Abstract
Tumor-Associated Carbohydrate Antigens (TACAs) are broad-spectrum targets for immunotherapy. Immunization with Carbohydrate Mimetic Peptides (CMPs) is a strategy to induce broad-spectrum TACA-reactive antibodies hypothesized to interfere with cellular pathways involved in tumor cell survival. A Phase I study was conducted with a first-in-man CMP referred to as P10s, conjugated to the Pan T cell carrier PADRE, along with MONTANIDE™ ISA 51 VG as adjuvant over a course of 5 immunizations. While designed as a safety and tolerability study, the potential for therapeutic impact was observed in a subject with metastatic lesions as evaluated before and after vaccine treatment. The subject received Vinorelbine and Trastuzumab (VT) for two months prior to study eligibility. PET scans showed partial response in the lungs and complete resolution of a previously enlarged subpectoral lymph node. Immunization with P10s vaccine resulted in responses to P10s, with serum and plasma antibodies reactive with and cytotoxic to human breast cancer cells in vitro, including the Trastuzumab-resistant HCC1954 cell line. However, the patient developed cystic masses in the brain parenchyma with no apparent evidence of metastases. The subject was switched to Docetaxel, Pertuzumab and Trastuzumab a year later, and her last PET scan showed a complete response in the lungs and lymph nodes. Incubation of cancer cells with a combination of vaccine-induced serum and docetaxel suggests that the induced antibodies sensitize tumor cells for more efficient killing upon administration of docetaxel. The data suggest that P10s-PADRE induces anti-tumor antibody response that in combination with chemotherapy can affect metastatic lesions in breast cancer patients.
Introduction
Molecular targeting of pathways that are pivotal to cellular homeostasis has great promise in cancer therapy. Tumor-Associated Carbohydrate Antigens (TACAs) are pan-targets on tumor cells because they are collectively and intimately involved in cell-death signaling pathways. Changes in glycosylation are often a hallmark of the transition from normal to inflamed or neoplastic tissue. TACAs affect a myriad of processes that correlate with poor prognosis of cancer because glycan signatures affect cell signaling and communication, cell motility and adhesion, angiogenesis, and organ tropism.Citation1,Citation2 A unique advantage in targeting TACAs is that multiple proteins and lipids on the cancer cell can be modified with the same carbohydrate structure, obviating a required definition of individual tumor-specific antigens. Thus, targeting TACAs broadens the spectrum of antigens recognized by the immune system. This is crucial for thwarting tumor escape since multiple antigens are targeted in an otherwise heterologous set of tumor cellsCitation3,Citation4 but also has potential clinical benefit as cell-death therapies.
Because of the broad-spectrum nature of antigens that express TACAs, antibodies to TACAs might have a functional characteristic associated with clinical benefit by interacting with TACA that regulate cell signaling pathways.Citation5,Citation6 For example, the neolactoseries antigen Lewis Y (LeY) regulates the phosphorylation and expression of some molecules involved the expression of cell cycle-related factors through EGFR/PI3K signaling pathways to promote cell proliferation.Citation7 LeY can influence the biological behavior of a tumor cell as an important composition of integrins α5 and β1 by some signal pathway, such as promoting cell adhesion and migration, that might involve Focal Adhesion Kinase (FAK) functioning upstream of PI3K/Akt, in transducing a β1 integrin viability signal on extracellular matrices. Antibodies directed against LeY side chains of ErbB receptors are capable of inhibiting ErbB-mediated signaling,Citation6 while other anti-LeY mAbs mediate the MAPK signaling pathway.Citation8 Likewise some gangliosides, such as GD2, can facilitate cell attachment.Citation9 Anti-GD2 mAb are noted to affect α2β1 integrin-mediated platelet adhesion to collagen and phosphotyrosine signaling of FAK.Citation10 Such results indicate that tumor gangliosides and LeY enhance adhesion to extracellular matrix by upregulating integrins mediated through FAK, thereby providing insight into how these TACAs may be involved in metastasis, and emphasizing the importance of targeting these moieties in cell-death therapies or in anti-adhesive therapies.
We have developed carbohydrate mimetic peptides (CMPs) that induce broad-spectrum humoral and cellular responses that inhibit tumor growth in preclinical studies.Citation11-Citation15 These CMPs were developed to interact with anti-LeY and anti-GD2 antibodies.Citation16,Citation17 We have moved one of these CMPs (referred to as P10s) into a Phase I clinical trial in stage IV breast cancer subjects to assess P10s vaccine safety and tolerability. P10s was designed, using reverse engineering concepts, to induce responses to several TACAs including LeY and selected gangliosides in preclinical models.Citation13,Citation18 Subjects immunized in our Phase I clinical trial were observed to mount an anti-P10s response, and the serum and plasma antibodies of post-immunized subjects mediate cytotoxicity of human breast cancer cell lines, including a cell line de novo-resistant to Trastuzumab, by as yet unknown mechanism(s). Here we report on the characterization of the antibody immune response in a patient with metastatic HER2+ Breast Cancer who had an unusual disease course and displayed a complete response in lung and lymph nodes after vaccination. These results suggest that the immune response to P10s-based vaccine has the potential for clinical benefit because the P10s vaccine induces TACA reactive antibodies with cytostatic or cytotoxic functionalities.
Results
Preclinical studies supported clinical evaluation of CMPs as a strategy to augment anti-TACA immune responses.Citation11-Citation15,Citation18 Consequently, we developed a CMP for clinical testing for vaccination of high-risk breast cancer patients. This CMP, referred to as P10s and having the sequence WRYTAPVHLGD, was reverse-engineered from knowledge of the crystal structures of anti-GD2 and anti-Lewis Y antibodies.Citation13 We have brought P10s from concept through proof of principle in animal studies, followed by GLP validation, filing of an IND (#14715), and into the clinic. P10s was developed as a more faithful mimic of TACAs compared with its parent peptide GVVWRYTAPVHLGDG (referred to as P10 [P10original]) shown to have anti-tumor activity in a mouse model.Citation15
P10s was synthesized with the Pan T cell peptide PADRE. The P10s-PADRE vaccine was developed for treatment of breast cancer patients with high risk of disease relapse. Six subjects were immunized 5 times over 23 wk. Among the subjects was a patient originally diagnosed with breast cancer at age 45 when a routine mammogram showed suspicious calcifications on the left breast, confirmed by a core biopsy as invasive ductal carcinoma. On 11/8/2006 she underwent bilateral skin-sparing mastectomy with sentinel lymph-node biopsy (SLNB) followed by bilateral saline implant reconstruction. Final pathology showed no cancer in the right breast and a 4 cm invasive ductal carcinoma in the left breast; grade III, with lymphovascular invasion. Margins were negative. Estrogen receptor (ER) was weakly positive and progesterone receptor (PR) was negative. HER2 was amplified by FISH. Left SLNB was negative. PET scan was negative for systemic metastases but paratracheal, paraesophageal and hilar lymph nodes were prominent. However, since there were calcified lymph nodes in the area it was believed that these were not related to metastatic disease. On January 4th 2007, she began adjuvant chemotherapy with dose-dense Adriamycin/Cyclophosphamide (60/600 mg/m2 every two weeks for four cycles followed by Paclitaxel (175 mg/m2) every two weeks for four cycles. Weekly Trastuzumab was started at the same time with Paclitaxel and continued for a total of one year. She was also started on Tamoxifen after the completion of chemotherapy and while she was taking the Trastuzumab. Yearly PET scans were obtained to check on the enlarged lymph nodes and showed complete regression.
In September 2009, she had a local recurrence with 1-cm mass on the left chest wall near the implant. This was resected to negative margins. The mass was ER-positive and PR-negative, but HER2 was still positive with CEP17/HER2 ratio of 15. She was given radiation therapy to the area. Staging scans showed no evidence of metastatic disease. However, in March 2011, a PET scan showed new small lung nodules scattered in both fields and a biopsy-positive left subpectoral lymph node. She was started on Vinorelbine and Trastuzumab (VT), and subsequent PET scans showed partial response in the lungs and complete resolution of the subpectoral lymph node. At that moment she was offered participation in the vaccine trial, as her disease was considered stable. She was deemed eligible for participation in the vaccine study and received her first subcutaneous injection of the vaccine of 300 µg on 9/16/2011 followed by similar doses on days 8, 15, 43, and 126 as per protocol, while continuing her standard treatment of VT.
Immune response to P10s-PADRE vaccine
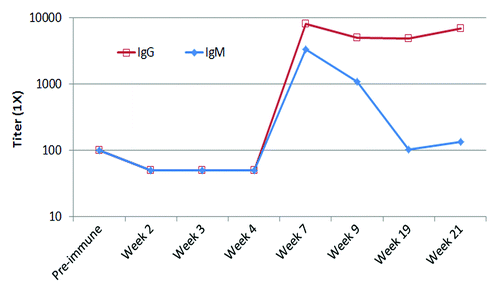
The most important feature of her immune response to P10s-PADRE vaccine is the observation that the immunized subject generated an immune response to P10s Multiple Antigen Peptide (MAP). The multivalent nature of MAP peptides allows for more faithful mimicry of the clustered structures of cell-surface glycans, which is extremely useful for testing glycan recognition in solid phase. ELISA assays revealed that the subject’s serum showed an increase in both IgM and IgG binding to the MAP peptide after immunization (). Both anti-peptide IgM and IgG peaked at week seven of the study. IgM was short-lived and returned back to the baseline by week 19; however, the activity of the IgG portion remained high.
Figure 1. IgM and IgG fractions of serum antibodies from immunized subject bind to MAP form of P10s in ELISA assays. ELISA plates were coated overnight and serum samples were diluted from 1:100 to 1:12 800. Reactivity was visualized using HRP-conjugated mouse anti-human antibody. Titers were estimated from absorbance- vs. -dilution curves by linear regression as described in the Methods section.
P10s-induced serum bound to the HCC1954 cell line (), and co-incubation with these cells led to significant stimulation of cell death (). P10s-immunized serum also inhibited the migration of HCC1954 cells (). We later tested the plasma in binding and observed a very similar pattern of reactivity (data not shown). Post-immune plasma was cytotoxic to both HCC1954 and MDA-MB-231 cell lines (). In a separate experiment, we harvested live cells after overnight incubation with sera and examined induction of apoptosis by detecting annexin expression. Apparently, the anti-P10s serum induced apoptosis in MDA-MB-231 cells (). Binding and cytotoxic effect of serum and plasma on aggressive triple-negative MDA-MB-231 and the de novo Trastuzamab-resistant HCC1954 cell lines is of clear clinical potential for this vaccine. In particular, the observed cytotoxicity against HCC1954 suggests a potential positive outcome for the patient tested. Further studies suggested that the induced antibodies could sensitize tumor cells to Docetaxel treatment ().
Figure 2. P10s-induced IgG serum antibodies from immunized subject bind to HCC1954 and stimulate cell death. (A) Cells were harvested with enzyme-free buffer, washed and incubated with preimmune and postimmune (week 7) sera. Binding was visualized with an FITC-conjugated mouse anti-human IgG. Filled histogram, secondary antibody only; dashed line histogram, preimmune serum; solid line histogram, serum collected at week 7. 1:100 dilution of the serum was used for reactivity. (B) P10s immunized serum kills HCC1954 breast cancer cells in vitro. Error bars represent SD based on three replications. The experiment was repeated three times. Two-tailed Student t test was used for comparison of means.
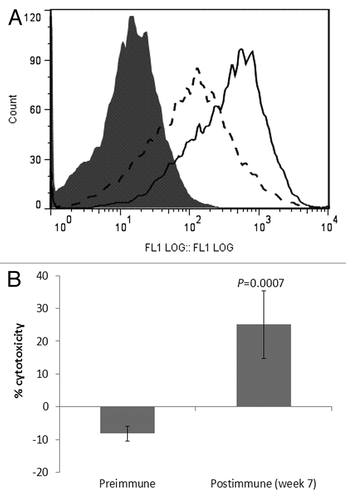
Figure 3. Anti-P10s serum from immunized subject inhibited migration of HCC1954 cells. Cells were incubated overnight with FBS or indicated sera on transwell membranes. Membranes were fixed, stained and those cells left on the surface were wiped out. Migrated cells were then visualized under a light microscope and counted. Average over three replications with SD are shown. P values were estimated by performing one-way ANOVA with Post-hoc Tukey analysis. N.S., not significant.
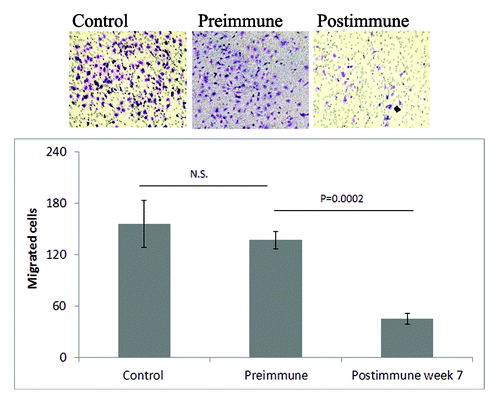
Figure 4. Postimmunization plasma kills breast cancer cells. (A) Cytotoxic effect of pre and postimmunization plasma (week 7) on HCC1954 and MDA-MB-231 cells. 5 × 104 were seeded in 24-well plates and incubated with the sera. Supernatants containing dead cells were removed and live cells were fixed and stained and images were taken. Representative images are shown. (B) Cytotoxicity in each cell line was also quantified by counting cells remained in triplicate wells and presented by bar graph. For each well 3 microscopic fields were counted and averaged. Percent toxicity was calculated based on cell number in control wells. P values are the result of comparing the effects of pre and postimmune sera on each cell line by two-tailed t tests.
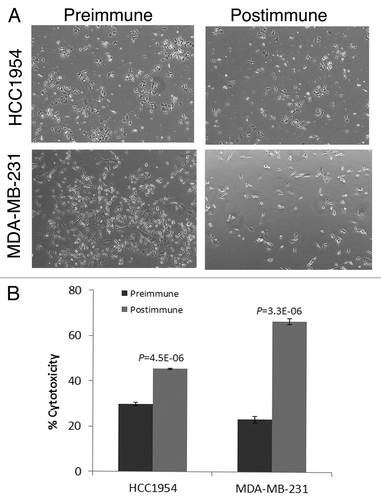
Figure 5. Anti-P10s serum induced apoptosis in MDA-MB-231 cells. Cells were incubated with 10% FBS (control) or indicated sera overnight and then harvested and stained with annexin V-FITC (FL1) and propidium iodide (FL3) using a live/dead assay kit from Invitrogen (Life Technologies, Grand Island, NY). U4, P4, and Q4 show apoptotic cells in control, preimmune treated and postimmune treated cells, respectively.
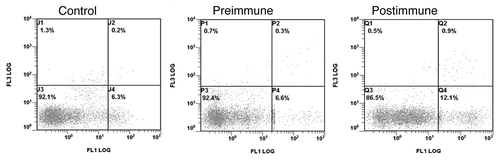
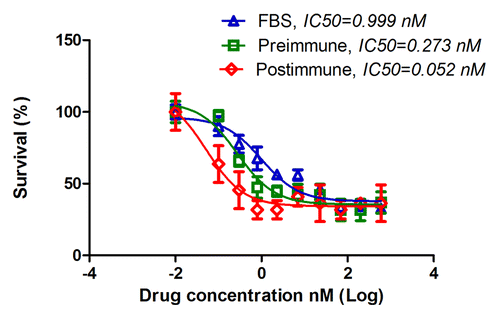
Figure 6. Preincubation with subject’s serum sensitized tumor cells to docetaxel toxicity. MDA-MB-231 cells were cultured in RPMI medium containing 10% FBS overnight. Medium was then replaced with one that contained pre or postimmune sera. FBS was used as control. After 5 h of incubation docetaxel was added in serial dilutions into wells. Twenty-four hours later wells were washed and live cells were fixed, stained and counted. Cell survival was determined and IC50 were estimated. Postimmune IC50 is significantly different that FBS and Preimmune IC50s, with P values of 6.21E-08 and 0.002, respectively.
Patient’s Clinical Status
Regression of lung metastases
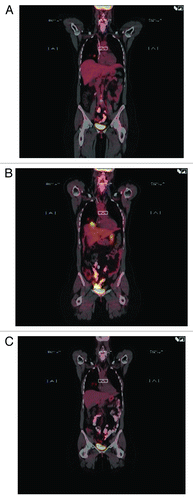
Other than local reactions with indurations at the site of injections, the vaccine did not have any side effects. After vaccination started, serial PET scans initially showed increased fluorodeoxyglucose (FDG) activity in the previously known lung metastases without increase in their size or number (; A, baseline; B, increase FDG uptake), then decrease of the FDG activity while her systemic treatment remained the same ().
Brain lesions
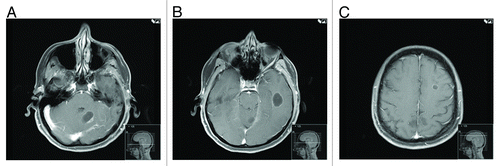
On May 18th 2012, she reported left arm weakness and ataxia. MRI of the brain showed three well-defined complex cystic masses in the brain parenchyma with no vasogenic edema around them (). On July 2nd 2012, she underwent left suboccipital craniotomy for resection of left cerebellar hemisphere lesion, and left frontotemporal craniotomy for resection of left inferior temporal cystic lesion. On July 12th 2012, she was started on fractionated stereotactic radiation therapy to the cystic lesions followed by whole brain radiation therapy.
Figure 8. MRI of the brain done on 5/11/2012. Cystic lesions were seen in the cerebellum (A), temporal (B), and frontal (C) lobes. Resection of the two large lesions showed no viable tumor on pathology specimens. PET scan done around the same time showed return of the lung lesions to baseline suggesting maximal response in the lung and possibly in the brain, which might explain the absence of viable tumor on pathology.
Specimens from the resection of the brain lesions showed mainly superficial cortical fragments, with scattered white matter fragments. Cytokeratin (AE1/AE3) and CAM 5.2 immunohistochemistry, performed on both specimen parts, were negative for epithelial cells. Multiple additional sections (levels ×3), obtained on both specimen parts, showed no other findings. Scattered CD3+ T-cells, mainly associated with vessel lumina and hemorrhagic areas, were present in the parenchyma, but the significance was not clear, though it likely did not indicate a significant inflammatory/infectious process. No CD20+ cells were observed, except for an occasional one in the vessels. Kappa and lambda light chains had high background, but there seemed to be a stronger staining in the vessels, likely associated with the plasma, probably as would be expected normally. Together with the virtual absence of CD20+ cells, no cellular staining was identified in the parenchyma. CD56 was tested for to identify any possible natural-killer cells, and was diffusely positive in neural tissue, making it very difficult to interpret; however, no significant cells that were positive for this antibody were identified. In addition, after reviewing the slides in the light of this new information, no inflammatory, infectious or reactive changes were identified. Specifically, no viral inclusions, microglial proliferation or nodules, macrophages, reactive astrocytes or necrotic cells were identified. Therefore, the current findings do not explain the nature of these cystic lesions described radiologically.
The subject’s neurologic deficit recovered for the most part but she still has some lack of coordination in her left arm and difficulty with word finding. Otherwise, she was able to go back to work fulltime, and her last MRI showed continued decrease in size of her lesions. She was switched to Docetaxel, Pertuzumab and Trastuzumab in September 2012 due to progression of her disease in the lungs. Her last PET scan from March 2013 showed complete response in the lungs and lymph nodes.
Discussion
The real goal of effective immune therapy targeting cancer-cell dissemination is to show that induced immune responses can result in tumor-cell death. Cells resistant to cell death metastasize, and overcoming this resistance is of therapeutic significance as a key cause of treatment failure. Despite the attention to T cells in cancer immunity, antibodies are hallmark components of anti-cancer activity. This raises expectations of therapeutic development of antibodies derived from the promise of increased biological potency beyond the stoichiometrically binding antibodies that just interfere with receptor binding. Preclinical studies support the hypothesis that antibody-induced responses against TACAs might have their greatest impact in the adjuvant setting, as such responses inhibit tumor outgrowth in metastatic models. Such observations suggest that sustained immunity against TACAs might have a beneficial effect on the course of malignant disease and long-term patient survival,Citation19,Citation20 because it is postulated that anti-carbohydrate antibodies are part of immune surveillance mechanisms.Citation21 The P10s-PADRE vaccine was developed as a broad-spectrum immunogen to induce responses to TACAs. The vaccine was shown to induce antibodies to P10s in subjects immunized with the vaccine and induced antibodies that bind to disparate representative human breast cancer cell lines, inhibiting migration, and mediating cytotoxicity.
Among the participants in the P10s vaccine study was one with an unusual disease course. Fourteen weeks after she received the vaccine, her PET scan showed increased uptake in her lung lesions without increase in their size. This was followed by improvement of her lung lesions six months later at the time that she was diagnosed with multiple brain lesions. An inflammatory response targeting the lung lesions and coinciding with the rise of the anti-P10 titers may have caused the increase in the uptake on PET scan in that area.
Anti-P10 IgM and IgG antibody titers peaked on week 7 after vaccination and remained high for the IgG while the IgM titers dropped to their baseline on week 19. At the time she was diagnosed with the brain lesions, her lung metastases had returned to their baseline. Resection of two of the brain lesions showed no evidence of cancer. The third lesion was small and more solid, and was treated by gamma knife. The changes in her lung lesions at that time were interpreted as not consistent with progression, since their size had not changed. The brain lesions did not show any evidence of malignancy, nor did they show evidence of inflammation.
Two hypotheses were formulated to explain this finding. First, this was a complication of the vaccine. All the other subjects on the study were evaluated with MRIs of the brain and none showed similar lesions. Animal data using this and vaccines of the same class have not shown any central nervous toxicity. Second, the lesions were related to breast cancer metastases that were immunologically cleared by the anti-P10 antibodies. But if this were the case, why did we not see any immune cells in the operative specimen? It is possible that the clearance of these metastases took place around the same time when the immune response peaked in the lung around week 14. If this is true, then the lesions that were found were the ghosts of the old metastases, and the symptoms were related to the enlargement of the residual cystic lesions.
If the antibodies were really cytotoxic, why did her disease progress? It is possible that the tumor had two compartments: one, primitive, which does not express the antigens targeted by the antibodies, and a second, more differentiated, which expresses the antigens targeted by the anti-P10 antibodies. Another explanation is that the IgM and IgG act in two different ways and once the IgM response subsided her tumor started growing again. Preclinical studies of CMP support a role for IgM antibodies because of their avidity. However, serum antibodies at week 19 and week 7 are functionally the same (data not shown). It is also possible that the amount of antibody produced is not sufficient to eradicate all tumor cells.
It is possible, though, that the IgG antibodies play a role in sensitizing tumor cells for more efficient activity of the DPT therapy. Docetaxel, Pertuzumab and Trastuzumab activity are known to be sensitive to phosphorylation status of FAK pathways. While it is unknown if the P10s-PADRE vaccine is beneficial in the present case, it is possible that P10s-reactive antibodies contribute to FAK silencing known to promote the in-vitro efficacy of docetaxel in both taxane-sensitive and taxane-resistant cell lines and may serve as a novel therapeutic approach. The same is observed for Pertuzumab and for Trastuzumab. Combining of antibodies induced by P10s-PADRE and at least docetaxel might have a clear beneficial result.
While TACA-directed vaccines are touted to inhibit micrometasteses, very little is known if vaccines actually help in overcoming tumor-cell survival through inhibition of signaling mechanisms lending to cytotoxicity. The P10s-Padre vaccine was able to induce antibodies to P10s that were cytotoxic in vitro, and very likely in vivo, in a patient with HER2 positive-metastatic breast cancer. These antibodies are speculated to have altered the natural history of the disease in this patient for some time. The P10s vaccine might prove beneficial as a novel approach to inducing a broad-spectrum response to work in combination with other therapies. We continue our work to characterize their exact pharmacodynamics and improve our ability to harness their power to achieve complete eradication of all cancer cells.
Patients and Methods
Study design
We designed our trial as a dose-escalation study using what have been called “standard” or “conventional” methods.Citation22 Subjects were treated in groups of three, the first at the initial dose of 300 µg P10s-PADRE per injection. If there were no dose-limiting toxicities (DLTs) in this first group, we planned to escalate to the final dose of 500 µg P10s-PADRE per injection, and treat the next group at this final dose. If there were exactly one DLT in the first group, we planned to vaccinate three more patients at the initial dose, and escalate to the final dose only if no additional DLTs were observed. Assessment of safety in this manner was the primary outcome. We deemed all other endpoints to be secondary, and their analysis to be exploratory and hypothesis-generating. All patients were included in the safety and efficacy analyses.
Peptide synthesis
P10s (WRYTAPVHLGDG) was covalently attached to the Pan T cell peptide PADRE (dAKchAVAAWTLKAAdA; AmbioPharm, Inc). The peptide was synthesized according to Good Manufacturing Practice guidelines.
Vaccine administration
Two cohorts of three stage IV breast cancer patients were enrolled. The peptide vaccine was administered in liquid form, emulsified with the adjuvant Montanide ISA-51VG, (SEPPIC, Inc), by subcutaneous (SC) injections on 5 separate occasions during Weeks 1, 2, 3, 7, and 19. Initially, a single cohort was administered P10s-PADRE (300 μg/mL) formulated with MONTANIDE™ ISA 51 VG. Doses of P10sPADRE admixed with MONTANIDE™ ISA 51 VG was administered to subjects subcutaneously in rotating injection sites in the abdomen.
ELISA assay
We assessed the presence of anti-P10s antibodies in the sera of patients before and after vaccination with ELISA. ELISA plates were coated with 1 µg/well of the MAP version of P10s in carbonate-bicarbonate buffer (Sigma-Aldrich) overnight. Serial 2-fold dilutions of plasma and serum samples, from 1:100 (dilution step 0) to a final dilution of 1:12 800 (dilution step 7), were added after blocking wells with PBS containing 0.5% FBS and 0.2% Tween 20 (Blocking buffer) for 1 h at 37 °C. Serum dilutions in blocking buffer were incubated for two h at 37 °C. After washing, wells were incubated with HRP-conjugated rabbit anti-human IgM (Jackson ImmunoResearch Laboratories, Inc) and goat anti-human IgG (Sigma-Aldrich) for 1 h at 37 °C. Then Tetramethylbenzidine substrate (Sigma-Aldrich) was added and the reaction was stopped after 20 min. Plates were read using an ELISA reader at 450 nM. The resulting absorbance-vs.-dilution curves were used to estimate normalized endpoint titers.
Cell lines and tissue culture
Human breast cancer cell lines were purchased from ATCC. Cells were cultured in a base medium supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies), 50 units/mL penicillin, and 50 μg/mL streptomycin. Base media for MDA-MB-231 and HCC1954 were DMEM and RPMI (both from Fisher Scientific), respectively.
Flow cytometry
To assess antibody-binding, cells were harvested with GIBCO® enzyme-free cell-dissociation buffer (Life Technolgies), washed with flow-cytometry buffer (PBS containing 1% BSA and 0.1% sodium azide) and incubated with pre-immune and post-immune (Week 7) sera in the same buffer. Binding was visualized with a FITC-conjugated mouse anti-human IgG (BD Biosciences). Acquisition and analysis of data was performed using EPICS®XL™ flow cytometer and EXPO32 ADC software (Beckman Coulter).
Cell toxicity assay
To assess the cytotoxicity of subjects’ samples towards the cell lines, 5 × 10^4 (24-well plate) of the respective cells were seeded in medium containing 10% FBS. After 24 h, the medium was refreshed with media containing 10% preimmune or postimmune sera. 48 h after addition of sera, supernatants were removed, live cells fixed and stained with Crystal violet. Plasma toxicity was determined following the same procedure except that the incubation time with pre- and postimmune plasma was reduced to 24 h. Percentage of cytotoxicity was calculated as 100% minus the percentage of cells surviving relative to the number of cells in control wells. All samples were assayed in triplicate, and the average of the triplicates was taken to be the sample’s measured cytotoxicity.
Migration assay
Cell migration was assayed in Corning® Transwell Permeable Supports (Corning Inc) with polycarbonate membranes (6.5 mm, 8-μm pore size). The bottom surfaces of the membranes were coated with fibronectin (20 μg/ml) in serum-free medium overnight. Cells were starved overnight, then harvested and mixed with pre- and postimmune sera and plated at 1 × 10^4 into the upper chambers of the transwell plates. The lower chamber of each well was filled with complete medium (10% FBS) containing 5 μg/ml of fibronectin as an adhesive substrate. Cells were incubated overnight, fixed and stained using the DIF-Quick kit. Non-migrating cells were removed with cotton swabs. Migrated cells were then visualized under a light microscope and enumerated in three randomly chosen fields using a light microscope.
Drug treatment
MDA-MB-231 cells were cultured in RPMI medium containing 10% FBS overnight. Medium was then replaced with one that contained pre or postimmune sera. FBS was used as control. After 5 h of incubation, docetaxel was added in serial dilutions into wells. Twenty-four hours later wells were washed and live cells were fixed, stained and counted. Percentage of survival was calculated as the ratio of cell number at a given drug dose to the cell number at the “0” drug dose. IC50 for drug alone and all combinations were calculated and compared.
Statistical analyses
ELISA assays were repeated three times independently, and each individual experiment designed to have three replications for each sample and dilution tested. Titers were estimated from absorbance-vs.-dilution curves by linear regression. The intercept of the regression line for each subject’s pre-immune serum was defined as the absorbance cutoff for determining end-point titer for that subject, and the dilution where each sample’s regression line crossed the subject’s absorbance cutoff was defined to be the sample’s end-point titer. Extrapolation beyond one dilution below the minimum or above the maximum actual dilutions was not allowed. This procedure normalized the subject’s pre-immune titer to be 1:100, and subjects with a 10-fold or greater increase in end-point titer over the 1:100 titer are considered responders. Other assays were repeated at least twice, and means and SDs were calculated based on at least two replications. Two-tail Student t test and one-way ANOVA with Tukey post-hoc comparisons were used to compare the means. SAS v9.3 (The SAS Institute), Excel (Microsoft Corporation) and Prism 5 (GraphPad Software, Inc) softwares were used for statistical analyses. Prism 5 was used to fit dose response curves using survival data, generated in drug/plasma combination experiments, and to calculate and compare IC50.
| Abbreviations: | ||
| TACAs | = | Tumor-Associated Carbohydrate Antigens |
| CMPs | = | Carbohydrate Mimetic Peptides |
| VT | = | Vinorelbine and Trastuzumab |
| LeY | = | Lewis Y |
| ER | = | Estrogen receptor |
| FAK | = | Focal adhesion kinase |
| SLNB | = | sentinel lymph-node biopsy |
| MAP | = | Multiple Antigen Peptide |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the subjects that participated in this trial. This work was supported by a Clinical Translational Award from the Department of Defense Breast Cancer Program (W81XWH-06-1-0542). The project described was also supported by the Translational Research Institute (TRI), grant UL1TR000039 through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences and the UAMS Center for Microbial Pathogenesis and Host Inflammatory Responses at UAMS, P20 GM103625. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- OnoM, HakomoriS. Glycosylation defining cancer cell motility and invasiveness. Glycoconj J2004; 20:71 - 8; http://dx.doi.org/10.1023/B:GLYC.0000018019.22070.7d; PMID: 14993838
- HakomoriS. Carbohydrate-to-carbohydrate interaction, through glycosynapse, as a basis of cell recognition and membrane organization. Glycoconj J2004; 21:125 - 37; http://dx.doi.org/10.1023/B:GLYC.0000044844.95878.cf; PMID: 15483378
- PashovA, Monzavi-KarbassiB, RaghavaGP, Kieber-EmmonsT. Bridging innate and adaptive antitumor immunity targeting glycans. J: 354068. Epub2010; 352010:354015
- PashovA, Monzavi-KarbassiB, Kieber-EmmonsT. Glycan mediated immune responses to tumor cells. Hum Vaccin2011; 7:Suppl156 - 65; http://dx.doi.org/10.4161/hv.7.0.14578; PMID: 21321481
- AixinjueluoW, FurukawaK, ZhangQ, HamamuraK, TokudaN, YoshidaS, UedaR, FurukawaK. Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies: roles of anoikis. J Biol Chem2005; 280:29828 - 36; http://dx.doi.org/10.1074/jbc.M414041200; PMID: 15923178
- KlingerM, FarhanH, JustH, DrobnyH, HimmlerG, LoibnerH, MuddeGC, FreissmuthM, SexlV. Antibodies directed against Lewis-Y antigen inhibit signaling of Lewis-Y modified ErbB receptors. Cancer Res2004; 64:1087 - 93; http://dx.doi.org/10.1158/0008-5472.CAN-03-2435; PMID: 14871842
- LiuD, LiuJ, LinB, LiuS, HouR, HaoY, LiuQ, ZhangS, IwamoriM. Lewis y Regulate Cell Cycle Related Factors in Ovarian Carcinoma Cell RMG-I in Vitro via ERK and Akt Signaling Pathways. Int J Mol Sci2012; 13:828 - 39; http://dx.doi.org/10.3390/ijms13010828; PMID: 22312289
- AzizF, QiuY. The role of anti-LeY antibody in the downregulation of MAPKs/COX-2 pathway in gastric cancer. Curr Drug Targets2014; 15:469 - 76; http://dx.doi.org/10.2174/1389450115666140217152042; PMID: 24533777
- ChereshDA, PierschbacherMD, HerzigMA, MujooK. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J Cell Biol1986; 102:688 - 96; http://dx.doi.org/10.1083/jcb.102.3.688; PMID: 3005335
- ChenYX, ChenXW, LiCG, YueLJ, MaiHR, WenFQ. Effect of tumor gangliosides on tyrosine phosphorylation of p125FAK in platelet adhesion to collagen. Oncol Rep2013; 29:343 - 8; PMID: 23117335
- Kieber-EmmonsT, LuoP, QiuJ, ChangTY, OI, Blaszczyk-ThurinM, SteplewskiZ. Vaccination with carbohydrate peptide mimotopes promotes anti-tumor responses. Nat Biotechnol1999; 17:660 - 5; http://dx.doi.org/10.1038/10870; PMID: 10404158
- Monzavi-KarbassiB, ArtaudC, JousheghanyF, HenningsL, Carcel-TrullolsJ, ShaafS, KorourianS, Kieber-EmmonsT. Reduction of spontaneous metastases through induction of carbohydrate cross-reactive apoptotic antibodies. J Immunol2005; 174:7057 - 65; http://dx.doi.org/10.4049/jimmunol.174.11.7057; PMID: 15905549
- Monzavi-KarbassiB, HenningsLJ, ArtaudC, LiuT, JousheghanyF, PashovA, MuraliR, HutchinsLF, Kieber-EmmonsT. Preclinical studies of carbohydrate mimetic peptide vaccines for breast cancer and melanoma. Vaccine2007; 25:3022 - 31; http://dx.doi.org/10.1016/j.vaccine.2007.01.072; PMID: 17303294
- Monzavi-KarbassiB, LuoP, JousheghanyF, Torres-QuiñonesM, Cunto-AmestyG, ArtaudC, Kieber-EmmonsT. A mimic of tumor rejection antigen-associated carbohydrates mediates an antitumor cellular response. Cancer Res2004; 64:2162 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-03-1532; PMID: 15026358
- WondimuA, ZhangT, Kieber-EmmonsT, GimottyP, SproesserK, SomasundaramR, FerroneS, TsaoCY, HerlynD. Peptides mimicking GD2 ganglioside elicit cellular, humoral and tumor-protective immune responses in mice. Cancer Immunol Immunother2008; 57:1079 - 89; http://dx.doi.org/10.1007/s00262-007-0439-4; PMID: 18157673
- QiuJ, LuoP, WasmundK, SteplewskiZ, Kieber-EmmonsT. Towards the development of peptide mimotopes of carbohydrate antigens as cancer vaccines. Hybridoma1999; 18:103 - 12; http://dx.doi.org/10.1089/hyb.1999.18.103; PMID: 10211797
- LuoP, AgadjanyanM, QiuJ, WesterinkMA, SteplewskiZ, Kieber-EmmonsT. Antigenic and immunological mimicry of peptide mimotopes of Lewis carbohydrate antigens. Mol Immunol1998; 35:865 - 79; http://dx.doi.org/10.1016/S0161-5890(98)00067-4; PMID: 9839555
- HenningsL, ArtaudC, JousheghanyF, Monzavi-KarbassiB, PashovA, Kieber-EmmonsT. Carbohydrate mimetic peptides augment carbohydrate-reactive immune responses in the absence of immune pathology. Cancers (Basel)2011; 3:4151 - 69; http://dx.doi.org/10.3390/cancers3044151; PMID: 24213131
- LloydKO. Carbohydrate vaccines for the immunotherapy of cancer. Drug News Perspect2000; 13:463 - 70; PMID: 12937618
- RagupathiG, LivingstonPO, HoodC, GathuruJ, KrownSE, ChapmanPB, WolchokJD, WilliamsLJ, OldfieldRC, HwuWJ. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin Cancer Res2003; 9:5214 - 20; PMID: 14614001
- VollmersHP, BrändleinS. Natural antibodies and cancer. N Biotechnol2009; 25:294 - 8; http://dx.doi.org/10.1016/j.nbt.2009.03.016; PMID: 19442595
- RosenbergerWF, HainesLM. Competing designs for phase I clinical trials: a review. Stat Med2002; 21:2757 - 70; http://dx.doi.org/10.1002/sim.1229; PMID: 12228889