Abstract
Development of a broad-spectrum synthetic vaccine against TB would represent an important advance to the limited vaccine armamentarium against TB. It is believed that the esx family of TB antigens may represent important vaccine candidates. However, only 4 esx antigens have been studied as potential vaccine antigens. The challenge remains to develop a vaccine that simultaneously targets all 23 members of the esx family to induce enhanced broad-spectrum cell-mediated immunity. We sought to investigate if broader cellular immune responses could be induced using a multivalent DNA vaccine representing the esx family protein members delivered via electroporation. In this study, 15 designed esx antigens were created to cross target all members of the esx family. They were distributed into groups of 3 self-processing antigens each, resulting in 5 trivalent highly optimized DNA plasmids. Vaccination with all 5 constructs elicited robust antigen-specific IFN-γ responses to all encoded esx antigens and induced multifunctional CD4 Th1 and CD8 T cell responses. Importantly, we show that when all constructs are combined into a cocktail, the RSQ-15 vaccine, elicited substantial broad Ag-specific T cell responses to all esx antigens as compared with vaccination with BCG. Moreover, these vaccine-induced responses were highly cross-reactive with BCG encoded esx family members and were highly immune effective in a BCG DNA prime-boost format. Furthermore, we demonstrate the vaccine potential and immunopotent profile of several novel esx antigens never previously studied. These data highlight the likely importance of these novel immunogens for study as preventative or therapeutic synthetic TB vaccines in combination or as stand alone antigens.
Introduction
Tuberculosis (TB) is a leading cause of death by an infectious agent resulting in 1.6 million deaths per year.Citation1 The increased co-morbidity of TB and HIV, along with the emergence of multidrug resistant strains, facilitates the TB epidemic, a pressing public health threat.Citation1,Citation2 The only available TB vaccine is the live-attenuated BCG vaccine, which was developed over 90 y ago. While the BCG vaccine has been demonstrated to be effective at reducing the risk of acute disseminated forms of TB, including TB meningitis, developed in exposed infants, its ability to prevent against adult and adolescent pulmonary TB, which accounts for most TB disease worldwide, remains unproven.Citation3,Citation4 Moreover, the vaccine is not recommended for immunocompromised individuals or children with HIV infection.Citation5 Clearly, there is an urgent need for a new, safe, and effective vaccine approaches that improve on the potential safety and immunity generated by BCG.
Currently several recombinant viral vectored vaccines and protein-based subunit vaccines co-administered with adjuvants are in clinical trials.Citation6 Most of these recombinant approaches target a limited number of Mycobacterium tuberculosis (Mtb) antigens, with Ag85A and Ag85B being the most targeted candidates. The recent disappointing results of the MVA85A trail which did not statistically improve the protective efficacy against TB in infants,Citation7 raises questions regarding the ultimate benefit of targeting a single antigen and if this approach will actually confer protection or impact disease in a heterogeneous human population. Vaccination with multiple immunodominant antigens from Mtb may be important to drive broad repertoire coverage to improve the protective effect of any new vaccination.Citation8,Citation9
The most prominent antigens described for induction of cell-mediated immunity have been members of the esx family.Citation10-Citation13 The esx family consists of 23 members that are approximately 100 amino acids in length, with 13 members that can be further divided into 3 distinct subfamilies based on high sequence homology between the members.Citation14 These proteins are attractive targets because they are potent T cell antigens that encode several immunodominant molecules, which are strongly recognized by the immune systems in both animals and humans.Citation12,Citation13,Citation15 These are small, secreted antigenic proteins that have been demonstrated to likely be required for the growth and pathogenicity of Mtb.Citation12 A few of the esx members are deleted in BCG, which has been associated with its attenuation and therefore, its low efficacy,Citation12 which also suggests they may represent important immune targets to alter pathogenicity. In addition, they have been demonstrated to serve as targets for not just CD4 but also CD8 T cells,Citation12 suggesting their potential to drive both a CD4 and CD8 T cell epitopic repertoire. Although immunizations with a single esx antigen preparation have been inadequate for controlling TB,Citation14,Citation16-Citation18 many studies have described that levels of protection and pathogen-specific cellular immunity can be enhanced by combining esx antigens with other TB-associated antigens.Citation8,Citation9,Citation14,Citation17-Citation22 For example, the animal challenge studies of the vaccine candidate Ag85B-ESAT6 have described that 2 Mtb antigens combined can be more effective than either antigen alone in protecting against TB infection.Citation22,Citation23 In addition, other esx candidates (esxH, esxV, esxW) have proved to be immunogenic in combination with other antigens.Citation12,Citation22,Citation24,Citation25 Accumulating evidence in the literature demonstrates the importance of the esx family as vaccine candidates.Citation26 We hypothesize that perhaps all members of the esx family, even those not yet investigated, may be important candidates for a TB vaccine cocktail. Yet, only 4 esx antigens have been studied in any detail, with the rest of the 19 esx members mostly immunologically uncharacterized. Therefore, we developed a strategy to design a polyvalent esx vaccine to study the immunogenicity of the entire family of esx antigens.
The duplication and conservation of nearly identical esx proteins within the Mtb genome suggests that they might be expressed under different physiological conditions.Citation23 If all of the esx members could be targeted, this could theoretically interrupt and provide protection against multiple stages in the bacterial life cycle. We took advantage of several important features of the esx family in these constructions to create a collection of antigens in a synthetic DNA format that when delivered by Electroporation (EP) would drive T cell immunity against all 23 esx family members.
In this report, we describe this novel multivalent esx vaccine approach and report that it is highly immunogenic and induces robust broad-spectrum T cell responses with the capability to boost BCG-induced esx-specific T cell responses both in stand alone or in prime boost studies using a BCG prime. Importantly we show that these vaccines drive much more robust T cell immunity than BCG alone. We present the immune profile of many esx family antigens not previously studied. Collectively these studies illustrate a simple strategy for induction of robust T cell immunity against important TB antigens.
Results
Construction and expression of the esx DNA vaccines
Based on diversity a total of 15 esx antigens were designed to be cross-reactive to all members of the esx family and these were distributed into groups of 3 fused antigens, for a total of 5 trivalent plasmids (). We have previously successfully cloned multiple vaccine antigens separated by endoproteolytic (furin) cleavage sites into a single plasmid for proper protein folding and to enhance CTL processing.Citation27 The same strategy was applied to our TB vaccines to reduce the number of plasmids in the vaccine from 15 to 5 plasmids, as displayed in .
Figure 1. Construction and expression of esx constructs. (A) Schematic representation of all 5 trivalent esx constructs encompassing a total of 15 esx antigens. All genes are cloned into the pVAX1 mammalian vector and are under the CMV promoter. The N-terminal IgE leader peptide, C-terminal HA tag, BGH polyA signal, kanamycin resistance gene and pUC origin are shown. (B) Expression of esx constructs in RD cells as analyzed by western blot analysis detected using an anti-HA mAb. Also shown is a loading control by staining for actin and relative sizes are indicated (KDa).
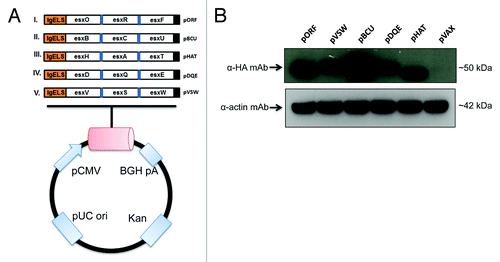
All 5 DNA transgenes were cloned into the pVAX1 mammalian expression vector and were genetically optimized to contain a human IgE leader sequence at the 5′ end in addition to species-specific codon and RNA optimization.Citation28 In addition, an HA tag was added to the 3′ terminus for immunodetection (). Following construction, protein expression of all 5 esx constructs was confirmed by immunoblotting. To determine the expression, human rhabdomyosarcoma (RD) cells were transfected separately with each esx construct or pVAX1 vector (negative control). Samples were harvested 48 h after transfection and assessed by Western immunoblotting (). The presence of a ~50kDa band was detected in the cell lysates of pORF, pHAT, pBCU, pDQE, and pVSW transfected RD cells using an anti-HA tagged monoclonal antibody (). For a comparative control, no protein band could be detected in the negative control pVAX empty vector-transfected cells.
Vaccination with esx DNA constructs generates strong Th1 responses in CB6F1 mice
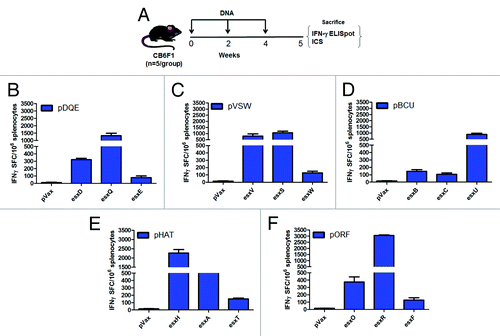
After expression of constructs in vitro, we next aimed to evaluate their immunogenicity in vivo. Since IFN-γ is a necessary component of anti-mycobacterial immunity, a quantitative IFN-γ ELISpot assay was used to determine whether plasmid immunization was capable of generating esx-specific IFN-γ cellular responses. Groups of CB6F1 mice (n = 5 per group) received 3 intramuscular (i.m.) immunizations at 2-wk intervals with 20 µg of each individual esx vaccine construct separately followed by EP (). One week after final immunization, the esx-specific IFN-γ responses were measured following peptide stimulation with overlapping peptide pools that span the length of their respective TB antigen. shows that our esx DNA vaccines generated antigen (Ag)-specific responses to each of the esx proteins encoded in the 5 vaccine constructs given separately. Of the encoded esx antigens, esxR was the most immunogenic with a mean of 3000 IFN-γ producing SFC/million splenocytes. The proportional order of the next immunogenic esx antigens was as followed: esxH (~2270 SFC), esxQ (~1324 SFC), esxS (~1065 SFC), esxU (~885 SFC), esxV (~793 SFC), and esxA (~559 SFC). The other 8 esx antigens stimulated modest, but significant, Th1 responses.
Figure 2. Immunogenicity of esx constructs compared. (A) Immunization schedule for DNA. Mice (n = 5 per group) were vaccinated plus EP with each individual DNA esx construct 3 times at 2-wk intervals and spleens were harvested 1 wk after last immunization to analyze the cellular immune responses (B–F). Esx-specific T cell responses in (B–F) were measured against a pool of peptides to their respective antigens by IFN-γ ELISpot. Error bars indicate SEM and experiments were performed independently at least 2 times with similar results.
Esx DNA vaccines induce potent CD4 and CD8 T cell responses
After confirming that immunization with each esx DNA constructs can elicit Ag-specific IFN-γ immune responses during vaccination, we next characterized the phenotype and cytokine production profile of the vaccine-induced Ag-specific T cells generated (). Given the importance of multifunctional CD4 and CD8 T cell immunity as an integral component of anti-mycobacterial immunity,Citation29,Citation30 we measured the ability of vaccine-induced Ag-specific T cell populations to secrete both IFN-γ and TNF-α in response to ex vivo Ag-specific pooled peptide stimulation 1 wk after the last immunization. Our gating strategy of intracellular cytokine flow-cytometry analysis is depicted in . By flow cytometry we observed a large population of cytokine producing antigen-specific CD4 T cells that could be isolated from the spleens. The CD4 T cells were directed against all encoded esx antigens, with the strongest responses to esxO, esxU, esxS, esxQ, esxH, and esxR (). All vaccinated mice induced esx-specific, total IFN-γ, and total TNF-α positive CD4 T cells in the spleens (). We also found that the majority of the vaccine-induced esx-specific CD4 T cells were IFN-γ/TNF-α double-positive producing cells (). In comparison, BCG vaccinated mice mainly showed no detectable responses of esx-specific CD4 T cells, except for the low frequency of esx-specific IFN-γ T cells of which the major phenotype were to the following esx antigens: esxS, esxR, and esxH (Fig. S1A). In terms of CD8 T cells, we observed potent vaccine-induced Ag-specific CD8 T cells producing total IFN-γ and total TNF-α to esxV, esxU, esxS, esxQ, esxH, and esxR antigens (). We also observed the induction of IFN-γ/TNF-α double positive CD8 T cells specific for esxV, esxU, esxS, esxQ, esxH, and esxR (). Although the ability of BCG to induce CD8 T cell responses remain controversial,Citation31-Citation33 we found no detectable vaccine-induced CD8 T cells secreting IFN-γ in the spleens of BCG vaccinated mice (Fig. S1B). Taken together, we demonstrate that vaccination with multivalent esx antigens broadens and induces potent Ag-specific CD4 and CD8 T cell responses in vivo.
Figure 3. Evaluation of the esx-specific CD4 and CD8 T cells responses following DNA vaccination. CB6F1 mice (n = 5) were immunized by i.m./EP with 3 injections at 2-wk intervals with 20 µg of each individual esx construct. Splenocytes were collected 1 wk after final vaccination, stimulated with their respective peptide pools and analyzed by flow cytometry following intracellular staining using antibodies against IFN-γ and TNF-α. (A) The gating strategy used to analyze the frequency of CD4 and CD8 T cells positive for IFN-γ and TNF-α cytokines. (B) Column graphs depicting esx-specific CD4 T cells releasing the cytokines IFN-γ (C) TNF-α and (D) double-positive producing cells (and pVAX control). (E) Column graphs show the esx-specific CD8 T cells releasing the cytokines IFN-γ (F) TNF-α and (G) double-positive producing cells (and pVAX control). Background staining from cells stimulated with medium alone has been subtracted. Error bars represent SEM of 5 mice per group. Experiments were performed independently at least 2 times with similar results.
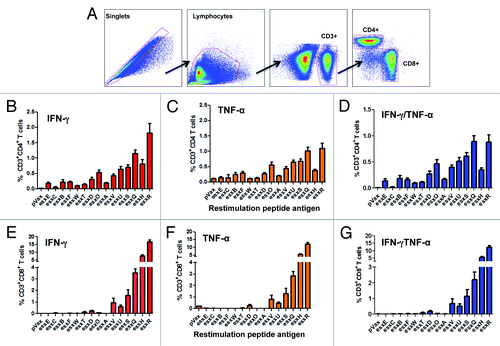
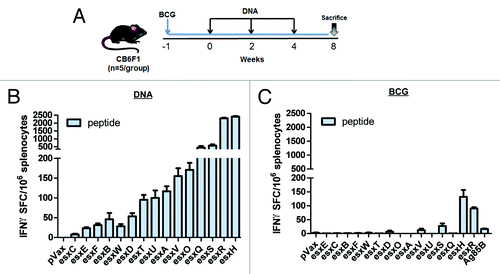
Figure 4. Immunization with RSQ-15 induces broader and stronger esx-specific Th1 immune responses compared with BCG. (A) Immunization schedule for DNA (black line) and BCG vaccination (blue line). CB6F1 mice (n = 5) were immunized 3 times at 2 wk intervals with all esx constructs co-delivered as a cocktail (RSQ-15 vaccine; 20 ug per esx construct) and 1 mo later, T cell responses were analyzed using splenocytes. (B) The frequency of esx-specific IFN-γ producing cells determined by IFN-γ ELISpot assay. (C) BCG mice were immunized by a single s.c. BCG injection (106 CFU) and splenocytes from BCG-primed mice were stimulated with all individual esx-specific peptide pools and IFN-γ production measured by ELISpot assay. Error bars indicate SEM and experiments were performed independently at least 2 times with similar results.
Co-delivered multivalent esx TB constructs (RSQ-15) generate broad esx-specific T cell responses
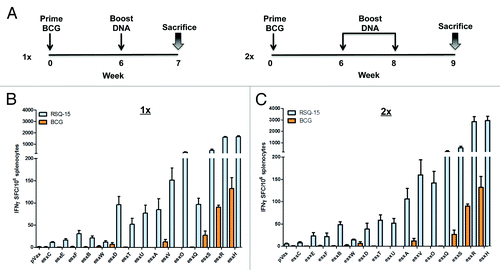
After having confirmed that the antigen cassettes induce significant breadth of Mtb-specific immune responses individually, we next examined the ability of the cassettes to drive immunity when delivered as a cocktail. This is an important issue which has been raised in several other vaccine systems. Mice (n = 5 per group) were vaccinated 3 times at 2-wk intervals with all 5 DNA vaccine cassettes pORF, pHAT, pBCU, pDQE, and pVSW (RSQ-15 vaccine), containing 20 µg of each esx vaccine. The quality of the T cell responses were assessed 1 mo post final vaccination. As shown in , the RSQ-15 vaccine elicited substantial antigen-specific IFN-γ SFC to all esx antigens and maintained a memory response. The most immunogenic esx antigens were esxH, esxR, esxS, and esxQ, respectively, while the other 11 esx antigens elicited a diversity of IFN-γ responses. For comparative purposes, a group of 5 CB6F1 mice were vaccinated subcutaneously (s.c.) with 106 CFU of M. bovis BCG Danish (BCG SSI),Citation9 6 wk prior to sacrificing the cohort on the same day as the DNA vaccinated mice (). The following esx members are known to be absent from BCG: esxO, esxV, esxP, esxW, esxA, and esxB.Citation34 As expected, in BCG-vaccinated mice, esxA, esxB, esxO, and esxW specific responses were not detected, however, there was a small response to esxV. This could have potentially been due to cross-reactivity, based on esxV high homology with the other Mtb9.9 subfamily members found in BCG.Citation13 In contrast to the 5 esx DNA vaccines, mice vaccinated with BCG only induced significant Th1 responses to 3 esx antigens: esxS (~27 SFC), esxH (~132 SFC), and esxR (~90 SFC) (). However, the esxS, esxH, and esxR responses elicited by the DNA esx constructs were approximately 21-, 20-, and 26–fold significantly higher than the BCG-induced responses. Antigen 85B (Ag85B), a highly immunogenic antigen known to be present in Mtb and BCG, was used as a control. Overall, the immunological responses to all the esx antigens was still detectable 1 mo after final vaccination and was still significantly higher than that of the mice vaccinated with BCG (). These data show that RSQ-15 vaccine generated broad and robust esx-specific T cell responses, which were higher than those elicited in BCG immunized mice.
Multivalent RSQ-15 vaccine boosts BCG induced esx immune responses
After determining that our RSQ-15 vaccine induces broad-spectrum immunity, we examined whether the RSQ-15 vaccine could specifically boost the weak esx Ag-specific T cell responses induced by BCG SSI. CB6F1 mice (n = 5 per group) were boosted with RSQ-15 up to 2 times with 2 wk intervals, 6 wk after s.c. vaccination with 106 CFU of BCG SSI (). The boosting of BCG with DNA vaccines significantly increased the esx-specific IFN-γ producing T cell responses in the spleen of BCG vaccinated mice. As shown in , the only BCG-induced esx responses following BCG vaccination were against esxS, esxR, and esxH. These T cell responses were substantially boosted by i.m. delivery of RSQ-15. The first (1×) boost alone yielded roughly comparable results to those generated by the second (2×) boost immunization. The only noticeable difference was a 4-fold increase seen in esxQ responses. Altogether, these data show that multivalent RSQ-15 vaccine can significantly boost and improve the esx immune responses to BCG. In addition, a single DNA boost is sufficient to elicit esx-specific T cell responses.
Figure 5. Prime-boost BCG vaccination with RSQ-15 DNA vaccine increases the esx-specific BCG-induced responses. (A) Immunization schedule for the 2 different prime-boost regimens. CB6F1 mice were immunized s.c. with 106 CFU of BCG SSI. Six weeks later, mice were boosted i.m once (1x) or boost immunized twice (2x) at 2 wk intervals with 100 µg (20 µg per esx construct) of the RSQ-15 vaccine. Seven days after the 1× (B) and 2× (C) boost, spleens were assayed by IFN-γ ELISpot. pVAX1 and BCG-only controls were included. Results represent SEM of 5 mice per group. Experiments were performed independently at least 2 times with similar results.
Esx DNA constructs induce esx antigen-specific cross-reactivity
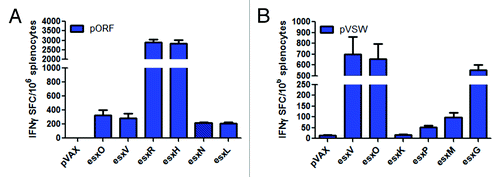
Next we sought to determine whether our approach could elicit cross-reactive T cells that simultaneously target the 8 esx antigens not encoded by our vaccine: esxN, esxL, esxP, esxM, and esxG (STable 1). As shown in supplementary , antigens esxV and esxO are 93–98% identical to esxN, esxI, and esxL; esxW is 98% identical to esxM, esxJ, esxK, and esxP, while esxS is ~96 identical to esxG. Thus, mice (n = 5 per group) were immunized 3 times at 2-wk intervals with 20 µg of pORF and pVSW. The cross-reactive esx-specific T cell responses were then measured in response to the appropriate esx-specific epitope peptides not covered by our vaccines. As shown in , pORF was stimulated with esxN and esxL, while pVSW immunized mice were stimulated with esxK, esxP, esxM, and esxG. As displayed in , the constructs generate strong cross-reactive cellular immune responses. Mice immunized with pORF not only elicited Ag-specific T cell responses to its specific encoded esx antigens, esxO and esxR, but also generated IFN-γ-producing T cell responses to its family orthologs, esxN, esxL, and even esxV and esxH (). The same cross-reactive trend was seen with the mice vaccinated with pVSW. displays that pVSW can induce cross-reactive T cell responses for esxP, esxM, and esxG, except for esxK. These data show that this DNA vaccination approach in combination with in vivo EP can immune cross reactivity to the other esx family members not encoded in the RSQ-15 vaccine, as well as the encoded antigens resulting in immune responses against all esx family members.
Figure 6. Esx DNA constructs elicit esx-specific cross-reactivity among their subfamily ortholog members. Mice were immunized with 20 µg of pORF or pVSW 3 times at 2 wk intervals and spleens were harvested 1 wk later and then stimulated with their respective esx-specific peptide pools to monitor the degree of cross-reactivity between esx antigens determined by IFN-γ ELISpot (A) Recognition of esxV, esxH, esxN, and esxL after pORF vaccination. (B) Recognition of esxO, esxK, esxP, esxM, and esxG after pVSW vaccination. Error bars indicate SEM and data shown are representative of 5 mice per group in 2 independent experiments that generated similar results.
Discussion
We have recently reported that highly optimized DNA vaccination with adaptive electroporation (EP) can elicit a strong Th1 and CD8 T cells, cellular immunity necessary to prevent diseae after exposure with Mtb,Citation28,Citation35-Citation39 thus suggesting it as a promising platform for the development of a TB vaccine. Although a few studies have shown that EP can enhance immune responses to a DNA TB vaccine,Citation40-Citation42 no current studies have tested this improved optimized DNA platform in the TB animal model. In this study, we show that all 5 esx optimized constructs we designed induced potent Ag-specific IFN-γ responses to all 15 encoded antigens by ELISpot. In addition, we observed that compared with naïve animals and the BCG vaccine group, all constructs induced relatively high levels of multifunctional CD4 and CD8 T cells expressing dual IFN-γ and TNF-α after immunization. Thus, establishing the validity of our DNA TB vaccines as a promising approach that can target a broad range of T cell epitopes, which increases the breadth of immune responses.
Despite the high sequence conservation in the esx family, there is heterogeneity, which has been described in T cell responses to the subset of esx antigen responses examined in humans.Citation43 Therefore to target a range of T cell epitopes and increase the breadth of immune responses we co-administrated all 5 esx constructs in a cocktail (RSQ-15 vaccine). Immunization with RSQ-15 plasmids induced a broad pattern of T cell reactivity to all 15 encoded esx members 1 mo after the last immunization (). Since BCG is currently the only available vaccine against TB, we aimed to compare our responses to those levels of esx-specific immunity generated by BCG vaccination the current standard. The immunization with the RSQ-15 vaccine plus EP were shown to generate significantly greater levels of esx-specific T cell immunity compared with BCG-induced esx responses, as well as induce a greater diversity of esx responses (). It is important that the RSQ-15 DNA vaccine drove a high reservoir of effector antigen-specific T cell responses as we observed, as such responses are likely important in pathogen control and clearance.Citation44 Moreover, we observed that the most immunodominant T cell responses induced by RSQ-15 were esxQ, esxS, esxR, and esxH. In agreement with previous studies, we find esxH to be highly immunogenic. However, our esxH-specific CD4 and CD8 cytokine secreting T cells was equivalent to or exceeded those of the other vaccine approaches, even without the inclusion of adjuvants or heterologous prime-boosting regimens.Citation24,Citation25,Citation45 Similarly, esxS, esxR, and esxH were the only dominant esx antigens that induced strong Th1 responses in BCG vaccinated mice. Interestingly, these 5 immunogenic esx antigens are categorized as part of the same esx Mtb locus family cluster (Esx3),Citation43 therefore, likely underlying the importance of this unique esx subfamily members as essential immunogenic esx antigens elicited by BCG and perhaps emphasizes their vital role in the efficacy of BCG.
The Esx-3 has been a focus of vaccine efforts because the Esx-3 type VII secretion system is a system that enables Mtb to secrete virulence factors, such as esxH, esxR and esxQ across its cell membrane, and has been shown to play a vital role in iron acquisition in Mtb.Citation46 However, esxH has been the only antigen of the TB10.4 esx subfamily targeted as a vaccine candidate because it is strongly recognized by BCG-vaccinated individuals and by 70% of TB patients.Citation23 Nevertheless, we show immunological evidence that simultaneously targeting all members of this subfamily in future vaccine approaches may provide better efficacy against TB. In addition, we also show for the first time the immunogenicity profile of other esx members (esxO, esxD, esxU, esxC, esxT, esxF, esxE) whose functions still remain unknown and until now had not been investigated in a vaccine setting. These other esx members have also been considered to be highly immunogenic and essential for bacterial survival,Citation12,Citation47 therefore targeting them through the induction of host immunity could impact bacterial survival in the host. Finally, although originally the esx family was considered to be as early secreted antigens, expressed only during the early stage of Mtb infection, new evidence suggests that this family of genes may be secreted and expressed at different stages of the life cycle of TB.Citation48-Citation50 Accordingly these studies suggest that the RSQ-15 vaccine may not only be studied as a prophylactic vaccine, but may also be examined as a post-exposure vaccine against latent TB.
Given the growing interest in heterologous prime-boost vaccination to enhance the immunity induced by initial BCG immunization, we sought to determine whether the RSQ-15 vaccine could augment comparatively weak esx responses induced by BCG (). Boosting BCG with the RSQ-15 vaccine significantly increased the Th1 responses to all esx antigens (). In addition, the 1x DNA boost immunization protocol had comparable effects with the 2× DNA boost immunization suggesting that 1× boost of our novel DNA approach is sufficient to enhance the immunogenicity of BCG. Therefore, this prime-boost approach may represent an effective future strategy for testing a new TB vaccine in adolescents. Notably, a relevant finding of this study is the potential of this multivalent esx vaccine approach to induce potent and highly cross-reactive cellular immune responses between members of the esx family. The ability of esx antigens from similar subfamilies to induce cross-reactivity was not due to the esx subfamilies sharing the same antigenic T cell epitopes, supporting that RSQ-15 can simultaneously drive T cells that target all esx family members.
While no true correlates are known for protection against TB, substantial evidence from both human and murine studies suggest that CD4 Th1 and CD8 IFN-γ/TNF-α double-positive producing cells are necessary to prevent disease after exposure and to maintain TB in a latent state.Citation29,Citation30,Citation44,Citation51-Citation53 These data highlight the importance of a vaccine that can induce optimal activation of both CD8 and CD4 T cells which could be the cornerstone for protective immunity against TB. In these studies we observe that compared with naïve animals and the BCG vaccine group, the RSQ15 vaccine induced relatively high levels of multifunctional CD4 T cells expressing dual IFN-γ and TNF-α after immunization, while esxV, esxU, esxS, esxQ, esxH, and esxR induced potent CD8 T cells secreting dual IFN-γ/TNF-α. This confirms RSQ15’s ability to induce a broad-spectrum of T cell immunity. This data supports previous studies reporting the esx antigens have the potential to induce responses to both CD4 and CD8 T cell epitopic repertoire.Citation12,Citation23,Citation54
In summary, we report development and immune study of a novel multivalent vaccine synthetic DNA approach. The multivalent RSQ-15 vaccine in combination with EP increased the breadth of immune responses, inducing broad potent esx-specific T cell responses that allows for T cell targeting of all members of the esx family of antigens. Moreover, we also illustrate for the first time the immunopotent profiles of many of the esx family gene members not previously reported, and underlying their value for future vaccine candidates. Together these studies demonstrate the advantage of combining family antigens for induction of broad TB immunity. Further investigation of this novel vaccine as a new tool for study in the context of prevention and immune therapy of TB infection could be important. As this study was focused on technology development it did not assess the impact of this novel ESX vaccine on protection from pathogenesis during infectious challenge. It is possible that such a vaccine could by itself impact either bacterial load in the lung or protect the lung from pathogenesis. It is also important to study this novel antigen complex as a cocktail with other important TB vaccines expressing antigens involved in latency or resussitation of Mtb. As a multistage TB vaccine will be ideal to target the pathogen at different pathogenic stages. In addition, we have reported on the use of genetic adjuvants to further enhance induced T cell immune responses in both preclinical as well as clinical studies.Citation55-Citation57 Such combination studies would likely be important. Future studies should also evaluate this vaccine as part of a BCG prime boost strategy in protection studies. Furthermore, prime boost studies against TB in combination with other vaccine platforms may be important to examine again in challenge studies. Studies on how these constructs confer protection against TB, is currently in progress.
Materials and Methods
Esx antigen selection for constructs
The esx family contains 23 low-mass proteins named alphabetically esxA through esxW with at least 15 being divided further into subfamilies due to high sequence-related homology.Citation12 One subfamily is the Mtb9.9 family, which consists of 5 open reading frames (ORF) with protein homology ranging from 92–98% (Table S1). Another subfamily, the QILSS subfamily, consists of 5 neighboring ORFs that share individual identity on the amino acid level of over 98%. The TB10.4 family consists of 3 proteins with homology ranging from 67 to 84%. Because many of these antigens have high homology, it is likely that they can potentially induce strong, highly cross-reactive cellular immune responses.Citation58,Citation59 Therefore, we selectively chose antigens to represent each of the subfamilies. We chose 2 antigens from the Mtb9.9 subfamily, one from the QLISS subfamily, and all 3 antigens from the more diverse TB10.4 subfamily (Table S1). In addition, 2 other esx proteins, esxS and esxG, share 96% homology; therefore, esxS will represent what we have designated the TB9.8 subfamily. The proteins where chosen on the basis that they met one or more of the following criteria:Citation1 known efficacy to reduce the bacterial load of Mtb in rodent modelsCitation2,Citation12,Citation26,Citation60 putatively secreted antigens;12,133 antigens known to be immunogenic in humans or cattle,Citation12 andCitation4 antigens known to be absent in BCG.Citation34 The remaining 8 esx genes have little homology to each other and, in order to target the entire family, were also incorporated (Table S1).
Plasmid construction
To generate the esx constructs, all of the 15 esx designed sequences were collected from GenBank (Mtb H37RV strain). After obtaining the sequences each construct was commercially synthesized, species-specific codon and RNA optimized and subsequently subcloned (Genscript) into the pVAX1 mammalian expression vector (Invitrogen) under the control of the cytomegalovirus immediate-early promoter. The esx plasmids (pORF, pHAT, pBCU, pDQE, and pVSW) contained multiple immunogens, each separated by a synthetic furin cleavage site (RGRKRSS) as previously described.Citation27 An HA tag was incorporated at the 3′end for each construct to facilitate analysis of expression. All constructs were commercially upscaled by Aldevron.
Transfection and immunoblotting
Human Rhabdomyosarcoma (RD) cell lines were cultured, transfected, and harvested as described previously.Citation54 Briefly, RD cells were cultured in 6-well plates and transfected with the constructs (pVAX as control) using LipofectamineTM2000 (Invitrogen) following the manufacturer’s protocol. Forty-eight hours later cells were lysed using modified RIPA cell lysis buffer and cell lysate was collected. Western blot analysis was performed with an anti-HA monoclonal antibody (Cell Signaling) and visualized with horseradish peroxidase (HRP)-conjugated anti-rat IgG (Cell Signaling) using an ECL western blot analysis systems (GE Amersham).
Animals
Female 8-wk-old CB6F1 mice were purchased from Jackson Laboratory. All animal experimentations were conducted and animals were maintained in accordance with the National Institutes of Health and the University of Pennsylvania IACUC guidelines for housing and care of laboratory animals.
Immunization/EP of mice
Mice were immunized in the tibialis anterior muscle 3 times, 2 wks apart by needle injection with 20 µg of each plasmid resuspended in sterile water (individually or mixed) and immediately followed by EP at the same site, using the CELLECTRA adaptive constant current EP device (Inovio Pharmaceuticals) as previously described.Citation28 Negative control mice received 3 equivalent doses of pVAX, and positive control mice were vaccinated subcutaneously with a single dose of 106 CFU BCG Statens Serum Institut (SSI) as previously described.Citation9 The BCG SSI was provided by Aeras. For the prime-boost studies, mice were first vaccinated with 106 CFU BCG, rested for 6 wk and then vaccinated once or twice with 100 μg (20 μg per plasmid) of RSQ-15.
ELISpot assays
For mice vaccinated with DNA, spleens were harvested 7 d following the final immunization as previously described.Citation28 For mice vaccinated only with BCG SSI, spleens were harvested 6 wk after vaccination. Briefly, spleens were collected in RPMI 1640 medium (supplemented with 10% FBS, 1X Antibiotic-Antimycotic, and 1X β-ME) and splenocytes were isolated by mechanical disruption of the spleen using a Stomacher machine (Seward Laboratory Systems). The resulting mashed spleens were filtered using a 40μm cell strainer, treated with ACK lysis buffer for 5 min to lyse the RBCs, washed in PBS and then resuspended in RPMI medium for use in ELISpot or Flow Cytometry assay. After spleens were harvested and processed IFN-γ ELISpot assays were performed to determine antigen-specific cytokine secretion from immunized mice as previously described.Citation61 The measurement of esx-specific T cell responses were assessed by stimulating splenocytes with pooled peptides (15-mers overlapping by 9 amino acids; 2.5 μg/mL final) spanning each individual esx TB antigen. All peptides were synthesized from GenScript. Concavalin A (Sigma-Aldrich) at 5 μg/mL was used as positive control and complete culture medium was used as negative control. Spots were enumerated using an automated ELISPOT reader (Cellular Technology).
Intracellular Cytokine staining for Flow Cytometry
Intracellular cytokine staining was performed as previously described.Citation28 Briefly, splenocytes were added to a 96-well plate (1 × 106/well) and were stimulated with their respective esx pooled peptides for 5–6 h at 37C/5% CO2 in the presence of Protein Transport Inhibitor Cocktail (Brefeldin A and Monensin) (ebioscience) according to the manufacturers instructions. The Cell Stimulation Cocktail (plus protein transport inhibitors) (phorbol 12-myristate 13-acetate (PMA), ionomycin, brefeldin A and monensin) (ebioscience) was used as a positive control and R10 media as a negative control. All cells were then stained for surface and intracellular proteins as described previously.Citation34 The cells were washed in FACS buffer (PBS containing 0.1% sodium azide and 1% FCS) before surface staining with flourochrome-conjugated antibodies. Cells were then washed with FACS buffer, fixed, and permeabilized using the BD Cytofix/Cytoperm TM (BD) according to the manufacturer’s protocol followed by intracellular staining. The following antibodies were used for surface staining: LIVE/DEAD Fixable Violet Dead Cell stain kit (Invitrogen), CD19 (V450; clone 1D3; BD Biosciences) CD4 (V500; clone RM4–5; BD Biosciences), CD8 (APC-Cy7; clone 53–6.7; Abcam). For intracellular staining the following antibodies were used: IFN-γ (APC; clone XMG1.2; Biolegend), TNF-α (PE; clone MP6-XT22; ebioscience), CD3 (PerCP/Cy5.5; clone 145–2C11; Biolegend). All data were collected using a LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star). T cells were gated for activated IFN-γ/TNF-α-producing T cells that were CD3+CD4+ or CD3+CD8+ and negative for CD19 and viability dye ().
Statistical analysis
The Student t test was applied to analyze statistical significance of all the quantitative cellular immune response data produced in this study. Error bars indicate SEM and all tests were performed using Prism Software and P < 0.05 were regarded as statistically significant compared with controls, pVAX, or BCG SSI.
Additional material
Download Zip (134.3 KB)Disclosure of Potential Conflicts of Interest
D.B.W. has grant funding, participates in industry collaborations, has received speaking honoraria, and fees for consulting. This service includes serving on scientific review committees and advisory boards. Remuneration includes direct payments or stock or stock options and in the interest of disclosure therefore he notes potential conflicts associated with this work with Inovio, where he is a member of the SAB, Bristol Myers Squibb, Roche, Ferring, Touchlight, oncosec, Merck, VGXI, and possibly others. Licensing of technology from his laboratory has created over 100 jobs in the private sector in the biotech/pharma industry. The other authors declare no competing financial interests. No writing assistance was utilized in the production of this manuscript.
Acknowledgments
This work was supported in part by AERAS and Inovio Pharmaceuticals.
The authors thank members of the Aeras Team and Megan C. Wise for their critical reading and helpful insights. The authors also thank Penn CFAR and ACC core for their support.
References
- Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med 2013; 368:745 - 55; http://dx.doi.org/10.1056/NEJMra1200894; PMID: 23425167
- Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, Hoffner S, Drobniewski F, Barrera L, van Soolingen D, et al, Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet 2009; 373:1861 - 73; http://dx.doi.org/10.1016/S0140-6736(09)60331-7; PMID: 19375159
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994; 271:698 - 702; http://dx.doi.org/10.1001/jama.1994.03510330076038; PMID: 8309034
- Brewer TF. Preventing tuberculosis with bacillus Calmette-Guérin vaccine: a meta-analysis of the literature. Clin Infect Dis 2000; 31:Suppl 3 S64 - 7; http://dx.doi.org/10.1086/314072; PMID: 11010824
- WHO. Use of BCG vaccine in HIV-infected infants. Wkly Epidemiol Rec 2010; 5:29 - 36
- Pitt JM, Blankley S, McShane H, O’Garra A. Vaccination against tuberculosis: how can we better BCG?. Microb Pathog 2013; 58:2 - 16; http://dx.doi.org/10.1016/j.micpath.2012.12.002; PMID: 23257069
- Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al, MVA85A 020 Trial Study Team. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013; 381:1021 - 8; http://dx.doi.org/10.1016/S0140-6736(13)60177-4; PMID: 23391465
- Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun 2004; 72:6148 - 50; http://dx.doi.org/10.1128/IAI.72.10.6148-6150.2004; PMID: 15385521
- Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 2011; 17:189 - 94; http://dx.doi.org/10.1038/nm.2285; PMID: 21258338
- Skjøt RL, Oettinger T, Rosenkrands I, Ravn P, Brock I, Jacobsen S, Andersen P. Comparative evaluation of low-molecular-mass proteins from Mtb identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect Immun 2000; 68:214 - 20; http://dx.doi.org/10.1128/IAI.68.1.214-220.2000; PMID: 10603390
- Louise R, Skjøt V, Agger EM, Andersen P. Antigen discovery and tuberculosis vaccine development in the post-genomic era. Scand J Infect Dis 2001; 33:643 - 7; http://dx.doi.org/10.1080/00365540110026971; PMID: 11669220
- Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R. ESAT-6 proteins: protective antigens and virulence factors?. Trends Microbiol 2004; 12:500 - 8; http://dx.doi.org/10.1016/j.tim.2004.09.007; PMID: 15488391
- Jones GJ, Gordon SV, Hewinson RG, Vordermeier HM. Screening of predicted secreted antigens from Mycobacterium bovis reveals the immunodominance of the ESAT-6 protein family. Infect Immun 2010; 78:1326 - 32; http://dx.doi.org/10.1128/IAI.01246-09; PMID: 20086089
- Kamath AT, Feng CG, Macdonald M, Briscoe H, Britton WJ. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun 1999; 67:1702 - 7; PMID: 10085007
- Mustafa AS, Skeiky YA, Al-Attiyah R, Alderson MR, Hewinson RG, Vordermeier HM. Immunogenicity of Mycobacterium tuberculosis antigens in Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle. Infect Immun 2006; 74:4566 - 72; http://dx.doi.org/10.1128/IAI.01660-05; PMID: 16861643
- Morris S, Kelley C, Howard A, Li Z, Collins F. The immunogenicity of single and combination DNA vaccines against tuberculosis. Vaccine 2000; 18:2155 - 63; http://dx.doi.org/10.1016/S0264-410X(99)00540-X; PMID: 10715531
- Khera A, Singh R, Shakila H, Rao V, Dhar N, Narayanan PR, Parmasivan CN, Ramanathan VD, Tyagi AK. Elicitation of efficient, protective immune responses by using DNA vaccines against tuberculosis. Vaccine 2005; 23:5655 - 65; http://dx.doi.org/10.1016/j.vaccine.2005.03.056; PMID: 16157425
- Brandt L, Elhay M, Rosenkrands I, Lindblad EB, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun 2000; 68:791 - 5; http://dx.doi.org/10.1128/IAI.68.2.791-795.2000; PMID: 10639447
- Li Z, Howard A, Kelley C, Delogu G, Collins F, Morris S. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect Immun 1999; 67:4780 - 6; PMID: 10456931
- Grover A, Ahmed MF, Singh B, Verma I, Sharma P, Khuller GK. A multivalent combination of experimental antituberculosis DNA vaccines based on Ag85B and regions of difference antigens. Microbes Infect 2006; 8:2390 - 9; http://dx.doi.org/10.1016/j.micinf.2006.04.025; PMID: 16962360
- Derrick SC, Yang AL, Morris SL. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine 2004; 23:780 - 8; http://dx.doi.org/10.1016/j.vaccine.2004.07.036; PMID: 15542202
- Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med 2010; 2:53ra74; http://dx.doi.org/10.1126/scitranslmed.3001094; PMID: 20944089
- Skjøt RL, Brock I, Arend SM, Munk ME, Theisen M, Ottenhoff TH, Andersen P. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect Immun 2002; 70:5446 - 53; http://dx.doi.org/10.1128/IAI.70.10.5446-5453.2002; PMID: 12228269
- Elvang T, Christensen JP, Billeskov R, Thi Kim Thanh Hoang T, Holst P, Thomsen AR, Andersen P, Dietrich J. CD4 and CD8 T cell responses to the M. tuberculosis Ag85B-TB10.4 promoted by adjuvanted subunit, adenovector or heterologous prime boost vaccination. PLoS One 2009; 4:e5139; http://dx.doi.org/10.1371/journal.pone.0005139; PMID: 19357780
- Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, Andersen P. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol 2005; 174:6332 - 9; http://dx.doi.org/10.4049/jimmunol.174.10.6332; PMID: 15879133
- Mahmood A, Srivastava S, Tripathi S, Ansari MA, Owais M, Arora A. Molecular characterization of secretory proteins Rv3619c and Rv3620c from Mycobacterium tuberculosis H37Rv. FEBS J 2011; 278:341 - 53; http://dx.doi.org/10.1111/j.1742-4658.2010.07958.x; PMID: 21134129
- Yan J, Reichenbach DK, Corbitt N, Hokey DA, Ramanathan MP, McKinney KA, Weiner DB, Sewell D. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine 2009; 27:431 - 40; http://dx.doi.org/10.1016/j.vaccine.2008.10.078; PMID: 19022315
- Shedlock DJ, Aviles J, Talbott KT, Wong G, Wu SJ, Villarreal DO, Myles DJ, Croyle MA, Yan J, Kobinger GP, et al. Induction of broad cytotoxic T cells by protective DNA vaccination against Marburg and Ebola. Mol Ther 2013; 21:1432 - 44; http://dx.doi.org/10.1038/mt.2013.61; PMID: 23670573
- Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 2009; 27:393 - 422; http://dx.doi.org/10.1146/annurev.immunol.021908.132703; PMID: 19302046
- Derrick SC, Repique C, Snoy P, Yang AL, Morris S. Immunization with a DNA vaccine cocktail protects mice lacking CD4 cells against an aerogenic infection with Mycobacterium tuberculosis. Infect Immun 2004; 72:1685 - 92; http://dx.doi.org/10.1128/IAI.72.3.1685-1692.2004; PMID: 14977976
- Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 2002; 46:709 - 17; http://dx.doi.org/10.1046/j.1365-2958.2002.03237.x; PMID: 12410828
- Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 2003; 9:533 - 9; http://dx.doi.org/10.1038/nm859; PMID: 12692540
- Murray RA, Mansoor N, Harbacheuski R, Soler J, Davids V, Soares A, Hawkridge A, Hussey GD, Maecker H, Kaplan G, et al. Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. J Immunol 2006; 177:5647 - 51; http://dx.doi.org/10.4049/jimmunol.177.8.5647; PMID: 17015753
- Gordon SV, Brosch R, Billault A, Garnier T, Eiglmeier K, Cole ST. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol Microbiol 1999; 32:643 - 55; http://dx.doi.org/10.1046/j.1365-2958.1999.01383.x; PMID: 10320585
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time?. Nat Rev Genet 2008; 9:776 - 88; http://dx.doi.org/10.1038/nrg2432; PMID: 18781156
- Villarreal DO, Talbott KT, Choo DK, Shedlock DJ, Weiner DB. Synthetic DNA vaccine strategies against persistent viral infections. Expert Rev Vaccines 2013; 12:537 - 54; http://dx.doi.org/10.1586/erv.13.33; PMID: 23659301
- Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 2012; 4:ra138; http://dx.doi.org/10.1126/scitranslmed.3004414; PMID: 23052295
- Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, De Rosa S, Carter DK, Rybczyk K, Frank I, et al, NIAID HIV Vaccine Trials Network. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid IL-12 and impact of intramuscular electroporation for delivery. J Clin Investigation. J Infect Dis 2013; 208:818 - 29; http://dx.doi.org/10.1093/infdis/jit236; PMID: 23840043
- Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, Betts MR, Draghia-Akli R, Weiner DB. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine 2008; 26:3112 - 20; http://dx.doi.org/10.1016/j.vaccine.2008.02.036; PMID: 18430495
- Tollefsen S, Tjelle T, Schneider J, Harboe M, Wiker H, Hewinson G, Huygen K, Mathiesen I. Improved cellular and humoral immune responses against Mycobacterium tuberculosis antigens after intramuscular DNA immunisation combined with muscle electroporation. Vaccine 2002; 20:3370 - 8; http://dx.doi.org/10.1016/S0264-410X(02)00289-X; PMID: 12213407
- Zhang X, Divangahi M, Ngai P, Santosuosso M, Millar J, Zganiacz A, Wang J, Bramson J, Xing Z. Intramuscular immunization with a monogenic plasmid DNA tuberculosis vaccine: Enhanced immunogenicity by electroporation and co-expression of GM-CSF transgene. Vaccine 2007; 25:1342 - 52; http://dx.doi.org/10.1016/j.vaccine.2006.09.089; PMID: 17052817
- Tollefsen S, Vordermeier M, Olsen I, Storset AK, Reitan LJ, Clifford D, Lowrie DB, Wiker HG, Huygen K, Hewinson G, et al. DNA injection in combination with electroporation: a novel method for vaccination of farmed ruminants. Scand J Immunol 2003; 57:229 - 38; http://dx.doi.org/10.1046/j.1365-3083.2003.01218.x; PMID: 12641651
- Uplekar S, Heym B, Friocourt V, Rougemont J, Cole ST. Comparative genomics of Esx genes from clinical isolates of Mycobacterium tuberculosis provides evidence for gene conversion and epitope variation. Infect Immun 2011; 79:4042 - 9; http://dx.doi.org/10.1128/IAI.05344-11; PMID: 21807910
- Derrick SC, Yabe IM, Yang A, Morris SL. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine 2011; 29:2902 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.02.010; PMID: 21338678
- Hervas-Stubbs S, Majlessi L, Simsova M, Morova J, Rojas MJ, Nouzé C, Brodin P, Sebo P, Leclerc C. High frequency of CD4+ T cells specific for the TB10.4 protein correlates with protection against Mycobacterium tuberculosis infection. Infect Immun 2006; 74:3396 - 407; http://dx.doi.org/10.1128/IAI.02086-05; PMID: 16714570
- Siegrist MS, Unnikrishnan M, McConnell MJ, Borowsky M, Cheng TY, Siddiqi N, Fortune SM, Moody DB, Rubin EJ. Mycobacterial Esx-3 is required for mycobactin-mediated iron acquisition. Proc Natl Acad Sci U S A 2009; 106:18792 - 7; http://dx.doi.org/10.1073/pnas.0900589106; PMID: 19846780
- Sutcliffe IC. New insights into the distribution of WXG100 protein secretion systems. Antonie Van Leeuwenhoek 2011; 99:127 - 31; http://dx.doi.org/10.1007/s10482-010-9507-4; PMID: 20852931
- Hoang TT, Nansen A, Roy S, Billeskov R, Aagaard C, Elvang T, Dietrich J, Andersen P. Distinct differences in the expansion and phenotype of TB10.4 specific CD8 and CD4 T cells after infection with Mycobacterium tuberculosis. PLoS One 2009; 4:e5928; http://dx.doi.org/10.1371/journal.pone.0005928; PMID: 19529765
- Bukka A, Price CT, Kernodle DS, Graham JE. Mycobacterium tuberculosis RNA expression patterns in sputum bacteria indicate secreted Esx factors contributing to growth are highly expressed in active disease. Front Microbiol 2011; 2:266; PMID: 22291682
- Bottai D, Di Luca M, Majlessi L, Frigui W, Simeone R, Sayes F, Bitter W, Brennan MJ, Leclerc C, Batoni G, et al. Disruption of the ESX-5 system of Mycobacterium tuberculosis causes loss of PPE protein secretion, reduction of cell wall integrity and strong attenuation. Mol Microbiol 2012; 83:1195 - 209; http://dx.doi.org/10.1111/j.1365-2958.2012.08001.x; PMID: 22340629
- Lalvani A, Brookes R, Wilkinson RJ, Malin AS, Pathan AA, Andersen P, Dockrell H, Pasvol G, Hill AV. Human cytolytic and interferon γ-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 1998; 95:270 - 5; http://dx.doi.org/10.1073/pnas.95.1.270; PMID: 9419365
- Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 1992; 89:12013 - 7; http://dx.doi.org/10.1073/pnas.89.24.12013; PMID: 1465432
- Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun 1998; 66:830 - 4; PMID: 9453650
- Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg TP, Vanonckelen A, Ooms J, Saman E, Ulmer JB, Content J, et al. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun 1998; 66:1527 - 33; PMID: 9529077
- Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, Betts MR, Draghia-Akli R, Weiner DB. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine 2008; 26:3112 - 20; http://dx.doi.org/10.1016/j.vaccine.2008.02.036; PMID: 18430495
- Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, De Rosa S, Carter DK, Rybczyk K, Frank I, et al, NIAID HIV Vaccine Trials Network. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis 2013; 208:818 - 29; http://dx.doi.org/10.1093/infdis/jit236; PMID: 23840043
- Villarreal DO, Wise MC, Walters JN, Reuschel EL, Choi MJ, Obeng-Adjei N, Yan J, Morrow MP, Weiner DB. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res 2014; 74:1789 - 800; http://dx.doi.org/10.1158/0008-5472.CAN-13-2729; PMID: 24448242
- Yan J, Corbitt N, Pankhong P, Shin T, Khan A, Sardesai NY, Weiner DB. Immunogenicity of a novel engineered HIV-1 clade C synthetic consensus-based envelope DNA vaccine. Vaccine 2011; 29:7173 - 81; http://dx.doi.org/10.1016/j.vaccine.2011.05.076; PMID: 21651948
- Laddy DJ, Yan J, Corbitt N, Kobasa D, Kobinger GP, Weiner DB. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine 2007; 25:2984 - 9; http://dx.doi.org/10.1016/j.vaccine.2007.01.063; PMID: 17306909
- Hogarth PJ, Logan KE, Vordermeier HM, Singh M, Hewinson RG, Chambers MA. Protective immunity against Mycobacterium bovis induced by vaccination with Rv3109c--a member of the esat-6 gene family. Vaccine 2005; 23:2557 - 64; http://dx.doi.org/10.1016/j.vaccine.2004.11.030; PMID: 15780437
- Shedlock DJ, Talbott KT, Wu SJ, Wilson CM, Muthumani K, Boyer JD, Sardesai NY, Awasthi S, Weiner DB. Vaccination with synthetic constructs expressing cytomegalovirus immunogens is highly T cell immunogenic in mice. Hum Vaccin Immunother 2012; 8:1668 - 81; http://dx.doi.org/10.4161/hv.22447; PMID: 23151448
- Yan J, Yoon H, Kumar S, Ramanathan MP, Corbitt N, Kutzler M, Dai A, Boyer JD, Weiner DB. Enhanced cellular immune responses elicited by an engineered HIV-1 subtype B consensus-based envelope DNA vaccine. Mol Ther 2007; 15:411 - 21; http://dx.doi.org/10.1038/sj.mt.6300036; PMID: 17235321
