Abstract
Quality control of Hemophilus Influenzae type b (Hib) conjugate vaccines is mainly dependent on physicochemical methods. Overcoming sample matrix interference when using physicochemical tests is very challenging, these tests are therefore only used to test purified samples of polysaccharide, protein, bulk conjugate, and final product. For successful development of a Hib conjugate vaccine, several ELISA (enzyme-linked immunosorbent assay) methods were needed as an additional tool to enable testing of in process (IP) samples.
In this paper, three of the ELISA's that have been very valuable during the process development, implementation and scaling up are highlighted. The PRP-ELISA, was a very efficient tool in testing in process (IP) samples generated during the development of the cultivation and purification process of the Hib-polysaccharide. The antigenicity ELISA, was used to confirm the covalent linkage of PRP and TTd in the conjugate. The anti-PRP IgG ELISA was developed as part of the immunogenicity test, used to demonstrate the ability of the Hib conjugate vaccine to elicit a T-cell dependent immune response in mice.
ELISA methods are relatively cheap and easy to implement and therefore very useful during the development of polysaccharide conjugate vaccines.
Abbreviations:
- Hib, Haemophilus Influenzae type b
- PRP, poly-ribosylribitol phosphate (Hib capsular polysaccharide)
- TTd, tetanus toxoid
- PRP-T, Hib vaccine (PRP conjugated to tetanus toxoid)
- BSA, bovine serum albumin
- PBS, phosphate buffered saline
- TMB, tetramethyl benzidine
- ELISA, enzyme-linked immuno sorbent assay
- NMR, nuclear magnetic resonance
- IPC, in process control
- QC, quality control
- IgG, immunoglobulin G
- HPSEC, high performance size exclusion chromatography
- RI, refractive index
- UV, ultraviolet
- tR, retention time
- Mw, weight-average molecular weight
- Mn, number-average molecular weight
- Mr, molecular weight
- kDa, kilo dalton
- ADH, adipic acid dihydrazide
- RIVM, The National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu)
- NVI, Netherlands Vaccine Institute
- Intravacc, Institute for Translational Vaccinology
- NIH, National Institutes of Health
- WHO, World Health Organization
- EP, European Pharmacopeia
- NIBSC, National Institute for Biological Standards and Control (UK)
Introduction
Enzyme-linked immunosorbent assay (ELISA) methods are widely used for the quantification of several types of polysaccharides as well as antibody responses after vaccination with polysaccharide protein conjugate vaccines.Citation1-5 For the measurement of anti-Hib antibody titers in human sera, commercially ELISA kitsCitation6 are also available. Within the framework of the development and technology transfer related to Hemophilus influenzae type b (Hib) conjugate vaccine at the Institute for Translational Vaccinology (Intravacc, originating from the former Vaccinology Unit of the National Institute of Public Health [RIVM] and the Netherlands Vaccine Institute [NVI]),Citation7 several ELISA's were developed in order to enable successful process development.
The PRP- (polyribosyl ribitol phosphate) ELISA was developed to determine the PRP content in in process (IP) samples generated during the cultivation of Hib organisms and purification of PRP. Quantification of PRP in the IP samples by colorimetric methods such as the Orcinol assayCitation8 is not possible because of interference with media components (e.g., glucose) and reagents used during purification (e.g., detergents). The Orcinol assay is only applicable on purified samplesCitation9,10. Pre-treatment of the samples is possible but very laborious, since multiple precipitation steps may be needed (data not shown).
The antigenicity-ELISA was developed to evaluate the covalent linkage of PRP and TTd (tetanus toxoid) on the conjugate during the development and implementation of the Hib conjugation process. Chromatographic methods help to visualize the presence of a high molecular entity (the conjugate) in the samples but do not demonstrate the presence of both the polysaccharide and carrier protein on the same molecule.
The anti-PRP IgG ELISA was used to test if the Hib conjugate is able to induce a T-cell dependent IgG antibody response against PRP in mice. A generally accepted potency test which reflects clinical efficacy is not available for Hib conjugate vaccines. Consequently such a test is not required by WHOCitation11 or EPCitation12 as lot release test. However, WHOCitation11 considers immunogenicity testing in animals meaningful during the development of Hib conjugate vaccines to demonstrate the ability of the conjugate to elicit a T-cell dependent immune response. Besides mice,Citation13 other animal models for example guinea pigs,Citation14,15 could also be used for immunogenicity testing of the new Hib conjugate vaccine. Mice were used for practical reasons.
After successful development of the Hib conjugate vaccine and transfer of the technology to several partners, the technology transfer partners implemented the PRP-ELISA and antigenicity-ELISA to test respectively for the identity of purified PRP and that of the final product.Citation11,12
In this paper the usefulness, applicability and limitations of three ELISA methods: PRP-, antigenicity-, and anti-PRP IgG ELISA are described. These ELISA's were indispensable during the development, implementation and scaling up stage of the Hib project. Qualification and validation data generated in collaboration with or by the individual partners during the technology transfer are outside the scope of this paper.
Results
The ELISA methods described were primary developed as an additional tool to allow process development and facilitate the implementation and scaling up of the process at partner's site. Some typical examples explaining how the different ELISA's were used during the development of the cultivation, purification and conjugation process are presented below.
PRP-ELISA
The PRP-ELISA was developed to monitor the PRP content in samples during the cultivation of Hib organisms and purification of Hib-polysaccharide from the culture supernatant.
shows a typical example of a PRP-ELISA curve. The concentration of the reference PRP sample was determined by the Orcinol test in advance, using D-(-)-ribose as a standard. All samples were pre-diluted to a concentration of approximately 20 ng/ml, in order to obtain a sigmoidal curve. The results of the reference PRP were used to determine the standard curve, describing the relation between the absorbance and the logarithm of the PRP concentration. The dilutions in the linear part of the standard curve were used to calculate the PRP concentration in the samples based on the absorbance measured for the specific sample dilution, using standard regression analysis in MS Excel. The results were multiplied with the dilution-factor to get the PRP concentration in the original sample.
Figure 1. PRP-ELISA, used to determine Hib capsular polysaccharide content in in process samples generated during the cultivation and purification process: 0.1% skimmed milk in phosphate buffered saline as a negative control (•), reference PRP sample as a positive control (▪) and an experimental PRP batch (▴).
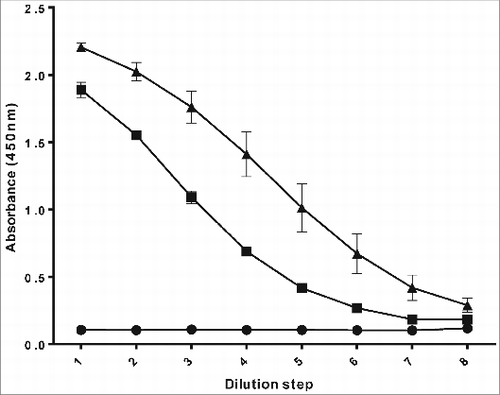
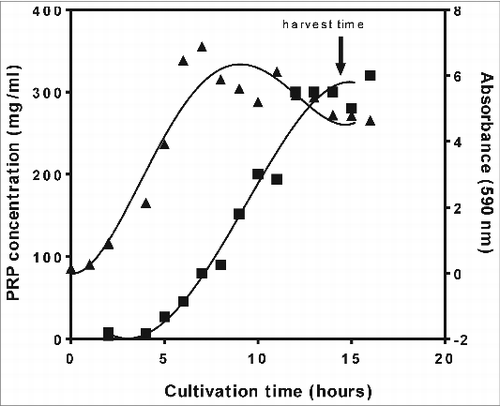
The PRP-ELISA was used to test in process samples from the cultivation process. Samples were taken every hour during a 40 L cultivation batch using an autosampler. A selection of these samples was tested; the results are shown in . During the exponential phase, it is clear that the secretion of the PRP in the supernatant lags behind biomass formation. After the exponential phase of the growth (as the absorbance [OD at 590nm] decreases), PRP continued to be released in the supernatant, indicating that an optimal optical density and thus biomass formation does not correspond to an optimal PRP concentration in the culture supernatant. So the PRP-ELISA results enabled to develop a protocol to achieve optimal harvests with high PRP concentration and low waste materials (for example cell debris, proteins, and nucleic acids)in the culture supernatant, based on physical parameters (especially pH) resulting in a very robust and easily scalable cultivation process.Citation16 Applying the PRP-ELISA during process development avoids harvesting too late with consequent accumulation of impurities in the culture supernatant and thus requiring an extensive purification process.
Figure 2. Cell growth and PRP concentration, determined by PRP-ELISA, as a function of cultivation time measured during the cultivation of Hemophilus Influenzae type b at a 40 L scale: absorbance (▴) at a wavelength of 590nm (OD590), reflecting cell growth, and PRP concentration (▪).
The PRP-ELISA was also used in the development of the PRP purification process. It was possible to quantify the amount of PRP in the presence of medium components during the concentration step. For example when two supernatant samples of a 3.5 L culture, one just after harvesting and the other 15 times concentrated were tested with the PRP-ELISA, a quantity of respectively 960 and 940 mg PRP was detected while in both the permeate sample and in the supernatant just after inoculation a quantity of ≤ 0.5 mg PRP was found.
Precipitation steps in the presence of a relatively low detergent percentage (for example cetavlon, cetyltrimethylammonium bromide) could be monitored by PRP-ELISA (). Supernatant and pellet samples precipitated in the presence of respectively 0.04, 0.08, or 0.16% w/v cetavlon were examined for PRP content. A mass balance of 85–109% was found in the example from . At relatively high cetavlon concentrations (>0.32%) it was clear that the presence of cetavlon disturbed the PRP-ELISA. While most of the PRP was expected to be present in the pellets, it was not possible to determine the anticipated content of PRP. Precipitation of the cetavlon pellets after dissolving in water for injection at 80% v/v ethanol, did help to determine the PRP content in the 0.32% cetavlon pellet. However for samples with a high percentage of detergent (>0.64%), one single ethanol precipitation step was clearly not enough to recover all PRP. Despite the detergent interference in the PRP-ELISA, it was possible to choose the right percentages of detergent and ethanol needed in the Hib purification process.
Table 1. PRP content in in process samples generated during the purification process of the Hib-polysaccharide, determined using PRP-ELISA. Effect of cetavlon on the PRP-ELISA results, before and after an additional ethanol precipitation
Antigenicity-ELISA
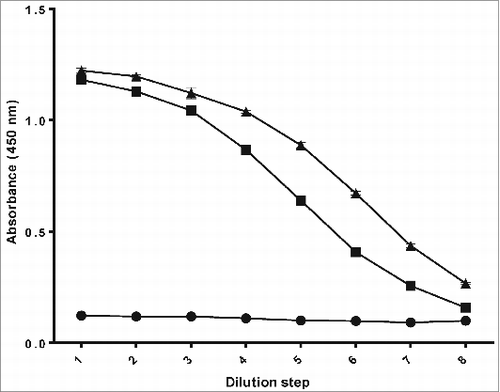
To confirm the covalent linkage of PRP and TTd, the antigenicity-ELISA was used to demonstrate the presence of PRP and TTd on the same molecule of the Hib conjugate. A typical example of the antigenicity-ELISA is given in . A mix of unconjugated PRP and TTd in a ratio equal to the test Hib conjugate vaccine was included as a negative control and a licensed Hib conjugate vaccine (PRP-T) as a positive control. Both Hib conjugate vaccines (test and positive control) consisting of PRP conjugated to Tetanus Toxoid gave a sigmoidal curve in this ELISA. As expected, the mixture of unconjugated PRP and TTd did not show a response in the test.
Figure 3. Antigenicity-ELISA, used to demonstrate the presence of antigenic sites of the Hib polysaccharide and carrier protein (TTd, tetanus toxoid) on the Hib conjugate: a sample containing a mix of unconjugated PRP and free tetanus toxoid (•) as a negative control, a licensed Hib conjugate vaccine (▪) as a positive control and the test Hib conjugate vaccine (▴).
High Performance Size Exclusion Chromatography (HPSEC) was used to determine the molecular weight of the PRP and to monitor the conjugation process (). Molecular weight was used as a measure for visualizing the conjugation reaction of PRP to TTd and thus the production of the conjugate. Detection of the conjugate (PRP-T) is done at various time points during the conjugation by UV (280 nm); gradual disappearance of TTd and appearance of conjugate is seen.
Figure 4. Monitoring of the conjugation process by HPSEC analysis. Formation of the conjugate (PRP-T; experimental batch) was done at various time points during the conjugation process by UV (280 nm; t0, ― black; 30 min, – – – blue; 60 min, — - pink; 120 min, - - - brown; end at 280 min. .. green). The gradual disappearance of unconjugated TTd (tR ∼17.5 min) occurred simultaneously to the formation of conjugate (tR 13–14 min, final Mr 1,411 kDa). ADH-modified PRP (PRP-AH) could also be detected using UV while unconjugated PRP had to be detected using Refractive Index (RI).
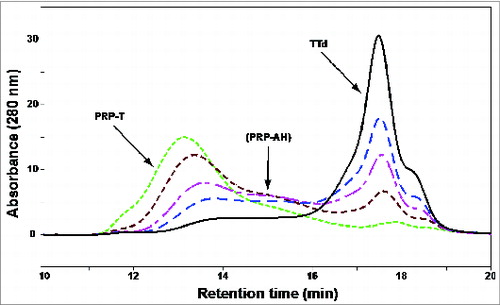
The added value of the antigenicity-ELISA in comparison with the HPSEC is the ability of this test to detect the PRP and TTd antigenic sites on the same conjugate molecule and thus in confirming that a PRP-T conjugate was produced.
Anti-PRP IgG ELISA
To examine the Hib conjugate for the ability to induce an immunological memory and consequently an IgG antibody response against PRP a mouse immunogenicity test was developed. A typical example is given in .
Figure 5. Anti-PRP IgG ELISA, used to test if the Hib conjugate is able to induce IgG antibodies against PRP in mice. Mice were immunized with PRP-T (▴) (test conjugate vaccine) and free PRP (▪) (unconjugated).
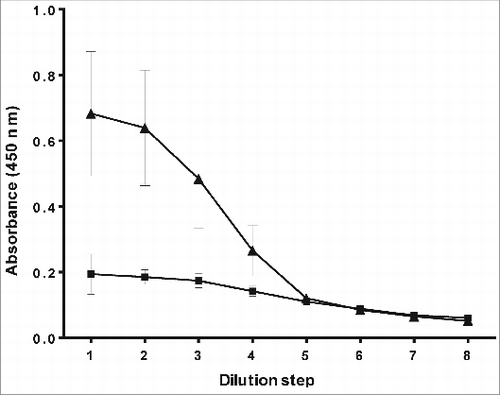
The results clearly show that immunization of mice with unconjugated PRP does not result in the production of IgG anti-PRP antibodies. However, immunization of mice with a Hib conjugate vaccine does result in a positive response. Five out of ten mice were identified as responders in this case. Although mice are not the best responders to Hib conjugate vaccine. The induction of an immunological memory and IgG anti-PRP antibodies could clearly be demonstrated. For that reason the mouse immunogenicity test and thus the anti-PRP IgG ELISA was considered to be useful in the development of the new Hib conjugate vaccine.
Discussion/Conclusion
Within the framework of the Hib technology transfer at Intravacc (originating from RIVM/NVI), a production process for a new Hib conjugate vaccine, including Quality Control (QC) tests, was developed. Knowhow related to the production process and QC was transferred to partners in Indonesia, India, and China.
Beside the development of all (physicochemical) tests required by WHO and EP for routine lot release of the new Hib conjugate vaccine,Citation11,12 a number of ELISA methods were developed and used to enable process development at Intravacc and process implementation, including further scaling up, at partner's site. Being highly sensitive, specific and relatively cheap; these ELISA methods were very useful in a successful process development.
The PRP-ELISA method was used to enable the development of the cultivation and purification process by detecting Hib-polysaccharide present in several in process samples generated during these processes. Medium components do not seem to interfere with this ELISA in contrast with the commonly used Orcinol test which can only be used to test pure polysaccharide samples. No extensive attempts were made to correlate the Orcinol test with the PRP-ELISA, as the primary objective of using this ELISA was to monitor the PRP content during individual cultivation and/ or purification batches. Consequently only relative values were relevant to optimize the cultivation and/or purification process. The PRP-ELISA seems to be disturbed by the presence of relatively high detergent concentrations. This didn't limit the use of the PRP-ELISA during the development of the purification process. Additionally it's advisable to study the impact of molecular weight on the test results, during the cultivation and purification of PRP the molecular weight of the polysaccharide may change.
The antigenicity-ELISA was used to test in process conjugation samples by demonstrating the presence of PRP and TTd antigenic sites on the same conjugate molecule. For that reason this ELISA was considered to have an added value in the demonstration of a covalent linkage between PRP and TTd. The antigenicity-ELISA may also be useful as an additional tool throughout stability and consistency testing. However these options were not extensively explored within the project as adequate tests are already available to monitor stability and consistency.
The anti-PRP IgG ELISA was very useful as a functional assay to demonstrate the ability of the conjugate to elicit an immunological memory and consequently induce IgG antibodies against PRP. For that reason mice were injected twice on days 0 and 14. A clear difference was seen with unconjugated PRP, which did not induce IgG antibodies against PRP. Sera generated in the mouse immunogenicity test were also used to test for the immunogenicity of TTd, carrier of the Hib conjugate, by using a Toxin Binding Inhibition (ToBI) assay.Citation17 The results showed that the TTd still had the ability to elicit anti-tetanus antibodies (data not shown).
The conjugation method used to prepare the coating antigen may be of importance because of the possible presence of non-functional epitopes in case a similar method is used to the test vaccine, this need to be further investigated.
In conclusion the reported antigenic and immunogenic characteristics show that the Hib conjugate vaccine production process developed by Intravacc leads to a PRP-T conjugate that expresses PRP antigens that are able to induce an immunological memory and elicit IgG antibodies against PRP. In general, ELISA methods are very useful during the development, implementation, and scaling up of polysaccharide protein conjugate vaccines. When properly qualified and validated the ELISA's can be used to test for the identity of the polysaccharide and conjugate.
Materials
Buffers: carbonate buffer (0.04 M, pH 9.6), phosphate buffered saline (PBS, 0.01 M, pH 7.2), and sodium acetate buffer (1.1M, pH 5.5). All buffers were prepared in-house.
Reagents: burro-anti-Hib (BAH) serum was kindly provided by Dr. J. RobbinsCitation18 (NIH), this serum was purified with a protein G column and then peroxidase-labeled resulting in Burro anti-Hib∼PO (BAH∼PO). The labeling or conjugation of the serum took place in-house by incubating the IgG fraction of the serum with activated peroxidase for 3 h (pH 9, room temperature). The conjugate was subsequently centrifuged and diafiltered against phosphate buffered saline. Equine anti-tetanus (HAT) was prepared in-house by immunizing horses with tetanus toxoid followed by tetanus toxin. This serum was peroxidase-labeled resulting in horse anti-tetanus∼PO (HAT∼PO). Biotinylated goat anti-mouse IgG: GE Healthcare, RPN1177, Streptavidin-horseradish peroxidase: GE Healthcare, RPN1231, and HbO-HACitation19 (Hib oligosaccharide linked to human albumin, available from NIBSC).
ELISA plates: Greiner (655092).
Diluent: 0.1% skimmed milk in PBS (phosphate buffered saline). Skimmed milk (BD Difco 232100).
Chemicals: tween-80 (Merck, 822187), ethanol (Merck, 1.00971), Hydrogen peroxide 30% (Merck, 107209), concentrated sulfuric acid (Merck, 100731), tetramethyl-benzidine (Sigma, T2885), bovine serum albumin (Serva, 11922), tween-20 (Merck, 822184).
Test Hib vaccine: A vaccine produced in-house by conjugating the Hib polysaccharide to tetanus toxoid, using the conjugation method originally described by John Robbins and collaborators (National Institutes of Health).Citation20
Reference PRP: WHO standard (NIBSC nr. 02/208) or an internal PRP standard (meeting all WHO and EP requirements).
Licensed Hib vaccine: A Hib conjugate vaccine licensed through RIVM (freeze-dried PRP-T).
Both Hib vaccines (test and licensed vaccine) were PRP-T, standalone and freeze-dried Hib conjugate vaccines.
Methods
High performance size exclusion chromatography (HPSEC)
HPSEC analysis of purified PRP samples and/ or samples generated during the conjugation reaction was performed on two OHpak SB-805 and SB-804 columns (300 × 8 mm, with the addition of an SB-G guard column, 50 × 6 mm; Shodex) mounted in series, with 10 mM phosphate-buffered saline (PBS, pH 7.2) as eluent, at 1 mL/min and 35°C. Elution was monitored with a RI and a multiple-wavelength UV detector from 215 to 280 nm. The Mr value of the polysaccharides was determined relative to the retention times (tR) of defined pullulan standards (Mw 5.8–853 kDa, Mw/Mn ∼1.1). The elution limits of the column set were determined by using high Mr dextran (Mr 5–40 × 10Citation6 kDa) and deuterium oxide (D2O). Detection of PRP was done by measurement of the RI signal. Impurities (proteins and nucleic acids) were detected by UV and RI.
PRP-ELISA
The PRP ELISA was used to monitor the PRP in culture supernatants and to monitor the purification process. The ELISA plates were coated with burro anti-Hib in carbonate buffer as catching antibody. To prevent non-specific binding of PRP samples, the plates were blocked with PBS containing 0.1% skimmed milk.
The samples to be tested including reference PRP were diluted 2-fold in series of eight dilutions using PBS containing 0.1% skimmed milk and 0.05% tween-80, making sure that the starting concentration was approximately 20 ng/mL. The plates were incubated for 2 h at room temperature. On each ELISA plate, a negative control (PBS with 0.1% skimmed milk) was included. The bound PRP was detected with peroxidase labeled burro anti-Hib (burro anti-Hib∼PO). Tetramethyl-benzidine was used as substrate; Tetramethyl-benzidine was being used as a replacement for carcinogenic compounds such as benzidine.Citation21,22 This reaction was stopped after ten minutes using 2 M sulfuric acid. The intensity of the color was directly related to the amount of PRP present in the sample. The absorbance was measured at 450 nm using an ELISA reader (Bio-tek Reader Elx808).
Intermediate washing steps were performed after each treatment except for the last incubation with the substrate. Washing could be done either manually using a multi-channel pipette or by means of a special washing apparatus, the plates were washed using a solution of 0.05% Tween-80 in PBS and turned upside down between the washing steps.
The PRP content in the samples was calculated by plotting the absorbance as a function of the sample dilution and by comparing the sample with the PRP reference (known PRP content) in a parallel line analysis in Excel. For this calculation only the linear part of the curve was used.
Antigenicity ELISA
The antigenicity ELISA was used to demonstrate covalent linkage of PRP and TTd in Hib conjugate. Unconjugated PRP and TTd were used as negative controls. A licensed Hib conjugate vaccine was used as positive control.
The ELISA plates were coated with burro anti-Hib in carbonate buffer as catching antibody. To prevent non-specific binding of samples, PBS containing 0.1% skimmed milk was used as a blocking agent. The samples tested were diluted 2-fold in series of eight dilutions with PBS containing 0.1% skimmed milk and 0.05% tween-80. The plates were incubated for 2 h at room temperature. Bound Hib conjugate was detected using peroxidase labeled antibodies directed against TTd (horse anti-tetanus∼PO). Intermediate washing steps were done as described under PRP ELISA. Tetramethyl-benzidine substrate was used to react with the peroxidase. This reaction was stopped after ten minutes using 2 M sulfuric acid. The absorbance was measured at 450 nm using an ELISA reader (Bio-tek Reader Elx808).
Anti-PRP IgG ELISA
The ability of the Hib conjugate vaccine to induce a T-cell dependent immune response was investigated in mice.
A group of 10 mice (NIH mice, 10–14 g, same sex) was injected subcutaneously with 1/10 of a human dose of the test Hib conjugate vaccine corresponding to 1 μg PRP, on day 0 and 14. The mice were bled on day 28 (eye extraction under anesthesia). Serum was prepared by allowing blood to coagulate for approximately 2 h at 37°C and overnight at 4°C. After coagulation, the serum was separated by centrifugation. The serum samples were stored at –30°C after inactivation for 30 min at 56°C.
The individual serum samples were analyzed for IgG anti-PRP antibodies. A licensed Hib conjugate vaccine was included as positive control in each test. Unconjugated PRP was used as a negative control. Immunization of mice using either a licensed vaccine or unconjugated PRP was done following the same procedure as the one followed for the test vaccine.
The ELISA plates were coated with a solution of 1 μg/mL HbO-HA antigen in PBS. The serum samples were diluted 2-fold using PBS containing 1% BSA and 0.05% Tween-20 and incubated for 2 h at 37°C. To detect IgG anti-PRP antibodies, biotinylated goat anti-mouse IgG was added. Streptavidin-peroxidase was added and TMB used as a substrate. This reaction was stopped after ten minutes using 2 M sulfuric acid. The absorbance was measured at 450 nm using an ELISA reader (Bio-tek Reader Elx808).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are indebted to all Intravacc (originating from RIVM/ NVI) personal who contributed to the Hib technology transfer project especially Annemarie Bowman-Kelder, Robert van der Put and Rudy Hertroys for generating useful data and to Gideon Kersten for reviewing the manuscript.
Funding
The Hib technology transfer project was funded by the various partners (BF, BE, SII, and Glovax/ SIBP), through bilateral license agreements. In the beginning a seed capital from Intravacc was used to study the feasibility of the project and generate proof-of-concept data.
References
- Beuvery EC, van Delft RW, Tiesjema RH, Nagel J. The enzyme-linked immunosorbent assay of meningococcal and some related Escherichia coli polysaccharides. J Biol Stand 1983; 11:195-204; PMID:6350305; http://dx.doi.org/10.1016/S0092-1157(83)80006-7
- Afshar M, Esmaily F, Aminian M, Asli E, Haadi A, Torabi M, Hatami A. A study on haemophilus influenzae type b growth rate and capsule production in different media. Archives of Razi Institute 2012; 67:7-12.
- Saydam M, Rigsby P, Mawas F. A novel Enzyme-Linked Immuno-Sorbent Assay (ELISA) for the quantification of total and free polysaccharide in Haemophilus influenzae b-Tetanus toxoid conjugate vaccines in monovalent and combined vaccine formulations. Biologicals 2014; 42:29-33; PMID:24200313; http://dx.doi.org/10.1016/j.biologicals.2013.10.002
- Sutton A, Vann WF, Karpas AB, Stein KE, Schneerson R. An avidin-biotin based ELISA for quantitation of antibody to bacterial polysaccharides. J Immunol Methods 1985; 82:215-24; PMID:3900215; http://dx.doi.org/10.1016/0022-1759(85)90353-9
- Kreeftenberg JG, Hamidi A. Annex 5. Lessons learned in the transfer of Hib conjugate vaccine technology and the consequences for access to this vaccine in developing countries. World Health Organization (WHO); 2004. Report No.: WHO/IVB/04.21. Available from: http://whqlibdoc.who.int/hq/2004/WHOIVB 04.21(302KB).pdf.
- Arvas A, Gur E, Bahar H, Torun MM, Demirci M, Aslan M, Kocazeybek B. Haemophilus influenzae type b antibodies in vaccinated and non-vaccinated children. Pediatr Int 2008; 50:469-73; PMID:19143969; http://dx.doi.org/10.1111/j.1442-200X.2008.02591.x
- Beurret M, Hamidi A, Kreeftenberg H. Development and technology transfer of Haemophilus influenzae type b conjugate vaccines for developing countries. Vaccine 2012; 30:4897-906; PMID:22683521; http://dx.doi.org/10.1016/j.vaccine.2012.05.058
- Ashwell G. Colorimetric analysis of sugars. Methods Enzymol 1957; 3:73-105; http://dx.doi.org/10.1016/S0076-6879(57)03350-9
- Lemercinier X, Jones C, Corbel MJ, Yost S. Analytical procedures used in the control of bacterial glycoconjugate vaccines. Pharm Pharmacol Commun 1997; 3:19-23.
- Cuervo ML, Pérez LR, Oviedo M, Costa L, Perdomo V. Relationships among physico-chemical and biological tests for a synthetic Hib-TT conjugate vaccine. Vaccine 2007; 25:194-200; PMID:17161239; http://dx.doi.org/10.1016/j.vaccine.2005.05.041
- WHO Expert Committee on Biological Standardization. Forty-ninth report. Annex 1. Recommendations for the production and control of Haemophilus influenzae type b conjugate vaccines. WHO Technical Report Series 2000; 897:27-60.
- Haemophilus type b conjugate vaccine. Eur Pharmacopoeia 2005; 5.0: 662-4.
- Chu C, Schneerson R, Robbins JB, Rastogi SC. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun 1983; 40:245-56; PMID:6601061
- Gupta RK, Anderson R, Cecchini D, Rost B, Griffin P Jr., Benscoter K, Xu J, Montanez-Ortiz L, Siber GR. Development of a guinea-pig model for potency/immunogenicity evaluation of diphtheria, tetanus acellular pertussis (DTaP) and Haemophilus influenzae type b polysaccharide conjugate vaccines. Dev Biol Stand 1996; 86:283-96; PMID:8785957
- Siber GR, Anderson R, Habafy M, Gupta RK. Development of a guinea pig model to assess immunogenicity of Haemophilus influenzae type b capsular polysaccharide conjugate vaccines. Vaccine 1995; 13:525-31; PMID:7483772; http://dx.doi.org/10.1016/0264-410X(94)00042-L
- Hamidi A, Beurret M. De Staat der Nederlanden, Process for producing a capsular polysaccharide for use in conjugate vaccines. US 7 582 459; 2004.
- Hendriksen CF, van der Gun JW, Marsman FR, Kreeftenberg JG. The use of the in vitro toxin binding inhibition (ToBI) test for the estimation of the potency of tetanus toxoid. Biologicals 1991; 19:23-9; PMID:2049173; http://dx.doi.org/10.1016/1045-1056(91)90020-K
- van Alphen L, Eijk P, Käyhty H, van Marle J, Dankert J. Antibodies to Haemophilus influenzae type b polysaccharide affect bacterial adherence and multiplication. Infect Immun 1996; 64:995-1001; PMID:8641812
- Toraño G, Toledo ME, Baly A, Fernandez-Santana V, Rodriguez F, Alvarez Y, Serrano T, Musachio A, Hernandez I, Hardy E, et al. Phase I clinical evaluation of a synthetic oligosaccharide-protein conjugate vaccine against Haemophilus influenzae type b in human adult volunteers. Clin Vaccine Immunol 2006; 13:1052-6; PMID:16960118; http://dx.doi.org/10.1128/CVI.00144-06
- Chu C, Schneerson R, Robbins JB, Rastogi SC. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun 1983; 40:245-56; PMID:6601061
- Yang J, Wang H, Zhang H. One-pot synthesis of silver nanoplates and charge-transfer complex nanofibers. J Phys Chem C 2008; 112:13065-9; http://dx.doi.org/10.1021/jp802604d
- Holland VR, Saunders BC, Rose FL, Walpole AL. A safer substitute for benzidine in the detection of blood. Tetrahedron 1974; 30:3299; http://dx.doi.org/10.1016/S0040-4020(01)97504-0
