Abstract
Cancer immunotherapy is a rapidly growing field in oncology. One attractive feature of cancer immunotherapy is the purported combination of minimal toxicity and durable responses. However such treatments are often very expensive. Given the wide-spread concern over rising health care costs, it is important for all stakeholders to be well-informed on the cost and cost-effectiveness of cancer immunotherapies. We performed a comprehensive literature review of cost and cost-effectiveness research on therapeutic cancer vaccines and monoclonal antibodies, to better understand the economic impacts of these treatments. We summarized our literature searches into three tables by types of papers: systematic review of economic studies of a specific agent, cost and cost-effectiveness analysis. Our review showed that out of the sixteen immunotherapy agents approved, nine had relevant published economic studies. Five out of the nine studied immunotherapy agents had been covered in systematic reviews. Among those, only one (rituximab for non-Hodgkin lymphoma) was found to be cost-effective. Of the four immunotherapy drugs not covered in systematic reviews (alemtuzumab, ipilimumab, sipuleucel-T, ofatumumab), high incremental cost-effectiveness ratio (ICER) was reported for each. Many immunotherapies have not had economic evaluations, and those that have been studied show high ICERs or frank lack of cost-effectiveness. One major hurdle in improving the cost-effectiveness of cancer immunotherapies is to identify predictive biomarkers for selecting appropriate patients as recipients of these expensive therapies. We discuss the implications surrounding the economic factors involved in cancer immunotherapies and suggest that further research on cost and cost-effectiveness of newer cancer vaccines and immunotherapies are warranted as this is a rapidly growing field with many new drugs on the horizon.
Introduction
Cancer immunotherapy or immune-oncology was first noted by William B. Coley in the 19th century when bacterial toxins injected into cancer patients led to occasional cures. The field remained dormant until the late 20th century when tumor-associated antigens and tumor-infiltrating lymphocytes were discovered and non-specific pro-inflammatory cytokines like interleukin-2 (IL-2) were noted to generate robust immune responses against some neoplasms.Citation1 The development of advanced biotechnology techniques over the last decade has accelerated our understanding of the immune system. It has recently been proposed that one of the eight hallmarks of malignant growth of tumors is the evasion of cancer cells from attack and elimination by the immune system.Citation2,3
Although there is no one standard definition of cancer immunotherapy, over the years three very broad categories have evolved. The first category consists of non-specific cytokines such as IL-2 and interferons (IFN) that have been shown to have modest success in melanoma and renal cell carcinoma, but drugs in this category carry significant toxicities and are infrequently used today.Citation4,5 The second category comprises of cancer vaccines, a form of active immunotherapy, designed to actively kill neoplasms via the body's own induction of tumor antibodies or T cells.Citation6
The third and largest class of cancer immunotherapeutics includes monoclonal antibodies (mAbs) which are a form of passive immunotherapy and are the most commonly used form of immunotherapy in oncology.Citation7 Monoclonal antibodies can be further subdivided into five categories: (1) mAbs directed against a tumor-specific antigen such as rituximab (CD-20) or alemtuzumab (CD-52); (2) mAbs directed against cellular receptors such as cetuximab (anti-EGFR) or trastuzumab (anti-Her2 receptor); (3) mAbs that target soluble growth factors such as bevacizumab that interacts with vascular endothelial growth factor (VEGF); (4) mAbs that are immunoconjugates, combining a chemotherapy or radioisotope agent with an anti-immune antibody such as T-DMI (trastuzumab with emtansine) or ibritumomab tiuxetan; and (5) mAbs that are directed against negative regulators of the immune system such as ipilimumab (checkpoint inhibitors).
The field of cancer immunotherapy is witnessing a renaissance in the past 5 years, with rapid development of novel monoclonal antibodies and therapeutic cancer vaccines for multiple tumor types. For example, the first two immunotherapeutics to be FDA approved based on improvements in overall survival in large phase III trials have both been approved since 2010: sipuleucel-T (Provenge®), a therapeutic cancer vaccine for castrate-resistant prostate cancer and ipilimumab (Yervoy), a T cell potentiating monoclonal antibody for metastatic melanoma.Citation8,9 With further phase III development of therapeutic vaccines (e.g., clinicaltrials.gov NCT01582672, NCT01322490) and exciting early clinical results using the anti-programmed cell death (anti-PD-1) and anti-programmed cell death ligand (anti-PD-L1) checkpoint inhibitor mAbs, cancer immunotherapy is bound to become a more prominent pillar of oncologic care.
As the oncology community celebrates therapeutic innovations made possible from modern cancer immunotherapy, the high price tag associated with these drugs raises concern over the affordability of cancer care.Citation10,11 mAbs such as rituximab (the first FDA approved mAb in oncology in 1997), trastuzaumab and bevacizumab have through the years been top-selling cancer drugs, generating billions of dollars in revenues for drug manufacturers.Citation12 The high cost associated with new oncologics, many of which are immunotherapy agents, has cast doubts on the ‘value’ of these novel agents and triggered widespread debates over their cost and cost-effectiveness throughout the world.Citation13 A prime example is sipuleucel-T, approved by the FDA in 2010, with a cost of $93,000 for three injections and a median overall survival benefit of 4.1 months.Citation9,Citation14 The cost of sipuleucel-T sparked a media outcry.Citation15 The Centers for Medicare and Medicaid Services (CMS) conducted its own evaluation of the drug, a very unusual move for a cancer drug already approved by the FDA, and issued a ruling in March of 2011 that they will allow national coverage.Citation16 The drug, although approved in the European Union as of 2013, is currently only beginning to be marketed in Europe, and the National Institute for Health and Care Excellence (NICE) is currently working on an analysis of sipuleucel-T to be released in 2015. Whether it will be paid for by EU countries remains unclear. A common tactic of pharmaceutical companies is to benchmark prices of new oncologics against existing products and to set the price at a level that is at least as high as the highest priced competing product.Citation17,18 Thus, unless therapeutic gains of future immunotherapies are significantly better than sipuleucel-T, similar questions and concerns will likely be raised again and again.
In an era of clearly limited resources and widespread concern over rising health care costs, the debate over the value of immunotherapeutics in oncology care will continue among all stakeholders – clinicians, payers, patients and their family members. The objective of our paper was to review the current status of knowledge on the cost and cost-effectiveness of cancer immunotherapeutics. Due to their high toxicity and limited use in current practice, we excluded non-specific cytokines from our review and focused on therapeutic cancer vaccines and mAbs. Also excluded were prophylactic vaccines because of our focus on immunotherapy for treatment purposes.
Results
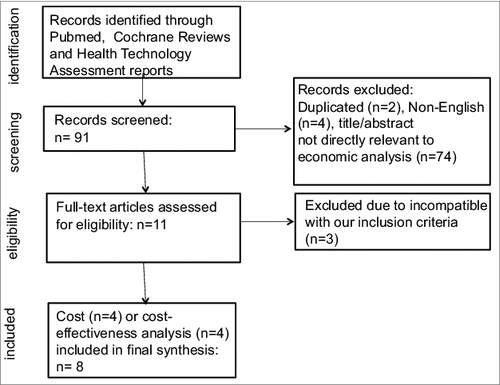
and depict the flowchart of the literature search process as suggested by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).Citation19 In brief, our phase I search () identified five systematic reviews of economic evaluations for five drugs: trastuzumab, rituximab, cetuximab, bevacizumab, and panitumumab.Citation20-24 The second-phase search excluded the above five agents and focused on the following eleven drugs: alemtuzumab, brentuximab vedotin, catumaxomab, ibritumomab tiuxetan, ipilimumab, obinutuzumab, ofatumumab, pertuzumab, sipuleucel-T, tositumomab, and trastuzumab emtansine. As shown in , we identified 91 studies in our initial screening from the phase II search. After reviewing title/abstract, we removed duplicated, non-English studies, and those irrelevant to economic analysis. Full-text review for the remaining studies led to the exclusion of studies incompatible with our inclusion criteria. Eight studies examining the costs or cost-effectiveness of alemtuzumab, ipilimumab, sipuleucel-T, or ofatumumab, were included in the final analyses.Citation25-32
Figure 1. Phase 1 search flow chart to identify systematic review(s) regarding the cost or cost-effectiveness of therapeutic cancer vaccines and immunotherapy published after January 1, 2012.
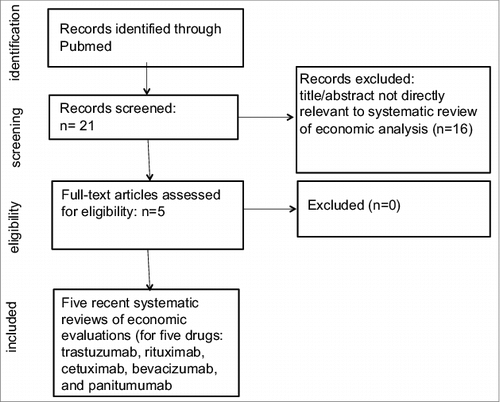
Figure 2. Phase 2 search flow chart to identify studies regarding the cost or cost-effectiveness of therapeutic cancer vaccines and immunotherapy based on our modified drug list.
summarizes findings of review articles identified from the phase I search regarding the cost-effectiveness of therapeutic cancer vaccines and immunotherapy in the current literature. As shown, rituximab combined with chemotherapy was considered to be cost-effective for non-Hodgkin's lymphoma (NHL) when compared to chemotherapy alone.Citation20,21 Trastuzumab was largely considered to not be cost-effective for HER2 positive metastatic breast cancer when compared to conventional chemotherapy, hormonal therapy, or best supportive care (BSC).Citation22 Cetuximab (+/- chemotherapy) was mainly considered not cost-effective for metastatic colorectal cancer when compared to conventional chemotherapy or BSC.Citation23,24 Bevacizumab (plus chemotherapy) was considered not cost-effective for mCRC when compared to chemotherapy alone.Citation24 Panitumumab was also considered not cost-effective for mCRC when compared to BSC alone.Citation23,24 However, Kirsten ras oncogene (KRAS) mutation testing before treatment with cetuximab or panitumumab was considered to be cost-effective compared to no testing.Citation24
Table 1. List of all immunotherapy agents approved (bolded therapies were reviewed; italicized therapies were summarized; rest have no published data)
Table 2. Summary of systemic review of economic evaluation for cancer immunotherapy/vaccine published since 2012
There were four cost papers identified from the phase II search, covering alemtuzumab (for chronic lymphocytic leukaemia, CLL), ipilimumab (for metastatic melanoma) and sipuleucel-T (for castration-resistant prostate cancer, CRPC) ().Citation25-28 Scott et al reported that alemtuzumab was cost saving ($11,181/patient) in New Zealand when compared to chemotherapy.Citation27 Jarkowski et al reported that ipilimumab with dose rounding was cost saving ($7,503/patient) in the United States when compared to ipilimumab without dose rounding.Citation28 Two commentary-type papers calculated the incremental drug cost ($98,780 for a full course) of sipuleucel-T and commented on the high cost of this immunotherapy agent.Citation25,26
Table 3. Summary of cost studies
Four cost-effectiveness studies were identified from the Phase II search, covering alemtuzumab (for T-cell prolymphocytic leukemia, T-PLL), ipilimumab (for previously treated advanced melanoma), sipuleucel-T (CRPC), and ofatumumab (for CLL) ().Citation29-32 Lu et al reported that while alemtuzumab (vs. chemotherapy) was likely to be cost-effective for T-PLL, especially if used earlier in the course of treatment, the cost-effectiveness of alemtuzumab is highly uncertain (ICER $42,710 ∼ $119,701/QALY among four scenarios).Citation29 Barzey et al reported that the probability that ipilimumab was cost-effective relative to BSC at willingness-to-pay (WTP) of $146,000 USD/QALY was 95% for patients with previously treated advanced melanoma. It should be noted that while the authors concluded that ipilimumab was cost-effective, the ICER estimated from their study was $140,811/QALY, which was higher than commonly accepted cost-effectiveness thresholds (i.e., $50,000 ∼ $100,000/QALY).Citation30 Holko et al reported that sipuleucel-T plus standard treatment was not cost-effective (ICER $289,964/QALY) for CRPC when compared to standard treatment alone.Citation31 In an appraisal of a manufacturer's submission, Hoyle et al reported that ofatumumab for CLL was associated with a high ICER ($126,756/QALY) when compared to BSC.Citation32
Table 4. Summary of cost-effectiveness studies
Discussion
To our knowledge this is the most up-to-date comprehensive systematic review of economic evaluations of immunotherapies in cancer in the recent era. We a priori excluded studies examining non-specific cytokines (IL-2 and IFN) and non-therapeutic vaccines, focusing our review on therapeutic cancer vaccines and mAbs. This left sixteen agents, all but one of which is a form of a monoclonal antibody. Among those, we identified economic studies for nine immunotherapy agents, leaving both costs and cost-effectiveness of seven agents unexplored. We reached the following conclusions from our review of economic studies of cancer immunotherapeutics.
First, we found that for around one-third (5/16) of agents (rituximab, trastuzumab, bevacizumab, cetuximab and panitumumab) systematic reviews regarding their cost-effectiveness have already been performed since 2012. Most (4/5) of these agents were considered as not cost-effective in the systematic reviews () with the noted exception of rituximab which has revolutionized the field of lymphoma.Citation33 Nevertheless, all are widely used in oncology practice throughout the world (and in particular in the United States) and clearly have provided clinical benefit for some (but not all) patients who are treated with them. The degree of that clinical benefit varies, and the lack of robust predictive biomarkers (for example, patients who are HER2 positive or KRAS wildtype do not all respond similarly to trastuzumab or cetuximab) makes it impossible to predict a response for an individual patient with any certainty, thus leading to widespread use of the drugs for all eligible patients. Inappropriate use of these targeted agents could be detrimental to patients, as exemplified by an increased risk of heart disease among cancer patients who received traztuzumab without documented evidence of HER2-testing.Citation34
Second, among the remaining 11 immunotherapy drugs, only four (alemtuzumab, ipilimumab, sipuleucel-T, ofatumumab) had been studied for either their cost or cost-effectiveness. Four of the 7 drugs not studied have been approved after 2011 (), and therefore their exclusion is not surprising. Of the remaining three, one (catumaxomab) has never been approved by the FDA while the other two (tositumomab and ibritumomab tiuxetan) are rarely used in practice and in fact as of this writing tositumomab has been withdrawn from the market by GlaxoSmithKline due to its current limited use and continual projected decline given the other treatment options available for patients with non-Hodgkin lymphoma. We expect an increasing number of economic evaluation studies of immunotherapy agents as time goes by. It should be noted that due to the paucity of literature in this area, our review also included several commentaries that touched on the economics of immunotherapy agents, which is a major limitation of this review.
Third, in our review of the cost-effectiveness analyses mentioned above, all drugs were associated with a high ICER, with the highest ICER found in sipuleucel-T vs. standard treatment for castration-resistant prostate cancer. The ICERs were in line with many of the modern targeted oncologic drugs,Citation35 whether IV or oral, and support the thesis that modern day oncologic care is expensive with often times mild gains for significant prices.Citation10,Citation36 In part this is due to the simple premise that cancer can be a deadly diagnosis for which for decades physicians had limited tools. Thus, when new therapies become available in the market both patients and doctors want to use them even though many of them may at best improve overall survival by only a few months, with some notable exceptions such as imatinib. As such, an increasing number of cancer patients are reported to experience financial hardship.Citation37,38 Concerns of financial and emotional distress from the use of costly new cancer drugs that offer marginal benefits, especially among patients with incurable cancers, have prompted the IOM Committee on the Quality of Cancer Care to recommend providers to communicate information on both total and out-of-pocket costs with patients and to promote early communications of supportive care.Citation39
Over the last several years, immunotherapy has experienced a revival. This has been driven by the approval of agents such as ipilimumab and sipuleucel-T, and also by a host of early phase trials with extremely promising results using both vaccines and mAbs.Citation40-42 In particular, the elucidation of the importance of immune checkpoint pathways in the progression of cancer has led to the development of novel mAbs aimed and disinhibiting the immune system in the presence of cancer. A prime example is the development of PD-1 agents targeting T-cell activation, which has shown exciting results in many solid tumors.Citation42-44 Several other “checkpoint” pathways are now being targeted in the clinical pipeline, as well as the development of a host of other antibody-drug conjugates, adoptive cell therapies and therapeutic vaccines.Citation45,46 Recent attempts to develop therapeutic HBV (e.g., clinicaltrials.gov NCT01374308) or HPV (e.g., clinicaltrials.gov NCT01957878) vaccines for use in already infected individuals have also showed early promise and may pave the way to the interruption of the infection-to-cancer progression.Citation47,48
Part of the allure of immunotherapy is the purported combination of minimal toxicity with durable responses due to immunologic memory, the specificity of the binding mAbs to a single target antigen, and the absence of mutation-induced resistance that has made it attractive both to scientists and the lay press.Citation49, 50 However, as with any anti-cancer therapy, immunotherapy carries possible side-effects, and these occasionally can be quite significant, eliciting for example a robust auto-immune reaction. Thus, their capacity to be used in practice outside carefully monitored clinical trials remains to be carefully defined.
Despite the excitement, there are many concerns about the cost-effectiveness and budget impact of the coming wave of checkpoint inhibitors.Citation13 Given their apparent efficacy, pharmaceutical companies are likely to seek prices as high or higher even than those for recent novel drugs. With innovator firms seeking regulatory approvals for combinations of checkpoint inhibitors with other immunotherapies and with targeted oral therapies, and for indications in multiple high prevalence cancers including melanoma and lung cancer and for extended or even chronic care use,Citation51 overall expenditures on these inhibitors threaten to be unsustainable.
Competition among the new therapies offers some measure of hope for payers attempting to restrain prices. Drug development costs may well be dropping as new clinical trial protocols such as the multi-agent, multi-sponsor lung cancer Master Protocol, combined with designation of many new drugs under the Breakthrough Therapy FDA approval pathway, lead to shorter development times.Citation52 As one example, Merck's MK-3475 checkpoint inhibitor moved from initial human testing to regulatory filing in under three years.Citation51 Lower development costs would be expected to attract more entrants and competition in this drug class. In addition, the historical willingness by oncologists to prescribe off-label combinations of drugs may offer opportunities to avoid very high pricing of regulator-approved drug combinations. Public payers in a number of countries frequently do not cover many high-priced therapies on grounds of cost-effectiveness.Citation53 However, private payers have shown only tentative signs of being willing to engage in tough management of novel oncologics, even for cancers with multiple therapeutic options offering room for price negotiations.Citation54 Finally, other potential ways to decrease the high cost of immunotherapy may be by reducing the number of doses administered in a particular treatment regimen or even by reducing the actual dose amount per administration of drug. For example, it is currently unknown how much or for how long one needs to receive ‘checkpoint inhibition therapy’ in order to develop and sustain the appropriate immunologic response and future trials will be necessary to address this question. Simply continuing therapy indefinitely once a response is attained may not be necessary when being treated with novel immunotherapy agents as opposed to the more traditional chemotherapy drugs.
Finding robust predictive biomarkers that would help select appropriate drugs for specific patients could be of major help in reducing costs & improving treatment effectiveness, but faces major obstacles. This challenge can be clearly illustrated by the example of sipuleucel-T. With a cost of close to $100,000 per patient and a median overall survival advantage of 4.1 months in those with metastatic castration resistant prostate cancer, the drug at first glance appears not to be cost-effective. The challenge then is whether a subset of patients can be identified prior to therapy initiation who may be predicted to derive significant benefit from the vaccine, while not subjecting those predicted to obtain minimal to no benefit to the treatment. Restricting the drug to the appropriately targeted subset of patients would lower the ICER and theoretically make the intervention cost-effective. Carefully matching patients with therapies based on their biomarkers will continue to be a focus of future trials and should hopefully help to improve the cost-effectiveness of immunotherapy in the future. The success of using biomarkers to enhance the cost-effectiveness of immunotherapy will hinge upon the accuracy as well as costs of tests for biomarkers.
In summary, our systematic review has demonstrated that with the exception of rituximab, most immunotherapies in oncology today either have not had an economic evaluation or have a relatively high ICER. In several instances, mAbs have been found not to be cost-effective. Given that oncology is just beginning to explore and evaluate modern day immunotherapies such as checkpoint inhibitors and therapeutic vaccines, their role may indeed prove to be vital and cost-effective in the coming years, but further evaluation is required.
Methods
We performed a comprehensive literature search for articles examining the cost or cost-effectiveness of therapeutic cancer vaccines and immunotherapy. Given the lack of standard definition of cancer immunotherapy, we determined the drugs to be included in our review as those that had been approved by the Food and Drug Administration (FDA)Citation55 or the European Medicines Agency (EMA)Citation56 as therapeutic cancer vaccines or mAbs before 2014. The master list of drugs included: trastuzumab, pertuzumab, cetuximab, panitumumab, bevacizumab, rituximab, ofatumumab, alemtuzumab, obinutuzumab, ipilimumab, tositumomab, ibritumomab tiuxetan, trastuzumab emtansine, brentuximab vedotin, sipuleucel-T, and catumaxomab (). Because some drugs on the list have been on the market for several years, there are recently published systematic reviews of economic evaluations of these drugs. Our approach was to avoid replicating information already available in the literature and to focus on economic studies of immunotherapy agents that have not been covered in review articles. However, we did provide a brief summary of findings from the systematic reviews so as to inform readers of the current state of knowledge on the economics of selected cancer immunotherapeutics. Thus, we conducted our searches in two phases. Phase I identified immunotherapy agents that have already been discussed in systematic reviews published after January 1, 2012, so as to restrict to recently published systematic reviews. We used balanced search filters for costs or economics in the PubMed ((“costs and cost analysis”[MeSH] OR costs[Title/Abstract] OR cost effective*[Title/Abstract]) OR (cost*[Title/Abstract] OR “costs and cost analysis”[MeSH:noexp] OR cost benefit analysis*[Title/Abstract] OR cost-benefit analysis[MeSH] OR health care costs MeSH : noexp)) as used in the literatureCitation57-59 and “cancer” and the above drug list in PubmedCitation60 and limited to systematic reviews published after January 1, 2012. After excluding the subset of the drugs identified from phase I (decided by consensus between two authors (Chien & Shih)), we then proceeded to phase II in which we performed a comprehensive literature search using the remaining drugs on the master list (the modified list) AND the above search filters for costs or economics AND cancer in Pubmed on Feb 6th, 2014. We revised the search terms (any drugs on the modified list) AND cancer AND cost* in title/abstract/keywords for Cochrane databaseCitation61 and also searched for drugs on the modified drug list in ‘Title’ in the advanced search of Health Technology Assessment in UK.Citation62 After removing duplicated and non-English studies, potentially relevant studies were selected by two independent reviewers (Chien & Shih) who reviewed titles and/or abstracts. Disagreement was resolved by discussion between the reviewers to reach a final consensus. Further review of the full paper of the above searches led to the decision on studies to be included in the final analyses, again by two independent reviewers (Chien & Shih). Disagreement was resolved by consensus after discussion.
We summarized studies identified from phases I and II searches in tables, categorized by type of papers: systematic review, cost, and cost-effectiveness. For systematic reviews, we synthesized information retrieved from the papers as follows: authors and year of publication, drug(s) and disease and treatment setting, numbers of studies included, results, and authors’ conclusion. For cost or cost-effectiveness papers, we synthesized information as follows: authors and year of publication, country, target population & intervention, study details (including perspective, cost type, reference year for cost, time horizon, parameters sources, discount rate and approach), results (including cost and/or effectiveness, and findings from one-way sensitivity analysis if available), and conclusions (including incremental cost or incremental cost-effectiveness ratio (ICER), probabilistic sensitivity analysis (PSA) if available, and the main conclusion from the authors). When multiple outcomes (such as life-year (LY) or quality-adjusted life (QALY)) were reported, we mainly extracted the one(s) that allowed for comparison with other studies. For reference year for cost, we used the number as reported in the paper or based on the most relevant year of data used or the year of publication if not clearly specified in the studies. Cost was expressed in 2013 USD by applying the local consumer price index and purchasing power parity index (PPP)Citation63,64 For the threshold value reported in probabilistic sensitivity analyses based on currency other than $US, we cited the original value as well as $US converted using PPP in 2013.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Drs. Shih and Smieliauskas are supported by a grant from the Agency for Healthcare Research and Quality (R01 HS018535), and Dr. Shih as well by The University of Chicago Cancer Research Foundation Women's Board. Dr. Chien is supported by a grant from the China Medical University Hospital (DMR-103-043), and the Ministry of Health and Welfare (MOHW 103-TD-B-111-03). Dr. Geynisman is in part supported by an independent grant from Pfizer, Inc. # 11703561.
References
- Finn OJ. Cancer immunology. The New England journal of medicine 2008; 358:2704-15.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74.
- Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2011; 29:4828-36.
- Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. The New England journal of medicine 1998; 338:1272-8.
- Eggermont AM, Suciu S, Testori A, Santinami M, Kruit WH, Marsden J, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012; 30:3810-8.
- Darcy PK, Neeson P, Yong CS, Kershaw MH. Manipulating immune cells for adoptive immunotherapy of cancer. Current opinion in immunology 2014; 27C:46-52.
- Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010; 28:4390-9.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 2010; 363:711-23.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine 2010; 363:411-22.
- Shih YC, Ganz PA, Aberle D, Abernethy A, Bekelman J, Brawley O, et al. Delivering high-quality and affordable care throughout the cancer care continuum. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2013; 31:4151-7.
- Smith TJ, Hillner BE. Bending the cost curve in cancer care. The New England journal of medicine 2011; 364:2060-5.
- Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008; 26:1774-7.
- Carroll J. New immunotherapies for cancer yield exciting results but high cost. Managed care (Langhorne, Pa) 2013; 22:54-6.
- Simoens S. Pharmaco-economic aspects of sipuleucel-T. Human vaccines & immunotherapeutics 2012; 8:506-8.
- http://www.washingtonpost.com/wp-dyn/content/article/2010/11/07/AR2010110705205.html.
- Centers for Medicare and Medicaid Services. Proposed decision memo for autologous cellular immunotherapy treatment of metastatic prostate cancer (CAG-00422N). March 30, 2011. (http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=247&ver=10&NcaName=Autologous+Cellular+Immunotherapy+Treatment+of+Metastatic+Prostate+Cancer&bc=BEAAAAAAEAAA&.).
- Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. The New England journal of medicine 2009; 360:626-33.
- Bach PB. Reforming the payment system for medical oncology. JAMA: the journal of the American Medical Association 2013; 310:261-2.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 2009; 6:e1000097.
- Papaioannou D, Rafia R, Rathbone J, Stevenson M, Buckley Woods H, Stevens J. Rituximab for the first-line treatment of stage III-IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic review and economic evaluation. Health technology assessment (Winchester, England) 2012; 16:1-253, iii-iv.
- Auweiler PW, Muller D, Stock S, Gerber A. Cost effectiveness of rituximab for non-Hodgkin's lymphoma: a systematic review. PharmacoEconomics 2012; 30:537-49.
- Parkinson B, Pearson SA, Viney R. Economic evaluations of trastuzumab in HER2-positive metastatic breast cancer: a systematic review and critique. The European journal of health economics : HEPAC : health economics in prevention and care 2014; 15:93-112.
- Hoyle M, Crathorne L, Peters J, Jones-Hughes T, Cooper C, Napier M, et al. The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No.150 and part review of technology appraisal No. 118): a systematic review and economic model. Health technology assessment (Winchester, England) 2013; 17:1-237.
- Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, von der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. European journal of cancer 2014; 50:40-9.
- Klemm J, Mehr SR. Prostate cancer. The American journal of managed care 2012; 18:SP119-21.
- Vasani D, Josephson DY, Carmichael C, Sartor O, Pal SK. Recent advances in the therapy of castration-resistant prostate cancer: the price of progress. Maturitas 2011; 70:194-6.
- Scott WG, Scott HM. Economic evaluation of third-line treatment with alemtuzumab for chronic lymphocytic leukaemia. Clinical drug investigation 2007; 27:755-64.
- Jarkowski A, 3rd, Nestico JS, Vona KL, Khushalani NI. Dose rounding of ipilimumab in adult metastatic melanoma patients results in significant cost savings. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners 2014; 20:47-50.
- Lu L, Peters J, Roome C, Stein K. Cost-effectiveness of alemtuzumab for T-cell prolymphocytic leukemia. International journal of technology assessment in health care 2012; 28:241-8.
- Barzey V, Atkins MB, Garrison LP, Asukai Y, Kotapati S, Penrod JR. Ipilimumab in 2nd line treatment of patients with advanced melanoma: a cost-effectiveness analysis. Journal of medical economics 2013; 16:202-12.
- Holko P, Kawalec P. Economic evaluation of sipuleucel-T immunotherapy in castration-resistant prostate cancer. Expert review of anticancer therapy 2014; 14:63-73.
- Hoyle M, Crathorne L, Garside R, Hyde C. Ofatumumab for the treatment of chronic lymphocytic leukaemia in patients who are refractory to fludarabine and alemtuzumab: a critique of the submission from GSK. Health technology assessment (Winchester, England) 2011; 15 Suppl 1:61-7.
- Maloney DG. Immunotherapy for non-Hodgkin's lymphoma: monoclonal antibodies and vaccines. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005; 23:6421-8.
- Shih YC, Xu Y, Dong W, Smieliauskas F, Giordano S, Shen Y. First do no harm: population-based study shows non-evidence-based trastuzumab prescription may harm elderly women with breast cancer. Breast cancer research and treatment 2014; 144:417-25.
- Shih YC, Halpern MT. Economic evaluations of medical care interventions for cancer patients: how, why, and what does it mean? CA: a cancer journal for clinicians 2008; 58:231-44.
- Malin JL. Wrestling with the high price of cancer care: should we control costs by individuals’ ability to pay or society's willingness to pay? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010; 28:3212-4.
- Ramsey S, Blough D, Kirchhoff A, Kreizenbeck K, Fedorenko C, Snell K, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health affairs 2013; 32:1143-52.
- Ubel PA, Abernethy AP, Zafar SY. Full disclosure–out-of-pocket costs as side effects. The New England journal of medicine 2013; 369:1484-6.
- IOM. Delivering high-quality cancer care: Charting a new course for a system in crisis. Washington, DC: Institute of Medicine, 2013.
- Long-Term Survival Achieved in Unfavorable Risk mRCC Patients Treated With a Combination of Autologous Immunotherapy (AGS-003) Plus Sunitinib A. Amin, A.Z., Dudek, G., Jha2, T., Logan, R.S., Lance, J.M., Holzbeierlein, V.A., Master, S.K., Pal, J.J., Knox, L., Karsh, D., Plessinger, C.A., Nicolette, R.A. Figlin, AGS-003-006 Study Group. ASCO 2014 Genitourinary Cancers Symposium – May 30-June 3, 2014 – Chicago, IL.
- Lubaroff DM. Prostate cancer vaccines in clinical trials. Expert review of vaccines 2012; 11:857-68.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine 2012; 366:2455-65.
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine 2013; 369:122-33.
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine 2013; 369:134-44.
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews Immunology 2012; 12:269-81.
- Eggermont A, Robert C, Soria JC, Zitvogel L. Harnessing the immune system to provide long-term survival in patients with melanoma and other solid tumors. Oncoimmunology 2014; 3:e27560.
- Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Science translational medicine 2014; 6:221ra13.
- Liu J, Kosinska A, Lu M, Roggendorf M. New therapeutic vaccination strategies for the treatment of chronic hepatitis B. Virologica Sinica 2014; 29:10-6.
- Promising New Cancer Drugs Empower the Body's Own Defense System. ANDREW POLLACK. Published: June 3, 2013 New York Times.
- Breaking Through Cancer's Shield. GINA KOLATA Published: October 14, 2013.
- Plumridge, Hester. “Drug Firms Focus on Advanced Melanoma,” Wall Street Journal 03/23/2014
- McCaughan, Michael. “Testing A New Model For Personalized Drug Development,”, The RPM Report December 2013.
- Cheema PK, Gavura S, Migus M, Godman B, Yeung L, Trudeau ME. International variability in the reimbursement of cancer drugs by publically funded drug programs. Current oncology 2012; 19:e165-76.
- Maas, Angela. “Advanced RCC Gains Scrutiny as More Drugs Are Approved For It,” Specialty Pharmacy News 9(4): April 2012.
- http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- http://www.ema.europa.eu/ema/.
- Glanville J, Paisley S. Identifying economic evaluations for health technology assessment. International journal of technology assessment in health care 2010; 26:436-40.
- Health Information Research Unit, McMaster University, http://hiru.mcmaster.ca/hiru/HIRU_Hedges_MEDLINE_Strategies.aspx, accessed on 2014/1/11.
- Shen C, Chien CR, Geynisman DM, Smieliauskas F, Shih YC. A review of economic impact of targeted oral anticancer medications. Expert review of pharmacoeconomics & outcomes research 2014; 14:45-69.
- http://www.ncbi.nlm.nih.gov/pubmed, accessed 2014/1/28.
- http://www.thecochrancelibrary.com/view/0/index/html, via institutional account, accessed 2014/2/6.
- http://www.journalslibrary.nihr.ac.uk/search?collection=netscc-journals&form=advanced&, accessed 2014/2/6
- Consumer Price Index. [cited 2014 Apr 12]; Available from: http://www.bls.gov/cpi/.
- IMF. World Economic Outlook Database. [cited 2014 Apr 12]; Available from: http://www.imf.org/external/pubs/ft/weo/2014/01/weodata/index.aspx.
