Abstract
To elicit potent humoral immunity and produce adequate neutralizing antibody especially in the genital tract and eventually to promote its immunogenicity, we designed an Eppin B-cell-dominant-epitope-based mimovirus vaccine with an RGD motif which can be nasally inoculated into male mice. Our results indicate that this immune strategy successfully generated a high antibody response with significantly higher anti-Eppin IgA in the genital tract, and eventually achieve significant inhibition of fertility without any interference with testis function and alteration in structural integrity. The fertility rate of the females mating with the vaccinated males declined and the progeny size was greatly reduced, but the contraceptive efficacy was still far from that of immunocontraceptives for human use. However, the research showed a new contraceptive vaccine construction and inoculation avenue, that is, mimovirus vaccine delivered nasally. Further investigation geared toward improving fertility inhibition efficacy using this inoculation strategy still remains to be explored.
Introduction
The burden of family planning should be shouldered by both men and women. However, the contraceptive options available to men are limited and considered either inconvenient or irreversible. Therefore, it is of great importance to explore new male contraceptives that are safe, convenient, and reversible as well. Contraceptive vaccine is taken as an attractive addition to the currently available family planning methods.
Many studies laid their emphasis on sperm-specific molecules and epididymis and testis-specific proteins in the last 2 decades. Immunization with these candidate molecules resulted different inhibition of fertility in animals, but the fertility inhibition efficacy is far from that of a successful contraceptive agent.Citation1 In addition, the previous immunization routes of contraceptive vaccine were limited to intramasucular injection, subcutaneous injection and intraperitoneal injection, which was invasive and inconvenient.
SPINLW1, known as epididymis protease inhibitor (Eppin), has an important role for sperm maturationCitation2 and sperm motility.Citation3,Citation4 Recombinant Eppin protein immunization showed a more satisfactory contraceptive effect (78%) in male monkeys,Citation5 thus cast a light on developing a promising male contraception agent. On considering that full-length protein immunization was potentially not safe, we previously studied the the immunodominant B-cell epitope of Eppin and demonstrated that SPINLW1 amino acids103–115, MFVYGGCQGNNNN, induced an epitope specific immune response and suppressed fertility in mice.Citation3 However, it only achieved a limited fertility inhibition with 23.3% of females fertile when caged with the inoculated males.. Therefore, introduction of new immunization strategies to stimulate potent epitope-specific immune response, especially in the reproductive tract, is key to promoting contraceptive efficacy.
Nasal immunization, as a noninvasive approach, is considered to be an ideal way to stimulate mucosal immunity and humoral immune response.Citation6,Citation7 Previous studies have shown that nasal immunization successfully induced a high level specific mucosal IgA response in the genital tract in mice, monkeys, and humans.Citation6 M cell is a specialized epithelial cell in the upper respiratory tract mucosa, which enables the efficient uptake of particulate antigen and delivery to the antigen-presenting cells. Delivery of DNA vaccine targeting M cell via respiratory tract mucosa was feasible.Citation8 Recent studies demonstrated that the RGD peptide motif (Gly-Arg-Gly-Asp-Ser) could effectively target M cells.Citation9,Citation10 Therefore, DNA vaccines with RGD targeted to M cells of nasal mucosa are expected to enhance the efficiency of antigen by nasal delivery. We had previously explored a novel strategy in antigen formation known as ‘mimovirus vaccine’Citation11-Citation14. In the present study, such a nanoparticle delivery system was constructed and delivered nasally by targeting to M cells with a RGD motif.
In order to promote immune response, many gene adjuvant candidates have been studied.Citation15-Citation17 IL-4 had been identified to have an important role in stimulating Th2 polarization response in vivo.Citation18,Citation19 Herein, we designed and constructed a recombinant dual plasmid simultaneously expressing both IL-4 and an epitope gene fragment. We then synthesized a polylysine (TAT49–57)-linker-cyclic RGD fusion peptide that would self-assemble with the plasmid to form nanoparticles, thereby exposing the flexible cyclic RGD ligand outside of the nanoparticle (referred to here as ‘mimovirus’). The biological features and fertility suppression of a mimovirus vaccine were investigated.
Materials and Methods
Animals
Eight- to ten-week-old specific pathogen-free BALB/c mice were obtained from the Animal Research center of the Third Military Medical University (Chongqing, China). Animals were housed in a temperature- and light-cycle controlled animal facility at the Institute of Immunology, PLA, Third Military Medical University. The mice were randomly divided into 4 groups (10 mice each group) and ear coded. All experiments were conducted according to the guidelines of the Chinese Animal Care for Laboratory Animals, and the protocols were approved by the Animal Care and Use Committee at the Third Military Medical University.
Plasmids
Plasmid pVITRO2-mcs and pORF-mIL-4 were purchased from InvivoGen Corporation. Plasmid pUC57-SP-E103–115-ThPADRE, containing epitope E103–115 and its signal peptide (SP, Eppin1–21) as well as Pan DR T Helper Epitopes (PADRE) was ordered from Sangon, and was designated here as pUC57-Pc. Primers for mIL-4 were as follows: sense, 5′- GCGGGATCCA CCATGGGTCT CAACCCC-3′ and antisense, 5′-GCGACGCGTG TACTACGAGT AATCCATT-3′. Primers for Pc were as follows: sense, 5′-CCGGAATTCA TGGGATCTTC TGGACTT-3′ and antisense, 5′-CCCCTCGAGT CAAGCGGCAG CCTTCA-3′. The resulting PCR products were cloned into pVITRO2-mcs according to the manufacturer’s protocol, and the plasmid pVITRO2-mcs-Pc/mIL-4 was constructed. Plasmids were verified by appropriate restriction enzyme digestion protocols and subsequent gel electrophoresis. The correctness of the generated sequences was confirmed by nucleotide sequence analysis. The plasmids were transfected into CHO cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After transfection, cells were incubated at 37 °C for 48 h. Then, the expression products of the transfected cells were identified by RT-PCR and western blotting.
Mimovirus
To prepare the mimovirus, the cell-penetrating peptide (TAT49–57, RKKRRQRRR) fused with flexible linker GGG and RGD containing peptide GRGDS were synthesized by GL Biochem, that is, GRGDS-GGG- TAT49–57 (designated as GR17). The purity of each peptide reached greater than 95%.
Construction of mimovirus was described previously.Citation11 Briefly, the mimovirus was prepared in microcentrifuge tubes by the addition of plasmid to peptide with the different molar ratios (0, 0.5, 1, 2, 4, 8, and 16) in HEPES-buffered saline (HBS) containing 10 mM HEPES and 150 mM NaCl. The final concentration of the DNA plasmid in all of the samples was identical (1000 μg/mL). The input molar ratio of peptide to DNA plasmid (r) was the ratio of moles of lysine to nucleotide (NH4+/PO3). Mimovirus was verified by gel retardation assay, DNase protection assay, transmission electron microscope and particle size analysis as described previously.Citation11,Citation14 Mimovirus was produced by a combination of the cationic peptides, GRGDS-GGG-TAT49–57 and pVITRO2-mcs-Pc/mIL-4.
Immunization schedule
Sexually mature Balb/C male mice were divided into 4 groups consisting of 10 mice per group. The mice were inoculated 4 times at 2-wk intervals with different modalities (). Each mouse was given nasal immunization with 100 μL of solution containing the mimovirus which composed of 60 μg of peptide and 20 μg of plasmid in group 4, or 100 μL PBS in group 1. However, each mouse received 20 μg of plasmid diluted in 100 μL PBS intramuscularly (in the anterior tibialis muscle on 2 legs) in group 3 and nasally in group 2 at the same interval and dosage. Immunizations were performed without anesthesia.
Table1. Treatment groups as designated by inoculation regimen
Antibody detection in the sera of immunized mice
Serum samples were collected at the indicated time points. To detect specific anti-Eppin antibodies, the plates were coated with optimized concentrations of rhEppin (100 ng in 100 μL of PBS in each well) at 4 °C in a humidified atmosphere overnight. Standard ELISA procedures were followed.Citation20,Citation21 If the ratio between the OD at 405nm of a well and the negative control exceeded 2.1, the sample in the well was defined as positive. Among the serial and positive serum samples, the maximum dilution ratio was the antibody titer.
Mating for fertility assays in vivo
To detect inhibition of fertility in vivo, each immunized male mouse was caged with three 6- to 8-wk-old Balb/C female mice for 1 wk, and checked for vaginal plugs each morning. The male mouse was kept separately and the litter sizes were recorded. The mean progeny size was calculated with total pup mumber against the number of pregnant mice. Two such mating trials were performed at 1 wk and 3 wk respectively after the final immunization. Male mice were maintained without further immunizations for 20 wk to test their ability to recover fertility after immunization. In the next week after the final fertility inhibition assay (4 wk after final immunization) or restoration test (20 wk after final immunization), 5 male mice of each group were randomly euthanized respectively and sera, epidydimis, epidydimal fluid lavages were collected for subsequent studies.
Evaluation of epididymal sperm
The right cauda epididymidis was carefully removed and used to collect epididymal sperm as described previously.Citation22 The sperm collected was centrifuged at 225 g for 10 min. The pellet was resuspended in 1.0 mL of F-10 medium. The total sperm count was counted and expressed as 106/mL. Evaluation of motility of epididymal sperm was done as described previously.Citation23 The live and dead spermatozoa were determined using 1% tryphan blue.Citation24 Simultaneously, hypo-osmotic swelling test (HOS), which had been proven as a better predictor of fertilizing capacity than motility,Citation25 was employed to evaluate the functional activity of the sperm membrane as described previously.Citation26 We also used the spermatozoa acrosin activity quantitative assay to determine sperm function according to operation instructions in the kit (Shen Zhen Hua kang Co., LTD).
Detection of IgA in epididymis lavages of immunized mice
The left cauda epididymidis was prepared as mentioned previously.Citation27 Briefly, the cauda epididymidis was separated, trimmed free of fat, and placed in 200 μL PBS containing 0.1% BSA. A 1 mL syringe was used to suck the PBS and flush the epididymis lumens thrice, and then express the residue fluid in the epidydimal lumens. The rhEppin-specific IgA antibodies in epidydimis lavage were assayed by an indirect ELISA.
Detection of serum testosterone levels
Male mice were anaesthetized and 1 mL of blood was obtained by cardiac puncture at 4 wk after finial immunization (after fertility assay) or 20 wk after final immunization (after restoration test). Serum testosterone (T) was measured using a radioimmunoassay (RIA) kit obtained from North Biological technology Research Institute (Beijing, China), according to operation instructions in the kit. The intra-assay coefficient of variation was 10% and the sensitivity reached 0.02 ng/mL. All the samples were assayed at the same time to minimize inter-assay variation.
Histologic examination
To detect any possible histological changes, the mice were euthanized at 4 wk after final immunization and the testis and epididymis were removed, then fixed in 4% (w/v) paraformaldehyde (Sangon) and embedded in paraffin. Tissue slices (4–5μm) were cut and deparaffinized by being immersed in xylene for 5 min 3 times, in 95% ethanol for 5 min 3 times and then in 70% ethanol for 5 min 3 times. The slices were stained with hematoxylin-eosin (HE) and observed under a light microscope.
Statistical analysis
Data were expressed as mean ± SEM. Statistical significance between 2 groups was determined by independent-samples t test and between 3 or more groups by one-way analysis of variance (ANOVA). The Pearson chi-square test was used to ascertain significant differences in fertility rates. Statistical analyses were performed with Statistical Product and Service Solutions (SPSS) Windows19.0 Statistical Software. Statistical significance was defined as P < 0.05.
3. Results
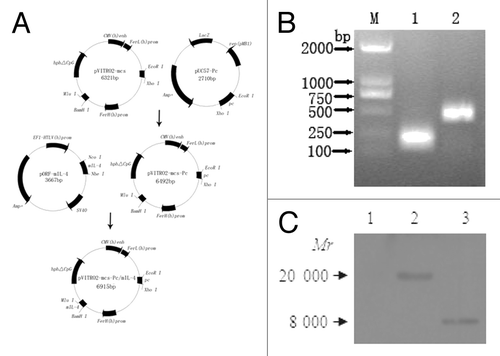
Construction and identification of DNA plasmids
As shown in , Pc gene was cloned into the eukaryotic expression vector pVITRO2-mcs, thus pVITRO2-mcs-Pc was constructed. mIL-4 gene was subsequently cloned into pVITRO2-mcs-Pc, resulting in plasmid pVITRO2-mcs-Pc/mIL-4. Thereafter, pVITRO2-mcs or pVITRO2-mcs-Pc/mIL-4 was transiently transfected into CHO cells, and the in vitro expression of the plasmids was analyzed by RT-PCR and western blotting. The ~170 bp and 460 bp in the case of plasmid pVITRO2-mcs-Pc/mIL-4 were detected in lane 1 and lane 2 respectively (). However, we did not detect bands in the supernatants of the cells transfected with plasmid pVITRO2-mcs (lane 1, ). In addition, the ~8 kDа and ~20 kDа molecular weight bands in lane 2 and lane 3 coincided with the theoretical molecular weights of mIL-4 protein and Pc protein respectively ().
Figure 1. Schematic representation of plasmid construction and in vitro expression of plasmid. (A) Construction of eukaryotic expression plasmid pVITRO2-mcs-Pc/mIL-4. (B) RT-PCR analysis of expressed products in the culture supernatant of CHO cells transfected with pVITRO2-mcs-Pc/mIL-4. M: DNA marker; 1: RT-PCR production for Pc gene; 2: RT-PCR production for mIL-4 gene. (C) Western blot analysis of expressed products in the culture supernatant of CHO cells transfected with different plasmids. Lane 1: CHO cells transfected with pVITRO2-mcs; Lane 2: CHO cells transfected with pVITRO2-mcs-Pc/mIL-4; Lane 3: CHO cells transfected with pVITRO2-mcs-Pc/mIL-4.
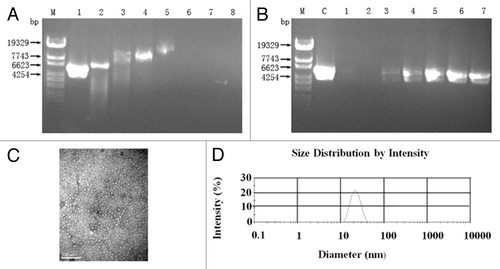
Formation of the mimovirus
The mimovirus was prepared when the plasmid self-assembled with the cationic peptide (GRGDSGGGRKKRRQRRR) as previously described. Then the properties of the mimovirus particles were investigated with the gel retardation assay, DNase I protection assay and TEM at different charge ratios. As shown in , no migration of the plasmid DNA band was observed in the gel retardation assay when r ≥ 4 (lane 6). This indicated that the negative charge of the plasmid was fully neutralized by the cationic peptide and the peptide/nucleic acid nanoparticle formation may be constructed. With the formation of nanoparticles, the plasmid DNA was expected to be protected from digestion by DNase I. DNase I protection assay were employed to evaluate the viability of each complex (). The plasmid DNA was partially protected at r = 2 (lane 4) and almost completely protected at r = 4 (lane 5). When r = 4, the peptide/plasmid particles showed a stable and relatively homogeneous shape () with diameters ranged between 10 nm and 45 nm (peak value of 22.57 nm) ().
Figure 2. Identification of the mimovirus. (A) DNA retardation assay. Mimovirus was prepared at the input molar ratio of peptide to DNA (r), r = 0, 0.25, 0.5, 1, 2, 4, 8, and 16 (lanes 1–8, respectively), then was analyzed by electrophoresis on a 1% agarose gel stained with goldview. (B) DNase Idigestion assay. Mimovirus2 samples at r = 0, 0.5, 1, 2, 4, 8, and 16 (lanes 1–7, respectively) were subsequently digested with DNase I. The remaining DNA was extracted and analyzed on a 1% agarose gel stained with goldview. (C) TEM observation. Mimovirus particles were condensed at r = 4 and were examined using a transmission electron microscopy at a magnification of 80 000. (D) Particle size analysis of mimovirus.
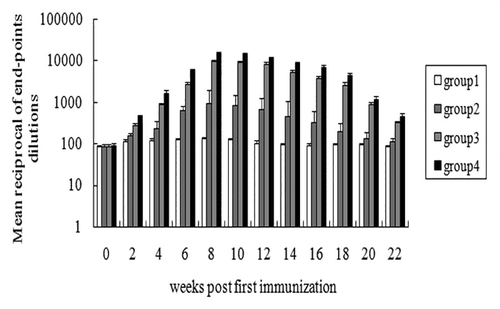
Antibody responses induced by mimovirus
We detected serum anti-rhEppin IgG of the vaccinated mice by standard ELISA. As shown in , the anti-rhEppin antibody levels could be detected after the first immunization and reached the peak at week 8 in the test groups. Importantly, the antibody responses in the mice nasally inoculated with mimovirus (group 4) were present earlier than in the other groups, and at the peak time, the mean reciprocal of end-point titers in this group were significantly higher than that of the group1 and group 2 (P < 0.05). However, the antibody titer of mice intramuscularly inoculated with plasmid (group 3) was also significantly higher than those of group 1 and group 2, although lower than that of group 4, but showed no significant difference.
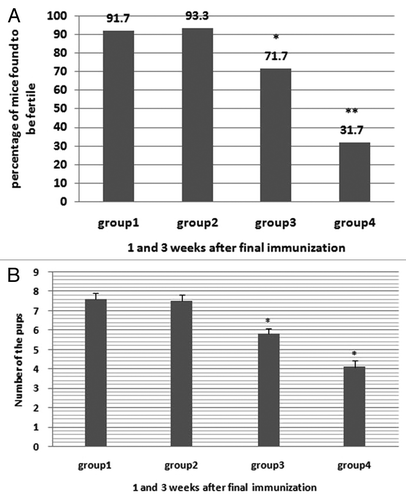
Fertility assays
Immunized males were mated twice with sexually mature virgin females at 1 and 3 wk after the final immunization, respectively. All female mice put up for mating showed a vaginal plug in 1 wk. Data were pooled for statistical analyses according to the inoculation groups (data at 1 and 3 wk respectively see supplementary figures). As shown in , the fertility rate of the females mating with males of group 4 was 31.7%, which was significantly lower than that of any other group (P < 0.05). Group 3 showed higher fertility rate when compared with group4, which was still significantly lower than the control group PBS (P < 0.05). However, as shown in , the mean progeny size in both group 3 (5.8 ± 0.29) and group 4 (4.1 ± 0.34) showed significant differences against group PBS (7.61 ± 0.33, P < 0.005), and there also existed significant difference between group 3 and group 4(P < 0.05). These data suggest that the vaccines reduced both the progeny size and fertility rate of the females. However, the fertility rate as well as the mean of progeny size of the females mating with the males showed no difference among groups 20 wk later (data see supplementary section).
Figure 4. Fertility assay of immunized mice. (A) The fertility rate of female mice mated with immunized males at 1 and 3 wk after the final immunization. (B) Progeny size of female mice mated with immunized males at 1 and 3 wk after the final immunization. Asterisk indicates a significant difference from the PBS group (*P < 0.05; **P < 0.001).
Sperm analysis of immunized mice
To further investigate the mechanism of reduced fertility in vaccinated male mice, sperm obtained from the cauda epididymidis were analyzed with respect to sperm count, motility, sperm membrane functional integrity and spermatozoa acrosin activity. As shown in , the sperm counts showed no significant difference among groups (P > 0.05). However, sperm motility and HOS were greatly suppressed in group 4 and group 3 when compared with group PBS (P < 0.01), with group 4 even lower. Spermatozoa acrosin activity was also greatly inhibited in group 4 and group 3 compared with group PBS (P < 0.05). There was no significant difference in all the above mentioned indicators among groups 20 wk post final immunization (data see supplementary section).
Table 2. Effects of mimovirus vaccine on the seminal parameters of immunized mice 4 wk after final immunization
IgA in epididymis fluid
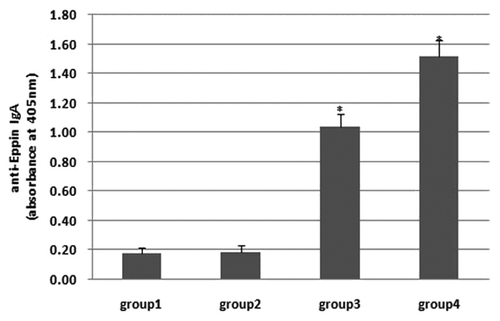
To detect the local IgA concentration in genital tract, we assayed it in the epidydimis fluid by indirect ELISA. Both plasmid injection (group 3) and mimovirus nasal immunization (group 4) successfully induced IgA response in the epidydimis, which were siginificantly higher than those of the PBS group (). However, group 4 still showed a significantly higher IgA concentration than those of group 3. Plasmid intranasal application (group 2) failed to induce a high IgA response in the epidydimis (P > 0.05).
Figure 5. Detection of the local IgA concentration in epididymis 4 wk after final immunization. Mimovirus intranasal inoculation as well as plasmid injection induced a higher IgA concentration (P < 0.05) when compared with the PBS group, while the former was much higher than the latter. Plasmid intranasal application failed to induce a high IgA response (P > 0.05).
Determination of serum testosterone levels
Serum testosterone concentrations were detected by RIA. The results exhibited no significant difference between groups with an intra-assay of 7%. The serum testosterone concentrations levels were 2.42 ± 0.04 (group 2), 2.41 ± 0.03 (group 3), 2.34 ± 0.09 (group 4). When compared with group 1(2.43 ± 0.11) there was no significant difference. When repeatedly assayed 20 wk after final immunization, the serum testosterone levels were generally decreased with no difference between the groups (data provided as supplemental figures).
Histological analysis
Histological examination was performed to exclude any possible histological changes of the testis and the epididymis. The testis and epididymis were stained with hematoxylin-eosin and observed under a light microscope. Results showed no histological difference between test groups and the control group (data not shown). Under light microscopy, testis sections exhibited no signs of infiltration and showed normal seminiferous tubule diameter and spermatids. Epididymal tubules showed no evidence of leukocyte infiltration and contained morphologically mature sperm in their lumen.
Discussion
Due to the shortcomings of existing contraceptive measures and the limited choices for men, it is of great importance to develop new methods for male contraception. In the past few decades, contraceptive vaccines were considered to be a promising approach for male contraception and were widely explored. But until now, none of them has been proven to be a successful contraceptive agent because of their limited fertility suppression efficacy.Citation1
Eppin, as a sperm specific antigen, was a promising target for developing safer and more effective contraceptive vaccine.Citation5 Antibodies to Eppin in immunized male monkeys have been shown to provide effective and reversible fertility inhibitionCitation5and these antibodies could inhibit sperm motility in vitro.Citation3 Our earlier study demonstrated that a fragment of Eppin (MFVYGGCQGNNNN) located at 103–115 amino acids has the ability to suppress fertility.Citation3 However, the fertility inhibition efficacy achieved still cannot meet needs of a contraceptive agent. Therefore, it is important to further investigate the construction of an epitope-based vaccine of Eppin and its effects on fertility inhibition.
Mimovirus vaccines, with sizes similar to viruses, can induce both cellular immunityCitation28,Citation29 and humoral immune responses.Citation14 These studies suggest that mimovirus vaccines could be a promising contraceptive vaccine modality. However, in our previous study, the mimovirus vaccine was inoculated intramuscularly, which was invasive and inconvenient, more importantly, the results showed only a moderate fertility inhibition effectCitation14 presumably because of its limited mucosal immune response. The aim of this study was to explore a more convenient and effective inoculation modality of mimovirus vaccine.
The nasal cavity is easily accessible and equipped with M cells that enable the efficient uptake of particulate antigens and delivery of them to the antigen-presenting cells. On considering that nasal immunization is noninvasive and can stimulate both mucosal immunity and humoral immune response,Citation6 we have designed a mimovirus vaccine that could easily be delivered nasally. In order to promote its efficiency, an RGD-based strategy was applied. The cyclic RGD containing peptide can specifically bind to β1-Integrin presenting at the surface of M cells in the upper respiratory tract. Therefore, the RGD-containing particulates are expected to enhance the efficacy of antigen. By using positive and negative charges interactions, we constructed a mimovirus vaccine containing a recombinant dual plasmid and a cation peptide, the former encoding both IL-4 and Eppin B-cell epitope, and the latter was a polylysine (TAT49–57)-linker-cyclic RGD fusion peptide. When the charge was fully neutralized, migration of the plasmid DNA was completely restrained and the DNA plasmid was completely protected from the activity of DNase. In this case, the stable and homogeneous nanoparticles formed and the diameter of most particles ranged between 10 nm and 45 nm, with a peak value of 22.57 nm ().
In this study, the dual plasmid, expressing both Eppin B-cell epitope and IL-4 as a molecular adjuvant, when delivered by intramuscularly injection (group 3), is able to elicit a much higher serum antibody response than that of plasmid alone delivered nasally (group 2). However, if the dual plasmid was coated with a cationic peptide containing an RGD motif and formed nano-particulates (mimovirus), it had the potential to elicit an even more potent immune response. That is to say, plasmid alone delivered by nose failed to generate potent immune response because of its limited uptake, whereas an RGD-based mimovirus antigen delivery system provides an efficient inoculation strategy.
With regard to the anti-fertility mechanism of Eppin, the results presented here were in agreement with our previous findingsCitation3,Citation27 and another report.Citation30 Anti-eppin antibody could leads to a reduction of fertility mainly by interfering with the function of sperm, but not the function of the testis. The total sperm motility and viability obtained from group 3 and group 4 were greatly inhibited when compared with the PBS group, while the sperm counts, serum testerone level as well as the testis and epididymic structures showed no significant difference. Although both the mimovirus delivered nasally (group4) and the plasmid intramuscularly injection (group3) successfully elicited a potent anti-Eppin antibody in serum, the fertility rate, and the progeny size in females mating with males in group 4 was even greatly reduced, presumably because mimovirus immunogen delivery system could produce considerable neutralizing antibodies especially in the genital tract, as was shown in the IgA response in the epidydimis. Such non-mucosa innoculation as intramuscular injection was good at inducing systemic immunization, while mucosa immunity might be comparably weak,Citation31 therefore, the antibody titer in genital tract was not so high. However, because of the commmon mucosal immune system (CMIS), vaccines inoculated through mucosa might induce both local and remote multi-mucosa immunity, and elicit humoral response simultaneously. In this study, pregnancy rate dropped to about 31.7%, which was similar to that seen in other researches.Citation3,Citation27 This would suggest that there must be some difficulties in completely inhibiting the rodent sperm motility.
In conclusion, this study explored a new construction of mimovirus vaccine based on an Eppin B-cell-dominant-epitope; and employed a new delivery method, that is, per nose. Such a design succeeded in generating a high antibody response especially in the genital tract, and eventually achieved a moderate inhibitory effect on fertility without interfering with testis function. However, aimed at a single epitope, it was hard to produce enough neutralizing antibody and completely inhibit millions of sperms, the contraceptive efficacy was still far from the standard of an immunocontraceptives for human use. Furthermore, the side-effect should be further studied because Eppin has several other functions except for sperm motility inhibition.Citation4 Even so, immunocontraception for males is still a novel method with some potential. Further investigation geared toward targeting multiple sperm antigens using this inoculation strategy still deserves to be explored.
Additional material
Download Zip (234.3 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was funded by grants from National Natural Science Foundation of China (No. 30901607 and No.81270758). The authors would also like to thank Dr Dev Sooranna, Imperial College London, for helping to edit the manuscript.
References
- Naz RK. Antisperm immunity for contraception. J Androl 2006; 27:153 - 9; http://dx.doi.org/10.2164/jandrol.05192; PMID: 16474022
- Richardson RT, Sivashanmugam P, Hall SH, Hamil KG, Moore PA, Ruben SM, French FS, O’Rand M. Cloning and sequencing of human Eppin: a novel family of protease inhibitors expressed in the epididymis and testis. Gene 2001; 270:93 - 102; http://dx.doi.org/10.1016/S0378-1119(01)00462-0; PMID: 11404006
- Chen Z, He W, Liang Z, Yan P, He H, Tang Y, Zhang J, Shen Z, Ni B, Wu Y, et al. Protein prime-peptide boost as a new strategy induced an Eppin dominant B-cell epitope specific immune response and suppressed fertility. Vaccine 2009; 27:733 - 40; http://dx.doi.org/10.1016/j.vaccine.2008.11.025; PMID: 19041353
- O’Rand MG, Widgren EE, Hamil KG, Silva EJ, Richardson RT. Functional studies of eppin. Biochem Soc Trans 2011; 39:1447 - 9; http://dx.doi.org/10.1042/BST0391447; PMID: 21936831
- O’rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, VandeVoort CA, Ramachandra SG, Ramesh V, Jagannadha Rao A. Reversible immunocontraception in male monkeys immunized with eppin. Science 2004; 306:1189 - 90; http://dx.doi.org/10.1126/science.1099743; PMID: 15539605
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6:148 - 58; http://dx.doi.org/10.1038/nri1777; PMID: 16491139
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6:148 - 58; http://dx.doi.org/10.1038/nri1777; PMID: 16491139
- Wu Y, Wang X, Csencsits KL, Haddad A, Walters N, Pascual DW. M cell-targeted DNA vaccination. Proc Natl Acad Sci U S A 2001; 98:9318 - 23; http://dx.doi.org/10.1073/pnas.161204098; PMID: 11459939
- Gullberg E, Keita AV, Salim SY, Andersson M, Caldwell KD, Söderholm JD, Artursson P. Identification of cell adhesion molecules in the human follicle-associated epithelium that improve nanoparticle uptake into the Peyer’s patches. J Pharmacol Exp Ther 2006; 319:632 - 9; http://dx.doi.org/10.1124/jpet.106.107847; PMID: 16914557
- Garinot M, Fiévez V, Pourcelle V, Stoffelbach F, des Rieux A, Plapied L, Theate I, Freichels H, Jérôme C, Marchand-Brynaert J, et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Control Release 2007; 120:195 - 204; http://dx.doi.org/10.1016/j.jconrel.2007.04.021; PMID: 17586081
- Wu YZ, Zhao JP, Wan Y, Jia ZC, Zhou W, Bian J, Ni B, Zou LY, Tang Y. Mimovirus: a novel form of vaccine that induces hepatitis B virus-specific cytotoxic T-lymphocyte responses in vivo. J Virol 2002; 76:10264 - 9; http://dx.doi.org/10.1128/JVI.76.20.10264-10269.2002; PMID: 12239302
- Yang Z, Wang L, Wang H, Shang X, Niu W, Li J, Wu Y. A novel mimovirus vaccine containing survivin epitope with adjuvant IL-15 induces long-lasting cellular immunity and high antitumor efficiency. Mol Immunol 2008; 45:1674 - 81; http://dx.doi.org/10.1016/j.molimm.2007.10.026; PMID: 18035418
- Tian Z, Wang H, Jia Z, Shi J, Tang J, Mao L, Liu H, Deng Y, He Y, Ruan Z, et al. Tumor-targeted inhibition by a novel strategy - mimoretrovirus expressing siRNA targeting the Pokemon gene. Curr Cancer Drug Targets 2010; 10:932 - 41; http://dx.doi.org/10.2174/156800910793357907; PMID: 20879980
- Shen ZG, He W, Zhang J, He HY, Yang X, Chen ZQ, Yang P, Li J, Liang ZQ, Wu YZ, et al. Induction of specific immune response and suppression of fertility by B-cell-epitope-based mimovirus vaccine. Reproduction 2011; 142:659 - 66; http://dx.doi.org/10.1530/REP-11-0161; PMID: 21908656
- Hirschhorn-Cymerman D, Perales MA. Cytokine-FC fusion genes as molecular adjuvants for DNA vaccines. Methods Mol Biol 2010; 651:131 - 55; http://dx.doi.org/10.1007/978-1-60761-786-0_9; PMID: 20686965
- Fagone P, Shedlock DJ, Bao H, Kawalekar OU, Yan J, Gupta D, Morrow MP, Patel A, Kobinger GP, Muthumani K, et al. Molecular adjuvant HMGB1 enhances anti-influenza immunity during DNA vaccination. Gene Ther 2011; 18:1070 - 7; http://dx.doi.org/10.1038/gt.2011.59; PMID: 21544096
- Li DJ, Wang HM, Li L, Zhao XR, Wang MY, Zhu Y, Meng Y, Yuan MM. Gene fusion of molecular adjuvant C3d to hCGbeta enhances the anti-hCGbeta antibody response in DNA immunization. J Reprod Immunol 2003; 60:129 - 41; http://dx.doi.org/10.1016/j.jri.2003.09.001; PMID: 14638440
- Ghochikyan A, Vasilevko V, Petrushina I, Movsesyan N, Babikyan D, Tian W, Sadzikava N, Ross TM, Head E, Cribbs DH, et al. Generation and characterization of the humoral immune response to DNA immunization with a chimeric beta-amyloid-interleukin-4 minigene. Eur J Immunol 2003; 33:3232 - 41; http://dx.doi.org/10.1002/eji.200324000; PMID: 14635031
- Frazer ME, Hughes JE, Mastrangelo MA, Tibbens JL, Federoff HJ, Bowers WJ. Reduced pathology and improved behavioral performance in Alzheimer’s disease mice vaccinated with HSV amplicons expressing amyloid-beta and interleukin-4. Mol Ther 2008; 16:845 - 53; http://dx.doi.org/10.1038/mt.2008.39; PMID: 18388924
- Pascual DW, Hone DM, Hall S, van Ginkel FW, Yamamoto M, Walters N, Fujihashi K, Powell RJ, Wu S, Vancott JL, et al. Expression of recombinant enterotoxigenic Escherichia coli colonization factor antigen I by Salmonella typhimurium elicits a biphasic T helper cell response. Infect Immun 1999; 67:6249 - 56; PMID: 10569734
- van Ginkel FW, McGhee JR, Liu C, Simecka JW, Yamamoto M, Frizzell RA, Sorscher EJ, Kiyono H, Pascual DW. Adenoviral gene delivery elicits distinct pulmonary-associated T helper cell responses to the vector and to its transgene. J Immunol 1997; 159:685 - 93; PMID: 9218583
- Tayama K, Fujita H, Takahashi H, Nagasawa A, Yano N, Yuzawa K, Ogata A. Measuring mouse sperm parameters using a particle counter and sperm quality analyzer: a simple and inexpensive method. Reprod Toxicol 2006; 22:92 - 101; http://dx.doi.org/10.1016/j.reprotox.2005.11.009; PMID: 16431076
- Reddy PS, Pushpalatha T, Reddy PS. Reduction of spermatogenesis and steroidogenesis in mice after fentin and fenbutatin administration. Toxicol Lett 2006; 166:53 - 9; http://dx.doi.org/10.1016/j.toxlet.2006.05.012; PMID: 16806747
- Talbot P, Chacon RS. A triple-stain technique for evaluating normal acrosome reactions of human sperm. J Exp Zool 1981; 215:201 - 8; http://dx.doi.org/10.1002/jez.1402150210; PMID: 6168732
- Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 1984; 70:219 - 28; http://dx.doi.org/10.1530/jrf.0.0700219; PMID: 6694140
- Yan P, He W, Liang Z, Chen Z, Shang X, He H, Tang Y, Ni B, Zhang J, Shen Z, et al. A novel dominant B-cell epitope of FSHR identified by molecular docking induced specific immune response and suppressed fertility. Gynecol Endocrinol 2009; 25:828 - 38; http://dx.doi.org/10.3109/09513590903015536; PMID: 19906003
- Chen Z, He W, Wu Y, Yan P, He H, Zhang J, Yang X, Shen Z, Liang Z, Li J. A highly specific antibody response after protein prime-peptide boost immunization with Eppin/B-cell epitope in mice. Hum Vaccin 2011; 7:849 - 55; http://dx.doi.org/10.4161/hv.7.8.16279; PMID: 21817855
- Wu YZ, Zhao JP, Wan Y, Jia ZC, Zhou W, Bian J, Ni B, Zou LY, Tang Y. Mimovirus: a novel form of vaccine that induces hepatitis B virus-specific cytotoxic T-lymphocyte responses in vivo. J Virol 2002; 76:10264 - 9; http://dx.doi.org/10.1128/JVI.76.20.10264-10269.2002; PMID: 12239302
- Yang Z, Wang L, Wang H, Shang X, Niu W, Li J, Wu Y. A novel mimovirus vaccine containing survivin epitope with adjuvant IL-15 induces long-lasting cellular immunity and high antitumor efficiency. Mol Immunol 2008; 45:1674 - 81; http://dx.doi.org/10.1016/j.molimm.2007.10.026; PMID: 18035418
- O’Rand MG, Widgren EE, Beyler S, Richardson RT. Inhibition of human sperm motility by contraceptive anti-eppin antibodies from infertile male monkeys: effect on cyclic adenosine monophosphate. Biol Reprod 2009; 80:279 - 85; http://dx.doi.org/10.1095/biolreprod.108.072942; PMID: 18945989
- Cerutti A. The regulation of IgA class switching. Nat Rev Immunol 2008; 8:421 - 34; http://dx.doi.org/10.1038/nri2322; PMID: 18483500