Abstract
Vaccination rates among United States (US) adults are suboptimal, resulting in morbidity, mortality, and financial burden attributable to potentially vaccine-preventable diseases (VPDs). Unadjusted annual incidence proportions of VPDs were estimated for Medicaid and commercially insured adults aged 19–64 years using 2006–2010 claims, along with age/gender-adjusted incidence proportions for 2010. In 2010, 1.6 million Medicaid adults (mean age 34 ± 12 years; 73.4% female) and 33 million commercially insured (mean age 42 ± 13 years; 52.2% female) were included. Age/gender-adjusted incidence proportions (per 100 000) in 2010 among Medicaid vs commercially insured adults for meningococcal disease were 26.2 (95% CI 22.9–29.8) vs 2.0 (1.9–2.2) (P < 0.001); hepatitis B 88.9 (82.6–95.6) vs 17.5 (17.0–17.9) (P < 0.001); pneumococcal disease 98.2 (91.7–105.1) vs 21.1 (20.7–21.6) (P < 0.001); hepatitis A 19.8 (16.9–23.1) vs 4.5 (4.3–4.7) (P < 0.001); mumps 2.1 (1.3–3.3) vs 1.4 (1.3–1.6) (P = 0.14); measles 0.3 (0.1–1.0) vs 0.3 (0.2–0.3) (P = 0.38); herpes zoster (60- to 64-year-olds only) 459 (408–515) vs 473 (466–481) (P = 0.35); varicella (19- to 39-year-olds only) 6.5 (4.8–8.5) vs 8.0 (7.5–8.5) (P = 0.12); influenza 586 (573–598) vs 633 (631–636) (P < 0.001); and pertussis 1.8 (1.1–2.8) vs 3.2 (3.0–3.4) (P < 0.001). Research is needed to fully understand the causes of the disparity of the coded incidence of some VPDs in adult Medicaid population than commercially insured adults in the US.
Although vaccines are one of the greatest public health achievements, millions of United States (US) adults do not receive recommended vaccinations.Citation1 For example, the Centers for Disease Control and Prevention (CDC) estimated that, in 2011, only 13% of people aged 19–64 y had received Tdap (tetanus, diphtheria, and acellular pertussis) since it was licensed in 2005, and only 16% of those aged ≥60 y had been vaccinated against herpes zoster.Citation2 It has been estimated that 40 000–50,000 adults die annually from potentially vaccine-preventable diseases (VPDs) in the US,Citation1 and that the financial burden of adult VPDs in the US is at least $10 billion annually.Citation3
Barriers to vaccination in the US include lack of patient awareness of vaccine availability and risks of contracting VPDs; lack of physician knowledge about adult vaccination recommendations and of appropriate recommendations from physicians to patients; financial barriers for patients and reimbursement issues for medical practices; failure to update vaccinations during office visits; logistics issues at the office level (e.g., limited refrigerator/freezer space and up-front vaccine costs); and a variable supply of vaccines.Citation1,Citation4-Citation8 The 2010 Patient Protection and Affordable Care ActCitation9 may remove some of these barriers and increase the use of preventive services in the US.Citation10 Under healthcare reform, commercial insurers are required to provide first dollar coverage for vaccines, but for Medicaid, the requirements are unclear and may not be determined.
There are no published papers reporting VPD incidence proportions among adults covered by Medicaid and those who are commercially insured using similar methodologies. This analysis attempts to quantify the incidence proportions of various VPDs in these 2 insured populations, which will serve as baselines for evaluating the impact of Affordable Care Act in these populations.
Results
Populations
This analysis was based on individuals aged 19–64 y reported for the years 2006–2010, ranging from 1.4 to 2.0 million Medicaid enrollees per year and from 23 to 33 million commercially insured individuals per year. contains population demographics as mean values over the 5 y and for 2010 alone. Demographics for each annual sample were similar within the commercially insured and Medicaid populations (data not shown). Compared with the commercially insured population, the Medicaid population was on average younger (mean age 34 ± 12 vs 42 ± 13 y) and included more females (74.1% vs 52.3%) over the 5 y ().
Table 1. Population demographics as mean values for the 5 y from 2006 to 2010 and for 2010 alone
Unadjusted and age/gender-adjusted results for 2010
Unadjusted and age/gender-adjusted (to the US 2010 censusCitation11) incidence proportion results for 2010 are shown in . Adjusted incidences of varicella, herpes zoster, measles, and mumps were not significantly different between the 2 populations; but incidences of pneumococcal disease, meningococcal disease, hepatitis A, and hepatitis B were significantly higher in the Medicaid population, while incidences of influenza and pertussis were significantly lower.
Table 2. Mean unadjusted and age/gender-adjusted VPD incidence proportions (95% confidence intervals) (per 100 000) among Medicaid and commercially insured adults (aged 19–64 y) for 2010
Unadjusted results (5-y mean values)
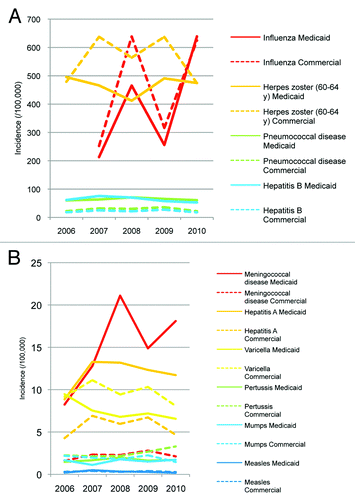
Mean unadjusted VPD incidences over the 5 y (or 4 influenza seasons) are shown in . The highest incidences were for herpes zoster (50–64 or 60–64 y only) and influenza, while there were few cases of measles and mumps. Unadjusted mean incidences of many of the VPDs were similar in the Medicaid and commercially insured populations, but incidences of pneumococcal and meningococcal diseases, hepatitis A, and hepatitis B were higher in the Medicaid population.
Table 3. Mean unadjusted VPD cases (incidence proportions [per 100,000]) (95% confidence intervals) for 2006–2010 among Medicaid and commercially insured adults aged 19–64 y
and show mean 5-y unadjusted VPD incidences stratified by age and gender for the Medicaid and commercially insured populations, respectively. Pneumococcal disease, meningococcal disease, hepatitis A, and hepatitis B incidences increased with age in both populations. Incidences of influenza, herpes zoster, and pertussis were higher among females than males in both populations; while incidences of hepatitis A and hepatitis B were generally higher among males than females.
Table 4. Mean VPD incidence proportions (per 100 000) over the 5 y (4 influenza seasons) by age range and gender for Medicaid patients
Table 5. Mean VPD incidence proportions (per 100 000) over the 5 y (4 influenza seasons) by age range and gender for commercially insured patients
Unadjusted results per year
shows how the incidences of the different VPDs varied over the 5 y (4 influenza seasons) of the study. Influenza had the largest variation between years. Absolute patient numbers, incidences per 100 000 and 95% CIs for each year in the Medicaid and commercially insured populations are shown in Table S2 and S3, respectively.
Discussion
This analysis, which quantifies the incidences of diagnostic codes for the various VPDs among Medicaid and commercially insured populations, found an appreciable burden of many VPDs in both populations.
Compared with 2010 national notifiable disease incidences reported by the CDC,Citation12 we identified similar incidences (per 100 000) of pertussis (adjusted Medicaid and commercial [ages 19–64 y] 1.8 and 3.2; CDC [ages 15–64 y]Citation1 3.9) and mumps (2.1 and 1.4; CDC 0.7), but higher incidences of measles (0.3 and 0.3; CDC 0.02), hepatitis A (19.8 and 4.5; CDC 0.6), hepatitis B (88.9 and 17.5; CDC 1.5), pneumococcal disease (98.2 and 21.1; CDC 6.5), and meningococcal disease (26.2 and 2.0; CDC 0.2). Our results are different from those from the CDC because the methodology used to capture the CDC rates is different to ours; for example the CDC can only count reported cases (and reporting of notifiable diseases is likely incompleteCitation13,Citation14), and the CDC do not count ‘probable’ cases for measles, hepatitis A, hepatitis B, or pneumococcal disease,Citation12 while we relied on the accuracy of coding in administrative claims databases.
Herpes zoster is not a notifiable disease, but our results (roughly 400–600/100 000 in ) are in a similar range to incidence rates derived from outpatient claims from over 10 million insured persons from MarketScan® databases, where incidences of herpes zoster among the relevant age groups were 340–830/100 000 during 2006.Citation15 Our results are also in a similar range to those from the placebo groups of 2 large, prospective herpes zoster vaccination trials (1079/100 000 among 60- to 69-y-olds during 1998–2004Citation16 and 657/100 000 among 50- to 59-y-olds during 2007–2010Citation17). Our influenza results are similar to those from a 2009–2010 study of over 200 000 adults, of whom 8.1% self reported influenza-like illness.Citation18 Of these, 40% reported seeking healthcare, and 26% of those who sought healthcare reported receiving a diagnosis of influenza,Citation18 for an incidence of around 840/100 000; roughly similar to our incidence of around 600/100 000 in 2010.
Our age/gender-adjusted results from 2010 showed that incidences of VPDs for which vaccination was only recommended for adults with risk factors (pneumococcal and meningococcal diseases, hepatitis A, hepatitis B) were higher in the Medicaid population, while incidences of most other VPDs were higher among commercially insured patients. The reasons for the differences in incidences between the 2 populations are unclear. Because of the large difference in the distribution of age and gender between commercially insured and Medicaid populations, we adjusted for age and gender while comparing the VPD incidence. Differences in VPD incidence between the 2 populations remained after the adjustment, which suggest other factors have played a role. Examples of such factors could include access to healthcare, occupation, life style, risk behaviors, nutritional status, personal hygiene, characteristics of residence, and presence of chronic medical conditions as well as others. Information on most of these factors is not available in the administrative claims databases and cannot be assessed in this study. Another potential explanation for the higher incidences of some VPDs among Medicaid adults, and vice versa, could be lower vaccination rates in one of the populations. Higher rates of vaccination, in accordance with ACIP recommendations, would help reduce VPD burden and the associated mortality, morbidity, costs to the healthcare system, and diminished productivity.Citation1 According to surveys of insurance companies and Medicaid programs, a vast majority of Medicaid programs and private insurance cover all ACIP recommended vaccines. Depending on the programs enrolled in, enrollees may be requested to pay a copay or deductible to get a vaccine. Because the database does not contain information on the cost-sharing structure of individual plans, we are unable to assess its impact on vaccine coverage
Possible differences in geographic distribution between the Medicaid and commercially insured populations may also account for the differences in the VPDs because infectious diseases are not randomly distributed across the US. However, per data use agreement, information on geographic residence of Medicaid enrollees is not accessible to the study team. Thus, the effect of geographic differences cannot be explored.
There is a general consensus that vaccination rates can be increased in both Medicaid and privately insured populations.Citation19,Citation20 Many strategies and mechanisms aiming at improving vaccination rates are being designed and tested, such as utilizing health information technology to send reminders to both providers and enrollees, reducing or eliminating patient copayment, and using of combination vaccines.Citation21-Citation23 On the other hand, insufficient Medicaid reimbursement for vaccination and counselling to healthcare providers, insecure vaccine supplies, and financial risk of stocking vaccines has been cited as the top barriers and need to be addressed as well.Citation21,Citation22
Limitations and strengths
There are limitations with the use of any claims databases. We relied on the use of International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnostic codes to identify individuals with the different VPDs and did not have access to medical charts or laboratory results to confirm the diagnoses. Furthermore, although the ICD-9-CM patient diagnostic coding system is often used in claims database research, this can be problematic because billing personnel may use more global and unspecified codes;Citation23,Citation24 and this type of diagnostic information may not always be reliable or valid.Citation23 As noted above our results differ substantially in some disease states in incidences determined by other methodologies, so there are methodological issues that need to be considered in interpreting the disparate data. To minimize the misidentification of VPDs via ICD-9-CM codes, we conducted an extensive search of published literature and compiled a list of ICD-9-CM codes used in previous studies to identify VPD cases.Citation15,Citation25-Citation32Two clinicians reviewed and finalized the list ICD-9-CM codes for the identification of VPD cases in this study. In addition, the diagnostic codes do represent the claims against the 2 types of health plans for the diagnosis, treatment, and care of VPDs, and therefore provide important information on the use of inpatient and outpatient care. However, we recognize that this study has not identified all VPDs in the 2 populations, as individuals would have to seek medical care and/or treatment for their condition to be counted, and milder cases may not be reported; and the utilization of healthcare by these different populations may be influenced by the severity of illness. Also, some VPD cases may be cared for outside the plan system. Furthermore, while we classified the diseases as vaccine preventable, it is recognized that not all the diseases can be avoided and vaccines are not universally effective.
To reduce impact the probable miscoding of zoster and varicella on the validity of our findings, we reclassified a subgroup of patients (i.e., patients aged ≥40 y, immunocompromised, or with post-herpetic neuralgia) initially coded as having varicella to cases with herpes zoster; and did not include the cases (14 in Medicaid and 125 in commercial) that were dual coded with both varicella and herpes zoster on the same day in the incidence estimation of varicella or herpes zoster. In addition, we did not consider the claims with an ICD-9-CM ‘diagnosis’ of a VPD and a vaccination code for the same VPD on the same day toward a VPD event since the claims were probably related to vaccination. The reclassifcation of claims may have resulted in an underestimation of the true disease burden, but the effect on both populations should be similar and the comparison between the populations should still be valid.
During the years of the study, measles, mumps, pneumococcal, meningococcal, influenza, hepatitis A, and hepatitis B vaccines were only recommended for adults with a risk factor in some or all age groups.Citation33-Citation36 As it was not possible to determine who had the relevant risk factors, we included all eligible enrollees in the analysis. It is possible that the proportions of people with such risk factors were different between the Medicaid and commercially insured populations.
Another limitation is that the confounding factors between individuals with Medicaid and commercial insurance cannot be fully adjusted while comparing the incidence of VPDs. Because the selection of insurance type was mainly based on income, Medicaid population may differ from commercially-insured population in many aspects, such as living conditions, access to care, and education level which cannot be assessed in the claims data. Also, the length of continuous enrollment in each type of insurance may be different. There is also a lack of knowledge of vaccination coverage rates in these different populations, making the proportions of susceptible patients in the 2 populations unknown. Since we only adjusted results for age and gender, there are likely other confounding factors that were not taken into consideration due to data limitations.
Another clinical issue that remains unclear from this study is that the incidence of some diseases in the 2 population is as high as 4 to 10 times different. These differences would be unlikely in populations living in a similar geographic area and having no extreme lifestyle.
Although our study included around 7% of the 24 million adults aged 18–64 y covered by MedicaidCitation37 and approximately 21% of the 132 million commercially insured adults aged 18–64 y in the US,Citation38 the findings may not be generalizeable to all Medicaid enrollees and commercially insured individuals. Also, we had access to Medicaid data from 12 US states. Each state has its own Medicaid eligibility criteria and coverage; there may be variations within the Medicaid cohort concerning provider access, co-pays, formularies, regional practice patterns, characteristics of the Medicaid population and other factors that vary across states and Medicaid plans.Citation23
However, the strength of this study is that it is the first to examine VPD incidences in Medicaid and commercially insured populations using the same methodology for both populations. In addition, we examined data over 5 y in a very large population and the incidences of VPDs are based on claims data and can be used to estimate the economic burden to the healthcare system.
Conclusions
This study demonstrates sizeable incidences of VPDs in Medicaid and commercially insured populations, with higher incidence proportions of meningococcal disease, pneumococcal disease, hepatitis B, and hepatitis A in the Medicaid population after adjustment for age and gender differences using the methodology in this study. If these findings are confirmed the information will be useful for public health authorities to formulate future public health policies to target specific vaccinations among adults who are not vaccinated in the 2 populations.
Materials and Methods
The objective of this study was to estimate VPD incidence proportions among Medicaid and commercially insured adults. The primary endpoint was the age/gender-adjusted incidences of VPDs in the 2 populations; secondary endpoints were the unadjusted incidences of VPDs overall and in different age/gender groups.
Populations
VPD incidences in Medicaid and commercially insured adults aged 19–64 y (inclusive) were examined in this cross-sectional study using data from the Truven MarketScan® Medicaid and commercial databases, which are constructed from paid medical and prescription drug claims. The Medicaid database represents 12 states and contains the pooled healthcare experience of approximately 13 million enrollees. The commercial database represents private sector health data from approximately 100 payers (i.e., large employers, health plans, and government and public organizations) and contains the pooled healthcare experience of approximately 126 million enrollees. We identified enrollees aged 19–64 y from both databases for the years 2006–2010; for the Medicaid population, we excluded those dually eligible for Medicare.
All data were de-identified and Health Insurance Portability and Accountability Act compliant. As no patient-identifiable information was used for this study, no Institutional Review Board approval was required.
VPDs
The VPDs studied – influenza, pertussis, varicella, herpes zoster, measles, mumps, pneumococcal disease, meningococcal disease, hepatitis A, and hepatitis B—were chosen based on the recommendations for adult vaccinations from the Advisory Committee for Immunization Practices (ACIP).Citation36 During the timeframe encompassed in this analysis, some vaccinations (influenza, measles, mumps, pneumococcal, meningococcal, hepatitis A, and hepatitis B) were only recommended for adults with risk factors (e.g., medical, occupational, lifestyle) in some or all age groups,Citation33-Citation36 but since these factors could not be determined from the databases, we analyzed data of all eligible adults.
Each case of a VPD was identified in the database using ICD-9-CM diagnosis codes (Table S1). The primary and first secondary diagnosis codes were used to identify VPDs. Claims with an ICD-9-CM “diagnosis” of a VPD on the same day as a Current Procedural Terminology vaccination code for the same VPD were not counted as a VPD event since such claims were possibly related to vaccination rather than true VPD cases. Claims with an ICD-9-CM “diagnosis” of a VPD and a laboratory code without subsequent claims for confirmation of diagnosis or clinical treatment were not counted as VPD cases either.
Patients were eligible to be in the numerator (i.e., have a VPD event) if they had 1 y of continuous coverage prior to their diagnosis, to ensure that it was an incident occurrence of the condition. Patients were eligible to be in the denominator if they had 1 mo of coverage during the study year.
Herpes zoster immunization is licensed for use among those aged ≥50 y, but only recommended for those aged ≥60 y. Therefore, we analyzed incidences among those aged 50–64 and 60–64 y, respectively. Due to the possibility of miscoding for diagnoses of varicella and herpes zoster,Citation39 patients aged ≥40 y with a diagnosis of varicella were reassigned to herpes zoster, as were younger people who were immunocompromised (human immunodeficiency virus, cancer, organ transplant recipient, rheumatoid arthritis, inflammatory bowel disease, lupus, multiple sclerosis, or psoriasis) or had post-herpetic neuralgia. Patients aged ≥50 y who were reassigned to herpes zoster were included in the respective age group analyses. Patients aged <40 y without immune compromise or post-herpetic neuralgia kept their original diagnosis code. If a patient was coded for both varicella and zoster (on different dates), only the earliest VPD was included (using the algorithm listed above). If a patient was coded for both varicella and zoster on the same date, they were not included in the numerator for the incidence estimation or both diseases.
Statistical analysis
For all VPDs (except influenza), cases were identified from the first occurrence of the disease for a patient; and VPD incidences were calculated by dividing the number of patients with the disease by the eligible population and multiplying by 100 000 to produce yearly incidences per 100 000. The incidences for influenza are reported for each influenza season, which was taken to be from July 1 to June 30, as influenza strains vary by year and patients may develop influenza in separate years. Only one influenza event was counted per patient for each influenza season.
To make the results more comparable between the Medicaid and commercially insured populations, we calculated age/gender-adjusted incidences estimates using the US Census population in the year 2010Citation11 as the standard. This was done by dividing the Medicaid and commercially insured populations into 8 groups (males and females aged 19–34, 35–44, 45–54, and 55–64 y) and extrapolating the VPD incidences to the numbers of people in these groups from the 2010 US census.Citation11
Ninety-five percent confidence intervals (95% CIs) for unadjusted incidences were calculated using a standard method for proportions.Citation40 Due to the small number of cases of studied VPDs, 95% CIs of age/gender-adjusted incidences were calculated using a method based on the gamma distribution,Citation41 except for 95% CIs for the incidence of influenza, which were calculated using a method based on the normal distributionCitation42 since the number of cases was large and the variance was small. The incidences in the 2 insured populations were compared using two-sided tests and P values were calculated using methods described in Rothman and Greenland.Citation43 A difference with a P value ≤ 0.05 was considered statistically significant. Assumptions of statistical methods were validated prior to applying the methods to the VPD incidences.
| Abbreviations: | ||
| ACIP | = | Advisory Committee for Immunization Practices |
| CDC | = | Centers for Disease Control and Prevention |
| CI | = | confidence interval |
| ICD-9-CM | = | International Classification of Diseases, 9th Revision, Clinical Modification |
| US | = | United States |
| VPD | = | vaccine-preventable disease |
Additional material
Download Zip (155.5 KB)Disclosure of Potential Conflicts of Interest
G.K., C.C., J.P., B.A., and S.B. are full-time employees of the GlaxoSmithKline Group of Companies and hold restricted shares in the GlaxoSmithKline Group of Companies as part of their employment. M.L. (and/or his institution) has received funding from the GlaxoSmithKline Group of Companies to complete the work disclosed in this manuscript and fees for participation in review activities for an adjudication committee. M.L. also declares to have received consulting fees from Merck, Sharpe and Dohme for an Advisory Board and grant support for studies from the GlaxoSmithKline Group of Companies and Merck, Sharpe and Dohme. Additionally, M.L. receives royalties from Merck, Sharpe and Dohme for a patent.
Acknowledgments
We thank Dr Jenny Lloyd of Compass Medical Communications Ltd. who provided medical writing services on behalf of the GlaxoSmithKline Group of Companies; Heather Santiago (GlaxoSmithKline publication manager) for editorial assistance and manuscript coordination; Ning Wu for editorial assistance on behalf of GlaxoSmithKline Group of Companies; Aaron Rak for his contribution to the study idea and defining the methods for the study; and Dr Hoa Le for help with the age/gender-adjustment methodology and validation of this project analysis.
Role of the funding source: GlaxoSmithKline Biologicals SA funded this study/research and was involved in all stages of study conduct, including study design; collection, analysis, and interpretation of the data; writing the report; and the decision to submit the paper for publication. GlaxoSmithKline Biologicals SA also paid all costs associated with the development and publication of this manuscript.
Contributions: G.K.: scientific input, methods selection, literature review, acquisition of data, statistical analysis, and support for the statistical report. C.C.: assisted with the development of the methodology, programmed all analyses, and reviewed the codes and manuscript. J.P.: helped with the development of the methodology and programming support, project management, review of codes, and review of the manuscript. B.A.: methodology selection, model population, sensitivity analysis, review of the study report. S.B.: scientific input in data evaluation and study report. M. Levin: scientific input, literature review, statistical analysis, scientific input into the study report. All authors had full access to the data, agreed with the submission of the publication, and approved the final article.
References
- Robert Wood Johnson Foundation, Trust for America’s Health, Infectious Disease Society of America. Adult Immunization: Shots to Save Lives. [cited 2010 Feb]. Available from: http://healthyamericans.org/report/73/adult-immunization-2010
- Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2011. MMWR Morb Mortal Wkly Rep 2013; 62:66 - 72; PMID: 23364272
- National Immunization Program. Strategic Plan 2000‒2005. A Blueprint for Sustained Success. Available from: http://www.immregistries.org/resources/NIP_strategic_plan.pdf
- Miller BL, Kretsinger K, Euler GL, Lu PJ, Ahmed F. Barriers to early uptake of tetanus, diphtheria and acellular pertussis vaccine (Tdap) among adults-United States, 2005-2007. Vaccine 2011; 29:3850 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.03.058; PMID: 21459173
- Lu PJ, Euler GL, Jumaan AO, Harpaz R. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine 2009; 27:882 - 7; http://dx.doi.org/10.1016/j.vaccine.2008.11.077; PMID: 19071175
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1 - 64; PMID: 21293327
- Hurley LP, Lindley MC, Harpaz R, Stokley S, Daley MF, Crane LA, Dong F, Beaty BL, Tan L, Babbel C, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010; 152:555 - 60; http://dx.doi.org/10.7326/0003-4819-152-9-201005040-00005; PMID: 20439573
- Dempsey AF, Davis MM. Overcoming barriers to adherence to HPV vaccination recommendations. Am J Manag Care 2006; 12:Suppl S484 - 91; PMID: 17203992
- U.S. Government Printing Office. The Patient Protection and Affordable Care Act. 2010.
- Stewart AM, Richardson OL, Cox MA, Hayes K, Rosenbaum S. The Affordable Care Act: U.S. Vaccine Policy and Practice. Department of Health Policy, School of Public Health and Health Services, The George Washington University Medical Center, 2010.
- United States Census Bureau. Age and Sex Composition: 2010.
- Centers for Disease Control and Prevention (CDC). Summary of notifiable diseases--United States, 2010. MMWR Morb Mortal Wkly Rep 2012; 59:1 - 111; PMID: 22647710
- Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol 2002; 155:866 - 74; http://dx.doi.org/10.1093/aje/155.9.866; PMID: 11978592
- Centers for Disease Control and Prevention (CDC). Automated detection and reporting of notifiable diseases using electronic medical records versus passive surveillance--massachusetts, June 2006-July 2007. MMWR Morb Mortal Wkly Rep 2008; 57:373 - 6; PMID: 18401332
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011; 52:332 - 40; http://dx.doi.org/10.1093/cid/ciq077; PMID: 21217180
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al, Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271 - 84; http://dx.doi.org/10.1056/NEJMoa051016; PMID: 15930418
- Schmader KE, Levin MJ, Gnann JW Jr., McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922 - 8; http://dx.doi.org/10.1093/cid/cir970; PMID: 22291101
- Biggerstaff M, Jhung M, Kamimoto L, Balluz L, Finelli L. Self-reported influenza-like illness and receipt of influenza antiviral drugs during the 2009 pandemic, United States, 2009-2010. Am J Public Health 2012; 102:e21 - 6; http://dx.doi.org/10.2105/AJPH.2012.300651; PMID: 22897525
- Williams WW, Lu PJ, O’Halloran A, Bridges CB, Pilishvili T, Hales CM, Markowitz LE, Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults - United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 63:95 - 102; PMID: 24500288
- Yoo BK. How to improve influenza vaccination rates in the U.S. J Prev Med. Public Health 2011; 44:141 - 8
- Association of State and Territorial Health Officials. Increase in Vaccine Administration Rates. Summary of State Stakeholder Meetings. Arlington, VA. July 2012. Available at http://www.astho.org/Programs/Immunization/Increase-in-Vaccine-Administration-Rates/. Accessed on April 30, 2014.
- Yoo BK, Berry A, Kasajima M, Szilagyi PG. Association between Medicaid reimbursement and child influenza vaccination rates. Pediatrics 2010; 126:e998 - 1010; http://dx.doi.org/10.1542/peds.2009-3514; PMID: 20956412
- Motheral BR, Fairman KA. The use of claims databases for outcomes research: rationale, challenges, and strategies. Clin Ther 1997; 19:346 - 66; http://dx.doi.org/10.1016/S0149-2918(97)80122-1; PMID: 9152572
- Strom BL, Carson JL. Use of automated databases for pharmacoepidemiology research. Epidemiol Rev 1990; 12:87 - 107; PMID: 2286228
- Shen AK, Hunsaker J, Gazmararian JA, Lindley MC, Birkhead GS. Role of health insurance in financing vaccinations for children and adolescents in the United States. Pediatrics 2009; 124:Suppl 5 S522 - 31; http://dx.doi.org/10.1542/peds.2009-1542L; PMID: 19948584
- Hunsaker J, Veselovskiy G, Gazmararian JA. Health insurance plans and immunization: assessment of practices and policies, 2005-2008. Pediatrics 2009; 124:Suppl 5 S532 - 9; http://dx.doi.org/10.1542/peds.2009-1542M; PMID: 19948585
- Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci 2009; 123:1315 - 24; http://dx.doi.org/10.1037/a0017616; PMID: 20001115
- Kesner RP, Hunsaker MR. The temporal attributes of episodic memory. Behav Brain Res 2010; 215:299 - 309; http://dx.doi.org/10.1016/j.bbr.2009.12.029; PMID: 20036694
- Ramos JM, Davis GJ, Hunsaker JC 3rd, Balko MG. Sudden death in a child with Carpenter Syndrome. Case report and literature review. Forensic Sci Med Pathol 2009; 5:313 - 7; http://dx.doi.org/10.1007/s12024-009-9128-2; PMID: 19924577
- Hatabu H, Hunsaker AR. The cost and consequence of “uncertainty”. Acad Radiol 2009; 16:1307 - 8; http://dx.doi.org/10.1016/j.acra.2009.09.001; PMID: 19835788
- Burge M, Hunsaker JC 3rd, Davis GJ. Death of a toddler due to ingestion of sulfuric acid at a clandestine home methamphetamine laboratory. Forensic Sci Med Pathol 2009; 5:298 - 301; http://dx.doi.org/10.1007/s12024-009-9127-3; PMID: 19936975
- Kesner RP, Hunsaker MR, Ziegler W. The role of the dorsal CA1 and ventral CA1 in memory for the temporal order of a sequence of odors. Neurobiol Learn Mem 2010; 93:111 - 6; http://dx.doi.org/10.1016/j.nlm.2009.08.010; PMID: 19733676
- Centers for Disease Control and Prevention (CDC). Recommended adult immunization schedule - United States, October 2006-September 2007. JAMA 2006; 296:2430 - 3; http://dx.doi.org/10.1001/jama.296.20.2430
- Advisory Committee on Immunization Practices. Recommended adult immunization schedule: United States, October 2007-September 2008. Ann Intern Med 2007; 147:725 - 9; http://dx.doi.org/10.7326/0003-4819-147-10-200711200-00187; PMID: 17947396
- Advisory Committee on Immunization Practices. Recommended adult immunization schedule: United States, 2009. Ann Intern Med 2009; 150:40 - 4; http://dx.doi.org/10.7326/0003-4819-150-1-200901060-00008; PMID: 19124819
- Advisory Committee on Immunization Practices. Recommended adult immunization schedule: United States, 2010. Ann Intern Med 2010; 152:36 - 9; http://dx.doi.org/10.7326/0003-4819-152-1-201001050-00008; PMID: 20048270
- U.S. Census Bureau. Public Health Insurance Status By Sex By Age. Universe: Civilian noninstitutionalized population. 2008-2010 American Community Survey 3-Year Estimates.
- U.S. Census Bureau. Private Health Insurance Status By Sex By Age. Universe: Civilian noninstitutionalized population. 2008-2010 American Community Survey 3-Year Estimates.
- Mahamud A, Marin M, Nickell SP, Shoemaker T, Zhang JX, Bialek SR. Herpes zoster-related deaths in the United States: validity of death certificates and mortality rates, 1979-2007. Clin Infect Dis 2012; 55:960 - 6; http://dx.doi.org/10.1093/cid/cis575; PMID: 22715169
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17:857 - 72; http://dx.doi.org/10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E; PMID: 9595616
- Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med 1997; 16:791 - 801; http://dx.doi.org/10.1002/(SICI)1097-0258(19970415)16:7<791::AID-SIM500>3.0.CO;2-#; PMID: 9131766
- Curtin LR, Klein RJ. Direct standardization (age-adjusted death rates). Healthy People 2000 Stat Notes 1995; 1 - 10; PMID: 11762384
- Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia: Lippincott-Raven, 1998.