Abstract
In Italy, the introduction of Universal Varicella Vaccination (UVV) has been decided but postponed, as a national programme, until 2015, when data from Regions which have already implemented it will be available. Starting from 2003, eight Italian Regions (Basilicata, Calabria, Friuli Venezia Giulia, Apulia, Sardinia, Sicily, Tuscany and Veneto) have progressively introduced UVV, in their immunization programme, with different schedules in children aged 13–15 months and 5–6 years, currently a two-dose schedule is adopted by all Regions. In June 2013, an Interregional Group on Varicella Vaccination (IGVV) has been established in order to assess the effectiveness of varicella vaccination with standardized and shared tools. The aim of this study was to evaluate the impact of varicella vaccination on the incidence and hospitalizations due to varicella and its complications in the period 2003–2012 in order to support the Italian decision makers on the future national adoption. Preliminary data showed that a general reduction of incidence and hospitalization rates was observed in the study period, resulting in relevant savings for the National Health Service. Immunization coverage with first dose at 24 months of age was high in all Regions (84%–95%) in 2012. Adverse events due to varicella vaccines were rare and without permanent sequelae. Underreporting of varicella cases and delays in the administration of the first dose of varicella vaccines were the main critical issues. In conclusion, solid evidences in support of universal UVV arise from the experiences available today in Italy.
Introduction
Varicella is an acute, highly contagious viral disease with worldwide distribution. Mostly a mild disorder in childhood, varicella develops with a more prolonged course, and more severe complications and more disabling symptoms in adults.Citation1,Citation2 Therefore, the severity of varicella hospitalizations increases with age. The most frequent complications are skin and soft tissue superinfections, followed by neurological and pulmonary complications. Varicella disease may be fatal, especially in neonates and in immunocompromized persons. In the United States of America, prior to availability of varicella vaccines, about 4 million cases of varicella occurred each year from 1990 to 1994. Of these cases, approximately 10 000 required hospitalization. From a public opinion point of view, varicella is not commonly perceived as an important public health problem, but the socioeconomic consequences, in terms of disease burden and costs of hospitalization in industrialized countries should not be underestimated.Citation3,Citation4
Varicella vaccines
Monovalent and combined varicella vaccines are highly immunogenic and efficacious in preventing varicella disease. Efficacy is very high against severe varicella.Citation5,Citation6
A two-dose vaccination regimen results in higher seroconversion rates and vaccine efficacy. A second dose given 4–12 wk after primary immunization elicits comparable antibody responses to those following administration of a second dose at 3–6 y, however the optimal timing of the second dose is still under discussion. A recent study by Bonanni et al. suggests that a short interval between two doses might be preferable for reducing breakthrough varicella (BV).Citation7 Some other open issues remain concerning the persistence of immunity, the risk of complications in BV cases many years after vaccination,Citation8 the requirement and optimal scheduling for additional booster doses or the effects of varicella vaccination in the long-term (e.g., transfer of maternal antibodies to newborns from varicella-vaccinated mothers).
Varicella vaccination policy against varicella in European countries
With regard to varicella vaccination recommendations in EU/EEA countries, as of 2012 only five countries endorsed Universal Varicella Vaccination (UVV) for children at national level (Cyprus, Germany, Greece, Latvia, Luxemburg) and two countries at regional level (Spain, Italy).Citation9
In Italy, since the approval of the National Health Plan 2003–2005, it was recognized that the availability of effective vaccines against varicella allowed the start of preventive vaccination initiatives aimed at reducing the incidence of this important disease.Citation10 In the National Plan for Vaccination 2005–2007, varicella vaccination was recommended for persons at high risk of complications (contacts of immunocompromized subjects; health care workers (HCWs); childbearing age women; school personnel) and susceptible adolescents. UVV was only limited to Regions with specific vaccination programmes: in order to avoid a possible shift of infection towards adulthood, only Regions likely to guarantee a level of Vaccination Coverage (VC) over 80%, were recommended to introduce varicella vaccination.Citation11
To date, the introduction of UVV has been decided but postponed, as a national programme, until 2015, when data from Regions which already implemented it will be available. At the national level, varicella vaccination was recommended in adolescents not previously vaccinated and anamnestically negative for varicella infection, in particular the administration of two doses one month apart from each other is suggested.Citation12
In Italy, only eight Regions, out of 21, started a UVV programme at the time of our data collection: Apulia, Basilicata, Calabria, Friuli Venezia Giulia, Sardinia, Sicily, Tuscany, and Veneto.Citation13-Citation20
The population belonging to those eight Regions amounted to almost 23 Million people, about 40% of the Italian resident population in 2012.Citation21
In June 2013, an Interregional Group on Varicella Vaccination (IGVV) was spontaneously established with the aim to share epidemiological data to measure the impact of the UVV programmes started in the above mentioned Italian Regions. The aim of the IGVV is to provide and spread regional and aggregated data within the scientific community and institutions; to exchange experiences on the varicella vaccination; to compare vaccination schedules and strategies adopted; above all, to provide additional elements for the assessment of the epidemiological impact of the UVV programmes in order to support the Italian decision makers on the future national adoption.
The specific aims of this study were to show the impact of UVV programmes on varicella incidence trends and on hospitalizations due to varicella complications and to assess the total annual costs of hospitalization due to varicella in eight Italian Regions, in the period 2003–2012. Furthermore, vaccination coverage data and available adverse event reporting rates following varicella vaccinations, were also provided.
Results
Starting from 2003, the Italian Regions of Apulia, Basilicata, Calabria, Friuli Venezia Giulia, Sardinia, Sicily, Tuscany, and Veneto, have progressively introduced UVV, in their immunization programme, with different schedules in children aged 13–15 mo and 5–6 y. Currently, a two-dose regimen for varicella vaccination is implemented in the 8 Regions. In these Regions varicella vaccination was universally offered free of charge. Some differences could be found in the schedules adopted by the 8 Italian Regions. ()
Table 1. Year of Universal Varicella Vaccination introduction and schedules adopted in 8 Italian Regions
Since the introduction of UVV programme in each Region, a decrease in incidence rates has been observed. In varicella Regional incidence rates are reported comparing the relative time (in years) before and after the UVV programme introduction, corresponding to the time “zero”, in each Region. The reduction of varicella incidence was higher in Regions which started the programme earlier, namely in Sicily, Veneto, Apulia, and Tuscany. In those Regions the decrease of varicella incidence was almost linear, since the time “zero” onward. However, even in Basilicata and Calabria, the positive effect of the immunization programme is measurable after only two years of varicella vaccination introduction. On the other hand, in Sardinia and in Friuli Venezia Giulia, it is too early to measure a clear impact of UVV programme on incidence rates, because these two Regions implemented varicella vaccination only recently. As a matter of fact, in Sardinia only 6 out of 8 Local Health Units (LHUs) started the UVV programme, and in FVG incidence data for the first year of UVV introduction (2013), were not included in the observation period of this survey.
Figure 1. Varicella incidence rates (×1000) in 8 Italian Regions, before and after the time “zero” of Universal Varicella Vaccination introduction (time “zero” being the year of start of varicella vaccination implementation, which is different in the different Regions).
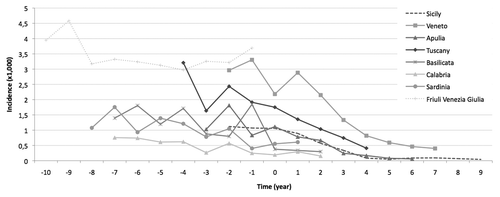
Reported incidence rates were higher in Regions of North and Central Italy (Friuli Venezia Giulia, Veneto, and Tuscany) while in the other Regions, belonging to Southern and insular areas, reported incidence rates were lower, possibly reflecting varying under-notification rates in the different areas of the country.
In , aggregated data obtained summing up all varicella official notifications reported in the 8 Regions are shown. Besides, incidence rates and 95% CI were also calculated for each year from 2003 to 2012. A downward trend in incidence rates is clearly observed in the entire period of observation. The highest incidence rate was observed in 2004 when only Sicily Region had already implemented the UVV programme. From 2006 onward, in the IGVV Regions, the incidence rates decreased constantly up to 2012, when varicella incidence rate reached the lowest annual level of all the period.
Figure 2. Varicella notifications, incidence rates and estimated incidence rates, assuming a 5-fold underreporting rate in the IGVV Regions (2003–2012). (Note: 95% CI for estimated incidence rates are reported in square brackets).
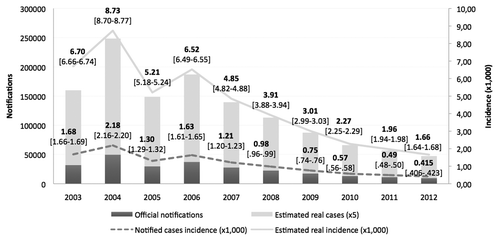
Moreover, based on the most recent estimates provided by the Italian sentinel networks of primary care pediatricians (SPES, Sentinel Pediatric Surveillance), we have assumed an average varicella underreporting rate of 5 for mandatory notification for the whole period and for all age groups.Citation22,Citation23 ()
As far as age specific incidence rates are concerned, all Regions reported the highest percentages of notifications in individuals under 15 y of age. In those age groups, percentages of varicella notifications varied between Regions but were >80% of the overall notified cases in all of them (data not shown).
The number of hospitalizations due to varicella complications is underestimated in our study with a high likelihood because some Regions did not provide data for the entire period (Tuscany in 2003 and Calabria in 2003 and 2004). However, the reduction of hospitalizations, and therefore of the hospitalization rates, from 2004 to 2012 was almost 75%. ()
Figure 3. Hospitalizations and annual hospitalization rates due to varicella complications (×100 000) in 8 Italian Regions (2003–2012). [Tuscany: data not available for 2003; Calabria: data not available for 2003 and 2004] (Note: 95% CI for hospitalization rates are reported in square brackets).
![Figure 3. Hospitalizations and annual hospitalization rates due to varicella complications (×100 000) in 8 Italian Regions (2003–2012). [Tuscany: data not available for 2003; Calabria: data not available for 2003 and 2004] (Note: 95% CI for hospitalization rates are reported in square brackets).](/cms/asset/64280d8c-df04-4c81-a19e-f5423ec2ed64/khvi_a_984111_f0003.gif)
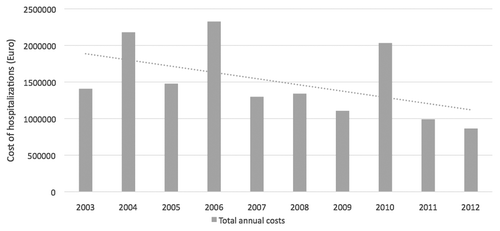
All these hospitalizations accounted for a high total cost in Euro. The total annual costs of hospitalizations due to varicella in 6 of the 8 Italian Regions, as primary and secondary diagnoses, are presented in . An almost linear decrease trend was registered after the UVV implementation. Indeed, costs of hospitalizations are underestimated since for two Regions (Sardinia and Calabria) data were not available for the entire period. Moreover, for two other Regions (Tuscany and Veneto) data were not available for the first year (2003). However, considering available data, a decreasing trend could be observed in the costs sustained by the Regional Health Service in the period of observation. In 2012, the annual costs settled to a value under 1 Million Euro, more than a half less of the value reported in 2004, when only Sicily Region had already implemented UVV. Comparing total annual hospitalization costs in 2004 and 2012, a general reduction of 60% was observed. Higher percentages of costs reduction were observed in Apulia (86%), Sicily (83%), Tuscany (77%), Veneto (75%), and Basilicata (71%), while in Friuli Venezia Giulia the reduction was modest, only 10%, because it was the last Region implementing a UVV programme.
Figure 4. Total annual costs of hospitalizations due to varicella, as primary and secondary diagnoses, in 6 Italian Regions (2003–2012) (Tuscany and Veneto: data not available for 2003; Sardinia and Calabria: data not available for the entire period).
Immunization coverage with varicella vaccines at 24 mo of age reached high levels in 2012 in all Italian Regions. The highest VC levels were registered in Apulia and Basilicata (over 90%); in Tuscany, Veneto and Sicily VC settled between 84% and 88%, while for Sardinia and Calabria only incomplete data were available. Data on VC in 2 out of 8 LHUs of Sardinia were available and settled at 72% and 82% in the LHU of Sassari and LHU of Cagliari, respectively. Finally, the UVV programme started in Friuli Venezia Giulia Region between March and April 2013, therefore, data on immunization coverage will be only available from 2015. As far as VC in Friuli Venezia Giulia is concerned, in only compliance to vaccination for the birth cohort 2012 was available and updated on April 29, 2014.
Table 2. Vaccination coverage for varicella vaccination at 24° months in 8 Italian Regions (2010 cohort), in 2012
Some Regions provided also data on adverse events following varicella immunization (AEFI) for the calculation of the adverse events reporting rate. () Veneto reported the highest rates in the entire period and Apulia the lowest. In Veneto, an active network of pharmacovigilance for monitoring vaccination safety was available since 1993.
Table 3. Reporting rate of adverse events following varicella immunization (AEFI) in 4 Italian Regions (2003–2012) [Data not available for Calabria, Friuli Venezia Giulia, Sardinia, Sicily]
Discussion
In Italy, during the last decade, Regions adopted different policies for vaccines not universally recommended at the national level. In particular, recommendations on varicella vaccination were heterogeneous and led to a marked discrepancy in vaccination coverage observed through the country.
The results of three surveys promoted by the Italian National Institute of Health (ISS) showed that Regions changed their recommendations between 2006 and 2010. In 2010, 7 Regions had adopted a UVV programme targeting children and susceptible adolescents. The remaining Regions offered varicella vaccination free of charge only to specific risk groups (such as child-bearing age women, health and school workers, household of individuals with immunodeficiency, chronic renal failure, HIV infection).Citation24
According to the results of the current study, the impact of a UVV programme on incidence rates was higher in Regions which started the programme earlier, i.e., Sicily, Veneto, Apulia, and Tuscany. In those Regions, an almost linear trend of varicella incidence decrease was observed, since the year “zero” of UVV introduction and up to 2012.
Sicily has been the first Italian Region to adopt in 2003 a one-dose UVV programme, with a coverage that, in a first period, ranged from 40% in 2003 to 60.5% in 2005. Both the initial suboptimal vaccine coverage and the one-dose strategy could explain the relatively slight decline in varicella morbidity that we have observed in the first years after the immunization introduction. Conversely, since 2007 vaccination coverage increased to 70% with a significant reduction of attributable morbidity that, as reported by other authors, in subjects under 15 y, passed from 95.7 to 9 cases/1000 person year. Accordingly, an important decrease in hospitalization due to varicella with complications (75% less) and without complications (80% less) was also observed.Citation25,Citation26 From 2010, the schedule was changed into a two-dose scheme and varicella immunization coverage increased steadily above 80%, being associated with a further reduction of varicella incidence to less than 0.1 cases/1000 person year.
In Veneto, a rapid implementation of the UVV programme allowed a drastic reduction of varicella incidence in all age groups. In 2004, there were about 60 600 new cases of varicella in Veneto, while the estimated number in 2008 dropped to 23 600 with an estimate of about 37 000 prevented cases. VC increased from 68% in 2006 to 79% in 2010. Hospitalization rates in Veneto decreased from 18.7/100 000 in 2000 to 8.4/100 000 in 2008.Citation27,Citation28 According to the results provided within this study, in Veneto in 2012 the VC at 24 mo increased again reaching values close to 90% (87.8%), and the decreasing trend in hospitalization rates continued until 2012, when it dropped to 0.8/100 000.
Since 2006, in Apulia, a decrease in incidence rates based on official notifications was observed, passing from 4500 varicella cases in 2006 to about 230 cases in 2012, corresponding to incidence rates of 1.1/1000 and 0.56/1000, respectively in 2006 and 2012. Even hospitalization rates decreased in the entire period of observation. All these results can be surely attributed to the high VC for varicella vaccines at 24 mo, that in 2012 reached 91.1%. Moreover, in Apulia, data on VC at 5–6 y of age and at 15 y were also provided. In 2012, VC for varicella reached the value of 64.8% and 28.8% for the birth cohorts 2005 and 1997, respectively.
In Tuscany VC at 24 mo for varicella reached 75% in 2010 and 84% in 2012. A reduction in notifications of 60% after four years of UVV implementation was observed. Therefore, the average incidence for varicella halved passing from a value of 2.3/1000 before varicella introduction (2004–2007) to a value of 1/1000 inhabitants in the vaccination period (2009–2012). The reduction of cases was observed in all age groups. After the introduction of the UVV programme in Tuscany, even hospitalizations and costs due to hospitalization for varicella decreased rapidly. Moreover, two further results were observed in Tuscany; a reduction in the severity of varicella complications because cases of post-varicella encephalitis halved in the vaccination period, and the number of haemorrhagic pneumonia due to varicella decreased to one fifth, besides an increase in the appropriateness of hospital admissions was also observed, due to decrease of varicella uncomplicated cases hospitalized.Citation29,Citation30
In Basilicata, VC at 24 mo for varicella reached the objective of 95% already in 2012. Therefore, incidence and hospitalization rates decreased already two years after UVV introduction, and the number of encephalitis cases due to varicella dropped to zero in the two-year vaccination period.
In the first year of UVV introduction in Calabria (2010), the adherence to varicella vaccination was 71%, but a decline in varicella vaccination compliance in a LHU of Calabria was observed the following year (55% in the birth cohort 2011). The recommendation from the National Drug Agency (AIFA) in November 2011Citation31 stating that the quadrivalent MMRV vaccine should no longer be recommended for the first dose administration resulted in an initial confusion of both parents and health care workers (hygienists and pediatricians) which resulted in a decreased adherence to vaccination. Fortunately, the problem was overcome through the application of a model of vaccination counseling involving most parents on the choice of the quadrivalent or monovalent vaccine since the first dose. In Calabria, the main concern regards the lack of activation of a computerized registry for vaccination, making it extremely difficult to monitor vaccine supply and to recall subjects for the first dose as well as for the second dose.
In Sardinia, 13 000–16 000 cases of varicella were estimated each year, among them 10 000 cases occurred in pediatric age. Besides, varicella can present with complications in 3.5% of healthy children under 15 y of age.Citation32 A clear impact on varicella incidence and hospitalization rates could not be observed in our study, because of the very recent introduction of UVV. Moreover, at the end of 2011, as a consequence of AIFA recommendationCitation32 the MMRV vaccine was no longer recommended as first dose in most of LHUs and a decrease in VC was temporarily observed. Only in the LHU of Cagliari the programme was started and actively continued with MMRV as first dose.
In Friuli Venezia Giulia, 4000 varicella cases were annually registered and 20–40 hospitalizations due to varicella can be observed annually. The incidence rate for varicella in 2012 was 3.4/1000 inhabitants, and the hospitalization rate was 2.1/100 000 inhabitants. Having introduced only recently a UVV, Friuli Venezia Giulia can be considered epidemiologically similar to a Region without vaccination.
The results of our study showed a decrease in costs of hospitalization due to varicella in the period of observation. Since a change in the system of attribution of hospitalization codes did not occur during the study period, such clear decreasing trend is attributable to an increase in the use of varicella vaccines. Moreover, hospitalization costs are probably underestimated, mainly because not all the 8 Regions collected data for the entire period. Second, a certain degree of underestimation in hospitalization costs, based on DRG, was already assessed in Italy by Azzari et al.Citation33 In addition, in the same study by Azzari et al., the highest rate of hospitalization (85.1%) was found in children <4 y of age and the largest number of complications (87.2%) occurred in previously healthy children. Considering that >85% of varicella cases occurred in subjects under 15 y, who are the main target of varicella vaccination, all Italian children and adolescents could receive a great benefit from UVV introduction at the national level because varicella vaccines have already demonstrated to protect from infection and from severe complications.
The persistence of varicella hospitalizations in the 8 Regions indicates the presence of a greater number of cases than those reported, and the need to better quantify the degree of under-notification, especially in some areas of the country.
In 1976–96, the degree of underreporting in Italy was estimated to be 7.7: approximately only 1 case of varicella out of 8 was notified in subjects between 6 m–20 y. Differences in underreporting rates were found between North, Centre, and South areas. Our results could likely reflect the degree of under-notification rates in the different areas of the country. For highly infectious diseases, like varicella, nearly all individuals will have been infected before adulthood, and, assuming a stable population, the number of births roughly corresponds to the number of cases. In Italy, during the second half of the Nineties, the average annual number of notified varicella cases was approximately 100 000 while the number of births in the same period was over 500 000 accounting for an underreporting ratio close to 5.Citation23
Our results are consistent with these estimates, in fact, considering the number of official varicella notifications in 2004 (when only Sicily had implemented UVV and all the 8 Italian Regions provided notification data), after the application of an underreporting factor of 5 to data collected in this study, the estimated number of varicella notifications reached a value close to 250 000 cases, a bit less than a half of a birth cohort of Italian population. Considering that the resident population in the 8 Regions participating to this study is near to 40% of the national population, a correspondence between our estimates and those already published can be found.
As a matter of fact, UVV programmes started in the 8 Italian Regions are likely to have prevented a number of cases almost 5-fold higher than that provided by official varicella notifications.
In Italy, a certain concern was raised on varicella vaccines, and in particular on the use of the MMRV vaccine after recommendations from the advisory vaccination committees in USA and Germany, and from the Italian Ministry of Health of preference for the use of monovalent vaccines for the first dose.Citation34,Citation35 In this study, a decrease of VC in specific areas was observed as a consequence of such recommendations.
Although the objectives set by the regional plans for VC have not yet been achieved, the use of the tetravalent MMRV vaccine has contributed substantially to the improvement of vaccine coverage, in some Regions (data not showed). The administration of varicella vaccine in the same vaccinating session of MMR, or better, the use of the quadrivalent MMRV vaccine avoids the delays of varicella immunization connected with the need to separate vaccination sessions when more than 3 injections should be given at the same time. Considering the delay for V vaccination, it might be useful to register routinary VC also at 36 mo of age (as in Veneto Region). In addition, registration of VC for the second dose at 6 y and in the adolescence at 16 y for 1st and 2nd dose should be introduced.
A limitation of our study is implied in the fact that the 8 Italian Regions adopted a UVV programme with different schedules and in different years, and therefore the periods before and after UVV introduction are different in length. Besides, collection of data was not completed for the entire period by all the 8 Regions, likely leading to an underestimation of the real burden of disease, especially concerning hospitalization. Furthermore, the information collected in this study does not allow to describe the post-marketing safety profile of the combination vaccine MMRV, with particular reference to the uncertainties on the increased risk of developing febrile seizures. More exhaustive investigations should be performed in order to assess the problem of the adverse events following varicella immunization for which in this analysis only a crude AEFI reporting rate was calculated from data obtained by half of the Regions included in this study.
Conclusion
A general reduction of incidence and hospitalization rates in all Regions after the UVV introduction is evident in our study. Despite mandatory varicella notification, likely underreporting in Italy continues to be high. A more sensitive surveillance system could reduce the degree of underreporting. Sentinel surveillance systems, such as the now discontinued pediatric surveillance network SPES, are more precise but their implementation is difficult and expensive.
Economic savings for the National Health Service, in terms of hospitalization reductions, were relevant but they are underestimated. In 2012, high varicella vaccination coverage rates at 24 mo were observed in all 8 Italian Regions. Adverse events due to MMRV (Measles-Mumps-Rubella-Varicella) vaccine were rare and without permanent sequelae, nevertheless, monitoring of varicella vaccination safety through an active pharmacovigilance system is necessary in order to increase the compliance to vaccination in the population.
Solid evidences in support of UVV arise from the experiences of the 8 Italian Regions where data are accumulating and/or are available to support the positive impact of UVV programmes.
The hope is that, in 2015, active free-of-charge UVV will be confirmed for all Italian children.
In such perspective, the MMRV vaccine is an essential tool, providing an organizational simplification and a reduction of accesses to immunization services.
Italian Regions are ready to implement a surveillance system including all the key elements suggested by ECDC. According to ECDC recommendations, the strategic elements to survey varicella epidemiology should be the monitoring of vaccine coverage, vaccine effectiveness, the occurrence of AEFI, the availability of age-specific disease incidence for varicella and age-specific incidence of severe disease (i.e., needing hospitalization). Surveillance for zoster is also needed to assess the impact of varicella vaccination on herpes zoster (HZ) and Italian Regions are moving forward to close the gap of knowledge in this field, too.Citation9,Citation36 Results provided by the IGVV members represent a further tool available for decision makers to assess the effectiveness of the UVV programme and to encourage (with data “on field”) the extension of universal varicella vaccination nationwide.
Materials and Methods
Members of the IGVV adopted a methodology for data collection from the regional archives of each participating Region. A common tool for data collection was delivered to all IGVV members on December 2013, in order to collect the same epidemiological data in the period 2003–2012. In particular, each participant was invited to fill in some work sheets produced in advance to automatically calculate: incidence rates based on varicella mandatory notifications, by year and by age group; hospitalization rates of complications due to varicella; the total costs of hospitalization due to varicella or its complications, as primary and secondary diagnoses; adverse events following varicella immunization. Moreover, vaccination coverages (VC) at 24 mo of age were also provided by IGVV members.
Incidence data
Varicella is subject to mandatory notification in Italy, and data provided by IGVV members were routinely collected at Regional level and reported to the Italian infectious diseases surveillance system.Citation37 Data on mandatory notifications, for the calculation of the incidence rates, were collected from all 8 Regions in the whole period (excluding Tuscany in 2003). Incidence rates were presented separately, by Region and as aggregated data, summing up all varicella notifications reported in the 8 Regions. Incidence rates are reported comparing the relative time (in years) before and after the UVV programme introduction, corresponding to the time “zero”, in each Region. Moreover, the number of estimated notifications was calculated applying an underreporting factor of 5 to all official notifications, in order to calculate a possible and probably more realistic scenario of varicella burden of disease. This is probably a conservative figure because in different age groups and geographic areas the underreporting factor could be higher, especially in older subjects and in the Southern Regions of Italy.Citation22,Citation23 95% CI for incidence rates were calculated for each year from 2003 to 2012.
Hospitalization data
Hospitalization data, covering the period 2003–2012, were collected by IGVV members of the 8 Regions, and all hospitalized cases for varicella or its complications, as a primary or secondary discharge diagnosis, were examined. In particular all hospital discharge records with the ICD9-CM code 052 (052.0, post-varicella encephalitis; 052.1: haemorragic pneumonia due to varicella; 052.2, myelitis post-varicella; 052.7, varicella with other specified complications; 052.8, varicella with other complications not specified; 052.9, varicella without mention of complication) were included in the analysis. 95% CI were calculated for hospitalization rates for each year from 2003 to 2012. Annual hospitalization rates were calculated on the resident population of the 8 Regions.Citation21
Costs of hospitalization
In Italy, hospital discharge records represents the regional reimbursement document for the hospitals or health facilities, thus each hospitalization is associated with a cost, based on DRG (Diagnosis Related Groups), which is covered by the Regional/National Health Service. Costs of hospitalization due to varicella (ICD9-CM code 052) were collected in the period 2003–2012. Data on costs of hospitalization were collected only from 6 out of 8 Regions in the whole period. Sardinia and Calabria did not provide data on costs of hospitalization while for Tuscany and Veneto data for 2003 were not available.
Vaccination coverage
Each IGVV member provided data on available VC at 24 mo for all the available years since UVV introduction.
Adverse events following immunization
Adverse events following varicella vaccines were collected from the national pharmacovigilance system and a crude AEFI reporting rate was calculated on the number of administered varicella vaccine doses, by year.
| Abbreviations: | ||
| IGVV | = | Interregional Group on Varicella Vaccination |
| UVV | = | Universal varicella vaccination |
| MMRV | = | Measles-Mumps-Rubella-Varicella quadrivalent vaccine |
| V | = | varicella monovalent vaccine |
| HCW | = | Health Care Worker |
| LHU | = | Local Health Unit |
| SPES | = | Sentinel Pediatric Survellance |
| PCV | = | Pneumococcal conjugate vaccine |
| Men C | = | Meningococcal conjugate C vaccine |
| HA | = | Hepatitis A vaccine |
| DRG | = | Diagnosis Related Groups |
| HZ | = | Herpes zoster |
| AE | = | Adverse Event |
| AEFI | = | Adverse event following immunization |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The Authors are grateful to the Regional Health Authorities of the 8 Regions included in this study for supplying data useful for the manuscript preparation.
References
- MillerE, VardienJ, FarringtonP. Shift in age in chickenpox. Lancet1993; 341:308 - 9; http://dx.doi.org/10.1016/0140-6736(93)92663-E; PMID: 8093945
- GuessHA, BroughtonDD, MeltonLJ3rd, KurlandLT. Population-based studies of varicella complications. Pediatrics1986; 78:723 - 7; PMID: 3763290
- FairleyCK, MillerE. Varicella-zoster virus epidemiology--a changing scene?. J Infect Dis1996; 174:Suppl 3S314 - 9; http://dx.doi.org/10.1093/infdis/174.Supplement_3.S314; PMID: 8896538
- WHO. Varicella vaccines. WHO position paper. Wkly Epidemiol Rec1998; 73:241 - 8; PMID: 9715106
- HeiningerU, SewardJF. Varicella. Lancet2006; 368:1365 - 76; http://dx.doi.org/10.1016/S0140-6736(06)69561-5; PMID: 17046469
- Gershon A, Takashaki M, Seward J. Varicella Vaccine. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 1596 6th Edition ed. Philadelphia: Saunders- Elsevier; 2013. p. 837-69.
- BonanniP, GershonA, GershonM, KulcsárA, PapaevangelouV, RentierB, Sadzot-DelvauxC, UsonisV, VesikariT, Weil-OlivierC, et al. Primary versus secondary failure after varicella vaccination: implications for interval between 2 doses. Pediatr Infect Dis J2013; 32:e305 - 13; http://dx.doi.org/10.1097/INF.0b013e31828b7def; PMID: 23838789
- AsanoY. Varicella vaccine: the Japanese experience. J Infect Dis1996; 174:Suppl 3S310 - 3; http://dx.doi.org/10.1093/infdis/174.Supplement_3.S310; PMID: 8896537
- European Centre for Disease Prevention and Control. Varicella vaccine in the European Union Stockholm: ECDC; 2014
- Ministry of Health. National Health Plan 2003-2005. [Piano Sanitario Nazionale 2003-2005.] Italian Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_948_allegato.pdf
- State-Regions Conference. National Plan for Vaccination 2005-2007. [Conferenza Stato-Regioni. Nuovo Piano Nazionale Vaccini 2005–2007. Gazzetta Ufficiale n.86 del 14-04-2005. Supplemento ordinario n. 63.] . Italian Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_543_allegato.pdf
- Ministry of Health. National Plan for Vaccination Prevention 2012-2014. [Piano Nazionale Prevenzione Vaccinale (PNPV) 2012-2014. Gazzetta Ufficiale n. 60 del 12.03.2012 (Supplemento Ordinario n.47)]. Italian Available from: http://www.salute.gov.it/imgs/c_17_pubblicazioni_1721_allegato.pdf
- Regione Sicilia. Circolare regionale del 22 luglio 2002, n. 1087. Gazzetta Ufficiale Regione Siciliana, 16 agosto 2002. Italian
- Regione Puglia. Deliberazione della giunta regionale 30 dicembre 2005, n. 2037. Piano Regionale della Prevenzione 2005-2007. Approvazione del Piano regionale Vaccini triennio 2005-2007. Bollettino Ufficiale della Regione Puglia n. 12 del 24.01.2006. Italian.
- Regione Sardegna. Deliberazione n.24/51 del 27.6.2013. Recepimento del “Piano Nazionale Prevenzione Vaccinale 2012-2014”. Italian
- Regione Basilicata. Deliberazione Giunta regionale. Calendario Vaccinale Regione Basilicata. Bollettino Ufficiale della Regione Basilicata n. 46 del 16.11.2010. Italian.
- Regione Calabria. Decreto Regionale n. 11096 del 29.7.2010 “Approvazione Calendario vaccinale regionale per l’età evolutiva (0-18 anni)”. Bollettino Ufficiale della Regione Calabria n. 34 del 27.8.2010. Italian
- Regione Toscana. Aggiornamento direttive regionali in materia di vaccinazioni. Revoca delibere n. 1249 del 24/11/2003, n.379 del 7/3/2005 e n.1060 del 10/10/2000. Modifica delibera n. 1386 del 17/12/2001. Deliberazione Giunta Regionale n. 1020 del 27.12.2007. Bollettino Ufficiale della Regione Toscana, n. 2 del 9.01.2008. Italian
- Regione Veneto. Delibera della Giunta Regionale Deliberazione Giunta Regionale n.411 del 28.02.2008 “Approvazione del Calendario Vaccinale della Regione Veneto”. (DGR n.4403 del 30.12.2005 “Modifiche e integrazioni”). Italian
- Regione Friuli Venezia Giulia. Deliberazione Giunta Regionale n° 1311 del 25.7.2012. Estensione dell’offerta vaccinale nella Regione Friuli Venezia Giulia. Bollettino Ufficiale della Regione autonoma FVG n. 36 del 5.09.2012. Italian
- ISTAT. Geodemo. Italian resident population (2003-2012). Available from: http://demo.istat.it/.
- Ciofi degli Atti ML. Sentinel Pediatric Surveillance in Italy: Results from 2000. BEN Notiziario ISS 2001; 14. Italian
- Ciofi degli AttiML, RotaMC, MandoliniD, BellaA, GabuttiG, CrovariP, SalmasoS. Assessment of varicella underreporting in Italy. Epidemiol Infect2002; 128:479 - 84; http://dx.doi.org/10.1017/S0950268802006878; PMID: 12113493
- AlfonsiV, D’AnconaF, GiambiC, NaccaG, RotaMC, Regional Coordinators for Infectious Diseases and Vaccinations. Current immunization policies for pneumococcal, meningococcal C, varicella and rotavirus vaccinations in Italy. Health Policy2011; 103:176 - 83; http://dx.doi.org/10.1016/j.healthpol.2011.10.002; PMID: 22030308
- GiammancoG, CiriminnaS, BarberiI, TitoneL, Lo GiudiceM, BiasioLR. Universal varicella vaccination in the Sicilian paediatric population: rapid uptake of the vaccination programme and morbidity trends over five years. Euro Surveill2009; 14:19321; PMID: 19728978
- Cuccia M, Pollina Addario S, Cernigliaro A, Palmigiano V. Ospedalizzazione per varicella in Sicilia dopo l'introduzione della vaccinazione. Ben Notiziario ISS 2009;22(3). Italian
- BaldoV, BaldovinT, RussoF, BusanaMC, PiovesanC, BordignonG, GilibertiA, TrivelloR. Varicella: epidemiological aspects and vaccination coverage in the Veneto Region. BMC Infect Dis2009; 9:150; http://dx.doi.org/10.1186/1471-2334-9-150; PMID: 19737419
- PozzaF, PiovesanC, RussoF, BellaA, PezzottiP, Emberti GialloretiL. Impact of universal vaccination on the epidemiology of varicella in Veneto, Italy. Vaccine2011; 29:9480 - 7; http://dx.doi.org/10.1016/j.vaccine.2011.10.022; PMID: 22015389
- Bechini A, Boccalini S, Levi M, Bonanni P. Universal varicella vaccination programme in Tuscany region (Italy), 2008-2011: Impact on disease incidence, immunization coverage and adverse reactions. Abstract reference number: A-534-0044-00814. ESPID, 32nd Annual Meeting, Milan 2013
- Boccalini S, Bechini A, Levi M, Bonanni P. Impact of universal varicella vaccination on hospitalisation in children under 15 years in Tuscany region, Italy. Abstract reference number: A-534-0044-00803. ESPID, 32nd Annual Meeting, Milan 2013
- Working Group Pediatrico AIFA. Raccomandazioni in relazione all’utilizzo dei vaccini MPRV. 14/11/2011. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/raccomandazione_vaccino_mprv_14_novembre_2011.pdf
- Sotgiu G, Castiglia P, Solinas G, Desole M, Mela MG, Maida A. Aspetti epidemiologici della Varicella in Sardegna. Atti IX Conferenza Nazionale di Sanità Pubblica, Parma (Italy), 13-15 Ottobre 2005.
- AzzariC, MassaiC, PoggiolesiC, IndolfiG, SpagnoloG, De LucaM, GervasoP, de MartinoM, RestiM. Cost of varicella-related hospitalisations in an Italian paediatric hospital: comparison with possible vaccination expenses. Curr Med Res Opin2007; 23:2945 - 54; http://dx.doi.org/10.1185/030079907X242610; PMID: 17937842
- MarinM, BroderKR, TemteJL, SniderDE, SewardJF, Centers for Disease Control and Prevention (CDC). Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep2010; 59:rr-31 - 12; PMID: 20448530
- Robert-Koch-Institut. Zur Kombinationsimpfung gegen Masern, Mumps,Röteln und Varizellen (MMRV). Epidemiol Bull2011; 38:352 - 3
- Drwal-KleinLA, O’DonovanCA. Varicella in pediatric patients. Ann Pharmacother1993; 27:938 - 49; PMID: 8395918
- SIMI. Sistema Informatizzato Malattie Infettive. Available from: www.simi.iss.it
