JEV is expanding its geographic distribution and epidemic intensity worldwide. JEV vaccination prevents morbidity and mortality, however, recent publications highlighted JE cases in individuals who have received vaccination, raising serious concerns about vaccine efficacy. In the present letter, serum samples from a small cohort of vaccinees (n = 16) were subjected to standard PRNT assays against the vaccine strain and heterologous circulating strains. Our results demonstrated that there is a significant reduction in protective antibodies against JEV circulating strains especially heterologous GI strains in adults immunized with SA14–14–2. Our findings deserve special concern in context of vaccine development and clinical trials evaluation.
Japanese encephalitis (JE) remains the most important cause of viral encephalitis in Asia and the Pacific region, and an estimated 50 000–70 000 human cases resulted in about 10 000 deaths worldwide annually.Citation1 Vaccination has been well proven the most successful strategy to reduce morbidity and mortality. The Chinese live attenuated vaccine derived from Japanese encephalitis virus (JEV) strain SA14–14–2 has just achieved the World Health Organization prequalification and now been recommended for use in all JE endemic countries.Citation2 To date, more than 300 million children have been immunized with the SA14–14–2 vaccine, and ideal safety and efficacy profile is well demonstrated in different countries.Citation3,4 However, independent groups from China and India reported a number of JE cases in individuals who have received SA14–14–2 vaccination.Citation5,6 These unexpected results arouse special concerns about the vaccine efficacy and cross protection against heterologous circulating JEV strains.
All circulating JEV strains can be divided into 5 genotypes (GI, GII, GIII, GIV, and GV) based on the genome sequence of envelope gene.Citation7 During past decades, GI strains gradually replaced GIII strains as the dominant circulating strain in Asia.Citation1 However, all JE vaccine strains including SA14–14–2 belong to GIII, whether there is any reduction in cross-protection capacity against heterologous JEV strains is being critically important.
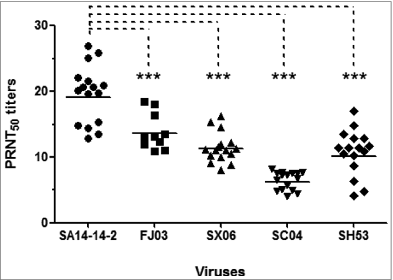
Here, we assessed the potency of SA14–14–2-induced neutralizing antibody against the predominant circulating JEV strains in China. A panel of diluted serum samples (n = 16) was collected from healthy adults who have received vaccination with the SA14–14–2 vaccine. Neutralizing antibody titers against the vaccine strain SA14–14–2 (GIII strain) and a number of circulating strains including FJ03 (GIII strain isolated in Fujian, China, 2003), SC04 (GI strain isolated in Sichuan, China, 2004), SH53 (GI strain isolated in Shanghai, China, 2001), and SX06 (GI strain in Shanxi, China, 2006), were determined by using the standard 50% plaque reduction neutralization test (PRNT), respectively.
As shown in , the PRNT50 titers against the 4 circulating strains were significantly lower than that against SA14–14–2. Notably, the PRNT50 titer against SC04–17 (<1:10) was significantly lower than the other JEV strains. Our preliminary results in vaccinated population demonstrated that there is a significant reduction in protective antibodies against JEV circulating strains especially heterologous GI strains. Previously, mice challenge experiments indicated that the SA14–14–2 vaccine could confer protection against the challenge of different JEV isolates,Citation8 while neutralizing antibodies against selected strains also decreased. Recent results from vaccinees with inactivated vaccines also suggested potential reduction in neutralization capacity against heterologous JEV genotypes.Citation9,10
Figure 1. Neutralizing antibody titers against homologous and heterologous JEV strains in vaccinees with the live vaccine SA14–14–2. PRNT50 are determined in BHK21 cells. The statistical significance (P < 0.05) was determined by one-way ANOVA with GraphPad Prism 5 and are indicated by asterisks.
Although the population size is relatively small, these results deserve extensive concerns. For most JE vaccine clinical trials, only neutralizing antibody against the homologous strain is detected. This probably would lead to unfaithful results in areas where the circulating strains were quite different from the vaccine virus. Heterologous circulating strains should be included for immunogenicity and efficacy studies in any immunogenicity and efficacy trials. Furthermore, efforts should be warranted to identify the potential genotype-specific neutralizing epitopes of JEV, which have been extensively investigated for dengue viruses.Citation11,12 Especially, structural and reverse genetic studies would help reveal the relevance between protection and antigenic variations among JEV genotypes.
Finally, a novel vaccine that derived from genotype I or multivalent vaccine that includes several genotypes of JEV strains may be beneficial in the context of vaccine development in the future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31300151), National Basic Research Project of China (No.2012CB518904), and the National Major Special Program of Science and Technology of China (No.2013ZX10004-805). C.F.Q. was supported by the Beijing Nova Program of Science and Technology (No.2010B041).
References
- Pan XL, Liu H, Wang HY, Fu SH, Liu HZ, Zhang HL, Li MH, Gao XY, Wang JL, Sun XH, et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J Virol 2011; 85:9847-53; PMID:21697481; http://dx.doi.org/10.1128/JVI.00825-11
- Yu Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine 2010; 28:3635-41; PMID:20226891; http://dx.doi.org/10.1016/j.vaccine.2010.02.105
- Bista MB, Banerjee MK, Shin SH, Tandan JB, Kim MH, Sohn YM, Ohrr HC, Tang JL, Halstead SB. Efficacy of single-dose SA 14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet 2001; 358:791-5; PMID:11564484; http://dx.doi.org/10.1016/S0140-6736(01)05967-0
- Kumar R, Tripathi P, Rizvi A. Effectiveness of one dose of SA 14-14-2 vaccine against Japanese encephalitis. N Engl J Med 2009; 360:1465-6; PMID:19339732; http://dx.doi.org/10.1056/NEJMc0808664
- Sarkar A, Banik A, Pathak BK, Mukhopadhyay SK, Chatterjee S. Envelope protein gene based molecular characterization of Japanese encephalitis virus clinical isolates from West Bengal, India: a comparative approach with respect to SA14-14-2 live attenuated vaccine strain. BMC Infect Dis 2013; 13:368; PMID:23927571; http://dx.doi.org/10.1186/1471-2334-13-368
- Hu Q, Chen B, Zhu Z, Tian J, Zhou Y, Zhang X, Zheng X. Recurrence of Japanese encephalitis epidemic in Wuhan, China, 2009-2010. PLoS One 2013; 8:e52687; PMID:23326348; http://dx.doi.org/10.1371/journal.pone.0052687
- Uchil PD, Satchidanandam V. Phylogenetic analysis of Japanese encephalitis virus: envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am J Trop Med Hyg 2001; 65:242-51; PMID:11561712
- Liu X, Yu Y, Li M, Liang G, Wang H, Jia L, Dong G. Study on the protective efficacy of SA14-14-2 attenuated Japanese encephalitis against different JE virus isolates circulating in China. Vaccine 2011; 29:2127-30; PMID:21237278; http://dx.doi.org/10.1016/j.vaccine.2010.12.108
- Erra EO, Askling HH, Yoksan S, Rombo L, Riutta J, Vene S, Lindquist L, Vapalahti O, Kantele A. Cross-protective capacity of Japanese encephalitis (JE) vaccines against circulating heterologous JE virus genotypes. Clin Infect Dis 2013; 56:267-70; PMID:23074319; http://dx.doi.org/10.1093/cid/cis883
- Fan YC, Chen JM, Chiu HC, Chen YY, Lin JW, Shih CC, Chen CM, Chang CC, Chang GJ, Chiou SS. Partially neutralizing potency against emerging genotype I virus among children received formalin-inactivated Japanese encephalitis virus vaccine. PLoS Negl Trop Dis 2012; 6:e1834; PMID:23029592; http://dx.doi.org/10.1371/journal.pntd.0001834
- Austin SK, Dowd KA, Shrestha B, Nelson CA, Edeling MA, Johnson S, Pierson TC, Diamond MS, Fremont DH. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog 2012; 8:e1002930; PMID:23055922; http://dx.doi.org/10.1371/journal.ppat.1002930
- Brien JD, Austin SK, Sukupolvi-Petty S, O’Brien KM, Johnson S, Fremont DH, Diamond MS. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J Virol 2010; 84:10630-43; PMID:20702644; http://dx.doi.org/10.1128/JVI.01190-10
