Abstract
We sought to develop an IL-33 vaccine and evaluate its efficacy in a mouse model of asthma. The full-length molecules of putative mature IL-33 were inserted into the immunodominant epitope region of hepatitis B core antigen using gene recombination techniques. The expressed chimeric protein presented as virus-like particles (VLPs) under observation using an electron microscopy. To investigate immunization characteristics of the VLPs, mice were immunized by using different doses, adjuvants, and routes. The VLPs induced sustained and high titers of IL-33-specific IgG and IgA even without the use of a conventional adjuvant, and the lowered ratio of IgG1/IgG2a in vaccinated mice indicated a shift from Th2 to Th1-like responses. To assess the vaccine effects on blocking the signaling of IL-33/ST2 pathway, mice receiving 3 vaccinations subjected to intraperitoneal sensitization and intranasal challenge with ovalbumin (OVA). Control animals received carrier or PBS in place of the vaccine. Immunization with the VLPs significantly suppressed inflammatory cell number and IL-33 level in BALF. OVA -induced goblet cell hyperplasia and lung tissue inflammatory cell infiltration were significantly suppressed in vaccinated mice. Our data indicate that IL-33 molecule-based vaccine, which may block IL-33/ST2 signaling pathway on a persistent basis, holds potential for treatment of asthma and, by extension, other diseases where overexpressed IL-33 plays a pivotal role in pathogenesis.
Introduction
Asthma is a severe inflammatory airway disease, and its pathological mechanisms haven’t been sufficiently understood. Recently accumulated data indicated that interleukin (IL)-33 played an important role in allergic airway inflammation. A genome-wide association study has identified that polymorphism of IL-33 gene is associated to susceptibility of asthma.Citation1 Elevated IL-33 level was detected in the patients with severe asthma.2In animal models, administration of IL-33 induced airway hyperresponsiveness and goblet cell hyperplasia in the lungs,Citation3 exacerbated eosinophil-mediated airway inflammation,Citation4 and amplified the polarization of alternatively activated macrophages that contribute to airway inflammationCitation5; on the other hand, ovalbumin (OVA)-treated ST2-deficient mice showed a significant reduction of airway resistance in response to methacholine stimulation,Citation6 moreover, delivery of soluble ST2 or anti-IL-33 antibody attenuated OVA-induced allergic inflammation in mice.Citation7,Citation8 It is thought that allergen-induced responses of Th2 cells and their cytokines and related adaptive immunity are main factors contributing to the development of allergic asthma, and IL-33 acts its pathological effects through provoking Th2-like immune responses. Recently, some other cell types responding to IL-33 stimulation are found involved in the asthma pathogenesis. For examples, IL-13-producing type 2 innate lymphoid cells (ILCs) expanded promptly upon IL-33 stimulation and caused airway contraction, and the ILCs-mediated airway hyperreactivity is independent of adaptive immunity responses.Citation9 Furthermore, TNF-α upregulated IL-33 expression in cultured airway smooth muscle cells isolated from severe asthma patients,Citation2 and IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma,Citation10 indicating a possible important role of IL-33 in refractory asthma. Taken together, accumulated data strongly suggest that IL-33 is a new potential target for treatment of asthma.
Anti-cytokine vaccine is emerging as a new and promising approach in the treatment of disorders characterized by maladaptive immunoregulatory responses, such as allergic and autoimmune diseases.Citation11 Vaccines targeting a variety of cytokines, such as TNF-α,Citation12 IL-13,Citation13 IL-9,Citation14 IL-1β,Citation15and IL-17,Citation16 have proven to be significantly effective in animal models of a variety of human diseases. The vaccine strategy may prevail over monoclonal antibodies on some features, such as, providing persistent intervening effects with only several doses of immunization which provides distinct convenience to treatment of chronic diseases. Hepatitis B virus core antigen (HBcAg), which has a capability of assembling efficiently into virus-like particles (VLPs) in E.coli cells, has shown to be highly immunogenic and broadly used as a powerful vaccine carrier for interested antigens.Citation17 In the current study, we sought to develop a highly efficient VLPs vaccine with full-length molecules of mature IL-33 presenting on the surface, and using a mouse model of asthma, to evaluate its efficacy of suppressing IL-33 pathological roles and its potentials in treatment of asthma.
Results
Recombinant chimeric protein HBcAg-33 was efficiently expressed and assembled into VLPs
The recombinant chimeric protein HBcAg-33 was expressed efficiently in E. coli cells analyzed by SDS-PAGE, and the expected band was recognized specifically by commercially derived polyclonal anti-IL-33 antibody in immunoblotting (). Analyzed by SDS-PAGE, vaccine HBcAg-33 and carrier HBcAg had an identical pattern on optiprep gradient ultracentrifugation (only HBcAg-33 was shown in ), i.e., both of the recombinant proteins presented mostly in the collected fractions 9 to 14, whereas most of the bacterial proteins even with larger molecular weight in SDS-PAGE appeared in fractions 1 to 10, which indicated that HBcAg-33 assembled into VLPs similar to that of HBcAg. The VLPs structure was further identified with electron microphotographic techniques (). The difference between the 2 VLPs was observed: the surface of the vaccine VLPs is quite rough owing to mature IL-33 molecules with a length of 158 aa was inserted into HBcAg, whereas that of the carrier HBcAg VLPs is relatively smooth, as shown by the magnified inserts in .
Figure 1. Expression and assembly of rHBcAg-33. (A) The map of recombinant plasmid pHBcAg33. (B) SDS-PAGE analysis (left panel) and immunoblotting with anti-IL-33 (right panel) identification for the expression of chimeric protein HBcAg-33; Arrows point to recombinant HBcAg-33. Lane 1: bacteria without induction; lane 2: bacteria after induction with IPTG . (C) Samples from Optiprep gradient ultracentrifugation were analyzed by 12% SDS-PAGE. (D) Prepared VLPs were photographed using a transmission electron microscope (original magnification is 30.0k×), and a bar representing 100 nm is shown. Small panels present magnified VLPs (magnification is about 60.0k×) showing the difference on the surface between the 2 VLPs.
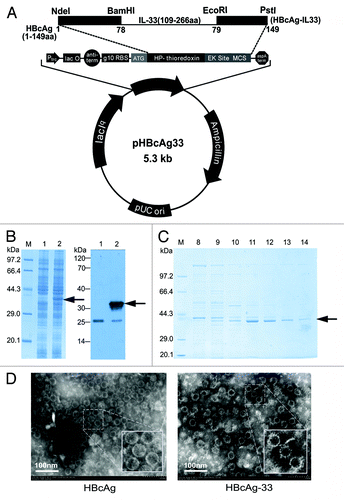
In addition, there were both hollow and solid particles existing in prepared VLPs, probably attributing to inclusion or exclusion of possible components of cytoplasm such as RNA molecules in the particles.
IL33-specific antibody responses to immunization with IL-33 vaccine
We first tested the ability of the vaccine (HBcAg-33) VLPs alone or in the presence of adjuvant (Alum or complete/incomplete Freund’s adjuvant, CFA/IFA) to induce an IL-33-specific IgG response. Mice immunized with vaccine alone produced a strong IL-33-specific antibody response, and the titer was up to 25 6000 after the second booster (week 5) which is comparable to those induced by vaccine emulsified with the adjuvants tested in this study. In all vaccinated groups, specific antibodies sustained at a high level for at least 3 mo (). Freund’s adjuvant group has higher and longer lasting response level, whereas Alum group seems decrease the most quickly in all the groups. There is no statistical difference for IgG titers found between groups except for at week 13 (P < 0.05).
Figure 2.IL33 specific antibody responses to immunization with IL-33 vaccine. (A) Vaccination without the use of conventional adjuvants induces sustained and high titer of IL-33–specific IgG, which is similar to those induced with the use of adjuvants Alum or FA/IFA at week 5. Subsequently, alum group showed a faster decrease than FA/IFA group and VLPs alone group.*P < 0.05 for comparison with FA/IFA group; #P < 0.05 for comparison with VLPs alone group (B) The antibody responses to different doses of vaccination.*P < 0.05 and **P < 0.01 for comparison with 5 µg group; #P < 0.05 for comparison with 10 µg group (C) Intranasal immunization induced specific IgA responses and a similiar IgG level to that of subcutaneous immunization. The serum dilutions are 1:50 and 1:1000 for IgA and IgG measurements, respectively. The statistical analyses were performed with unpaired Student t test.
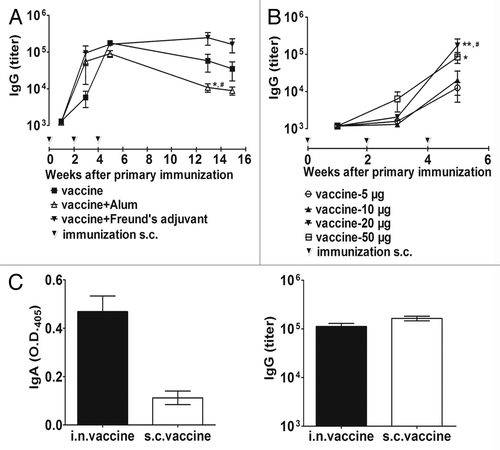
The antibody responses elicited by immunization with the vaccine VLPs showed kind of but not strictly a dose-dependent manner from 5 µg to 50 µg (). Immunization of 5 µg or 10 µg of VLPs induced an apparent production of specific IgG at a similar level, and 20 µg or 50 µg of VLPs elicited a significantly higher response after the second booster (week 5). Notably, 20 µg of VLPs reached a similar level to that induced by 50 µg dose. Comparing different immunization routes, i.n. immunization induced both mucosal and systemic IgG and IgA responses, and the strength of serum IgG response was close to that produced by s.c. immunization at the same dose of 20 µg VLPs ().
HBcAg-specific IgG1 and IgG2a responses to immunization with IL-33 vaccine
HBcAg-specific IgG1 and IgG2a responses were analyzed using ELISAs (). Compared with the carrier, different doses of IL-33 vaccine showed a trend of increased IgG2a levels but had no apparent effects on IgG1 levels. The ratios of IgG1/IgG2a in vaccine group decreased significantly as compared with those in the carrier group, which indicated an increased Th1-like IgG responses.
Figure 3. HBcAg –specific IgG1and IgG2a responses to immunization with IL-33 vaccine. (A) HBcAg –specific IgG1 and IgG2a responses to different doses of IL-33 vaccine or carrier. (B) HBcAg –specific IgG1 and IgG2a responses to intranasally (i.n.) or subcutaneously (s.c.) immunized vaccine with or without the use of adjuvants. The serum dilution used for this assay is 1:1000. The P value was determined by unpaired Student t test. *P < 0.05, **P < 0.01, ***P < 0.001.
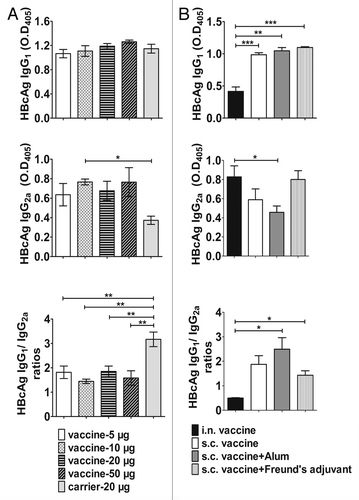
We also investigated the effects on IgG1 and IgG2a responses of intranasal (i.n.) immunization approach and also subcutaneous (s.c.) immunization approach with the use of different adjuvants. As shown in , all the groups receiving s.c. immunization with or without the adjuvants induced IgG1 response at a similar level, whereas immunization via i.n. approach decreased significantly IgG1 level. IgG2a responses displayed different levels among the 4 groups, and the results showed that i.n immunized group and Freund’s adjuvant group produced the strongest IgG2a responses whereas Alum group got the lowest IgG2a level. There was a statistical significance reached between i.n immunized group and the Alum group (P < 0.05). The ratio of IgG1/IgG2a in i.n. immunized group is significantly lower than those of s.c. immunized groups (P < 0.05).
Vaccine suppresses airway inflammation and mucus overproduction
Mice were administrated with vaccine, carrier or PBS, and subsequently sensitized/challenged with OVA as the protocol (). Analysis of cytospin samples of Bronchoalveolar lavage fluid (BALF) showed that cells in BALF are mainly comprised of eosinophils, lymphocytes, monocytes, and neutrohpils. BALF eosinophilia was reduced significantly in vaccinated mice (n = 8) as compared with mice receiving carrier (n = 8) (P < 0.001) and mice receiving PBS (n = 12) (P < 0.01) (). IL-33 levels in BALF in each group were analyzed using ELISA (). The results showed that the mean level of IL-33 in the vaccinated s.c. groups (n = 8) were suppressed significantly when compared with that in either the carrier group (n = 8) (P < 0.01) or the PBS group (n = 8) (P < 0.01).
Figure 4. Vaccine reduces allergen-induced airway inflammatory responses. (A) The protocol used for assessing vaccine efficacy in an asthma model. (B) Vaccine reduces BALF eosinopilia; **P < 0.01; ***, P < 0.001. (C) Vaccine downregulates IL-33 accumulation in BALF. *P < 0.05; **P < 0.01. (D) Vaccine suppresses lung inflammation and goblet-cell hyperplasia through histological analysis of the lung. Representative images were stained with hematoxylin and eosin (H&E) and periodic acid–Schiff (PAS), respectively. Arrows point to accumulated inflammatory cells, and arrowheads point to goblet cells within the respiratory epithelium. The inserts are powered magnifications showing respectively infiltrated inflammatory cells (mainly eosinophils and lymphcytes) or goblet cells. (E) Semiquantitative analysis of histology. Peribronchiolar/perivascular accumulation of inflammatory cells was assessed using an indexed scale. Goblet-cell abundance was measured as the percentage of PAS-positive cells in the total airway epithelia of medium-sized airways (90–150 mm in diameter). The data were expressed as score values. *P < 0.05. All the statistical analyses were performed with One-way ANOVA followed by Newman-Keuls multiple-comparisons test. Original magnification, ×200.
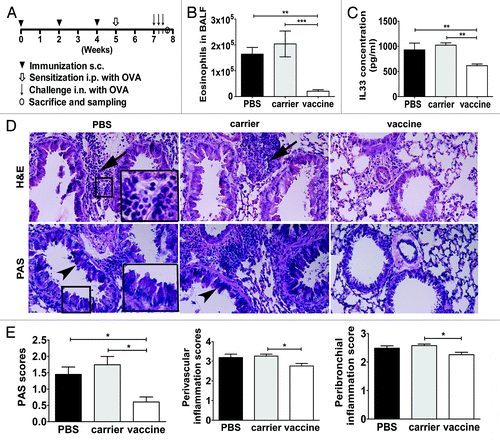
In formalin-fixed lungs, H&E staining was performed to assess tissue inflammation, which is a common manifestation of allergic airways responses. In control mice receiving carrier or PBS, there was evidence of obvious perivascular and peribronchial accumulation of inflammatory cells, which appeared to be mainly comprised of eosinophils and lymphocytes, as shown by the magnified insert of the representative photomicrograph (). Notably, consistent with BALF cytology, H&E staining of lung sections confirmed that the accumulation of inflammatory cells in the airways was suppressed in mice that received IL-33 vaccine (). Airway goblet-cell abundance was analyzed on PAS-stained slides. Representative images analysis () correlated well with lung tissue immune cell infiltration. In mice that received carrier or PBS, OVA challenges led to a marked increase in goblet cells hyperplasia. Mice that received IL-33 vaccine showed dramatically fewer goblet cells after allergen sensitization/challenges. Semiquantitative analyses of goblet cells and tissue inflammation were performed in 4 mice in each group. The results further confirmed that IL-33 vaccine immunization significantly suppressed goblet cell hyperplasia and lung tissue inflammation (P < 0.05) ().
Discussion
Innovative application of anti-cytokine vaccine concepts represents a promising approach to treatment of severe, often lifelong, chronic human diseases which dominated by maladaptive immunoregulatory responses. Although blocking only one molecule may be ineffectiveCitation18,Citation19 or insufficient to attain significant benefit in all clinical manifestations in all patients,Citation20-Citation22 the clinical experience provides proof of concept about the potential utility of targeting key molecule(s) for therapy.Citation23
Several peptide epitopes from inflammatory cytokines which are key mediators in pathogenesis of asthma or inflammatory bowel diseases have been successfully presented onto the surface of HBcAg VLPs, and immunization with these VLPs ameliorated significantly the development of diseases manifests in animal models.Citation13,Citation24-Citation26 However, linear epitopes may be not powerful enough to elicit efficient neutralizing antibodies (our unpublished data). Moreover, based on our previous experience, the assembling ability of HBcAg might be destroyed even if a short peptide is inserted, and sometimes even an amino acid alteration within the successfully inserted peptide would hamper the VLPs assembly (our unpublished data). It is worth mentioning, in this current study, full-length molecules of putative mature IL-33 were successfully presented on the HBcAg VLPs, which may be evidenced from that vaccine can induce IL-33 specific antibodies and vaccine VLPs present much rougher surface than carrier VLPs. The vaccine is believed to be highly immunogenic and potent due to simultaneously possess 2 key virtues of an optimal vaccine, i.e., the highly organized structure of VLPs and the presentation of target molecules showing native conformation. Our results encouragingly suggest that even a large molecule, which has important epitopes with three-dimensional structure required, might be presented efficiently by HBcAg VLPs. We speculate that a chimeric protein with a large target molecule inserted into HBcAg carrier might have chance to assemble into VLPs if the target molecule can be expressed alone as soluble form in E.coli cells,. Besides, both N and C-terminals of the target molecule locate naturally at the same side of the three-dimensional structure and are adjacent to each other, might also increase the possibility of VLPs assembly.
Considering a full-length molecule-based vaccine may theoretically have more chances to evoke cross-reactive antibodies against other self-proteins and cause autoimmune diseases than a peptide-based vaccine, a BLAST comparison was performed by using tools in NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). The result showed that there were no any other molecules with high homogeneity to IL-33 found. Although risks of causing autoreactivity cannot be excluded completely by this analysis, to some extent, it does increase benefits on safety of IL-33 full-length molecules-based vaccine.
The immunization characteristics of the VLPs vaccine were investigated in this study by using different vaccine doses, immunization routes, and immunization adjuvants. We found that the serum IgG intensity induced by i.n. immunization was close to that of s.c. immunization when the same vaccine dose was used. The observation indicates that i.n. administration of a VLPs vaccine is a viable approach when the establishment of a specific mucosal immune is important. We speculated that inflammatory cytokines accumulated on mucosal surface of airway may have significantly contributed to the development of allergen-induced allergic inflammatory responses. If i.n. immunization with vaccine evoked mucosal antibodies responses, it may add extra benefits to control airway allergic responses by directly neutralizing IL-33 accumulated on mucosal surface. However, we found that i.n. immunization caused infiltration of immune cells into BALF (data not shown), which may interfere with the assessment of vaccine efficacy on suppressing airway inflammation in an acute model like we used here. And thus, we chose to use s.c immunization. In this current study, there was not a strict dose-dependency of antibody responses found, and, the antibodies evoked by VLPs with Alum adjuvant decreased even faster than that induced by VLPs alone, both of which were kind of unexpected to us. The possible explanations might due to small numbers of mice used in the study, individual variation of mice, as well as the experimental operation variations.
Asthma is thought to be primarily driven by biased Th2 inflammatory responses. In this report, we checked the response patterns of antibody subclass to carrier to reflect the type of immune responses to some extent. Analyzing the ratio of IgG1 to IgG2a, we demonstrated that the reaction to the inserted IL-33 molecules led to a change in the responses to HBcAg; the Th2-biased responses were converted into more balanced levels of Th1 and Th2 responses in the mice receiving IL- 33 vaccine. Different doses of the vaccine showed a similar change of IgG1/IgG2a mainly by raising IgG2a levels. As expected, Freund’s enhanced IgG2a and Alum was on the contrary. The results are in consistent with the notion that IL-33 provokes Th2 responses. Interestingly, i.n. immunization produced a more profound effect on suppressing IgG1 and raising IgG2a than s.c. immunization, indicating that i.n. administration of the vaccine might be a viable alternative when mucosal immune responses and a shift to Th1 responses are required.
Interleukin-33 is a key mediator in inducing airway and tissue eosinophilia which is a cardinal feature of allergic asthma. Eosinophils are thought to contribute substantially to airway remodeling,Citation27,Citation28 and also acute responses such as goblet cell hyperplasia.Citation29 We performed total cell and eosinophil counts in BALF and histological examination of lung to detect the effects of IL-33 molecules-based vaccine on airway inflammation and mucus hyperplasia. Corresponding to suppressed IL-33 level in BALF, the accumulation of eosinophils in both the BALF and lung tissue were significantly reduced by vaccination, and we observed a dramatic suppression of OVA-induced mucous production, as indicated by reduced goblet cell number.
To be noticed, besides the possible pathogenic roles in many severe disorders like airway allergic inflammation, IL-33 has also other important effects depending on disease environments. For example, IL-33 acts as a key danger signal released by necrotic structural cells such as epithelial cells to alert immune system for developing immune responsesCitation30; IL-33 is capable of activating mast cells to be sensors of cell injuryCitation31 and probably play both beneficial and detrimental actions due to cause neutrophil influxCitation32; IL-33 may attenuate clinical sepsis by modulating the expression Of CXCR2 and chemo-taxis of neutrophilsCitation33; the application of anti-IL-33 antibodies attenuates intestinal mucositis and enhances effectiveness of tumor chemotherapy in mice.Citation34 IL-33/ST2 signaling indicated as an important protective pathway in various Cardiovascular (CV) diseasesCitation35,Citation36; IL-33 may have protective effects during atherosclerosis by inducing a Th1-to-Th2 switch of immune responsesCitation37; IL-33 presents protective effects in obesity and type 2 diabetes through affecting lipid metabolism via an increased production of Th2 cytokines and a switching of macrophage polarization from M1 to M2 phenotype.Citation38-Citation40 Taken together, IL-33 might play beneficial or detrimental roles depending on disease microenvironment.
Neutralization of IL-33 may raise concerns about the lack of normal physiological functions provided by IL-33. Actually, this issue has been discussed in depth in recent reviews.Citation41-Citation43 First, the success application of monoclonal antibodies in clinic have proven that targeting to self proteins may be efficacy and safe; second, under normal conditions, cytokine release occurs within a so-called ‘immune synapse’ that requires intimate association of the cells involved. Previous studies indicate that auto-antibodies induced by vaccine had little impact at the ‘immune synapse,’ and appear only to neutralize excess cytokine that accumulates ectopically in the extracellular compartment; third, local tissues of disorders have more abundant blood backflow which bring much more auto-antibodies than normal tissues. Finally and maybe more importantly, accumulated data from clinical trialsCitation41-Citation43 have shown that anti-self protein vaccines are well tolerated and safe in human. And thus, theoretical analyses and practical research data support that anti-cytokine vaccine are a safe and promising strategy, although caution is needed when a designated target molecule is concerned.
In summary, IL-33 is emerging as an important mediator in many severe human diseases. By using an acute asthma model, we demonstrated that a vaccine could efficiently suppress pathologic effects of IL-33. The results confirmed the proinflammatory roles of IL-33 in asthma and also indicated a promising strategy of suppressing IL-33 for the treatment of asthma and, by extension, other disorders in which elevated IL-33 are involved in the pathogenesis. However, safety and effectiveness of IL-33 vaccine still need further to be cautiously and adequately assessed in the future clinical trials. Furthermore, the vaccine was only assessed using a unnatural allergen OVA-induced model of acute airway allergic inflammation. Further studies are urgently required for employing vaccine to clarify the IL-33 functions and roles in asthma pathogenesis under different diseases microenvironments, by using different allergens-induced models and especially chronic models with sustained airway inflammation and remodeling.
Materials and Methods
Preparation of recombinant vaccine
Plasmid pHBcAg-33, which expressed a chimeric protein with putative mature IL-33 molecule inserted between amino acids 78 and 79 of the immunodominant epitope of truncated HBcAg (1–149 amino acid, GenBank accession number: GQ377581), was constructed by gene recombinant methods (). Briefly, gene encoding for truncated HBcAg was synthesized (Sangon Biotech, Ltd.) with a oligonucleotides linker containing BamH I and EcoR I recognization sequences engineered between codes for amino acids 78 and 79, and cloned into plasmid pThioHisA (Invitrogen) using endonuclease Nde I (TaKaRa Biotechnology Co., Ltd.) and PstI (TaKaRa Biotechnology Co., Ltd.). Consequently, plasmid pHBcAg was constructed, which expresses modified carrier protein HBcAg. And then, gene encoding for mature IL-33 (GenBank accession number: AY905582)was inserted into the plasmid pHBcAg using previously introduced BamH I and EcoR I. Obtained recombinant plasmid pHBcAg-33 was identified and transformed into CaCl2-treated competent DH5α cells. The bacteria were induced with 1 mmol/l IPTG (Sangon Biotech, Ltd.) for 4 h at 25 °C. The expression of recombinant protein was analyzed and identified by 12% denaturing SDS-PAGE and immunoblotting with polyclonal anti-IL-33 antibody (Santa Cruz Biotechnology). Recombinant protein was purified using a procedure consists of ultrasonication lysis, precipitation with 40% saturation ammonium sulfate, rinse with 20% saturation ammonium sulfate, ultracentrifugation with 27% to 39% optiprep (Sigma-Aldrich) density gradient and chromatography with Sepharose CL-4B (Sigma-Aldrich). Purified proteins were kept in –70 °C for the use of immunization. The presence of VLPs was confirmed using a transmission electron microscope.
Mice
Female BALB/c mice (6- to 8-wk-old) (SCXK [jing] 2012–0001; 16–18 g), purchased from Vital River Laboratory Animal Technology Co. Ltd., were raised and maintained in the Central Animal Care Services of the Institute of Medical Biology, CAMS, and PUMC, under specific pathogen free (SPF) condition. All protocols of animal experiments were approved by the Animal Ethics Committee of Institute.
Protocols for immunization of mice
To test the VLPs’ immunogenicity, 3 groups of mice (n = 4) were immunized subcutaneously (s.c.) with 20 μg vaccine (HBcAg-33) with aluminum adjuvant(Thermo Scientific), Freund’s adjuvants(Sigma-Aldrich), or without the use of any conventional adjuvant, respectively; and another group of mice (n = 4) was immunized s.c. with 20 μg carrier (HBcAg) as control. In order to determine appropriate immunization dose, 5 μg, 10 μg, 20 μg, and 50 μg of vaccine were used to immunize s.c. mice without the use of any adjuvant, respectively. And another group of mice were immunized intranasally (i.n.) with 20 μg vaccine. The different doses of vaccine were prepared by diluting the same batch of purified VLPs, which guaranteed the increasing dose of vaccine correspond to a proportionally increasing immunologically active dose of IL-33. There were 4 mice in each group. The mice were immunized 3 times at 2 wk intervals. For the study of using Freund’s adjuvants, the VLPs were mixed with complete Freund’s adjuvant for primary immunization and with incomplete Freund’s adjuvant for booster immunizations.
Animal model of allergic airway inflammation and sample collection
Four groups of mice were included: (1) vaccine: immunized s.c. with 20 μg vaccine; (2) carrier: immunized s.c. with 20 μg carrier; (3) control: injected with phosphate buffered saline (PBS, 0.02 mol/l with 0.15 mol/l NaCl, pH 7.4). All the groups were subjected to one intraperitoneal sensitization with 10 μg ovalbumin (OVA, grade IV; Sigma-Aldrich) and 2 mg Alum adjuvant mixed in 500 µl PBS and 3 continuous everyday i.n. challenges with 50 μg OVA in 40 µl PBS, following protein or PBS administration. One day after OVA challenge, mice were anaesthetized and blood samples were collected by cardiac puncture. In addition, lung lavage was collected with 1 mL cold PBS, flushing gently 3 times. The total cell numbers in BALF were counted using a hemocytometer. The supernatants after centrifugation were kept at –70 °C for cytokine measurements. The pellets were resuspended with PBS and then spinned onto glass slips for Wright’s-Giemsa stain (Nanjing JianCheng Technology Co., Ltd.) staining. The percentage of eosinophils was obtained by counting the numbers of eosinopjhlis and total cells with light microscope at 200× magnification. The number of eosinophils for each BALF sample was calculated by using the obtained percentage times the number of total cells.
Enzyme-linked immunosorbent assay (ELISA)
The IL-33 concentrations in BALF were detected with ELISA as described previously,Citation13 using paired capture and biotinylated detection antibodies purchased from eBioscience, Inc. (eBioscience) and according to supplier’s instructions. Avidin–phosphatase system was used to develop the reaction. Measurements for antigen-specific IgA, IgG, IgG1, and IgG2a antibodies were performed successively by coating microplates with antigens:0.5 μg/mL of IL-33 (Peprotech) or HBcAg, adding serum or BALF samples, incubating with specific primary and alkaline phosphatase-conjugated secondary antibodies, and developing with alkaline phosphatase substrate. The volume of working solution is 100 μL in this assay. All the antibodies were purchased from eBioscience Inc., and the coated IL-33 was commercially derived from Peprotech Inc. The titer of antiserum was determined as the reciprocal of the highest dilution of the sample in which the OD405 value was twice of that of the corresponding control serum pooled from carrier immunized mice when its OD405 was around 0.10. A serum from vaccinated mice with titer determined was taken as the reference serum, and a standard curve was prepared with the 2-fold serially diluted reference serum and taking OD405 values as y-axis and titers as x-axis. The titers of serum samples were obtained by referring the standard curve by OD405 values.
Histological analyses
Left lungs were fixed in PBS-buffered formalin, and then embedded in paraffin and serially sectioned (5 mm) sagittally at the anterior–posterior midline. Specimens were stained with hematoxylin and eosin (H&E) to examine inflammation, and with periodic acid–Schiff (PAS) to detect mucus production. Semiquantitative analyses for peribronchiolar/perivascular inflammation and airway goblet-cell hyperplasia were performed in a double-blinded fashion.Citation13 Briefly, peribronchiolar/perivascular inflammation in H&E-stained slides was assessed using an indexed scale: 0 = normal; 1 = infrequent inflammatory cells; 2 = a ring of inflammatory cells 1 cell layer deep; 3 = a ring of inflammatory cells 2 to 4 cells deep; 4 = a ring of inflammatory cells of more than 4 cells deep. The numerical scores for the abundance of PAS-positive goblet cells in each airway were determined as follows: 0: <5% goblet cells; 1: 5–25%; 2: 25–50%; 3: 50–75%; 4: >75%.
Statistical analysis
The significance of differences between experimental groups was analyzed using one-way analysis of variance (ANOVA), followed by Newman-Keuls multiple-comparisons test, or unpaired Student t test (GraphPad Prism 5.0, GraphPad Software Inc.). Values are reported as the mean ± standard errors of the mean.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by grants from Yunnan Provincial Science and Technology Department (2011FA023 and 2013IA005), National Natural Science Foundation of China (81072399), and Peking Union Medical College (2012–1001- 030).
The authors thank Mr. Cheng Guo and Jinxian Zhou for excellent assistance in electron microscopy, and acknowledge Mr Xi Wang, Ms Qingling Wang, and Ms Dong Shen for assistance in histological analyses.
References
- Grotenboer NS, Ketelaar ME, Koppelman GH, Nawijn MC. Decoding asthma: translating genetic variation in IL33 and IL1RL1 into disease pathophysiology. J Allergy Clin Immunol 2013; 131:856 - 65; http://dx.doi.org/10.1016/j.jaci.2012.11.028; PMID: 23380221
- Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemière C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol 2009; 183:5094 - 103; http://dx.doi.org/10.4049/jimmunol.0802387; PMID: 19801525
- Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol 2008; 20:791 - 800; http://dx.doi.org/10.1093/intimm/dxn037; PMID: 18448455
- Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol 2010; 185:3472 - 80; http://dx.doi.org/10.4049/jimmunol.1000730; PMID: 20693421
- Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol 2009; 183:6469 - 77; http://dx.doi.org/10.4049/jimmunol.0901575; PMID: 19841166
- Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol 2013; 132:933 - 41; http://dx.doi.org/10.1016/j.jaci.2013.05.012; PMID: 23810766
- Yin H, Li XY, Liu T, Yuan BH, Zhang BB, Hu SL, Gu HB, Jin XB, Zhu JY. Adenovirus-mediated delivery of soluble ST2 attenuates ovalbumin-induced allergic asthma in mice. Clin Exp Immunol 2012; 170:1 - 9; http://dx.doi.org/10.1111/j.1365-2249.2012.04629.x; PMID: 22943195
- Mizutani N, Nabe T, Yoshino S. Interleukin-33 and alveolar macrophages contribute to the mechanisms underlying the exacerbation of IgE-mediated airway inflammation and remodelling in mice. Immunology 2013; 139:205 - 18; http://dx.doi.org/10.1111/imm.12071; PMID: 23323935
- Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol 2012; 129:216 - 27, e1-6; http://dx.doi.org/10.1016/j.jaci.2011.10.036; PMID: 22119406
- Saglani S, Lui S, Ullmann N, Campbell GA, Sherburn RT, Mathie SA, Denney L, Bossley CJ, Oates T, Walker SA, et al. IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J Allergy Clin Immunol 2013; 132:676 - 85, e13; http://dx.doi.org/10.1016/j.jaci.2013.04.012; PMID: 23759184
- Delavallée L, Duvallet E, Semerano L, Assier E, Boissier MC. Anti-cytokine vaccination in autoimmune diseases. Swiss Med Wkly 2010; 140:w13108; PMID: 21043003
- Lee CK, Kim HS, Ye BD, Lee KM, Kim YS, Rhee SY, Kim HJ, Yang SK, Moon W, Koo JS, et al, Korean Association for the Study of Intestinal Diseases (KASID) Study. Patients with Crohn’s disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J Crohns Colitis 2014; 8:384 - 91; http://dx.doi.org/10.1016/j.crohns.2013.09.022; PMID: 24144611
- Ma Y, Halayko AJ, Basu S, Guan Q, Weiss CR, Ma AG, HayGlass KT, Becker AB, Warrington RJ, Peng Z. Sustained suppression of IL-13 by a vaccine attenuates airway inflammation and remodeling in mice. Am J Respir Cell Mol Biol 2013; 48:540 - 9; http://dx.doi.org/10.1165/rcmb.2012-0060OC; PMID: 23470628
- Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med 2012; 18:1248 - 53; http://dx.doi.org/10.1038/nm.2856; PMID: 22772464
- Guler R, Parihar SP, Spohn G, Johansen P, Brombacher F, Bachmann MF. Blocking IL-1α but not IL-1β increases susceptibility to chronic Mycobacterium tuberculosis infection in mice. Vaccine 2011; 29:1339 - 46; http://dx.doi.org/10.1016/j.vaccine.2010.10.045; PMID: 21093494
- Guan Q, Weiss CR, Qing G, Ma Y, Peng Z. An IL-17 peptide-based and virus-like particle vaccine enhances the bioactivity of IL-17 in vitro and in vivo. Immunotherapy 2012; 4:1799 - 807; http://dx.doi.org/10.2217/imt.12.129; PMID: 23240747
- Roseman AM, Borschukova O, Berriman JA, Wynne SA, Pumpens P, Crowther RA. Structures of hepatitis B virus cores presenting a model epitope and their complexes with antibodies. J Mol Biol 2012; 423:63 - 78; http://dx.doi.org/10.1016/j.jmb.2012.06.032; PMID: 22750730
- Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, Coyle AJ, Koike M, Spitalny GL, Kiener PA, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol 2010; 125:1237 - 44, e2; http://dx.doi.org/10.1016/j.jaci.2010.04.005; PMID: 20513521
- Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Carlsten C, Davis BE, Deschesnes F, Duong M, Durn BL, Howie KJ, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med 2011; 183:1007 - 14; http://dx.doi.org/10.1164/rccm.201008-1210OC; PMID: 21057005
- Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, Lal P, Arron JR, Harris JM, Busse W. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013; 187:804 - 11; http://dx.doi.org/10.1164/rccm.201208-1414OC; PMID: 23471469
- Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, Norris PS, Choi H, Scheu S, Pfeffer K, et al. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med 2011; 17:596 - 603; http://dx.doi.org/10.1038/nm.2356; PMID: 21499267
- Hardyman MA, Wilkinson E, Martin E, Jayasekera NP, Blume C, Swindle EJ, Gozzard N, Holgate ST, Howarth PH, Davies DE, et al. TNF-α-mediated bronchial barrier disruption and regulation by src-family kinase activation. J Allergy Clin Immunol 2013; 132:665 - 75, e8; http://dx.doi.org/10.1016/j.jaci.2013.03.005; PMID: 23632299
- Hansbro PM, Scott GV, Essilfie AT, Kim RY, Starkey MR, Nguyen DH, Allen PD, Kaiko GE, Yang M, Horvat JC, et al. Th2 cytokine antagonists: potential treatments for severe asthma. Expert Opin Investig Drugs 2013; 22:49 - 69; http://dx.doi.org/10.1517/13543784.2013.732997; PMID: 23126660
- Ma Y, Guan Q, Bai A, Weiss CR, Hillman CL, Ma A, Zhou G, Qing G, Peng Z. Targeting TGF-beta1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm Bowel Dis 2010; 16:1040 - 50; http://dx.doi.org/10.1002/ibd.21167; PMID: 19924805
- Ma Y, HayGlass KT, Becker AB, Fan Y, Yang X, Basu S, Srinivasan G, Simons FE, Halayko AJ, Peng Z. Novel recombinant interleukin-13 peptide-based vaccine reduces airway allergic inflammatory responses in mice. Am J Respir Crit Care Med 2007; 176:439 - 45; http://dx.doi.org/10.1164/rccm.200610-1405OC; PMID: 17556715
- Ma Y, Ma AG, Peng Z. A potential immunotherapy approach: mucosal immunization with an IL-13 peptide-based virus-like particle vaccine in a mouse asthma model. Vaccine 2007; 25:8091 - 9; http://dx.doi.org/10.1016/j.vaccine.2007.09.009; PMID: 17935839
- Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov 2013; 12:117 - 29; http://dx.doi.org/10.1038/nrd3838; PMID: 23334207
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 2013; 13:9 - 22; http://dx.doi.org/10.1038/nri3341; PMID: 23154224
- Tachdjian R, Al Khatib S, Schwinglshackl A, Kim HS, Chen A, Blasioli J, Mathias C, Kim HY, Umetsu DT, Oettgen HC, et al. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. J Allergy Clin Immunol 2010; 125:1128 - 36, e8; http://dx.doi.org/10.1016/j.jaci.2010.01.054; PMID: 20392476
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’?. PLoS One 2008; 3:e3331; http://dx.doi.org/10.1371/journal.pone.0003331; PMID: 18836528
- Enoksson M, Lyberg K, Möller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol 2011; 186:2523 - 8; http://dx.doi.org/10.4049/jimmunol.1003383; PMID: 21239713
- Enoksson M, Möller-Westerberg C, Wicher G, Fallon PG, Forsberg-Nilsson K, Lunderius-Andersson C, Nilsson G. Intraperitoneal influx of neutrophils in response to IL-33 is mast cell-dependent. Blood 2013; 121:530 - 6; http://dx.doi.org/10.1182/blood-2012-05-434209; PMID: 23093619
- Alves-Filho JC, Sônego F, Souto FO, Freitas A, Verri WA Jr., Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med 2010; 16:708 - 12; http://dx.doi.org/10.1038/nm.2156; PMID: 20473304
- Guabiraba R, Besnard AG, Menezes GB, Secher T, Jabir MS, Amaral SS, Braun H, Lima-Junior RC, Ribeiro RA, Cunha FQ, et al. IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol 2014; Forthcoming http://dx.doi.org/10.1038/mi.2013.124; PMID: 24424522
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007; 117:1538 - 49; http://dx.doi.org/10.1172/JCI30634; PMID: 17492053
- Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail 2009; 2:684 - 91; http://dx.doi.org/10.1161/CIRCHEARTFAILURE.109.873240; PMID: 19919994
- Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med 2008; 205:339 - 46; http://dx.doi.org/10.1084/jem.20071868; PMID: 18268038
- Saidi S, Bouri F, Lencel P, Duplomb L, Baud’huin M, Delplace S, Leterme D, Miellot F, Heymann D, Hardouin P, et al. IL-33 is expressed in human osteoblasts, but has no direct effect on bone remodeling. Cytokine 2011; 53:347 - 54; http://dx.doi.org/10.1016/j.cyto.2010.11.021; PMID: 21190867
- Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun 2009; 384:105 - 9; http://dx.doi.org/10.1016/j.bbrc.2009.04.081; PMID: 19393621
- Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, Xu D, Sattar N, McInnes IB, Liew FY. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res 2010; 107:650 - 8; http://dx.doi.org/10.1161/CIRCRESAHA.110.218867; PMID: 20634488
- Link A, Bachmann MF. Immunodrugs: breaking B- but not T-cell tolerance with therapeutic anticytokine vaccines. Immunotherapy 2010; 2:561 - 74; http://dx.doi.org/10.2217/imt.10.30; PMID: 20636009
- Le Buanec H, Bensussan A, Bagot M, Gallo RC, Zagury D. Active and passive anticytokine immune therapies: current status and development. Adv Immunol 2012; 115:187 - 227; http://dx.doi.org/10.1016/B978-0-12-394299-9.00007-2; PMID: 22608260
- Jennings GT, Bachmann MF. Immunodrugs: therapeutic VLP-based vaccines for chronic diseases. Annu Rev Pharmacol Toxicol 2009; 49:303 - 26; http://dx.doi.org/10.1146/annurev-pharmtox-061008-103129; PMID: 18851703
