Abstract
Respiratory infections caused by Pseudomonas aeruginosa are a major clinical problem globally, particularly for patients with chronic pulmonary disorders, such as those with cystic fibrosis (CF), non-CF bronchiectasis (nCFB) and severe chronic obstructive pulmonary disease (COPD). In addition, critically ill and immunocompromised patients are also at significant risk of P. aeruginosa infection. For almost half a century, research efforts have focused toward development of a vaccine against infections caused by P. aeruginosa, but a licensed vaccine is not yet available. Significant advances in identifying potential vaccine antigens have been made. Immunisations via both the mucosal and systemic routes have been trialled in animal models and their effectiveness in clearing acute infections demonstrated. The challenge for translation of this research to human applications remains, since P. aeruginosa infections in the human respiratory tract can present both as an acute or chronic infection. In addition, immunisation prior to infection may not be possible for many patients with CF, nCFB or COPD. Therefore, development of a therapeutic vaccine provides an alternative approach for treatment of chronic infection. Preliminary animal and human studies suggest that mucosal immunisation may be effective as a therapeutic vaccine against P. aeruginosa respiratory infections. Nevertheless, more research is needed to improve our understanding of the basic biology of P. aeruginosa and the mechanisms needed to upregulate the induction of host immune pathways to prevent infection. Recognition of variability in the host immune responses for a range of patient health conditions at risk from P. aeruginosa infection is also required to support development of a successful vaccine delivery strategy and vaccine. Activation of mucosal immune responses may provide improved efficacy of vaccination for P. aeruginosa during both acute exacerbations and chronic infection.
Introduction
Pseudomonas aeruginosa is an oxidase positive, glucose non-fermenting Gram negative bacillus that normally inhabits soil and aquatic environments.Citation1 Its large genome, adaptive regulatory systems, metabolic versatility and high intrinsic antibiotic resistance, facilitates its survival in a diverse range of habitats.Citation2P. aeruginosa is also an opportunistic human pathogen, causing acute life-threatening infections in patients with a damaged epithelial barrier (e.g., burns, intravascular and urinary catheters, traumatic and surgical wound sites and endotracheal tubes) or impaired immune function (e.g., cancer, human immunodeficiency virus infection, bone marrow, and organ transplantation) and resulting in bacteraemia, urinary tract infection or pneumonia.Citation3P. aeruginosa can also cause persistent infections within the lower respiratory tracts of patients with chronic pulmonary disorders. Chronic P. aeruginosa infection develops as a result of damaged or abnormal airway epithelium and compromised local pulmonary clearance mechanisms, and includes patients with cystic fibrosis (CF),Citation4 non-CF bronchiectasis (nCFB),Citation5 and chronic obstructive pulmonary disease (COPD).Citation6 The organism possesses an impressive array of virulence factors that enable it to cause acute infections, and the metabolic versatility to facilitate its ongoing persistence within the lung microenvironment, both of which have been reviewed extensively elsewhere.Citation7,Citation8 This brief review examines the current status of the development of a vaccine against P. aeruginosa.
Why is a vaccine needed?
The burden of disease caused by P. aeruginosa is substantial. In the United States, P. aeruginosa is the second most common pathogen causing acute healthcare-associated pneumonia in the critically ill, elderly and immunocompromised, while worldwide it is second only to Staphylococcus aureus as a cause of infections within intensive care units.Citation9,Citation10 Treatment is complicated by the organism’s resistance to multiple antibiotics and its capacity to form aggregates and biofilms on mucosal membranes and medical device surfaces, further increasing resistance to antibiotic action.Citation11,Citation12 For example, ventilator-associated P. aeruginosa pneumonia, in particular has a high attributable case facility rate.Citation13 A Canadian study reported the annual incidence of P. aeruginosa bacteraemia as 3.6/100,000 population, with only one in five cases being community-acquired.Citation14 The overall mortality rate in this population-based study was 29% and the study identified important risk factors for bacteraemia, including that its frequency increased with advancing age, underlying chronic disease and male gender.
The establishment of persistent P. aeruginosa infection in those with chronic pulmonary disorders is also of considerable importance. By adulthood, more than 70% of patients with CF will have a chronic P. aeruginosa infection, which leads to accelerated pulmonary decline, reduced quality of life and poorer survival.Citation15,Citation16 Similarly, the organism also acts as a marker of severe disease and poor prognosis in the 10–30% of infected adult patients with nCFB and COPD.Citation17,Citation18 Chronic pulmonary conditions are the fourth leading cause of death globally, with more than four million people dying prematurely each year, and more than 200 million people diagnosed with COPD worldwide.Citation19 Consequently, both acute and chronic P. aeruginosa infections are associated with significant morbidity, increased mortality and considerable cost to the health system and the community.
Despite advances in managing the critically ill, mortality from acute P. aeruginosa infections, such as sepsis and pneumonia, remains high,Citation20 while chronic infection in those with underlying pulmonary disorders and impaired airway clearance mechanisms is notoriously difficult to eradicate. The organism’s chromosomally encoded intrinsic resistance to antibiotics, its capacity to acquire resistance from mutations and horizontal gene transfer, and its capability to form self-protective biofilms, deep within the lower airways, are contributing factors to its persistence.Citation11,Citation21
Overall, the development of effective therapeutic regimes for those at risk of infection from this opportunistic pathogen remains challenging,Citation22 due to its multiple virulence mechanisms and the wide variation in the underlying causes of susceptibility to infection exhibited by various patient groups. Clearly, all individuals vulnerable to P. aeruginosa infection could potentially benefit from a P. aeruginosa vaccine.
Despite a substantial research effort over the past 50 years, a vaccine licensed for clinical use has not yet been delivered and several challenges remain to be addressed. For example, use of a prophylactic vaccine requires immunisation prior to P. aeruginosa colonisation of the airways and thus may be limited to the uninfected elderly and CF or nCFB patients who are already known to be at increased risk of P. aeruginosa infection. After P. aeruginosa colonisation and establishment of infection, a therapeutic vaccine is required in order to clear the organism. It is currently not known whether the same formulation will be effective for both scenarios. Furthermore, treatment of acute infections in patients resulting from mechanical ventilation, central vascular catheterization, chemotherapy-induced mucositis and neutropaenia, or from severe trauma, including burns, are increasingly reliant upon a passive immunotherapeutic approach. These patients are usually critically ill, have impaired systemic immunity, and their responses to active immunisation may be delayed or diminished.Citation23 In contrast, patients with chronic endobronchial infections may have impaired local immune responses and poor airway clearance, but retain relatively intact systemic immune responses.Citation24
The lung microenvironment in patients with CF provides an even greater challenge for successful vaccine development, due to its increased susceptibility to bacterial colonisation and acute exacerbation episodes during chronic infection. The lower airways of CF patients produce viscous mucus, while mucociliary clearance is decreased, effectively reducing the opportunity for pathogen clearance.Citation25 Innate immunity within the CF lung is impaired (reduced pH and defensins),Citation26 airway phagocytic function is abnormal and there is apparent mucosal immune dysregulation.Citation24 In addition to the opportunity for infection provided by the host’s compromised homeostasis, P. aeruginosa has an impressive armoury of weapons to facilitate successful colonisation and infection. For example, the organism produces several virulence factors that are able to impair or modulate local defenses (quorum sensing signaling molecules (QSSM), proteases and pyocyanin).Citation27 Furthermore, using mutations and genetic rearrangements, it is also able to downregulate the expression of highly immunogenic virulence factors (O-antigen, type III secretion systems, flagella)Citation28,Citation29 and upregulate other factors, including pyoverdine,Citation30 to produce aggregates and biofilms.Citation7,Citation12,Citation28,Citation29
P. aeruginosa vaccine antigens and vaccine immune responses
Several P. aeruginosa antigens have been identified as potential vaccine candidates, which, to date include lipopolysaccharide (LPS) O-antigen, polysaccharides, polysaccharide-protein conjugates, outer membrane proteins F and I, the type III secretion system component PcrV, flagella, pili, attenuated P. aeruginosa S.enterica SL326, DNA and whole killed cells,Citation31,Citation32 in addition to a number of non-integral outer membrane protein candidate antigens.Citation33
It is increasingly clear that development of strong opsonophagocytic antibody responses following immunisation is insufficient for successful vaccine development and that stimulation of T cell responses, including IFN-γ, IL-17 and GM-CSF secretion, is essential. These observations highlight the possibility that different vaccine formulations may need to be considered for defined infections and patient populations.Citation34,Citation35 Furthermore, a Th17 T cell response may provide evidence of a potential mechanism for enhanced endobronchial clearance of non-typeable Hemophilus influenzae (NTHi) in COPD patients following oral immunisation with a whole killed cell NTHi oral vaccine.Citation36 The proposed mechanism of this response is driven from oral vaccine stimulation of Peyer’s patches, followed by Th17 effector T cells circulating to the lungs via a mucosal immune network. Clearly, significantly more basic research is required to determine the type of immune responses needed to provide or enhance immunoprotection against P. aeruginosa infections and to encompass the diverse clinical presentations of infection caused by this organism, before an effective vaccine can be developed.
Vaccine outcomes to date
Currently, a licensed vaccine is not yet available against P. aeruginosa, despite many promising animal and preliminary clinical studies in humans. An excellent expert review by Priebe and Goldberg (2014) has recently addressed the “state-of-the-art” in vaccinology for P. aeruginosa and is recommended to the reader.Citation35 That review, however, did not report on research scaffolding the potential development of a mucosal vaccine against P. aeruginosa. For example, we have shown that a whole killed cell P. aeruginosa vaccine demonstrated considerable promise in an animal model of acute lung infection.Citation37 These studies have been successfully expanded to demonstrate that mucosal immunisation with cytosolic proteins can also lead to enhanced clearance from the lungs of animals acutely infected with P. aeruginosa.Citation33 Two of these antigens, catalase A (KatA) and amidase (Ad) were further studied.
P. aeruginosa possesses two heme-containing catalases,Citation38 with KatA being the principal catalase, which provides the first line of defense against osmotic stress and H2O2 attack by phagocytes.Citation39 KatA, the 'isoenzyme A' form, is located in the cytoplasm and periplasm and appears to also locate on the bacterial surface.Citation40 In the absence of KatA expression, biofilms are more easily killed by H2O2Citation41 and QSSMs mediate a significant component of biofilm resistance to H2O2.Citation42 In contrast, Ad is an enzyme found in the periplasm and has no known relationship to bacterial virulence. The P. aeruginosa amidase is a 6x38-kDa enzyme that catalyzes the hydrolysis of a small range of short aliphatic amides and belongs to the nitrilases, a 13-branch superfamily of thiol enzymes involved in natural product biosynthesis and post-translational modification.Citation43
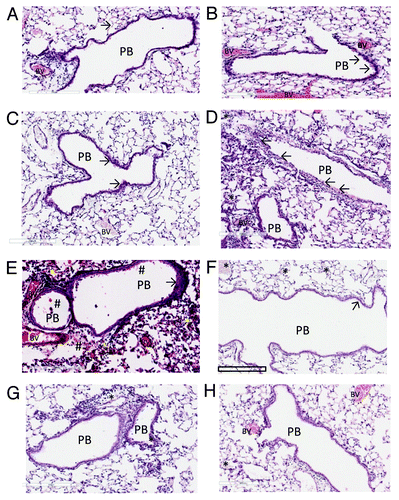
Parenteral immunisation studies have demonstrated that KatA appears to be just as effective in mice as the homologous killed P. aeruginosa whole cell vaccine, and slightly better than the leading vaccine candidate OprF-OprI (provided by Dr von Specht) (). We have also demonstrated that KatA combined with Ad is protective in a chronic lung infection model ( and ), significantly reducing the number of bacteria recovered 4 h after acute challenge with P. aeruginosa. In this model, there was visually much less epithelial thickening in the bronchiole wall, less cellular infiltration, less alveolar wall damage and less lung consolidation in immunised animals compared with non-immunised controls (). Indeed, the extent of epithelial damage 4 h after bacterial challenge was still evident 24 h after administration of the bacterial challenge, with the alveoli exhibiting significant damage and bleeding in non-immunised animals. The presence of clear mucus in some smaller bronchioles provides evidence of localized mucosal responses within the KatA/Ad immunised animals. Most importantly, this study demonstrated that, parenteral immunisation was effective against an acute exacerbation of a chronic lung infection in mice when immunisation occurred after the infection was firmly established (). In most chronic pulmonary disorders, individuals suffer episodes of acute exacerbations. Thus, in this model, both the day 7 and day 35 P. aeruginosa challenges represent acute exacerbation situations, with day 35 being a severe episode. These results show potential for candidate antigens, such as KatA and Ad, to enhance clearance of an acute P. aeruginosa-associated exacerbation of an established chronic P. aeruginosa infection.
Figure 1. Clearance of P. aeruginosa from the acutely infected lung mouse model, 4h post challenge with live bacteria. Mice (Balb/c) were parenterally immunised with 10 µg antigen (KatA or OprF-I) or 106 colony forming units (cfu) heat killed Pa in Incomplete Freund’s adjuvant (as indicated) on days 0 and 14. Acute P. aeruginosa lung infection involved direct intra-tracheal (IT) inoculation of live P. aeruginosa (106cfu) into the lungs while briefly sedated with alfaxan (12 mg/kg) on day 21. Clearance was assessed at 4 h post live challenge. *P < 0.05 compared with non-immune.
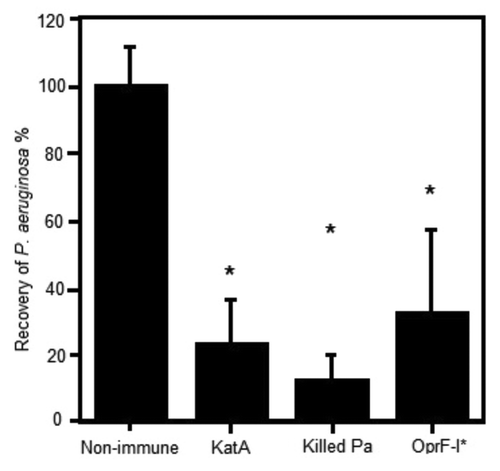
Figure 2. Clearance of live P. aeruginosa administered IT in agar. Mice were parenterally immunised as described in , except Kat A was mixed equally with Ad (10 µg each). Live challenge with P. aeruginosa via the trachea was administered on day 21 with 2x104 colony forming units in 0.5% agar. ⬜ Non-immune group (SEM = ± 44%); ⬤ KatA and Ad immunised group (SEM = ± 22%); ⬥ heat-killed P. aeruginosa immunised group (SEM = ± 12%). Data presented as the mean of n = 5–6 mice per group. S.E.Ms were too small to show graphically and are given above in the legend text.*P < 0.05 compared with the non-immune.
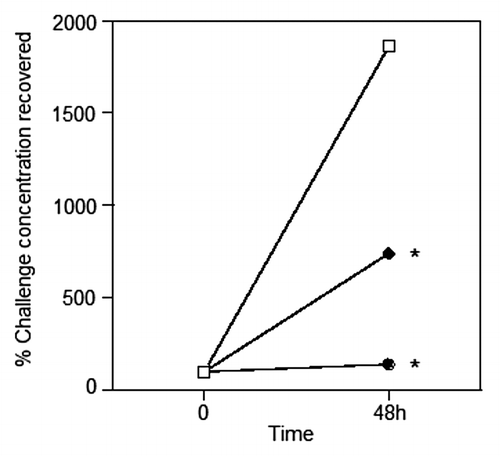
Figure 3. Vaccinating to clear an established chronic P. aeruginosa infection. This animal model was established in our laboratory to determine whether immunisation was effective in combating acute bacterial challenge in mice that had already been chronically infected with P. aeruginosa. (A) In this model, Balb/c mice received 104 colony forming units (cfu) of live P. aeruginosa in 0.5% agar intra-tracheally (IT) into the lungs on day 0. On day 7, they received a second dose of 104 cfu live P. aeruginosa in PBS (IT). One group of control non-immune mice⬜, received only agar on day 0 and PBS on Day 7, prior to acute live P. aeruginosa challenge at day 35, this provided a control group not previously exposed to an infection. The mice were then immunised sub-cutaneously on days 14 and 28 with either PBS (control mice) or vaccines formulated in IFA. On day 35, mice received an acute infection of 106cfu live P. aeruginosa (IT). The mice were killed at 4 h post this infection and bronchial lavage samples collected for testing. (B) This figure illustrates the clearance of P. aeruginosa in the bronchial lavage following acute bacterial IT challenge, 106cfu live P. aeruginosa or PBS, at day 35 in each of the groups. Non-immune mice received PBS (IT) and were sham immunised. All the filled symbol groups received P. aeruginosa in agar (IT) at day 0. ⬥Non-immunised control group (but did receive infections on days 0 and 7); ⬤KatA/Ad immunised group; ▲ heat-killed Pa. Kat A was mixed equally with Ad (10 µg each); and the heat killed P. aeruginosa (HKPa) group was immunised with heat-killed P. aeruginosa. Data presented as mean ± SEM of n = 5–6 mice per group.
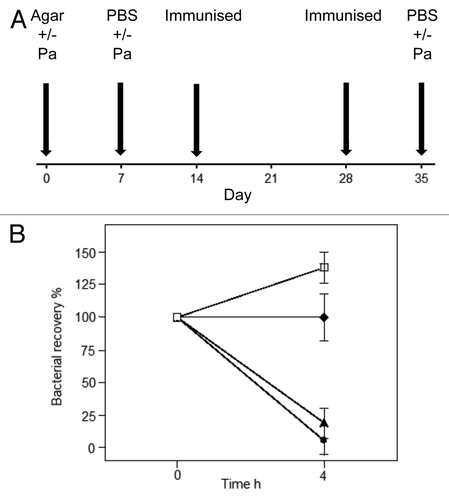
Figure 4. The histopathology of the lungs of Balb C mice immunised against Kat A/Ad, after acute exacerbation of chronic infection by P. aeruginosa. Immunised mice lungs showed reduced cellular recruitment, infiltration and lung consolidation and damage after the acute bacterial challenge compared with lungs from non-immunised, chronically infected control mice. (A) Shows the histological features of the lung in naïve mice, prior to chronic infection. Pulmonary bronchioles (PB) exhibit regular, intact epithelial surface and the epithelial walls of the alveolus (arrowed) encompass well defined expanded chambers, free of mucus. BV, blood vessel. (B) Illustrates the increased epithelial thickness (arrowed) and infiltration of nucleated cells adjacent to the alveoli resulting from establishment of chronic infection, within the mouse lungs prior to immunisation. (C) Illustrates similar characteristics resulting from the establishment of chronic infection in the lung in non-immunised mice, prior to bacterial challenge. As observed in (B), chronic infection has increased epithelial thickness (arrowed). (D) Shows the features of lung histology from chronically infected mice after immunisation against Kat A/Ad, prior to acute bacterial challenge. Although increased epithelial thickness is observed in the PB and there is evidence of localized cellular recruitment, the airways remain clear. (E) Demonstrates the damage induced in lung morphology from chronically infected mice in the absence of immunisation to Kat A/Ad, four hours after acute bacterial challenge. PB epithelia are thickened, alveolar walls and capillaries compromised leading to extravasation of blood into airways (#), increased cellular recruitment and consolidation of the lung through collapse of alveolar structure. (F) Demonstrates, in comparison to (E), that 4 h post bacterial challenge, the lung morphology of mice immunised against Kat A/Ad does not exhibit the epithelial damage, alveolar destruction, cellular recruitment or leakage of blood into the airways seen in non-immunised animals. The airways remain relatively clear, with minimal epithelial thickening (arrow). There is some evidence of increased mucus secretion (*) in smaller airways that have retained the mucus during histological processing of the lung. (G and H) Illustrates the lung histology, 24 h post bacterial challenge in non-immunised (G) and animals immunised against Kat A/Ad. In both groups, clearance of the acute damage observed in (E and F), has occurred, however the epithelial thickening, cellular infiltration and alveolar damage is more extensive in the lungs from non-immunised mice. Immunised mice still retain mucus within some of the airways however the extent of airway damage and consolidation appears less in this group.
Despite the enhanced clearance of an acute experimental P. aeruginosa exacerbation, there remains the challenge to translate the findings to human clinical presentations. For example, during acute exacerbations caused by P. aeruginosa in CF patients with chronic P. aeruginosa infection, bacterial load is not necessarily increased before-handCitation44,Citation45and while clinical improvement is associated with a fall in bacterial numbersCitation45 this is not a requirement in those with advanced disease.Citation46 Similarly, even if bacterial load is reduced by several orders of magnitude by the use of systemic and inhaled antibiotics, this effect can be independent of any clinical benefit in stable patients with chronic P. aeruginosa infection in those with CF or nCFB.Citation47,Citation48 In contrast, a single clinical study in nCFB patients reported correlation of increased bacterial load during stable clinical states with increased inflammation, exacerbation risk and severity of exacerbation episode.Citation49
Chronic P. aeruginosa infection is difficult to clear using antibiotics since P. aeruginosa can form aggregates or an organized exopolysaccharide biofilm matrix which can be up to 1000-fold more resistant to antibiotics than free-living planktonic cells.Citation12,Citation50,Citation51 Biofilm formation and the expression of a range of associated virulence genes is controlled by a network of transcriptional regulators and their cognate ligands, small signaling molecules or autoinducers, including several acyl homoserine lactones (AHLs).Citation52 This system has been termed “quorum sensing”. The importance of biofilm formation and quorum sensing in the pathogenesis of establishing P. aeruginosa infection has been demonstrated in a range of animal models where gene knockouts for one or more of the QSSMs or their receptors render the bacterium almost avirulent.Citation53,Citation54
A novel approach to P. aeruginosa vaccine development would be to use QSSM carrier protein conjugates as vaccine formulations. In our mouse model of acute lung infection, we have demonstrated that the lasIRrhlIR knockout strain showed equal persistence to wild type parental PAO1, induced equal or greater neutrophil infiltration to the lungs, and induced similar levels of expression of inflammatory cytokines in the lungs and antibody responses, both in terms of magnitude and isotype.Citation55 In contrast to previous reports, these results suggested that the lack of quorum sensing alone does not significantly affect the immunogenicity, infectiveness and persistence of P. aeruginosa. The most frequently produced QSSMs are N-3-(oxododecanoyl)-L-homoserine lactone (OdDHL) and butyryl L-homoserine lactone (BHL), collectively known as AHLs. It has been widely postulated that blocking quorum sensing and biofilm formation could have a therapeutic benefit by limiting the ability of P. aeruginosa to form biofilms. Together, these results suggest that aggregation of P. aeruginosa rather than biofilm formation may be of greater importance for establishment of acute infection for this organism.
Attempts to reduce the virulence of P. aeruginosa have used several strategies, including the use of structural analogs of QSSMs to block their function.Citation56 Previously, it has been shown that an immune response to QSSMs can be generated, when they are chemically conjugated to a large protein. Conjugation of AHLs to bovine serum albumin (BSA) or keyhole limpet hemocyanin was used to produce monoclonal antibodies in mice,Citation57 while immunisation of mice with BSA-conjugated OdDHL resulted in a partial protection against lung infection.Citation58 Recent studies in mice with OdDHL conjugated to KatA by Lazenby, Cooley and Kyd (unpublished data) also produced an effective immune response and together, these studies represent an ongoing direction for animal-based vaccine development research.
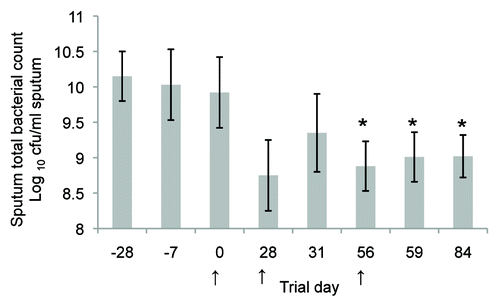
Expansion of animal research into mucosal vaccine development against P. aeruginosa in humans has been undertaken in the form of a safety and immunogenicity study, conducted on the whole killed cell P aeruginosa oral vaccine.Citation59 Twenty-five normal healthy subjects were studied and no vaccine-attributable adverse events were observed clinically or on haematology and biochemistry blood profiles. Increased serum P. aeruginosa specific antibodies and phagocytic indices were observed post-immunisation. A pilot open label study has also been conducted on nine patients with bronchiectasis.Citation60 Results from these patients showed a significant reduction in total P. aeruginosa counts in sputum cultures ().
Figure 5. Clinical trial results showing total sputum bacterial counts (calculated as the log10 colony forming units /mL sputum, mean ± SEM) for the nine patients with bronchiectasis from a pilot open label study. The mean sputum bacterial count is significantly lower (P < 0.05; unpaired t test) for days 56–84 compared with the pre-screening day (Day 28) and initial trial culture (Day 7). Oral immunisation commenced on Day 0, Day 28, or Day 56 as indicated by arrows and continued for a 3 d period. Each subject received 2 × 1011 formaldehyde killed P. aeruginosa per day as an enteric coated capsule. All subjects were fasting for at least 6 h prior to the administration of the capsule.Citation59 Reproduced with permission of the University of Sydney from: RL Clancy, G Pang, Dunkley M, and AW Cripps Control of bacterial colonisation of the respiratory tract mucosa in man. In: Mucosal Solutions: Advances in mucosal immunology.Volume 1. Editors: Alan J Husband, Kenneth W Beagley, Robert L Clancy, Andrew M Collins, Allan W Cripps and David L Emery. University of Sydney, Sydney, Australia. pp 261–268. 1997
Together, these animal and clinical studies demonstrate that mucosal immunisation holds promise for the development of a successful vaccine against P aeruginosa and that a range of antigen formulations are possible. Despite this progress, further studies on mucosal delivery systems for the protein antigens as well as the mechanisms of the immunity induced are needed. Much more developmental work is required, but if successful the rewards will be great for potentially millions of patients worldwide with chronic P. aeruginosa respiratory infections.
| Abbreviations: | ||
| Ad | = | amidase |
| AHL | = | acyl homoserine lactones |
| BHL | = | butyryl L-homoserine lactone |
| BSA | = | bovine serum albumin |
| CF | = | cystic fibrosis |
| CFU | = | colony forming units |
| COPD | = | chronic obstructive pulmonary disease |
| HKPa | = | heat killed P. aeruginosa |
| IFA | = | incomplete Freund’s adjuvant |
| IT | = | intra-tracheal |
| KatA | = | catalase A |
| LPS | = | lipopolysaccharide |
| nCFB | = | non-cystic fibrosis bronchiectasis |
| NTHi | = | non-typeable Haemophilus influenzae |
| OdDHL | = | N-3-(oxododecanoyl)-L-homoserine lactone |
| OprF-I | = | Recombinant outer membrane fusion protein I of P. aeruginosa |
| PBS | = | phosphate buffered saline |
| QSSM | = | quorum sensing signalling molecules |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Ms Penny Chapman for her editorial assistance with this manuscript.
References
- Kidd T, Whiley D, Bell S, Grimwood K. Pseudomonas. In: Liu D, ed. Molecular detection of human bacterial pathogens. Boca Raton, FL.: CRC Press, 2011:1009-21.
- KlockgetherJ, CramerN, WiehlmannL, DavenportCF, TümmlerB. Pseudomonas aeruginosa Genomic Structure and Diversity. Front Microbiol2011; 2:150; http://dx.doi.org/10.3389/fmicb.2011.00150; PMID: 21808635
- Pier G, Ramphal R. Pseudomonas aeruginosa. In: Mandell G, Bennett J, Dolin R, eds. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. Philadelphia: Churchill Livingstone, 2010:2835-60.
- CiofuO, HansenCR, HøibyN. Respiratory bacterial infections in cystic fibrosis. Curr Opin Pulm Med2013; 19:251 - 8; http://dx.doi.org/10.1097/MCP.0b013e32835f1afc; PMID: 23449384
- McShanePJ, NaureckasET, TinoG, StrekME. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med2013; 188:647 - 56; PMID: 23898922
- DesaiH, EschbergerK, WronaC, GroveL, AgrawalA, GrantB, YinJ, ParameswaranGI, MurphyT, SethiS. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc2014; 11:303 - 9; http://dx.doi.org/10.1513/AnnalsATS.201310-350OC; PMID: 24423399
- GellatlySL, HancockRE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis2013; 67:159 - 73; http://dx.doi.org/10.1111/2049-632X.12033; PMID: 23620179
- CrabbéA, LedesmaMA, NickersonCA. Mimicking the host and its microenvironment in vitro for studying mucosal infections by Pseudomonas aeruginosa. Pathog Dis2014; 71:1 - 19; http://dx.doi.org/10.1111/2049-632X.12180; PMID: 24737619
- SievertDM, RicksP, EdwardsJR, SchneiderA, PatelJ, SrinivasanA, KallenA, LimbagoB, FridkinS, National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol2013; 34:1 - 14; http://dx.doi.org/10.1086/668770; PMID: 23221186
- VincentJL, RelloJ, MarshallJ, SilvaE, AnzuetoA, MartinCD, MorenoR, LipmanJ, GomersallC, SakrY, et al, EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA2009; 302:2323 - 9; http://dx.doi.org/10.1001/jama.2009.1754; PMID: 19952319
- SunHY, FujitaniS, QuintilianiR, YuVL. Pneumonia due to Pseudomonas aeruginosa: part II: antimicrobial resistance, pharmacodynamic concepts, and antibiotic therapy. Chest2011; 139:1172 - 85; http://dx.doi.org/10.1378/chest.10-0167; PMID: 21540216
- StaudingerBJ, MullerJF, HalldórssonS, BolesB, AngermeyerA, NguyenD, RosenH, BaldurssonO, GottfreðssonM, GuðmundssonGH, et al. Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med2014; 189:812 - 24; http://dx.doi.org/10.1164/rccm.201312-2142OC; PMID: 24467627
- RelloJ, RuéM, JubertP, MusesG, SoñoraR, VallésJ, NiedermanMS. Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit Care Med1997; 25:1862 - 7; http://dx.doi.org/10.1097/00003246-199711000-00026; PMID: 9366771
- ParkinsMD, GregsonDB, PitoutJD, RossT, LauplandKB. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection2010; 38:25 - 32; http://dx.doi.org/10.1007/s15010-009-9145-9; PMID: 20012908
- SandersDB, LiZ, LaxovaA, RockMJ, LevyH, CollinsJ, FerecC, FarrellPM. Risk factors for the progression of cystic fibrosis lung disease throughout childhood. Ann Am Thorac Soc2014; 11:63 - 72; http://dx.doi.org/10.1513/AnnalsATS.201309-303OC; PMID: 24261460
- KeremE, VivianiL, ZolinA, MacNeillS, HatziagorouE, EllemunterH, DrevinekP, GulmansV, KrivecU, OlesenH, ECFS Patient Registry Steering Group. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS Patient Registry. Eur Respir J2014; 43:125 - 33; http://dx.doi.org/10.1183/09031936.00166412; PMID: 23598952
- LoebingerMR, WellsAU, HansellDM, ChinyanganyaN, DevarajA, MeisterM, WilsonR. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J2009; 34:843 - 9; http://dx.doi.org/10.1183/09031936.00003709; PMID: 19357155
- AlmagroP, SalvadóM, Garcia-VidalC, Rodríguez-CarballeiraM, CuchiE, TorresJ, HerediaJL. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration2012; 84:36 - 43; http://dx.doi.org/10.1159/000331224; PMID: 21996555
- FerkolT, SchraufnagelD. The global burden of respiratory disease. Ann Am Thorac Soc2014; 11:404 - 6; http://dx.doi.org/10.1513/AnnalsATS.201311-405PS; PMID: 24673696
- WilliamsBJ, DehnbostelJ, BlackwellTS. Pseudomonas aeruginosa: host defence in lung diseases. Respirology2010; 15:1037 - 56; http://dx.doi.org/10.1111/j.1440-1843.2010.01819.x; PMID: 20723140
- HøibyN. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC Med2011; 9:32; http://dx.doi.org/10.1186/1741-7015-9-32; PMID: 21463524
- HurleyMN, CámaraM, SmythAR. Novel approaches to the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. Eur Respir J2012; 40:1014 - 23; http://dx.doi.org/10.1183/09031936.00042012; PMID: 22743672
- VincentJL. Vaccine development and passive immunization for Pseudomonas aeruginosa in critically ill patients: a clinical update. Future Microbiol2014; 9:457 - 63; http://dx.doi.org/10.2217/fmb.14.10; PMID: 24810345
- CohenTS, PrinceA. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med2012; 18:509 - 19; http://dx.doi.org/10.1038/nm.2715; PMID: 22481418
- O’SullivanBP, FreedmanSD. Cystic fibrosis. Lancet2009; 373:1891 - 904; http://dx.doi.org/10.1016/S0140-6736(09)60327-5; PMID: 19403164
- HartlD, GaggarA, BrusciaE, HectorA, MarcosV, JungA, GreeneC, McElvaneyG, MallM, DöringG. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros2012; 11:363 - 82; http://dx.doi.org/10.1016/j.jcf.2012.07.003; PMID: 22917571
- LavoieEG, WangdiT, KazmierczakBI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect2011; 13:1133 - 45; http://dx.doi.org/10.1016/j.micinf.2011.07.011; PMID: 21839853
- YangL, JelsbakL, MolinS. Microbial ecology and adaptation in cystic fibrosis airways. Environ Microbiol2011; 13:1682 - 9; http://dx.doi.org/10.1111/j.1462-2920.2011.02459.x; PMID: 21429065
- FolkessonA, JelsbakL, YangL, JohansenHK, CiofuO, HøibyN, MolinS. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol2012; 10:841 - 51; http://dx.doi.org/10.1038/nrmicro2907; PMID: 23147702
- Mayer-HamblettN, RosenfeldM, GibsonRL, RamseyBW, KulasekaraHD, Retsch-BogartGZ, MorganW, WolterDJ, PopeCE, HoustonLS, et al. Pseudomonas aeruginosa in vitro phenotypes distinguish cystic fibrosis infection stages and outcomes. Am J Respir Crit Care Med2014; 190:289 - 97; PMID: 24937177
- DöringG, PierGB. Vaccines and immunotherapy against Pseudomonas aeruginosa. Vaccine2008; 26:1011 - 24; http://dx.doi.org/10.1016/j.vaccine.2007.12.007; PMID: 18242792
- Sedlak-WeinsteinE, CrippsAW, KydJM, FoxwellAR. Pseudomonas aeruginosa: the potential to immunise against infection. Expert Opin Biol Ther2005; 5:967 - 82; http://dx.doi.org/10.1517/14712598.5.7.967; PMID: 16018741
- ThomasLD, KydJM, BastinDA, DunkleyML, CrippsAW. Immunisation with non-integral OMPs promotes pulmonary clearance of Pseudomonas aeruginosa. FEMS Immunol Med Microbiol2003; 37:155 - 60; http://dx.doi.org/10.1016/S0928-8244(03)00073-7; PMID: 12832119
- WuW, HuangJ, DuanB, TraficanteDC, HongH, RisechM, LoryS, PriebeGP. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med2012; 186:420 - 7; http://dx.doi.org/10.1164/rccm.201202-0182OC; PMID: 22723292
- PriebeGP, GoldbergJB. Vaccines for Pseudomonas aeruginosa: a long and winding road. Expert Rev Vaccines2014; 13:507 - 19; http://dx.doi.org/10.1586/14760584.2014.890053; PMID: 24575895
- ClancyRL, DunkleyM. Acute exacerbations in COPD and their control with oral immunization with non-typeable haemophilus influenzae. Front Immunol2011; 2:7; http://dx.doi.org/10.3389/fimmu.2011.00007; PMID: 22566798
- CrippsAW, DunkleyML, ClancyRL. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect Immun1994; 62:1427 - 36; PMID: 8132349
- MacmillanH, NorimineJ, BraytonKA, PalmerGH, BrownWC. Physical linkage of naturally complexed bacterial outer membrane proteins enhances immunogenicity. Infect Immun2008; 76:1223 - 9; http://dx.doi.org/10.1128/IAI.01356-07; PMID: 18086812
- LeeJ-S, HeoY-J, LeeJK, ChoY-H. KatA, the major catalase, is critical for osmoprotection and virulence in Pseudomonas aeruginosa PA14. Infect Immun2005; 73:4399 - 403; http://dx.doi.org/10.1128/IAI.73.7.4399-4403.2005; PMID: 15972537
- ThomasLD, DunkleyML, MooreR, ReynoldsS, BastinDA, KydJM, CrippsAW. Catalase immunization from Pseudomonas aeruginosa enhances bacterial clearance in the rat lung. Vaccine2000; 19:348 - 57; http://dx.doi.org/10.1016/S0264-410X(00)00146-8; PMID: 10930690
- ElkinsJG, HassettDJ, StewartPS, SchweizerHP, McDermottTR. Protective role of catalase in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide. Appl Environ Microbiol1999; 65:4594 - 600; PMID: 10508094
- HassettDJ, MaJ-F, ElkinsJG, McDermottTR, OchsnerUA, WestSEH, HuangCT, FredericksJ, BurnettS, StewartPS, et al. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol Microbiol1999; 34:1082 - 93; http://dx.doi.org/10.1046/j.1365-2958.1999.01672.x; PMID: 10594832
- AndradeJ, KarmaliA, CarrondoMA, FrazãoC. Structure of amidase from Pseudomonas aeruginosa showing a trapped acyl transfer reaction intermediate state. J Biol Chem2007; 282:19598 - 605; http://dx.doi.org/10.1074/jbc.M701039200; PMID: 17442671
- StressmannFA, RogersGB, MarshP, LilleyAK, DanielsTW, CarrollMP, HoffmanLR, JonesG, AllenCE, PatelN, et al. Does bacterial density in cystic fibrosis sputum increase prior to pulmonary exacerbation?. J Cyst Fibros2011; 10:357 - 65; http://dx.doi.org/10.1016/j.jcf.2011.05.002; PMID: 21664196
- ReidDW, LathamR, LamontIL, CamaraM, RoddamLF. Molecular analysis of changes in Pseudomonas aeruginosa load during treatment of a pulmonary exacerbation in cystic fibrosis. J Cyst Fibros2013; 12:688 - 99; http://dx.doi.org/10.1016/j.jcf.2013.03.008; PMID: 23706827
- DeschaghtP, SchelstraeteP, Van SimaeyL, VanderkerckenM, RamanA, MahieuL, Van DaeleS, De BaetsF, VaneechoutteM. Is the improvement of CF patients, hospitalized for pulmonary exacerbation, correlated to a decrease in bacterial load?. PLoS One2013; 8:e79010; http://dx.doi.org/10.1371/journal.pone.0079010; PMID: 24312174
- TunneyMM, EinarssonGG, WeiL, DrainM, KlemER, CardwellC, EnnisM, BoucherRC, WolfgangMC, ElbornJS. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med2013; 187:1118 - 26; http://dx.doi.org/10.1164/rccm.201210-1937OC; PMID: 23348972
- QuonBS, GossCH, RamseyBW. Inhaled antibiotics for lower airway infections. Ann Am Thorac Soc2014; 11:425 - 34; http://dx.doi.org/10.1513/AnnalsATS.201311-395FR; PMID: 24673698
- ChalmersJD, SmithMP, McHughBJ, DohertyC, GovanJR, HillAT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med2012; 186:657 - 65; http://dx.doi.org/10.1164/rccm.201203-0487OC; PMID: 22744718
- DrenkardE, AusubelFM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature2002; 416:740 - 3; http://dx.doi.org/10.1038/416740a; PMID: 11961556
- KydJM, McGrathJ, KrishnamurthyA. Mechanisms of bacterial resistance to antibiotics in infections of COPD patients. Curr Drug Targets2011; 12:521 - 30; http://dx.doi.org/10.2174/138945011794751519; PMID: 21194403
- SchusterM, GreenbergEP. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int J Med Microbiol2006; 296:73 - 81; http://dx.doi.org/10.1016/j.ijmm.2006.01.036; PMID: 16476569
- PearsonJP, FeldmanM, IglewskiBH, PrinceA. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect Immun2000; 68:4331 - 4; http://dx.doi.org/10.1128/IAI.68.7.4331-4334.2000; PMID: 10858254
- RumbaughKP, GriswoldJA, IglewskiBH, HamoodAN. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect Immun1999; 67:5854 - 62; PMID: 10531240
- LazenbyJJ, GriffinPE, KydJ, WhitchurchCB, CooleyMA. A quadruple knockout of lasIR and rhlIR of Pseudomonas aeruginosa PAO1 that retains wild-type twitching motility has equivalent infectivity and persistence to PAO1 in a mouse model of lung infection. PLoS One2013; 8:e60973; http://dx.doi.org/10.1371/journal.pone.0060973; PMID: 23593362
- RasmussenTB, GivskovM. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol2006; 296:149 - 61; http://dx.doi.org/10.1016/j.ijmm.2006.02.005; PMID: 16503194
- KaufmannGF, SartorioR, LeeSH, MeeJM, AltobellLJ3rd, KujawaDP, JeffriesE, ClaphamB, MeijlerMM, JandaKD. Antibody interference with N-acyl homoserine lactone-mediated bacterial quorum sensing. J Am Chem Soc2006; 128:2802 - 3; http://dx.doi.org/10.1021/ja0578698; PMID: 16506750
- MiyairiS, TatedaK, FuseET, UedaC, SaitoH, TakabatakeT, IshiiY, HorikawaM, IshiguroM, StandifordTJ, et al. Immunization with 3-oxododecanoyl-L-homoserine lactone-protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J Med Microbiol2006; 55:1381 - 7; http://dx.doi.org/10.1099/jmm.0.46658-0; PMID: 17005787
- CrippsAW, PeekK, DunkleyM, VentoK, MarjasonJK, McIntyreME, SizerP, CroftD, Sedlak-WeinsteinL. Safety and immunogenicity of an oral inactivated whole-cell pseudomonas aeruginosa vaccine administered to healthy human subjects. Infect Immun2006; 74:968 - 74; http://dx.doi.org/10.1128/IAI.74.2.968-974.2006; PMID: 16428742
- Clancy R, Pang G, Dunkley M, Cripps A. Control of bacterial colonisation of the respiratory tract mucosa in man. In: Alan J Husband, Kenneth W Beagley, Robert L Clancy, Andrew M Collins, Allan W Cripps, Emery DL, eds. Mucosal Solutions: Advances in mucosal immunology. Sydney, Australia: University of Sydney, 1997:261-8.
