922955
- Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol 2010; 8:129; http://dx.doi.org/10.1186/1741-7007-8-129; PMID: 20920375
- Cooper PJ, Chico ME, Losonsky G, Sandoval C, Espinel I, Sridhara R, Aguilar M, Guevara A, Guderian RH, Levine MM, et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis 2000; 182:1199 - 206; http://dx.doi.org/10.1086/315837; PMID: 10979918
- Clemens JD, Sack DA, Harris JR, Van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 1990; 335:270 - 3; http://dx.doi.org/10.1016/0140-6736(90)90080-O; PMID: 1967730
- Anh DD, Canh G, Lopez AL, Thiem VD, Long PT, Son NH, Deen J, von Seidlein L, Carbis R, Han SH, et al. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine 2007; 25:1149 - 55; http://dx.doi.org/10.1016/j.vaccine.2006.09.049; PMID: 17055622
- Mahalanabis D, Lopez AL, Sur D, Deen J, Manna B, Kanungo S, von Seidlein L, Carbis R, Han SH, Shin SH, et al. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. [Electronic Resource] PLoS One 2008; 3:e2323; http://dx.doi.org/10.1371/journal.pone.0002323; PMID: 18523643
- Saha A, Chowdhury MI, Khanam F, Bhuiyan MS, Chowdhury F, Khan AI, Khan IA, Clemens J, Ali M, Cravioto A, et al. Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine 2011; 29:8285 - 92; http://dx.doi.org/10.1016/j.vaccine.2011.08.108; PMID: 21907255
- Kanungo S, Paisley A, Lopez AL, Bhattacharya M, Manna B, Kim DR, Han SH, Attridge S, Carbis R, Rao R, et al. Immune responses following one and two doses of the reformulated, bivalent, killed, whole-cell, oral cholera vaccine among adults and children in Kolkata, India: a randomized, placebo-controlled trial. Vaccine 2009; 27:6887 - 93; http://dx.doi.org/10.1016/j.vaccine.2009.09.008; PMID: 19761838
- Jertborn M, Svennerholm AM, Holmgren J. Intestinal and systemic immune responses in humans after oral immunization with a bivalent B subunit-O1/O139 whole cell cholera vaccine. Vaccine 1996; 14:1459 - 65; http://dx.doi.org/10.1016/S0264-410X(96)00071-0; PMID: 8994322
- Alam MM, Riyadh MA, Fatema K, Rahman MA, Akhtar N, Ahmed T, Chowdhury MI, Chowdhury F, Calderwood SB, Harris JB, et al. Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera. Clin Vaccine Immunol 2011; 18:844 - 50; http://dx.doi.org/10.1128/CVI.00562-10; PMID: 21346055
- Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, Sah B, Niyogi SK, Park JK, Sarkar B, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2013; 13:1050 - 6; http://dx.doi.org/10.1016/S1473-3099(13)70273-1; PMID: 24140390
- Khan IA, Saha A, Chowdhury F, Khan AI, Uddin MJ, Begum YA, Riaz BK, Islam S, Ali M, Luby SP, et al. Coverage and cost of a large oral cholera vaccination program in a high-risk cholera endemic urban population in Dhaka, Bangladesh. Vaccine 2013; 31:6058 - 64; http://dx.doi.org/10.1016/j.vaccine.2013.10.021; PMID: 24161413
- World Health Organization. Cholera vaccines: WHO position paper. Wkly Epidemiol Rec 2010; 85:117 - 28; PMID: 20349546
- Deen JL, von Seidlein L, Sur D, Agtini M, Lucas ME, Lopez AL, Kim DR, Ali M, Clemens JD. The high burden of cholera in children: comparison of incidence from endemic areas in Asia and Africa. [electronic resource] PLoS Negl Trop Dis 2008; 2:e173; http://dx.doi.org/10.1371/journal.pntd.0000173; PMID: 18299707
- Desai SN, et al. Study of Alternative Vaccination Schedule of Oral Cholera Vaccine. NCT01233362. in Vaccines and Enteric Diseases. 2013. Bangkok.
- Ryan ET, Calderwood SB, Qadri F. Live attenuated oral cholera vaccines. Expert Rev Vaccines 2006; 5:483 - 94; http://dx.doi.org/10.1586/14760584.5.4.483; PMID: 16989629
- Tacket CO, Losonsky G, Nataro JP, Cryz SJ, Edelman R, Kaper JB, Levine MM. Onset and duration of protective immunity in challenged volunteers after vaccination with live oral cholera vaccine CVD 103-HgR. J Infect Dis 1992; 166:837 - 41; http://dx.doi.org/10.1093/infdis/166.4.837; PMID: 1527420
- Richie EE, Punjabi NH, Sidharta YY, Peetosutan KK, Sukandar MM, Wasserman SS, Lesmana MM, Wangsasaputra FF, Pandam SS, Levine MM, et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 2000; 18:2399 - 410; http://dx.doi.org/10.1016/S0264-410X(00)00006-2; PMID: 10738097
- Calain P, Chaine JP, Johnson E, Hawley ML, O’Leary MJ, Oshitani H, Chaignat CL. Can oral cholera vaccination play a role in controlling a cholera outbreak?. Vaccine 2004; 22:2444 - 51; http://dx.doi.org/10.1016/j.vaccine.2003.11.070; PMID: 15193408
- Suharyono A, Simanjuntak C, Witham N, Punjabi N, Heppner DG, Losonsky G, Totosudirjo H, Rifai AR, Clemens J, Lim YL, et al. Safety and immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR in 5-9-year-old Indonesian children. Lancet 1992; 340:689 - 94; http://dx.doi.org/10.1016/0140-6736(92)92231-4; PMID: 1355798
- Gotuzzo E, Butron B, Seas C, Penny M, Ruiz R, Losonsky G, Lanata CF, Wasserman SS, Salazar E, Kaper JB, et al. Safety, immunogenicity, and excretion pattern of single-dose live oral cholera vaccine CVD 103-HgR in Peruvian adults of high and low socioeconomic levels. Infect Immun 1993; 61:3994 - 7; PMID: 8359923
- Simanjuntak CH, O’Hanley P, Punjabi NH, Noriega F, Pazzaglia G, Dykstra P, Kay B, Suharyono, Budiarso A, Rifai AR, et al. Safety, immunogenicity, and transmissibility of single-dose live oral cholera vaccine strain CVD 103-HgR in 24- to 59-month-old Indonesian children. J Infect Dis 1993; 168:1169 - 76; http://dx.doi.org/10.1093/infdis/168.5.1169; PMID: 8228350
- Perry RT, Plowe CV, Koumaré B, Bougoudogo F, Kotloff KL, Losonsky GA, Wasserman SS, Levine MM. A single dose of live oral cholera vaccine CVD 103-HgR is safe and immunogenic in HIV-infected and HIV-noninfected adults in Mali. Bull World Health Organ 1998; 76:63 - 71; PMID: 9615498
- Qadri F, Chowdhury MI, Faruque SM, Salam MA, Ahmed T, Begum YA, Saha A, Al Tarique A, Seidlein LV, Park E, et al, PXV Study Group. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine 2007; 25:231 - 8; http://dx.doi.org/10.1016/j.vaccine.2006.08.031; PMID: 16996172
- Qadri F. (personal communication with PI) Safety and Immunogenicity of Peru-15 Vaccine When Given With Measles Vaccine in Healthy Indian and Bangladeshi Infants. NCT00624975. 2013: Dhaka.
- Ratanasuwan W, Lee Y. A Randomized, Placebo-Controlled Trial to Evaluate the Safety and Immunogenicity of a Single Dose Regimen of Live Attenuated Oral Cholera Vaccine (Choleragarde®) in HIV-Seropositive Adults in Thailand. NCT00741637. in Presented at Vaccines and Enteric Diseases. 2013. Bangkok.
- Valera R, García HM, Jidy MD, Mirabal M, Armesto MI, Fando R, García L, Fernández R, Año G, Cedré B, et al. Randomized, double-blind, placebo-controlled trial to evaluate the safety and immunogenicity of live oral cholera vaccine 638 in Cuban adults. Vaccine 2009; 27:6564 - 9; http://dx.doi.org/10.1016/j.vaccine.2009.08.042; PMID: 19720365
- Garcia HM, et al. (2011) A single dose of live-attenuated 638 Vibrio cholerae oral vaccine is safe and immunogenic in adult volunteers in Mozambique. VacciMonitor Volume, 1-8.
- Mahalanabis D, Ramamurthy T, Nair GB, Ghosh A, Shaikh S, Sen B, Thungapathra M, Ghosh RK, Pazhani GP, Nandy RK, et al. Randomized placebo controlled human volunteer trial of a live oral cholera vaccine VA1.3 for safety and immune response. Vaccine 2009; 27:4850 - 6; http://dx.doi.org/10.1016/j.vaccine.2009.05.065; PMID: 19523608
- Jeuland M, Cook J, Poulos C, Clemens J, Whittington D, DOMI Cholera Economics Study Group. Cost-effectiveness of new-generation oral cholera vaccines: a multisite analysis. Value Health 2009; 12:899 - 908; http://dx.doi.org/10.1111/j.1524-4733.2009.00562.x; PMID: 19824189
- Sarker AR, Islam Z, Khan IA, Saha A, Chowdhury F, Khan AI, Qadri F, Khan JA. Cost of illness for cholera in a high risk urban area in Bangladesh: an analysis from household perspective. BMC Infect Dis 2013; 13:518; http://dx.doi.org/10.1186/1471-2334-13-518; PMID: 24188717
- International Vaccine Institute. An investment case for the accelerated introduction of oral cholera vaccines. Seoul, Korea.2012. [cited January 27, 2014]; Available from: http://www.ivi.int/web/www/04_03.
- Mogasale V, et al. Oral cholera vaccines to control endemic disease: an economic and epidemiological modelling analysis. Lancet 2013; 382:6; http://dx.doi.org/10.1016/S0140-6736(13)62167-4
- World Health Organization. Cholera, 2011. Wkly Epidemiol Rec 2012; 87:289 - 304; PMID: 22905370
- Chao DL, Halloran ME, Longini IM Jr.. Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. Proc Natl Acad Sci U S A 2011; 108:7081 - 5; http://dx.doi.org/10.1073/pnas.1102149108; PMID: 21482756
- Waldor MK, Hotez PJ, Clemens JD. A national cholera vaccine stockpile--a new humanitarian and diplomatic resource. N Engl J Med 2010; 363:2279 - 82; http://dx.doi.org/10.1056/NEJMp1012300; PMID: 21105832
- International Vaccine Institute. Cholera: Orissa mass vaccination Campaign. 2011 [cited February 1, 2013]; Available from: http://www.ivi.int/web/www/02_05_02.
- Ivers LC, Teng JE, Lascher J, Raymond M, Weigel J, Victor N, Jerome JG, Hilaire IJ, Almazor CP, Ternier R, et al. Use of oral cholera vaccine in Haiti: a rural demonstration project. Am J Trop Med Hyg 2013; 89:617 - 24; http://dx.doi.org/10.4269/ajtmh.13-0183; PMID: 24106187
- Luquero FJ, Grout L, Ciglenecki I, Sakoba K, Traore B, Heile M, Dialo AA, Itama C, Serafini M, Legros D, et al. First outbreak response using an oral cholera vaccine in Africa: vaccine coverage, acceptability and surveillance of adverse events, Guinea, 2012. PLoS Negl Trop Dis 2013; 7:e2465; http://dx.doi.org/10.1371/journal.pntd.0002465; PMID: 24147164
- Khatib AM, Ali M, von Seidlein L, Kim DR, Hashim R, Reyburn R, Ley B, Thriemer K, Enwere G, Hutubessy R, et al. Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet Infect Dis 2012; 12:837 - 44; http://dx.doi.org/10.1016/S1473-3099(12)70196-2; PMID: 22954655
- Bridging Study for Killed Oral Cholera Vaccine in Ethiopia. NCT01524640. 2012 [cited February 23, 2013]; Available from: http://clinicaltrials.gov/ct2/show/NCT01524640?term=oral+cholera+vaccine%2C+ethiopia&rank=1.
- Desai SN, et al. Evaluation of a Boosting Regimen With Oral Cholera Vaccine. NCT01579448. in Vaccines and Enteric Diseases. 2013. Bangkok.
- Coalition for Cholera Prevention and Control. Comprehensive Integrated Strategy for Cholera Prevention and Control. 2013.
- Walker RI, Steele D, Aguado T, Ad Hoc ETEC Technical Expert Committee. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 2007; 25:2545 - 66; http://dx.doi.org/10.1016/j.vaccine.2006.12.028; PMID: 17224212
- Rahman KM, Arifeen SE, Zaman K, Rahman M, Raqib R, Yunus M, Begum N, Islam MS, Sohel BM, Rahman M, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine 2011; 29:1347 - 54; http://dx.doi.org/10.1016/j.vaccine.2010.10.035; PMID: 21040694
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606 - 14; http://dx.doi.org/10.1016/S0140-6736(10)60889-6; PMID: 20692030
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289 - 98; http://dx.doi.org/10.1056/NEJMoa0904797; PMID: 20107214
- Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, et al. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis 2011; 5:e1289 - 1289; http://dx.doi.org/10.1371/journal.pntd.0001289; PMID: 22028938
- Levine MM, Ferreccio C, Cryz S, Ortiz E. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet 1990; 336:891 - 4; http://dx.doi.org/10.1016/0140-6736(90)92266-K; PMID: 1976928
- Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis 1991; 13:926 - 39; http://dx.doi.org/10.1093/clinids/13.5.926; PMID: 1660184
