Abstract
An overwhelming number of medicines on the market are oral medicine with the disadvantage of lower bioavailability universally. Newcastle disease (ND) has become a serious disease that threatens the poultry industries in many countries, and there are no treatments available for ND. The biodegradable materials could be surface modified and protect antigen or DNA from damage. Furthermore, nanoparticles are also a potential drug delivery with proper size. However, Newcastle disease virus (NDV) vaccines encapsulated in nanoparticles were widely used due to their proved a high safety and induced quicker and better mucosal and humoral immune responses. Here we review the results of mucosal immune delivery system for ND. Due to the safety, low toxicity, and better immunogenicity of the mucosal immune delivery system, our studies provide a clearly view that used the biodegradable materials to research and develop the human vaccines to save more patients’ lives. These promising results provide a foundation for testing the approach in humans.
Introduction
The appearance of proteins, vitamins and gene drugs has made a great contribution to the field of medicine. At the same time, researchers also paid more attention to study oral medicine which can be adopted by people and convenient in the field of biomedicine and molecular biology. However, these oral medications have some disadvantages such as protein drugs have a character of high molecular weight, bad penetrability of biomembrane and partially be disrupted by protease in vivo, vitamin pills could hardly adapt to the enteric pH and gut flora, easily degradation by the nucleases and even have low bioavailability.
Drug delivery system (DDS) has powerful features of delivering drugs into target site,Citation1 enhancing the stability of drugs and protecting antigen from degradation. Liposome, natural polymers, synthetic polymers and nanoparticles are served as potent adjutants of mucosal drug delivery system. Nano-drug delivery system is one of the most potential areas in nanomedicine. The advantage of nano-drug delivery system with high level of drug release, appropriate sizes and diversification of dosage form made it successfully used in clinical application.Citation2
Among all the available drug delivery materials, the biodegradable polymers become the best candidates because of their favorable biodegradability, biocompatibility and low toxicity. Chitosan is a natural biodegradable polysaccharide extracted from crustacean shells. It has been proven that chitosan nanoparticles can protect again biological degradation and sustained the release of drugs.Citation3,Citation4 We also modified the surface of chitosan to overcome the poor solubility in physiological pH. The results demonstrated that the derivatives of chitosan were capable of enhancing the bioavailability.Citation5,Citation6 Furthermore, as a novel drugs delivery carrier and tissue engineering applications, cationic Poly (D, L)-lactic-co-glycolic acid (PLGA) have a rising feature of promising DNA vector with sustained-releasing ability.Citation7
Newcastle Disease Virus Vaccine
Newcastle disease (ND) is a highly contagious viral disease of poultry and still one of the major problems of developing poultry industries in many countries.Citation8,Citation9 There are no treatments available for ND except vaccination. The NDV live vaccines and inactivated vaccines are used universally to control ND.Citation10 But, these conventional vaccines have some disadvantages generally. Live vaccines can be administrated by feeding, drinking, aerosol and spray. However, a major problem of live vaccine is the requirements for both biocontainment and cold chain,Citation11,Citation12 especially potential damage due to partial virus toxicity reservation.Citation13 Inactivated vaccines could not elicit an efficient antibody response after the single immunization.Citation14 Its poor immunogenicity and insecurity due to incomplete inactivation limitations the application of inactivated vaccines.Citation15 In recent years, scores of people pay close attention to DNA vaccines as an emerging vaccines,Citation16 which can induce perfect mucosal, and humoral immune responses.Citation17,Citation18
Advance of Vaccine Encapsulated in Nanoparticles
During the past few years, large quantities of laboratories have made great efforts to prepare effective drug delivery. Also, their works lay a foundation for the further development of vaccines and drugs encapsulated in nanoparticles. Furthermore, mucosal immune with dual functions of cellular and humoral immunity are very important in the immune system.Citation19 As the first line of defense against Newcastle disease virus (NDV) infection, mucosal immune has a large epithelial surface with highly vascularized mucosa facilitating absorption and ready accessibility.Citation20 We have already made contributions to developed a novel mucosal delivery system to enhance mucosal immune responses and protect chickens against ND.
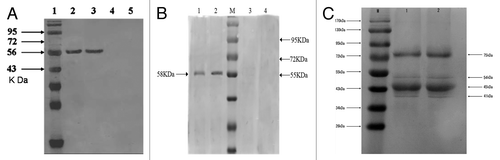
The NDV vaccines encapsulated in chitosan and PLGA nanoparticles we prepared which induced better immune responses compared with the naked plasmid DNA vaccines. Wei Li et al.Citation13 used the method of double emulsion-solvent evaporation to prepare PLGA-plasmid DNA nanoparticles (pFNDV-PLGA-NPs) with the advantage of sustained release effect and perfect immune effect. Zhang et al.Citation21 prepared F gene plasmid DNA of NDV encapsulated in chitosan nanoparticles (pFNDV-CS-NPs) based on complex coacervation. Gang Chen et al.Citation14 also did interrelated work and prepared novel mucosal delivery system as chitosan nanoparticles containing the lentogenic live-virus vaccine (NDV-CS-NPs) using ionic cross linking method. These nanoparticles were all in perfect characterization of spherical and polydisperse nature. TEM results () showed that the prepared nanoparticles had a good dispersion with regular round shape and did not have adhesion or subsidence damage in morphology. The average diameter was 433.5 ± 7.5nm, 199.5 nm and 371.1 nm for pFNDV-PLGA-NPs, pFNDV-CS-NPs and NDV-CS-NPs, respectively. The Zeta potential of these nanoparticles was +2.7 mV, +12.11 mV and +2.84 mV, respectively, which is high enough to combine plasmid DNA betterCitation22 and these nanoparticles are electrically stable. Then the western blotting analysis () was performed as previously describedCitation23 to detect the NDV structural proteins after encapsulation. The antigen expression ( and ) indicated that the plasmid DNA encapsulated in chitosan nanoparticles and the naked plasmid DNA could express the expected 58 kDa antigen, but blank nanoparticles and the cell control group had no expressed antigen. indicated that after encapsulation four NDV structural proteins (HN, F, P, and M) were still detected in NDV-CS-NPs, indicating that the ionic cross linking process of preparing NDV-CS-NPs had no significant effect on NDV proteins. The above results proved that the antigen unchanged after the production of the nanoparticles and also can express in vivo.
Figure 1. Transmission electron microscopy micrograph of the NDV vaccine nanoparticles. (A) pFNDV-PLGA-NPs(magnification 10,000 × ); (B) pFNDV-CS-NPs(magnification 30,000 × ); (C) NDV-CS-NPs (magnification 25,000 × ). Abbreviations: NDV, Newcastle disease virus; pFNDV-PLGA-NPs, the F gene of Newcastle disease virus encapsulated in PLGA nanoparticles; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles; NDV-CS-NPs, lentogenic live-virus vaccine against Newcastle disease virus.
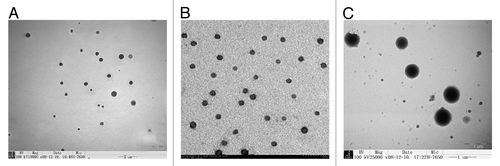
Figure 2. In vitro expression of the NDV vaccine nanoparticles by western blotting. (A) In vitro expression of the pFNDV-PLGA-NPs in BHK cells. Lane 1: protein marker; Lane 2: the naked plasmid DNA groups; Lane 3: the plasmid DNA from pFNDV-PLGA-NPs transfected group; Lane 4: blank PLGA-NPs group; Lane 5: BHK cells group as the negative control; (B) In vitro expression of the pFNDV-CS-NPs in 293T cells. Lane 1: pFNDV-CS-NPs transfected group; Lane 2: naked plasmid DNA groups; M: Protein marker; Lane 3: 293T cells as the negative control; Lane 4: blank CS-NP group; (C) Detection of the NDV structural proteins after encapsulation by western blot. M: Protein marker; Lane 1: Original NDV fluid; Lane 2: NDV recovered from the NDV-CS-NPs. Four positive reaction bands were detected at 75 kDa (HN), 54 kDa (F), 45 kDa (P), and 41 kDa (M). Abbreviations: CS, chitosan; NDV, Newcastle disease virus; pFNDV-PLGA-NPs, the F gene of Newcastle disease virus encapsulated in PLGA nanoparticles; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles; HN, hemagglutinin-neuraminidase protein; F, fusionprotein; P, phosphoprotein; M, matrix protein.
showed the IgG antibody levels after immunization .The chickens immunized with pFNDV-PLGA-NPs produced a higher level of IgG compared with the naked plasmid DNA immunization group (), and the IgG antibody titers which the chickens immunized with pFNDV-CS-NPs () and NDV-CS-NPs () also significantly different from the other immunization groups. Furthermore, the nanoparticles maintained the higher level antibody for a long time. The safety of the nanoparticles was also tested by in vitro cytotoxicological analysis and in vivo immunization in chickens. The safety assay results showed that the nanoparticles were safe for using and there were no nervous signs in immunization chickens, showing a high level of safety in chickens
Figure 3. IgG antibody titers in immune SPF chickens. (A) IgG antibody titers in serum of SPF chickens immunized with PBS (IM), blank PLGA-NPs (IM), blank PLGA-NPs (IN), and the naked plasmid DNA (IM), pFNDV-PLGA-NPs (IM), pFNDV-PLGA-NPs (IN) or pFNDV-PLGA-NPs (IM/IN); (B) IgG antibody titers in serum of SPF chickens immunized with PBS (IM), blank CS-NP (IM), blank CS-NP (IN), and the naked plasmid DNA (IM), pFNDV-CS-NPs (IM), pFNDV-CS-NPs (IN); (C) IgG antibody titers in serum of SPF chickens immunized with NDV-CS-NPs orally, NDV-CS-NPs intranasally, LaSota live vaccine intranasally, inactivated NDV vaccines, blank CS-NPs control, and physiological saline by oral route. Abbreviations: CS, chitosan; NDV, Newcastle disease virus; IM, intramuscularly; IN, intranasally; PBS, phosphate buffered saline; pFNDV-PLGA-NPs, the F gene of Newcastle disease virus encapsulated in PLGA nanoparticles; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles; HN, hemagglutinin-neuraminidase protein; F, fusionprotein; P, phosphoprotein; M, matrix protein.
Conclusions and Perspectives
Over the years, the worldwide epidemic disease ND not only injured public health seriously, but also affected economic development of poultry farming. Thus, the newly drug delivery systems were developed to enhance the ability of vaccines. Chitosan and PLGA based on their advantage of biocompatibility, biodegradability, and low toxicity have become the excellent gene and drugs delivery carriers.
In our studies, we had shown that the chitosan and PLGA nano-drug delivery systems are effective to encapsulate gene, protect antigen from degradation, and induce mucosal immune response. These nanoparticles have the advantages of live vaccines and inactivated vaccines, while compensating for their defects. The induction of significant mucosal immune responses after administration in chickens showed a range of potential applications. More studies need to be conducted to further optimize these chitosan and PLGA nanoparticles for use in commercial applications.
Alternatively, our studies could be a potent new research field in human vaccines. In recent years, the new types of bird flu appeared in China and made people panicky. We considered use the bio-materials to prepare the nanoparticles that coated the antigen of bird flu virus to control the epidemic disease. However, further studies need to be conducted to expand the nano-particles muscle drug delivery in human vaccines.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We gratefully acknowledge Ministry of Education for providing the facilities to carry out this work. This work was supported in part by the National Natural Science Foundation of China (31072119), Innovative Research Team for Agricultural Microbiology Fermentation Technology in Heilongjiang Provincial University (2012td009), Changiang Scholar Candidates Program for Provincial Universities in Heilongjiang (2014CJHB005), Scientific and Technological Key Project of Heilongjiang Province (GC13B403), Early Research and Development Cultivation Project of Scientific and Technological Achievements Industrialization for Provincial Universities in Heilongjiang (1253CGZH10), Innovation Foundation of Harbin (2013RFQXJ030 and 2011RFQXN039), and Education Committee Science and Technology Research General Projects of Heilongjiang Province (12521408).
References
- Bonifácio BV, Silva PB, Ramos MA, Negri KM, Bauab TM, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomedicine 2014; 9:1 - 15; PMID: 24363556
- Gao B, Wang J, Wang D, Zhu Z, Qiao Z, Yang G, Nie F. A novel preparation method for drug nanocrystals and characterization by ultrasonic spray-assisted electrostatic adsorption. Int J Nanomedicine 2013; 8:3927 - 35; http://dx.doi.org/10.2147/IJN.S48597; PMID: 24143097
- de Moura MR, Aouada FA, Mattoso LH. Preparation of chitosan nanoparticles using methacrylic acid. J Colloid Interface Sci 2008; 321:477 - 83; http://dx.doi.org/10.1016/j.jcis.2008.02.006; PMID: 18295778
- Cui Z, Mumper RJ. Chitosan-based nanoparticles for topical genetic immunization. J Control Release 2001; 75:409 - 19; http://dx.doi.org/10.1016/S0168-3659(01)00407-2; PMID: 11489327
- Günbeyaz M, Faraji A, Ozkul A, Puralı N, Senel S. Chitosan based delivery systems for mucosal immunization against bovine herpesvirus 1 (BHV-1). Eur J Pharm Sci 2010; 41:531 - 45; http://dx.doi.org/10.1016/j.ejps.2010.08.011; PMID: 20800678
- Gerdts V, Mutwiri G, Richards J, van Drunen Littel-van den Hurk S, Potter AA. Carrier molecules for use in veterinary vaccines. Vaccine 2013; 31:596 - 602; http://dx.doi.org/10.1016/j.vaccine.2012.11.067; PMID: 23219438
- Malhotra M, Lane C, Tomaro-Duchesneau C, Saha S, Prakash S. A novel method for synthesizing PEGylated chitosan nanoparticles: strategy, preparation, and in vitro analysis. Int J Nanomedicine 2011; 6:485 - 94; PMID: 21562608
- Arifin MA, Mel M, Abdul Karim MI, Ideris A. Production of Newcastle disease virus by Vero cells grown on cytodex 1 microcarriers in a 2-litre stirred tank bioreactor. J Biomed Biotechnol 2010; 2010:586363; http://dx.doi.org/10.1155/2010/586363; PMID: 20625497
- Tseng LP, Chiou CJ, Deng MC, Lin MH, Pan RN, Huang YY, Liu DZ. Evaluation of encapsulated Newcastle disease virus liposomes using various phospholipids administered to improve chicken humoral immunity. J Biomed Mater Res B Appl Biomater 2009; 91:621 - 5; http://dx.doi.org/10.1002/jbm.b.31437; PMID: 19582853
- Chimeno Zoth S, Gómez E, Carrillo E, Berinstein A. Locally produced mucosal IgG in chickens immunized with conventional vaccines for Newcastle disease virus. Braz J Med Biol Res 2008; 41:318 - 23; http://dx.doi.org/10.1590/S0100-879X2008000400010; PMID: 18392454
- Rauw F, Gardin Y, Palya V, Anbari S, Gonze M, Lemaire S, van den Berg T, Lambrecht B. The positive adjuvant effect of chitosan on antigen-specific cell-mediated immunity after chickens vaccination with live Newcastle disease vaccine. Vet Immunol Immunopathol 2010; 134:249 - 58; http://dx.doi.org/10.1016/j.vetimm.2009.10.028; PMID: 19939464
- Tretyakova I, Lukashevich IS, Glass P, Wang E, Weaver S, Pushko P. Novel vaccine against Venezuelan equine encephalitis combines advantages of DNA immunization and a live attenuated vaccine. Vaccine 2013; 31:1019 - 25; http://dx.doi.org/10.1016/j.vaccine.2012.12.050; PMID: 23287629
- Zhao K, Li W, Huang T, Luo X, Chen G, Zhang Y, Guo C, Dai C, Jin Z, Zhao Y, et al. Preparation and efficacy of Newcastle disease virus DNA vaccine encapsulated in PLGA nanoparticles. PLoS One 2013; 8:e82648; http://dx.doi.org/10.1371/journal.pone.0082648; PMID: 24386106
- Zhao K, Chen G, Shi XM, Gao TT, Li W, Zhao Y, Zhang FQ, Wu J, Cui X, Wang YF. Preparation and efficacy of a live newcastle disease virus vaccine encapsulated in chitosan nanoparticles. PLoS One 2012; 7:e53314; http://dx.doi.org/10.1371/journal.pone.0053314; PMID: 23285276
- Tseng LP, Liang HJ, Deng MC, Lee KM, Pan RN, Yang JC, Huang YY, Liu DZ. The influence of liposomal adjuvant on intranasal vaccination of chickens against Newcastle disease. Vet J 2010; 185:204 - 10; http://dx.doi.org/10.1016/j.tvjl.2009.05.019; PMID: 19570697
- Zhao K, Shi X, Zhao Y, Wei H, Sun Q, Huang T, Zhang X, Wang Y. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine 2011; 29:8549 - 56; http://dx.doi.org/10.1016/j.vaccine.2011.09.029; PMID: 21945253
- Abbas AO, Donovan MD, Salem AK. Formulating poly(lactide-co-glycolide) particles for plasmid DNA delivery. J Pharm Sci 2008; 97:2448 - 61; http://dx.doi.org/10.1002/jps.21215; PMID: 17918737
- Loke CF, Omar AR, Raha AR, Yusoff K. Improved protection from velogenic Newcastle disease virus challenge following multiple immunizations with plasmid DNA encoding for F and HN genes. Vet Immunol Immunopathol 2005; 106:259 - 67; http://dx.doi.org/10.1016/j.vetimm.2005.03.005; PMID: 15963824
- Slütter B, Plapied L, Fievez V, Sande MA, des Rieux A, Schneider YJ, Van Riet E, Jiskoot W, Préat V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J Control Release 2009; 138:113 - 21; http://dx.doi.org/10.1016/j.jconrel.2009.05.011; PMID: 19445980
- Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev 2005; 57:1640 - 65; http://dx.doi.org/10.1016/j.addr.2005.07.009; PMID: 16182408
- Zhao K, Zhang Y, Zhang X, Li W, Shi C, Guo C, Dai C, Chen Q, Jin Z, Zhao Y, et al. Preparation and efficacy of Newcastle disease virus DNA vaccine encapsulated in chitosan nanoparticles. Int J Nanomedicine 2014; 9:389 - 402; http://dx.doi.org/10.2147/IJN.S54226; PMID: 24426783
- Basarkar A, Devineni D, Palaniappan R, Singh J. Preparation, characterization, cytotoxicity and transfection efficiency of poly(DL-lactide-co-glycolide) and poly(DL-lactic acid) cationic nanoparticles for controlled delivery of plasmid DNA. Int J Pharm 2007; 343:247 - 54; http://dx.doi.org/10.1016/j.ijpharm.2007.05.023; PMID: 17611054
- Liu SW, Chen HY, Cao DJ, Lu JL. Effect on chicken lymphocytes by Newcastle disease virus. Chin J Animal Poult Infect Dis 1998; 20:140 - 2
