Abstract
Among currently available vaccine strategies for cancer, nucleotide-based vaccination is an appealing treatment modality. Curevacs’ mRNA containing vaccines (RNActive®) combine the beneficial properties of sufficient antigen-expression, autologous immune-stimulation and a high flexibility with respect to production and application. CV9103 and CV9104 are novel RNActive®-derived anticancer vaccines for the treatment of patients with prostate cancer. After successful phase I/II studies with documentation of good tolerability and favorable immune-activation of CV9103, the vaccine CV9104 is currently undergoing clinical testing in specific clinical settings such as castration resistant prostate cancer and as a neoadjuvant agent in men with high risk prostate cancer prior to surgery. This review discusses the available preclinical and clinical data on the anticancer vaccination treatment with RNActive®-derived anticancer-vaccines CV9103 and CV9104.
Introduction
Prostate cancer is the most frequent malignancy in men in western countries, with a lifetime incidence of about 15–20%.Citation1 While the management of localized, early stage prostate cancer is favorable and the survival rates after surgery or radiotherapy in these stages are high, with up to nearly 100% in five years, metastatic prostate cancer remains an incurable condition.Citation2 Apart from men primarily diagnosed with prostate cancer in a metastatic state, between 27% and 53% of all patients undergoing radical prostatectomy (RP) or radiation therapy develop local or distant recurrences within 10 y of initial treatment and 16–35% of patients receive second-line therapy within five years of initial therapy.Citation3-5
For patients that present with or progress to advanced metastatic stages, standard treatment consists of androgen deprivation and chemotherapy. However, after long-term application nearly all patients are no longer sensitive to these treatments. Progression or relapse happen even under a complete androgen blockage, where plasma concentrations of testosterone are suppressed to below 50 ng/dL by castration or gonadotropin-releasing hormone (GnRH) analogs, while the effects of the remaining androgens are blocked by androgen receptor (AR) antagonists. This situation is termed castration resistant prostate cancer (CRPC). Fortunately within the last years a multitude of novel substances for the treatment of CRPC have been studied, approved and introduced into daily clinical practice.Citation5 While the CYP-17 inhibitor abiraterone and the highly AR-affine novel anti-androgen enzalutamid represent new effective hormonal therapeutic approaches. Cabazitaxel is a classic cytotoxic (taxane) agent, and it is the first drug of this substance category to improve overall survival after prior doxetaxel treatment in CRPC.Citation6 For many years prostate cancer has been regarded as a non-immunogenic disease in contrast to other tumor entities, like renal cell carcinoma (RCC) or lymphoma. Nevertheless, with the slow progression of prostate cancer, and the availability of PSA as a surrogate parameter for therapeutic response, immunotherapy has been discussed as an appealing treatment option. Several phase II and III immunotherapy trials for prostate cancer have provided evidence for a benefit in terms of disease response, time to progression and overall survival in patients with advanced disease.Citation7,8
Anticancer-vaccine strategies in prostate cancer
With the advent of Sipuleucel-T, an active cellular immunotherapy consisting of autologous peripheral-blood mononuclear cells (PBMCs), including antigen-presenting cells (APCs), that have been activated ex vivo with a recombinant fusion protein (PA2024) consisting of a prostate antigen (prostatic acid phosphatase, PAP) that is fused to granulocyte–macrophage colony-stimulating factor (GM-CSF), prostate cancer immunotherapy has come into a sharper focus. In the clinical phase III trial of Sipuleucel-T vs. placebo, a prolonged survival among men with asymptomatic or minimally symptomatic metastatic CRPC was observed, whereas no significant effect on the time to objective disease progression was reported. The general toxicity of Sipuleucel-T in this trial was low.Citation9
Among the reasons why prostate cancer has been amenable to immune-based therapies is that several tumor-associated antigens (TAAs) are overexpressed on prostate cancer cells, including but not limited to, prostate-specific antigen (PSA), prostatic acid phosphatase (PAP), and prostate-specific membrane antigen (PSMA).Citation10-12 These TAAs can be specifically targeted by activated immune cells, such as cytotoxic T lymphocytes (CTLs) as part of an immune-mediated anti-tumor response.Citation13 Especially for patients after RP, such antigens offer ideal targets for immunotherapy as they are only present in tumor but not in healthy tissue.Citation14 As the production of the active Sipuleucel-T vaccine is an extensive procedure with leucaphersis of the patients PMBCs and ex vivo activation of the APCs, alternative modes of activation of the adaptive immune system against prostate cancer cells are of interest. With regard to this, an alternative approach to therapeutic cancer vaccines has been realized by PSA-TRICOM, a vector-based vaccine that consists of two poxviruses administered sequentially.Citation15,16 With the poxviruses used as a vector an in vivo immune response can be obtained to trigger an anti-tumor response. The large genome of the DNA viruses provides an optimal vector system for vaccine development in terms of safety and efficacy as it can integrate thousands of base pairs of foreign DNA without compromising infectivity or other essential functions. Replication is limited to the cytoplasm and there is no risk of mutation as the genome does not integrate into host DNA.Citation17 In PSA-TRICOM, transgenes for PSA and three T-cell co-stimulatory molecules (B7.1, ICAM-1, and LFA-3) have been inserted into the genome. After successful phase I/II trials for PSA-TRICOM, with demonstration of a good tolerability and -in analogy to the Sipuleucel-T trial- no change in median time to progression (TTP), but a significant improvement in overall survival was reported. Median survival time in the treatment arm was 25.1 mo vs. 16.6 mo for patients in the placebo arm (HR: 0.56, 95% CI, 0.37 to 0.85).Citation18 A randomized, placebo-controlled phase III trial of PSA-TRICOM is currently enrolling patients with asymptomatic or minimally symptomatic mCRPC.Citation19 The results of this trial are expected in 2016.Citation18,Citation20-23 gives an overview of prostate cancer vaccine strategies.
Table 1. Antigens encoded by CV9103
Nucleotide-based vaccination strategies for prostate cancer
An alternative route of in vivo cancer vaccination is via the application of synthetic nucleotide-based DNA or RNA vaccines. Immunization with plasmid DNA encoding tumor-associated antigens has shown to induce humoral and cellular immune responses.Citation24 A phase phase I/IIa trial with a DNA vaccine encoding human PAP in patients with formerly classified D0 prostate cancer, which corresponds to biochemical recurrence after definitive surgery and/or radiation therapy and no evidence of suspected lymph node, bone, or visceral metastatic disease on bone scans or CT scans, had the goal to elicit a sustainable immune response, able to eradicate a tumor or at least, restrain its growth. In this trial, 22 patients received pTVG-HP, a plasmid DNA encoding the full-length human PAP cDNA, in a dose-escalation manner with 100, 500, or 1,500μg of plasmid DNA, co-administered intradermally with 200μg GM-CSF, six times at 14-d intervals. Three of 22 (14%) patients developed PAP-specific IFN-γ-secreting CD8+ T-cells immediately after the treatment course. Nine of 22 (41%) patients developed PAP-specific CD4+ and/or CD8+ T-cell proliferation. The PSA doubling time was observed to increase from a median of 6.5 mo pretreatment to 8.5 mo on-treatment (P = 0.033), and 9.3 mo in the 1-y post-treatment period (P = 0.054).Citation25 However, obstacles regarding the application of DNA-based anti-cancer vaccines consist in the risk of integration into the host genome and subsequent malignant transformation, especially when compared with the properties of mRNA. It has been reported that mRNA molecules can activate the innate immune system inducing the maturation of professional antigen presenting cells and the release of cytokines.Citation26,27 Combining antigen presentation and immune stimulation, mRNA is considered as an attractive vector for vaccination.Citation28 Apart from the fact that mRNA is known as notoriously unstable being confronted with the virtually omnipresent ribonucleases in vivo, first experiences with mRNA as a therapeutic were reported in 1989 after the development of a broadly applicable in vitro transfection technique using a synthetic cationic lipid, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA), incorporated into a liposome (lipofectin).Citation29
Subsequently, mRNA was advocated as a vaccine platform, bringing together the immunological features of live attenuated vaccines such as endogenous antigen expression and T cell induction with those of killed or subunit vaccines like defined composition and safety.
As the minimal genetic construct, mRNA harbors only the elements directly required for expression of the encoded protein and offers strong safety advantages. mRNA does not interact with the genome, as recombination between single-stranded RNA molecules may only occur in rare cases.Citation26,30,31 A potentially detrimental genomic integration can therefore be excluded. This lack of genomic integration in combination with mRNA being non-replicative as well as metabolically decaying within a few days makes mRNA a merely transient carrier of information.Citation32
mRNA as the technological basis of therapeutics and vaccines offers a high flexibility regarding its production and application. Any protein can be encoded and expressed by mRNA, in principle enabling the development of prophylactic and therapeutic vaccines for infections and cancer, as well as protein replacement therapies. Since changes of the encoded protein alters the sequence of the RNA molecule, leaving its physicochemical characteristics largely unaffected, diverse products can be manufactured based on the same established production process without adjustment, a relevant property with regard to saving time and reducing costs. In terms of efficacy, mRNA-based therapeutics profit from the fact that they do not need to cross the nuclear envelope as opposed to DNA. In contrast to peptide vaccines, mRNA vaccines lack MHC haplotype restriction.Citation33 In addition, mRNA binds to pattern recognition receptors and mRNA vaccines may be designed to be self-adjuvanting, a property which peptide- and protein-based vaccines are missing.Citation34
One of the major reasons for limitations in cancer vaccination strategies is the influence of the tumor microenvironment. Interstitial pressure within large tumor masses, a lack of T-cell co-stimulatory molecules, and the exhaustion of T-cells chronically exposed to viral antigens have been reported to negatively influence the tumor microenvironment with regard to therapeutic cancer vaccination.Citation35 The fact that the inhibitory co-receptor programmed death 1 (PD-1) has been shown to be present on such exhausted T cells underlines the possible positive influence of PD1-inhibitors as an immune-adjuvant. Furthermore, the tumor microenvironment has been shown to contain immunosuppressive immune cell types including CD4+ regulatory T cells (Tregs), myeloid derived suppressor cells (MDSCs), suppressor CD8+ T cells, tumor-associated macrophages (TAMs), and regulatory natural killer (NK)/NKT cells as well as numerous soluble immunosuppressive factors, including TGF-β, IL-10, indoleamine-pyrrole 2,3 dioxygenase (IDO), and VEGF.Citation35,36 As with any phenotypic analysis of cell types within a tumor mass, many tumors display antigenic heterogeneity, which is reflected both at the level of antigen expression and at the level of the antigen–peptide MHC complex. Therefore, the goal of combining multiple tumor-associated antigens within one vaccine compound is to obtain more broad and efficient immunogenic response compared with single-antigen vaccination.
Another limitation in vaccination strategy might be the selection of peptides with an immunodominant vs. a cryptic character. For the activation of naive CD4+ T cells the kinetic stability of the peptide to the MHC class II complex seems to be important.Citation37 In addition CD4+ T cell activation appears to require multiple contact with antigen presenting cells, in contrast to CD8+ T cells, which need only a single encounter with APC to initiate expansion and differentiation.Citation38,39 In mRNA based vaccination the peptides are derived from the transcribed protein the mRNA molecule codes for. Thereby, the respective peptide sequences are unknown in this vaccination strategy, which on the other hand allows an HLA independent patient selection. Furthermore if multiple proteins are transcribed, the result will be the endogenously processing and subsequent display of multiple antigens on the respective MHC class I and II molecules. In this case of multiple peptides the immunodominant and not the cryptic peptides will lead to T cells activation.Citation37 In addition, there are indications that the immunodominance of a peptide will allow the peptide to successfully recruit CD4+ T cells independently of the context into which it is incorporated and its neighboring peptides.Citation37
RNActive® derived mRNA
As natural mRNA molecules are unstable, they exhibit insufficient efficacy for vaccination. This is further explained by the suboptimal translation into protein, and the low immunostimulating capacity of unformulated RNA. To address these issues the company CureVac (Tübingen, Germany) has developed a proprietary technology platform termed RNActive®. RNActive®-derived molecules are optimized and particularly formulated mRNA molecules for the direct application in human resulting in optimized efficacy in terms of translational activity and immune stimulation. At the level of the RNA molecule, RNActive® derived mRNA combines particular flanking sequences at the 5′ (Cap structure, Kozak sequence) and 3′end (Homo sapiens α-globin 3′ untranslated region, A70, C30) with a GC enrichment of the original coding sequence according to the degenerated genetic code. In order to increase the level and duration of protein expression, the coding and non-coding parts of the molecule are modified by in silico and experimental methods. Only the naturally occurring nucleotides are used in this process. The generated mRNAs further undergo a purification process to additionally increase total protein expression. The resulting increment of protein expression was measured with an extent of 4 to 5 orders of magnitude and the kinetics of expression were changed, resulting in a peak after 24h and significantly prolonged activity.Citation40 Despite these improvements of antigen expression, “naked” RNA was observed to be insufficient for immunostimulation.Citation41 To address this drawback, RNActive® derived mRNA is combined with protamine, which helps to arise Th1 T cell responses against antigens with the possible involvement of TLR7/TLR8.Citation27,42 As the protamine/mRNA complexes are very tight, the adjuvant effect comes at the cost of a weak antigen expression and depends strongly on the ratio between protamine and mRNA.Citation33,34 The components do not interact with each other and are both taken up by endocytic, yet distinct pathways into the cell as no colocalizaton was found.Citation33,34 However, the immunostimulatory effect of RNActive®-vaccine, is lost in TLR7−/− mice, indicating a pivotal role of TLR7 in the mechanism of this vaccine.Citation40
Only if the mRNA vaccine has reached the cytosol an immune response can be established. Therefore, the mRNA molecule has to cross the plasma membrane, which is mediated by either diffusion controlled mechanisms or diverse endocytic pathways, dependent of the respective cell type.Citation33 Some species show a vesicular localization in endocytic or lysosomal compartments and dendritic cells uptake the mRNA predominately by macropinocytosis upon intranodal injection. The protamine-comlexed mRNA vaccines reveal different routes and kinetics of uptake for the two components.Citation33 Additionally hydrodynamic pressure may contribute to target cell transfection in case of local injections.Citation33
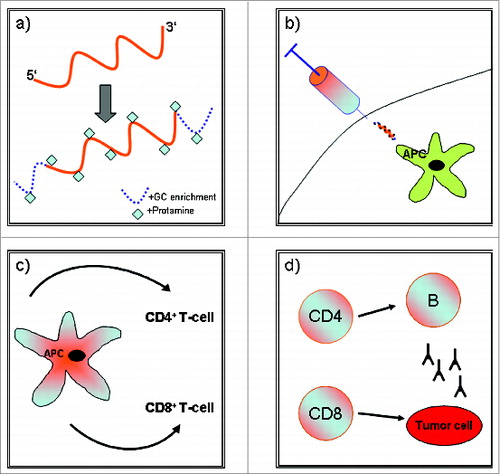
illustrates the mode of action of RNActive®-vaccine-derived CV9104.
Figure 1. (a) mRNA is complexed with protamine to achieve enhanced immune adjuvancy and flanked with GC-enrichment at the 5′ and 3′ end to increase antigen expression, (b) Intradermal application of the vaccine (APC = antigen presenting cell), (c) Antigen processing and concomitant activation of CD4+ and CD8+ T-cells, (d) Antitumor effects via cell-mediated and humural immune mechanisms.
Preclinical data
Compounds encoding ovalbumin or prostate specific membrane antigen (PSMA) were produced to perform an initial assessment of the immune response induced by RNActive®-vaccine based vaccination approaches. The ovalbumin vaccine induced a strong humoral immune response resulting in high IgG1 and IgG2a antibody titers suggesting both a Th2 and Th1 response, while only the activation of IFN-γ-secreting, functional cytolytic CD8+ T cells as well as IFN-γ-secreting CD4+ T cells was observed for both vaccines. Repeated vaccination substantially increased the frequency of IFN-γ-secreting CD8+ T cells without increasing the frequency of CD4+ regulatory T cells. Importantly, apart from the strong effector immune response of RNActive®-vaccines also a strong memory T-cell response by repeated vaccination with a PSMA encoding RNActive®-vaccine was observed.Citation40
To study the T-cell dependent anti-tumor activity of RNActive® vaccines a mouse model (E.G7-Ova model) was employed. Mice were either vaccinated prophylactically with an ovalbumin encoding RNActive® vaccine and then challenged with ovalbumin expressing E.G7 tumor cells, or inoculated with E.G7-Ova tumor cells first and then immunized with the ovalbumin RNActive® vaccine once the tumor had grown to around 80mm3. In the prophylactic model, a protective effect by delaying the tumor growth for about three weeks was reported. In the therapeutic model, even at the advanced tumor size treated, the vaccine suppressed tumor growth for more than a week. When assessed for possible reasons of tumor escape, escaping tumors had largely downregulated or even eliminated ovalbumin expression.Citation40
Further analysis of the mechanism of action of RNActive® vaccines revealed that CD4+ T cells were essential during the induction phase of the immune response, whereas the anti-tumor immune response depended on CD8+ T cells. Interestingly, an analysis of changes within the tumor showed a higher frequency and persistence of CD8+ T cells after as few as two vaccinations.Citation41 Close to 70 genes were upregulated in the tumors of vaccinated mice, among these NK-cell related genes, markers of activated, cytolytic T cells as well as those encoding chemokines, IFN-γ and IFN-γ-related genes.Citation41
Furthermore, synergistic effects by the co-administration of anti-CTLA-4 antibodies were reported, which is of special interest regarding possible combinatory therapy regimens.Citation41
RNActive® derived CV-9103 and CV-9104
CV9103 as the precursor vaccine of CV-9104 is based on four prostate-specific antigens: PSA, PSMA, PSCA, and STEAP (). Each of these antigens has been described as a potential target for the immunotherapy of prostate cancer, supported by the recruitment of antigen-specific cytotoxic T cells or antigen-specific antibodies.Citation43-48
Table 2. Overview of selected prostate cancer vaccines
In the first-in-man study, CV9103 was administered intra-dermally in 5 doses in weeks 1, 3, 7, 15 and 23. In Phase I, 3 dose escalation levels (256 μg, 640 μg and 1280 μg total mRNA) were investigated in cohorts of 3–6 patients. Short-interval vaccinations in weeks 1, 3 and 7 were intended to prime the adaptive antigen-specific immunity, whereas the subsequent long-interval vaccinations in Weeks 15 and 23 are designed to retain or boost antigen-specific immunity. This form of booster vaccination is common in clinical trials of prostate cancer vaccines, with the objective to overcome the potential immune suppressive mechanisms of tumors.Citation8
The intradermal route is effective in priming dendritic cells of the skin and antigen-presenting cells in general.
In the open, uncontrolled, multi-center, international, prospective trial of CV9103, after the enrollment of safety data (local, systemic tolerance, blood and laboratory blood chemistry) and a dose escalation step in the phase I, in which the recommended dose (RD) was established, the phase II part of the study confirmed the safety and explored the immunologic activity of that dose. 44 patients with castrate resistant PCA with rising PSA and predominantly existing metastases (>80%) were enrolled with safety as primary endpoint and immunogenicity as the secondary endpoint.Citation49
One dose limiting toxicity, urinary retention, was observed at the highest dose level, which was expanded to six patients. A maximum tolerated dose was not defined. Of the reported related adverse events, most were injection-site reactions or flu-like symptoms such as chills and fever. Serious adverse events were reported, very few were classified as possibly related with the vaccine, urinary retention being the most frequent (n = 3). After the establishment of the safety profile the high dose level was further expanded by additional 32 patients (phase IIa).Citation49
RNActive® vaccine cocktails showed very high immunogenicity rates in patients with prostate carcinoma. Antigen-specific T cells were detected in around 80% of prostate carcinoma patients independent of their HLA-background. A majority of immune responders, around 58%, reacted against multiple antigens and the responses were detected against all antigens independent of their cellular localization. The observed clinical courses were favorable, with individual patients showing prolonged stabilization of PSA-levels after initial rises. One patient had a greater than 85% drop in his PSA-level.Citation49
Clinical trials of CV 9104
As described above, the clinical phase I/II study with CV9103 showed a favorable safety profile and a potent induction of antigen-specific immune responses against all four antigen components. For the subsequent clinical evaluation of RNActive® anticancer vaccines additional mRNA molecules were included into the compound, namely encoding PAP and Mucin 1 (MUC1), a glycoprotein that is overexpressed and aberrantly glycosylated in various cancer types including PCA. These antigens were shown to be clinically effective in the clinical trial of Sipuleucel-T (PAP)Citation9 and in a number of trials mainly in patients with non-small cell lung cancer (MUC1). In a pilot phase II study in hormone-naive patients with PSA failure after radical prostatectomy vaccination with L-BLP25, a liposomal peptide vaccine targeting MUC1 resulted in a prolonged PSA doubling time.Citation50 The new composition of the vaccine was named CV9104. CV9104 is currently undergoing clinical testing in two randomized controlled clinical trials. The first study is enrolling patients with metastatic prostate cancer.Citation51 Patients with histologically confirmed castrate refractory metastatic adenocarcinoma of the prostate with progressive disease after surgical castration or during androgen suppression therapy including a GNRH agonist or antagonist and after at least one additional anti-hormonal manipulation with a serum testosterone level of <50 ng/dL or <1.7 nmol/L are included. Patients receive CV9104 at a starting dose of 1920 μg in weeks 1, 2 and 3. The vaccinations continue in weeks 5, 7, 9, 12, 15, 18 and 24, then every 6 wk for up to 12 mo after the first vaccination and then every 3 mo thereafter until treatment discontinuation. The primary endpoint of this trial is overall survival. Secondary endpoints consist in progression free survival from date of randomization (PFS1) and from start of the first subsequent systemic therapy (PFS2), percent change to maximal and to minimal PSA from baseline and before start of first subsequent systemic cancer therapy as well as analyses of cellular and humoral immune response rates against the six antigens encoded by CV9104.
Furthermore, quality of life analysis by absolute change from baseline FACT P score and EQ-5D score and pain sub-scores is subject to secondary analysis. Patient recruitment for the trial has been completed.
The second clinical scenario, where CV9104 is studied in a phase II trial, is in men with high-risk and intermediate-risk non-metastatic prostate cancer.Citation52 For these patients, radical prostatectomy is a standard treatment option. Nevertheless, the estimated risk for positive lymph nodes is high, with up to 40% and in case of positive surgical margins adjuvant radiotherapy is required. Therefore new adjuvant or neoadjuvant treatments that can prevent relapses after primary treatment for localized prostate cancer are highly needed. Since relapses arise from micrometastases remaining in the organism after intended curative treatment such an adjuvant or neoadjuvant therapy should ideally eradicate micrometastases and should be well tolerated without inducing severe or long irreversible side effects. With regard to this, tumor-vaccination seems to be a promising approach to prevent relapse of prostate cancer after primary local treatment inducing long lasting prevention of tumor recurrence via induction of immune memory.
Patients with histologically confirmed prostate cancer with intermediate risk disease or no more than one criterion for high risk disease based on the classification of the European Association of Urology (EAU)Citation5 who are scheduled to undergo radical prostatectomy are enrolled. Eligible patients must not have any clinical indication to perform urgent surgery that would not allow administration of the vaccine during the 6 wk period prior to radical prostatectomy. Apart from the objective to determine the value of the vaccine in earlier stages of prostate cancer in order to prevent relapse the trial is investigating a new needle free injection device in order to increase patient comfort and to reduce the risk of needle stick injuries of trial personnel and to perform further evaluation of the mechanism of action of CV9104. Another study objective is to determine whether vaccination with CV9104 can induce detectable changes in the immune infiltrate of prostate cancer patients and change the immunological environment to a prognostically more favorable one. Changes in the tumor tissue will further be correlated with changes of biomarkers in the peripheral blood. Answering these questions will help to understand the mechanism of action of RNactive®-based CV9104, but also identify potential factors in the tumor environment that may inhibit the beneficial immune effects of CV9104 against the tumor cells. These factors may include the infiltration with regulatory T cells, myeloid-derived suppressor cells, tumor-associated macrophages or expression of inhibitory checkpoint molecules like CTLA-4 and PD-1 and their ligands. Understanding the immunological environment of prostate cancer tissue after vaccination with CV9104 compared with the environment of non-vaccinated patients may help to develop combination therapies in the future, e.g., the combination with anti-CTLA-4, anti-PD-1 or PD-L1 antibodies, that may result in better anti-tumor activity.
Conclusions
Nucleotide based prostate cancer immunotherapy with RNActive® based compounds such as CV9104 offers a high specificity as only antigen positive tissues are subject to the therapeutic effect. Transient expression of RNActive®-based mRNA gives precise control of the pharmacokinetics and dose levels with high safety as RNActive®-based mRNA does not exhibit genomic integration. The self-adjuvanted prostate cancer vaccine induces a balanced immune response comprising of a dual activation of the immune system encoding antigens and simultaneously stimulate the innate immune system, effector and memory responses.
The first clinical studies of CV9103 indicate a favorable safety profile of RNActive® vaccines and prove their effectiveness. Ongoing clinical trials are set to demonstrate the utility of application in more defined clinical settings. Noteworthy, RNActive®-based vaccines can be produced in a highly flexible and versatile process.
Disclosure of Potential Conflicts of Interest
JB and SR are subinvestigator and AS is principal investigator of CV9103 and CV9104 clinical trials.
Funding
JB is supported by a grant by the Deutsche Forschungsgemeinschaft (DFG, SFB 685 C5)
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin 2006; 56:106-30; PMID:16514137; http://dx.doi.org/10.3322/canjclin.56.2.106
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012; 62:220-41; PMID:22700443; http://dx.doi.org/10.3322/caac.21149
- Bott SR. Management of recurrent disease after radical prostatectomy. Prostate Cancer Prostatic Dis 2004; 7:211-6; PMID:15278094; http://dx.doi.org/10.1038/sj.pcan.4500732
- Grossfeld GD, Stier DM, Flanders SC, Henning JM, Schonfeld W, Warolin K, Carroll PR. Use of second treatment following definitive local therapy for prostate cancer: data from the caPSURE database. J Urol 1998; 160:1398-404; PMID:9751363; http://dx.doi.org/10.1016/S0022-5347(01)62548-5
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2014; 65:467-79; PMID:24321502; http://dx.doi.org/10.1016/j.eururo.2013.11.002
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, et al.; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376:1147-54; PMID:20888992; http://dx.doi.org/10.1016/S0140-6736(10)61389-X
- Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol 2000; 18:3894-903; PMID:11099318
- Waeckerle-Men Y, Uetz-von Allmen E, Fopp M, von Moos R, Böhme C, Schmid HP, Ackermann D, Cerny T, Ludewig B, Groettrup M, et al. Dendritic cell-based multi-epitope immunotherapy of hormone-refractory prostate carcinoma. Cancer Immunol Immunother 2006; 55:1524-33; PMID:16612599; http://dx.doi.org/10.1007/s00262-006-0157-3
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al.; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/10.1056/NEJMoa1001294
- Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 1998; 82:2256-61; PMID:9610707; http://dx.doi.org/10.1002/(SICI)1097-0142(19980601)82:11£2256::AID-CNCR2233.0.CO;2-S
- Goldfarb DA, Stein BS, Shamszadeh M, Petersen RO. Age-related changes in tissue levels of prostatic acid phosphatase and prostate specific antigen. J Urol 1986; 136:1266-9; PMID:2430115
- Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol 1979; 17:159-63; PMID:89106
- Chakraborty NG, Stevens RL, Mehrotra S, Laska E, Taxel P, Sporn JR, Schauer P, Albertsen PC. Recognition of PSA-derived peptide antigens by T cells from prostate cancer patients without any prior stimulation. Cancer Immunol Immunother 2003; 52:497-505; PMID:12783216; http://dx.doi.org/10.1007/s00262-003-0377-8
- Madan RA, Gulley JL, Kantoff PW. Demystifying immunotherapy in prostate cancer: understanding current and future treatment strategies. Cancer J 2013; 19:50-8; PMID:23337757; http://dx.doi.org/10.1097/PPO.0b013e31828160a9
- Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med 2010; 363:479-81; PMID:20818868; http://dx.doi.org/10.1056/NEJMe1006300
- Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs 2009; 18:1001-11; PMID:19548854; http://dx.doi.org/10.1517/13543780902997928
- Kwak H, Hörig H, Kaufman HL. Poxviruses as vectors for cancer immunotherapy. Curr Opin Drug Discov Devel 2003; 6:161-8; PMID:12669450
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28:1099-105; PMID:20100959; http://dx.doi.org/10.1200/JCO.2009.25.0597
- Randomized A. Double-blind, Phase 3 Efficacy Trial of PROSTVAC-V/F +/- GM-CSF in Men With Asymptomatic or Minimally Symptomatic Metastatic Castrate-Resistant Prostate Cancer (Prospect). http://wwwclinicaltrialsgov/ct2/show/NCT01322490
- Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res 2000; 6:1632-8; PMID:10815880
- Gulley JL, Heery CR, Madan RA, Walter BA, Merino MJ, Dahut WL, Tsang KY, Schlom J, Pinto PA. Phase I study of intraprostatic vaccine administration in men with locally recurrent or progressive prostate cancer. Cancer Immunol Immunother 2013; 62:1521-31; PMID:23836412; http://dx.doi.org/10.1007/s00262-013-1448-0
- Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, Whiteside TL, Schlom J, Wilding G, Weiner LM. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2004; 22:2122-32; PMID:15169798; http://dx.doi.org/10.1200/JCO.2004.08.083
- Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, Pedicano JE, Gehan E, Peck RA, Arlen P, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol 2000; 18:3964-73; PMID:11099326
- Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, Bergman B, Egevad L, Hellström M, Kiessling R, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer 2004; 91:688-94; PMID:15280930
- McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol 2009; 27:4047-54; PMID:19636017; http://dx.doi.org/10.1200/JCO.2008.19.9968
- Pascolo S. Messenger RNA-based vaccines. Expert Opin Biol Ther 2004; 4:1285-94; PMID:15268662; http://dx.doi.org/10.1517/14712598.4.8.1285
- Scheel B, Teufel R, Probst J, Carralot JP, Geginat J, Radsak M, Jarrossay D, Wagner H, Jung G, Rammensee HG, et al. Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur J Immunol 2005; 35:1557-66; PMID:15832293; http://dx.doi.org/10.1002/eji.200425656
- Sorrentino S. Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell Mol Life Sci 1998; 54:785-94; PMID:9760987; http://dx.doi.org/10.1007/s000180050207
- Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci U S A 1989; 86:6077-81; PMID:2762315; http://dx.doi.org/10.1073/pnas.86.16.6077
- Chetverin AB. Replicable and recombinogenic RNAs. FEBS Lett 2004; 567:35-41; PMID:15165890; http://dx.doi.org/10.1016/j.febslet.2004.03.066
- Jäschke A, Helm M. RNA sex. Chem Biol 2003; 10:1148-50; PMID:14700622; http://dx.doi.org/10.1016/j.chembiol.2003.12.003
- Probst J, Weide B, Scheel B, Pichler BJ, Hoerr I, Rammensee HG, Pascolo S. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther 2007; 14:1175-80; PMID:17476302; http://dx.doi.org/10.1038/sj.gt.3302964
- Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Developing mRNA-vaccine technologies. RNA Biol 2012; 9:1319-30; PMID:23064118; http://dx.doi.org/10.4161/rna.22269
- Fotin-Mleczek M, Duchardt KM, Lorenz C, Pfeiffer R, Ojkić-Zrna S, Probst J, Kallen KJ. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J Immunother 2011; 34:1-15; PMID:21150709; http://dx.doi.org/10.1097/CJI.0b013e3181f7dbe8
- Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst 2012; 104:599-613; PMID:22395641; http://dx.doi.org/10.1093/jnci/djs033
- Mocellin S, Wang E, Marincola FM. Cytokines and immune response in the tumor microenvironment. J Immunother 2001; 24:392-407; http://dx.doi.org/10.1097/00002371-200109000-00002
- Sant AJ, Chaves FA, Leddon SA, Tung J. The Control of the Specificity of CD4 T Cell Responses: Thresholds, Breakpoints, and Ceilings. Front Immunol 2013; 4:340; PMID:24167504; http://dx.doi.org/10.3389/fimmu.2013.00340
- Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol 2000; 165:6833-9; PMID:11120806; http://dx.doi.org/10.4049/jimmunol.165.12.6833
- Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med 2005; 201:1555-65; PMID:15897273; http://dx.doi.org/10.1084/jem.20042521
- Kallen KJ, Heidenreich R, Schnee M, Petsch B, Schlake T, Thess A, Baumhof P, Scheel B, Koch SD, Fotin-Mleczek M. A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines. Hum Vaccin Immunother 2013; 9:2263-76; PMID:23921513; http://dx.doi.org/10.4161/hv.25181
- Fotin-Mleczek M, Zanzinger K, Heidenreich R, Lorenz C, Thess A, Duchardt KM, Kallen KJ. Highly potent mRNA based cancer vaccines represent an attractive platform for combination therapies supporting an improved therapeutic effect. J Gene Med 2012; 14:428-39; PMID:22262664; http://dx.doi.org/10.1002/jgm.2605
- Carralot JP, Probst J, Hoerr I, Scheel B, Teufel R, Jung G, Rammensee HG, Pascolo S. Polarization of immunity induced by direct injection of naked sequence-stabilized mRNA vaccines. Cell Mol Life Sci 2004; 61:2418-24; PMID:15378210; http://dx.doi.org/10.1007/s00018-004-4255-0
- Alves PM, Faure O, Graff-Dubois S, Cornet S, Bolonakis I, Gross DA, Miconnet I, Chouaib S, Fizazi K, Soria JC, et al. STEAP, a prostate tumor antigen, is a target of human CD8 +T cells. Cancer Immunol Immunother 2006; 55:1515-23; PMID:16622681; http://dx.doi.org/10.1007/s00262-006-0165-3
- Dannull J, Diener PA, Prikler L, Fürstenberger G, Cerny T, Schmid U, Ackermann DK, Groettrup M. Prostate stem cell antigen is a promising candidate for immunotherapy of advanced prostate cancer. Cancer Res 2000; 60:5522-8; PMID:11034097
- Kiessling A, Schmitz M, Stevanovic S, Weigle B, Hölig K, Füssel M, Füssel S, Meye A, Wirth MP, Rieber EP. Prostate stem cell antigen: Identification of immunogenic peptides and assessment of reactive CD8+ T cells in prostate cancer patients. Int J Cancer 2002; 102:390-7; PMID:12402309; http://dx.doi.org/10.1002/ijc.10713
- Machlenkin A, Paz A, Bar Haim E, Goldberger O, Finkel E, Tirosh B, Volovitz I, Vadai E, Lugassy G, Cytron S, et al. Human CTL epitopes prostatic acid phosphatase-3 and six-transmembrane epithelial antigen of prostate-3 as candidates for prostate cancer immunotherapy. Cancer Res 2005; 65:6435-42; PMID:16024648; http://dx.doi.org/10.1158/0008-5472.CAN-05-0133
- Rodeberg DA, Nuss RA, Elsawa SF, Celis E. Recognition of six-transmembrane epithelial antigen of the prostate-expressing tumor cells by peptide antigen-induced cytotoxic T lymphocytes. Clin Cancer Res 2005; 11:4545-52; PMID:15958640; http://dx.doi.org/10.1158/1078-0432.CCR-04-2235
- Todorova K, Ignatova I, Tchakarov S, Altankova I, Zoubak S, Kyurkchiev S, Mincheff M. Humoral immune response in prostate cancer patients after immunization with gene-based vaccines that encode for a protein that is proteasomally degraded. Cancer Immun 2005; 5:1; PMID:15641767
- Kübler H, Maurer T, Stenzl A, Feyerabend S, Steiner U, Schostak M, et al. Final analysis of a phase I/IIa study with CV9103, an intradermally administered prostate cancer immunotherapy based on self-adjuvanted mRNA. J Clin Oncol (Meeting Abstracts) 2011; 29.
- North SA, Graham K, Bodnar D, Venner P. A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J Urol 2006; 176:91-5; PMID:16753376; http://dx.doi.org/10.1016/S0022-5347(06)00494-0
- Trial of RNActive®-Derived Prostate Cancer Vaccine in Metastatic Castrate-refractory Prostate Cancer. http://wwwclinicaltrialsgov/ct2/show/record/NCT01817738
- An Open Label Randomised Trial of RNActive® Cancer Vaccine in High Risk and Intermediate Risk Patients With Prostate Cancer. http://wwwclinicaltrialsgov/ct2/show/NCT02140138
