Abstract
Today, immune compromised interferon-α-receptor deficient mice expressing hCD46 (IFNARCD46tg) are usually used for measles virus (MV) based vaccine characterization. However, for the development of MV-based recombinant vaccine candidates (rMV), an immune competent mouse model is desirable in order to induce and evaluate meaningful immune response. In this study, humoral and cellular immune response induced by rMV in immune competent mice expressing human MV receptor CD46 (hCD46tg) were compared with those induced in wild-type black/6, and IFNARCD46tg mice.
All three strains developed humoral and cellular response against MV, whereas only hCD46tg and IFNARCD46tg mice developed a humoral response against the transgene. Differences were observed in the magnitude of the response, where the IFNARCD46tg mice displayed the strongest immune responses, followed by the hCD46tg mice and the black/6 mice. Interestingly, hCD46tg and wt black/6 mice showed a predominant CD4+ T-cell response against MV-N, whereas IFNARCD46tg mice developed both, CD4+ and CD8+ T-cell response against MV-N. Analysis of the cytokine profile of MV-N specific CD4+ T-cells and transgene (SIVgag) specific CD8+ T-cells revealed qualitative differences of the T-cell responses; noticeably a significant reduction of the frequency of CD4+IL-2+ expressing cells in IFNARCD46tg mice as compared with hCD46tg or wt black/6 mice.
We show in this study significant quantitative and qualitative differences in immune responses between immune competent and immune-compromised mice. Our results therefore highlight the importance of the animal model and support the use of hCD46tg mice as mouse model for the characterization of the immunological profile induced by recombinant measles virus vaccine candidates.
Introduction
The live attenuated measles virus (MV) vaccine is one of few vaccines having an extensive safety and efficacy record, which is applicable in children and adults. The vaccine can be injected subcutaneously and/or dispensed as an aerosol.Citation1 These characteristics are very attractive for the development of MV as a candidate viral vector. The design, engineering and production of recombinant MV (rMV) from clinically approved vaccine strains is nowadays achieved using reverse genetic technology.Citation2,Citation3 Recombinant MV candidates expressing viral or non-viral antigens of pathogens including Hepatitis B,Citation4 Dengue,Citation5 HIV,Citation6 West Nile,Citation7 SIV,Citation8 SARS,Citation9 Malaria,Citation10 and HPVCitation11 have been generated and used as candidate vaccines in different animal models including transgenic mice (for review see ref. Citation12).
So far, rMV vaccine candidates were developed and assessed in MV-susceptible immune-compromised mice (IFNARCD46tg)Citation2,Citation7,Citation9,Citation13 or in non-human primate models.Citation4,Citation6,Citation14 The IFNARCD46tg mouse expresses the human cell surface receptor CD46Citation15 and lacks the interferon (IFN)-α-receptor subunit 1 (IFNAR), which renders this mouse very susceptible to viral infection. Indeed, the IFN-α/β cytokine family members share an ubiquitously expressed heterodimeric receptor composed of IFNAR1 and IFNAR2 that are necessary for signal transduction.Citation16 Type I IFNs orchestrate antiviral immune responses in many different ways, including the induction of cellular antiviral effectors,Citation17 by linking innate and adaptive immunity,Citation18 or by sustaining clonal CD4+ T-cell expansion.Citation19 The characterization of candidate vaccines in IFNARCD46tg mice might therefore lead to an incomplete immunological profile. The parameters that are routinely assessed for the characterization of rMV candidate vaccines such as humoral, cellular, and mucosal immunity, including memory response, epitope mapping, or T-helper phenotype may be affected by type I IFNs. Thus, it was necessary to use an immune competent animal model for the characterization of rMV candidate vaccines.
Several transgenic mice strains expressing the natural MV receptor [human CD46 or human Signaling Lymphocyte Activation Molecule (SLAM, also called CD150)] are available.Citation20–Citation24 Human SLAM has been described to function as receptor for most wild-type MV strains, whereas hCD46 is mostly used by the vaccine strains.Citation25,Citation26 hCD46tg mice were generated independently in 1996 by two different groups,Citation27,Citation28 and have mainly been used to study MV infection, pathology, tropism, and immunosuppression.Citation13,Citation29 The physio-pathological function of hCD46 has been studied in details demonstrating enhancement of infection in hCD46tg mice in vivo and in vitro.Citation13 The wild-type mice were usually not very susceptible to MV infection since neither CD46 nor SLAM is present or functional.Citation21,Citation30,Citation31 Although the efficiency of MV infection and replication in hCD46tg murine cells was slightly reduced compared with human cells, the potential of the hCD46tg mice as a model for the characterization of rMV vaccines remained.
We compared immune responses induced by rMV in three different mice strains, immune competent hCD46tg mice, wild-type black/6 mice and IFNARCD46tg mice. In this study, we demonstrate the importance of using an immune competent mouse model to assess the immunogenicity of rMV.
Results
Selection of the optimal hCD46tg mouse strain
Measles virus (MV) is known to infect human cells through docking to the hCD46 or through the Signaling Lymphocyte Activation Molecule (SLAM) receptor. These receptors were introduced into mice in order to render them susceptible to MV; the level of susceptibility is dependent on the expression of hCD46 at the surface of each cell.Citation15 Thus, we determined the cell surface expression of hCD46 by FACS on Peripheral Blood Mononuclear Cells (PBMCs) of three different hCD46tg mice strains, and compared them to black/6 wt mice and the commonly used immune compromised mouse model, the IFNARCD46tg mice. As presented in , hCD46tg-BCitation29 displayed the highest hCD46 expression with a median fluorescence intensity (fi) of 109, followed by hCD46tg-ACitation28,Citation32 mice PBMCs with a median of 89 fi, whereas hCD46tg-CCitation13 and IFNARCD46tgCitation13 mice showed lower hCD46 expression. Black/6 wt mice did not express the hCD46 receptor, corroborating previous reports.Citation22,Citation33 Interestingly, despite the differences between the mice strains, the hCD46 expression is remarkably homogeneous within each mouse strain.
Figure 1. Detection of hCD46 expression on murine PBMCs. Whole blood was isolated from individual mice of five different mouse stains (black/6, hCD46tg-A, hCD46tg-B, hCD46tg-C, and IFNARCD46tg) and stained against hCD46. Median fluorescence intensity was measured by FACS analysis.
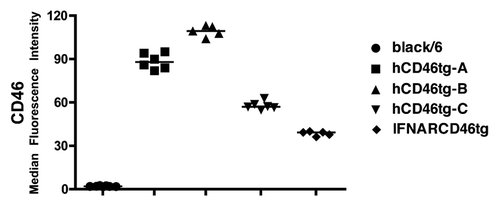
hCD46 expression was an important, but not the only, criteria for choosing the most suitable mouse model. The breeding capacity of each mouse strain was also evaluated and taken into consideration. Mouse strain hCD46tg-A was bred from a heterozygous hCD46tg-A male crossed with a wt black/6 female, which resulted in high breeding capacity. In contrast, homozygous hCD46tg-B and hCD46tg-C mice showed reduced breeding capacity and were prone to obesity (unpublished observation). Since hCD46 was highly expressed on the surface of PBMCs from hCD46tg-A mice, and due to high breeding capacity, we chose to continue this study with the hCD46tg-A strain.
Determination of the optimal immunization dose
Upon ensuring the replication capacity of rMV in hCD46tg-A mice (data not shown), optimal immunization dose was investigated. IFNARCD46tg mice are known to respond to a rMV immunization dose of 104 pfu/animal; however, this dose may not be optimal for immune competent black/6 wt or hCD46tg-A mouse strains. Optimal immunization dose was determined by a dose-response experiment. The animals were immunized i.m with either 103, 104, or 105 plaque-forming units (pfu) of rMVb. Sera were analyzed 6 wk post immunization for MV-IgG titers (). No significant humoral response was measured at an immunization dose of 103 or 104 pfu in both black/6 and hCD46tg-A mice. In contrast, IFNARCD46tg mice readily developed a significant humoral response against MV at 103 and 104 pfu/dose. However, a 105 pfu/dose was necessary to induce relevant immune responses in hCD46tg-A and to a less extent in black/6 wt mice.
Figure 2. Dose response experiment. A) black/6, hCD46tg-A, and IFNARCD46tg mice were immunized i.m with 103, 104, or 105 pfu rMVb. Anti-measles N humoral immune response was determined by ELISA 4 wk post immunization. ELISA positivity is defined as 3 × over negative control sera.
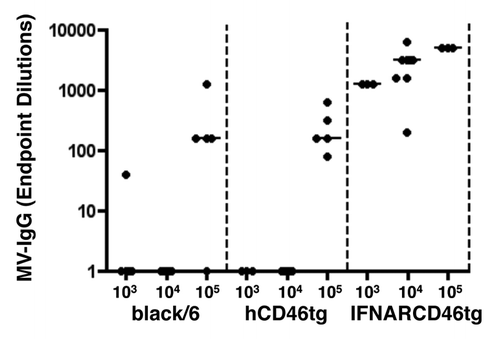
After a single immunization of 105 pfu/dose/animal, no significant difference was observed between MV-IgG titers of black/6 wt and hCD46tg-A mice. Yet, after a boost, hCD46tg-A mice showed a 51- fold increase of the median anti-MV IgG titers as compared with a 19-fold increase in black/6 mice, or a 9-fold increase in IFNARCD46tg mice (data not shown). The boosting capacity of the IFNARCD46tg mice was therefore reduced approximately by a 6-fold as compared with hCD46tg-A mice, which might suggest a saturation of the response. This prime-boost immunization regimen revealed not only quantitative differences, but also differences in the kinetics of the immune responses against MV between the different mouse strains. Finally, an immunization dose of 105 pfu rMV seemed to be optimal for hCD46tg-A mice and was thus used in subsequent experiments.
Analysis of the humoral immune response against rMVb2-HIVenv
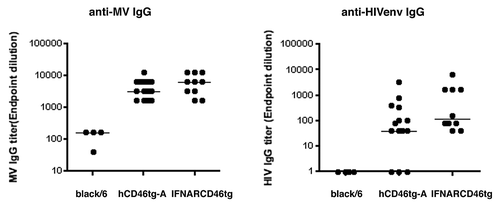
The humoral immune response (IgG) was characterized against the transgene (Env) expressed by rMVb2-HIVenv. All three mice strains were immunized intranasally (i.n) with 105 pfu rMVb2-HIVenv followed by an intramuscular (i.m) boost after 4 wk. Humoral responses against MV-N and HIVenv were assessed 6 wk post immunization (). hCD46tg-A and IFNARCD46tg mice developed comparable anti-MV-IgG titers, which ranged from 1:1000 to 1:100 000. Similarly, the humoral response against HIVenv was comparable between these two mice strains; however, the results were less homogenous, with anti-HIVenv titers ranging between 1:10 and 1:10 000. As expected, black/6 mice developed only minimal anti-MV-IgG and undetectable HIVenv humoral responses; indicating that rMV did not replicate well in black/6 mice.
Analysis of the cellular immune responses against rMV-SIVgag
Black/6, hCD46tg-A and IFNARCD46tg mice were immunized i.m, each with 105 pfu rMVb2-SIVgag. As shown in , each mouse strain developed cellular immune responses against the MV-N protein. IFNARCD46tg mice generated the largest number of MV-N specific IFNγ-secreting cells, with a median count of 1310 spot forming cells (SFC), followed by hCD46tg-A (271 SFC) and black/6 (156 SFC). The difference observed in numbers of IFNγ-secreting cell between black/6 and hCD46tg-A mice was significant (P = 0.003**).
Figure 4. Cellular immune response against rMV-SIVgag: Groups of eight mice of black/6 (black circle), hCD46tg-A (gray triangle), and IFNARCD46tg (gray square) mice were immunized i.m with 105 pfu rMV-SIVgag or 105 pfu UV inactivated rMV-SIVgag (UV-rMV-SIVgag). Cellular immune response was assessed by IFNγ ELISpot 2 wk post immunization. Median values are depicted as line. (A) Spot Forming Cells (SFC) against measles N. (B) SFC against SIVgag. *1 P = 0.003** / *2 P = 0.049* / *3 P = 0.015*
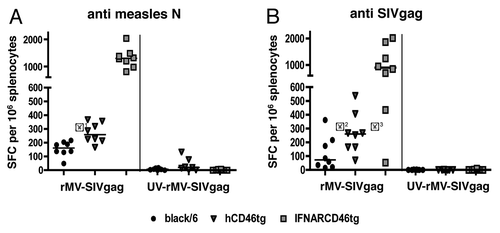
Cellular immune responses against the transgene SIVgag are shown in . Again, IFNARCD46tg mice generated the highest cellular immune response against SIVgag, with a median of 902 SFC, followed by hCD46tg-A (261 SFC), and black/6 (72 SFC). Differences observed between black/6 and hCD46tg-A, as well as differences between hCD46tg-A and IFNARCD46tg were statistically significant (P = 0.049* and P = 0.015*, respectively). Control immunization with UV-inactivated rMVb2SIVgag did not induce detectable immune responses against SIVgag, and only minor responses against MV-N.
In summary, significant differences in the magnitude of IFNγ-secreting cells were observed between all three mice strains for both, the MV-N and the SIVgag response. In general, IFNARCD46tg mice induced the highest cellular immune response, followed by hCD46tg-A, then black/6 mice.
Characterization of CD4+ and CD8+ response against recombinant measles vaccine expressing SIVgag
We determined more specifically the nature of the T-cell response involved against rMV and its transgene, i.e., whether it is a CD4+ or CD8+ T-cell specific response. Splenocytes of mice immunized with 105 pfu of rMVb2-SIVgag were restimulated in vitro in the presence of either MV-N or SIVgag peptide pools. IFNγ expression is shown for CD4+ or CD8+ T-cells (). Analysis of the immune response against MV-N revealed that all mice developed a CD4+ T-cell specific immune response. In contrast, only two out of five (2/5) black/6, 3/5 hCD46tg-A, but 5/5 IFNARCD46tg mice also developed significant MV-N-specific CD8+ T-cell responses. Similarly to the results obtained by ELISpot assay, IFNARCD46tg mice showed the highest frequency of MV-N-specific IFNγ-secreting CD4+ or CD8+ T-cells. As compared with IFNARCD46tg mice, hCD46tg-A and black/6 wt mice showed lower frequencies of IFNγ-secreting T-cells, with hCD46tg-A mice higher than black/6 wt mice ().
Figure 5. Intracellular cytokine expression profile of rMV-SIVgag induced by CD4+ and CD8+ T-cells against measles N or SIVgag as detected by intracellular cytokine FACS analysis. Five black/6, hCD46tg-A, and IFNARCD46tg mice were immunized with 105 pfu rMVb2-SIVgag. Splenocytes were isolated 2 wk post immunization and restimulated in vitro with either MV-N or SIVgag peptide pools. CD4+ and CD8+ T-cells were stained against IFNγ, TNFα, and IL-2. Negative controls, cultured with media alone, showed less than 0.04% double positive cells in the upper right quadrant when stained against IFNγ and CD4+ or CD8+ (data not shown). (A) Representative FACS results of the three mouse strains. Upper 2 figure lines show the response against MV-N. Lower 2 figure lines show the response against SIVgag. Percentages of IFNγ positive cells are calculated in relation to CD4+ or CD8+ positive cells only. (B) Percentile IFNγ, IL-2, TNFα cytokine distribution for CD4+ T-cells reactive against MV-N and CD8+ T-cells reactive upon SIVgag restimulation.
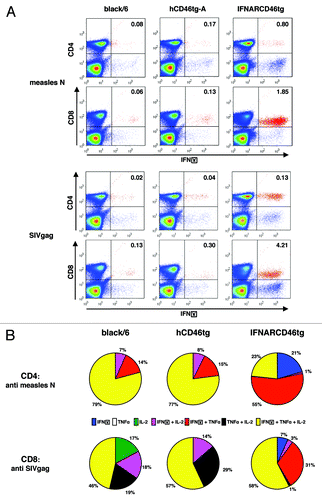
The cellular immune response against SIVgag was predominantly mediated by CD8+ T-cells after immunization with 105 pfu of rMVb2-SIVgag (). Only the IFNARCD46tg mice showed, additionally, a significant CD4+ T-cell response against SIVgag.
In summary, hCD46tg-A mice developed a predominant CD4+ T-cell response against the vector backbone (MV-N), but a predominant CD8+ T-cell response against the SIVgag transgene.
Intracellular cytokine expression profile of CD4+ T-cells reactive to MV-N and CD8+ T-cells reactive to SIVgag
To further characterize the predominant IFNγ-secreting T-cells, we determined the cytokine distribution of the CD4+ T-cells reactive to MV-N, and of the CD8+ T-cells reactive to SIVgag. 79% of all MV-N specific CD4+ T-cells in black/6 mice, or 77% hCD46tg-A mice co-expressed IFNγ, IL-2, and TNFα (). In IFNARCD46tg mice, this population was relatively small with 23%, but the predominant population of measles-N reactive CD4+ T-cells was IFNγ+/ TNFα+ double positive with 55% responders. Interestingly, it was the only mouse strain displaying solely IFNγ+ T-cells.
For the CD8+ T-cell reactive against SIVgag, the percentage of triple positive cells expressing IFNγ, IL-2, and TNFα was comparable between the three mouse strains with 46%, 57%, and 57% respectively. The second most predominant population in black/6 and hCD46tg-A mice was the IL-2+/TNFα+ double positive CD8+ T-cell, with 19% and 29%, respectively. Those were hardly detectable in IFNARCD46tg mice, while 31% of the CD8+ T-cell against SIVgag co-expressed TNFα+/ IFNγ+.
In conclusion, hCD46tg and black/6 mice showed comparable cytokine patterns of measles-N specific CD4+ T-cells and SIVgag specific CD8+ T-cells, whereas T-cells from IFNARCD46tg mice showed a different cytokine pattern with significantly less specific T-cells secreting IL-2.
Discussion
Recombinant measles vaccines (rMV) are being developed as vaccine candidates against several infectious diseases including HIVCitation3,Citation34 and Malaria.Citation35 Pre-clinical development of rMV includes the characterization of immune response in mice and/or primates; however, primate studies are logistically challenging and expensive. It is therefore highly desirable to first test vaccine candidates in a representative mouse model and study the reactions very carefully in preparation of primate studies. IFNARCD46tg mice, the current mouse model for rMV, lack the type I interferon (IFN) receptor, and are thus unresponsive to IFNα and IFNβ, and may not reflect the human immune system in an adequate manner.Citation36 The initiation of any host immune response against viral infections, however, strongly depends on the expression of type I IFNs.Citation37 We were thus convinced that a mouse model for the characterization of rMV should include type I IFN effects. Hence we established and optimized the use of an immune competent mouse model for the preclinical characterization of rMVs.
Since hCD46 is the cellular receptor for measles vaccine strains, we chose the hCD46tg-A strain for its high expression of hCD46tg, high breading capacity, and strain traceability.Citation28 Upon immunization, all three tested mouse strains, black/6, hCD46tg-A and IFNARCD46tg, developed an immune response against rMV. However, the strength of the immune response strongly varied depending on the strain and on the immunization dose. IFNARCD46tg mice developed a strong humoral immune response against rMV readily at low immunization titer of 103 pfu, whereas the optimal immunization dose for black/6 and hCD46tg-A was 105 pfu. Interestingly, anti-measles IgG titers in black/6 or hCD46tg mice were comparable after a single immunization. However, when assessed after a boost, measles IgG titer were significantly higher in hCD46tg mice compared with black/6. The greater boosting capacity in hCD46tg mice than in black/6 wt mice is likely due to a better replication of rMV in hCD46tg mice; contrary to IFNARCD46tg mice where the magnitude of immune response plateaued earlier.
Since measles vaccines have proven very effective by the intranasal/aerosol immunization route, we reproduced this immunization scheme in mice. In hCD46tg-A mice, an intranasal prime followed by and intramuscular boost led to 100% seroconversion for MV specific antibodies and 79% seroconversion for HIVenv specific antibodies. In this particular intranasal, intramuscular prime-boost immunization regimen, the antibody response in IFNARCD46tg mice was comparable to that of hCD46tg mice, both for the MV and the HIVenv responses. Black/6 mice, on the other hand developed only minimal MV response and undetectable HIVenv response. Prime-boost regimen are generally used to induce long-term humoral and memory immune responses.Citation38 Long-term humoral memory responses are difficult to assess in mice, unless challenge tests were performed. However, using the same rMV-HIVenv, a significant and long-term induction of neutralizing antibodies was observed in non-human primate model (unpublished data).
Cellular immune response against the vector and the transgene was assessed by ELISpot assays and intracellular cytokine FACS analysis. IFNγ ELISpot results showed the superiority of hCD46tg mice as compared with black/6 mice in the induction of anti-measles and anti-SIVgag specific cellular immune response. In contrast, the cellular immune response measured in IFNARCD46tg mice remained the strongest among the three mouse strains. Our FACS analysis revealed a CD4+ T-cell predominance in the immune response against measles N in black/6 and hCD46tg mice after a single immunization. The IFNARCD46tg mice developed both, a CD4+ and CD8+ T-cell response against measles N.In contrast, all three mouse strains developed a predominant CD8+ T-cell response against the SIVgag.
Assessement of the cytokine profile against the measles vector and its transgene revealed thatin all three mouse strains the immune response against measles N was predominant CD4+ T-cell whereas the immune response against SIVgag was predominant CD8+ T-cell. Differences were not only observed regarding the magnitude of immune response but also regarding the cytokine expression of T-cells. The cytokine profile of black/6 and hCD46tg-A mice was comparable for both measles specific CD4+ T-cell and SIVgag specific CD8+ T-cells. However, IFNARCD46tg mice showed generally reduced percentage of IL-2 producing specific T-cells resulting in reduced IFNγ+/TNFα+/IL-2+ triple positive T-cells. IL2 is involved in proliferation and maturation of specific CD8 T-cells and IL2 production from CD8 T-cells was shown to directly correlate with the effector cell differentiation of these cells.Citation39 This difference in the quality of the immune response highlights the importance of an intact immune system in order to induce a specific, mature, and long-lived immune response. For the development of a vaccine, the quality of the response might prevail on the quantity and it is important to define the characteristics of the cellular response induced by a vaccine.
Latest studies show that diverse immunization route lead to quantitatively and qualitatively different immune responses and that the immunization route as well as the immunization regimen probably require an approach tailored to the targeted virus.Citation40,Citation41 Here we disclosed significant quantitative and qualitative differences in immune responses between the immune competent hCD46tg-A mice and the immunocompromised IFNARCD46tg mice. This emphasizes the importance of the choice of the animal model in immunological studies and supports the use of immune competent mouse model.
In conclusion, hCD46tg-A mice developed cellular as well as humoral immune response against the MV vector and the transgene; thus, this mouse model can be recommended for investigating immune responses against rMVsvaccines.
Material and Methods
Viruses
Recombinant measles viruses were generated as previously described.Citation3,Citation34,Citation42 Briefly, 293–3-46 helper T-cells were transfected using a calcium phosphate protocol (Invitrogen) with full-length p(+)MV, and pEMC-La helper plasmid. The formation of syncytia, indicating successful rescue events, was monitored by microscopy. Single syncytia, representing individual clones of rMV, were picked and stored at −80 °C until use. Virus stocks were prepared on MRC-5 cells. The SIVgag gene was amplified from the p239SpSp5 plasmid received from Dr R AndinoCitation8 and the HIV antigens were obtained from Dr G Nable (VRC). Measles vector and transgene sequence stability were analyzed by RT-PCR after 10 passages on Vero cells. Transgene expression was confirmed by western blot and immunofluorescence.
Mice and immunization
B6.FVB-Tg(CD46)2Gsv mice, from here on called hCD46tg-A, were generated by Dr N Yannoutsos.Citation28 Both, black/6 wt and hCD46tg-A mice were obtained from Harlan BV.Citation32 hCD46tg-A mice were bred from heterozygous hCD46tg-A male crossed with black/6 wild-type females. Offspring carrying the hCD46 transgene was identified by PCR genotyping.
Human CD46tg-B homozygous mice were generated by Dr MB Oldstone,Citation29 and used here as a control. IFNARCD46tg and hCD46tg-C mice were generated by Dr B Mrkic,Citation13 and were maintained at Crucell, Berna. The animals were kept under optimal hygiene conditions according to the Swiss animal welfare regulations.
Mice were immunized intramuscularly (i.m.) or intranasally (i.n.) at the age of 6 to 12 wk with standard measles vaccine or recombinant measles virus (rMV) expressing Human Immunodeficiency Virus (HIV) clade B envelope protein (rMVb-HIVenv clade B) or rMV expressing the Simian Immunodeficiency Virus (SIV) gag protein (rMVb-SIVgag). The immunization dosage was typically 1 × 105 pfu or as indicated. Blood was collected retro bulbar at wk 4, 6, and 8.
Detection of hCD46
Mouse PBMCs were stained using a fluorochrome-conjugated human anti-CD46 mouse-specific antibody purchased from BD PharMingen. Samples were acquired on a FACS Calibur flow cytometer (BD Biosciences), and data were analyzed using the FlowJo software (TreeStar).
Briefly, whole blood was isolated from individual mice and added to 200 μl PBS 10 mM EDTA to prevent coagulation. Twenty microliters (20 μl) FITC anti-human CD46 antibody (BD PharMingen) was added to 100 μl blood-PBS solution and incubated for 30 min at 4 °C. Cells were fixed and erythrocytes lysed using a one-step approach with 3 ml BD FACS Lysis solution (BD Pahrmingen). Cells were washed twice with FACS Buffer (PBS, 2 mM EDTA, 1% FCS) prior acquisition.
Intracellular cytokine staining
The following antibodies and reagents were used for intracellular cytokine staining: Anti-CD4-FITC, anti-CD8-FITC, anti-TNFalpha-PE-Cy7, anti-IFNγ-PE, anti-IL-2-APC, anti-mouse CD16/CD32 Fc Block, anti-mouse CD49, anti-mouse CD28. All antibodies were obtained from BD Europe. BD Cytofix/Cytoperm Plus Kit (BD Europe) was used to fix and permeabilize the cells.
Two weeks post immunisation, spleens were meshed through a 70 µm cell strainer (BD Falcon) and rinsed with PBS containing 2% FCS. Erythrocytes were lysed using 1.5 ml ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA). Splenocytes were transferred to RPMI (RPMI1640 with L-Glutamine, 25 mM HEPES, Gibco) media containing 5% FCS (Gibco), 0.1 mM Non-Essential Amino Acids (Gibco), 1mM Sodium Pyruvate (Gibco), 0.5 mM 2-Mercaptoethanol (Gibco), 100 U/ml PenStrept (Gibco). One × 106 splenocytes per 96 well were restimulated with peptide pools in the presence of 1 µg/ml mouse anti-CD49 and 1 µg/ml mouse anti-CD28. After an incubation of 1 h at 37 °C, BD GolgiPlug was added according to the manufacturer’s instructions and incubated for an additional 4 h. After Fc receptor blocking, cells were stained against CD4 or CD8, fixed, permeabilized, and stained for 45 min against TNFα, IFNγ and IL-2 at 4 °C. Splenocytes were then washed twice in FACS buffer and fixed in BD cell fix (BD PharMingen). FACS analysis was performed within 24 h.
Peptides
Different peptide pools were used to restimulate splenocytes for FACS and ELISpot assays.
Peptides covering the SIVgag and HIVenv regions were obtained from the NIH AIDS Reagent and Reference Reagent Program. For SIVgag, SIVmac 239 Gag (15-mer) peptides were used at a final concentration of 250 nM per peptide; for HIVenvB, HIV-1 Con Subtype B Env (15-mer) peptides; Complete Set was used. Either a pool including all peptides was used at a concentration of 142 nM per peptide, or a pool of 40 peptide was used at a concentration of 750 nM per peptide. For the measles-N peptides, 15-mers spanning the whole measles N protein and overlapping by 10 aa were used at a final concentration 100 nM per peptide (Anawa Trading SA). Similarly, Np6 and Np9 peptidesCitation43 (Anawa) were used at a concentration of 4 µM per peptide.
ELISA assays
Anti-MV antibody titers were determined according to standard protocols.Citation35 Anti-HIVenv clade B IgG titers were determined using a commercial ELISA assay kit (Genscreen HIV1/2 ELISA Kit, Bio-Rad). Value three times above the mean of negative sera was set as cut-off. Titers are represented as log10 of the reciprocal end-dilution.
ELISPOT assay
Splenocytes were extracted as described above. IFN-γ ELISPOT assay was performed according to manufacturer’s instructions (BD IFNγ ELISPOT Set). Duplicates of 0.5 Mio or 0.2 Mio splenocytes per well were subsequently restimulated for 17–18 h at 37 °C in 5% CO2 with either an equal volume of medium (negative control), medium containing peptide pools, or medium containing PMA (20 ng/ml) Ionomycin (1 µM) as positive control. ELISPOT development was performed according to BD AEC Substrate Reagent Set. ELISpot plates were evaluated on an AID Viruspot Reader (Pharma Consulting Marion Senn GmbH). Values 3 × above background were considered positive. Test was considered valid with medium negative controls lower than < 50 spots per million splenocytes.
| Abbreviations: | ||
| fi | = | Fluorescence intensity |
| hCD46 | = | Human CD46 |
| HIV | = | Human immunodeficiency virus |
| IFNAR | = | Interferon-α/β receptor |
| MRC-5 | = | Human fetal lung fibroblast cells |
| MV | = | Measles virus |
| PBMC | = | Peripheral Blood Mononuclear Cell |
| pfu | = | Plaque forming units |
| rMV | = | Recombinant measles virus |
| SARS | = | Severe acute respiratory syndrome |
| SFC | = | Spot forming cells |
| SIV | = | Simian immunodeficiency virus |
| SLAM | = | Signalling lymphocyte activation molecule |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We would like to thank Kaspar Scherler, Jorge Barcos, Orhan Ilter, and Amanda Brogli for excellent technical support and animal care; Angelique Lemckert and Katarina Radosevic for organizing the hCD46tg-A mice; Armando Zuniga and Marian Wiegand for producing rMVs. We achnowledge the NIH AIDS Reagent and Reference Program for HIVand SIV anitbodies and peptides. This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract No. HHSN266200600018C to HYN.
References
- GriffinDE, PanCH, MossWJ. Measles vaccines. Front Biosci2008; 13:1352 - 70; http://dx.doi.org/10.2741/2767; PMID: 17981635
- CombredetC, LabrousseV, MolletL, LorinC, DelebecqueF, HurtrelB, McClureH, FeinbergMB, BrahicM, TangyF. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J Virol2003; 77:11546 - 54; http://dx.doi.org/10.1128/JVI.77.21.11546-11554.2003; PMID: 14557640
- ZunigaA, LinigerM, MorinTN, MartyRR, WiegandM, IlterO, WeibelS, BilleterMA, KnuchelMC, NaimHY. Sequence and immunogenicity of a clinically approved novel measles virus vaccine vector. Hum Vaccin Immunother2013; 9:607 - 13; http://dx.doi.org/10.4161/hv.23242; PMID: 23324616
- del ValleJR, DevauxP, HodgeG, WegnerNJ, McChesneyMB, CattaneoR. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J Virol2007; 81:10597 - 605; http://dx.doi.org/10.1128/JVI.00923-07; PMID: 17634218
- BrandlerS, Lucas-HouraniM, MorisA, FrenkielMP, CombredetC, FévrierM, BedouelleH, SchwartzO, DesprèsP, TangyF. Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus. PLoS Negl Trop Dis2007; 1:e96; http://dx.doi.org/10.1371/journal.pntd.0000096; PMID: 18160988
- LorinC, MolletL, DelebecqueF, CombredetC, HurtrelB, CharneauP, BrahicM, TangyF. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J Virol2004; 78:146 - 57; http://dx.doi.org/10.1128/JVI.78.1.146-157.2004; PMID: 14671096
- DesprèsP, CombredetC, FrenkielMP, LorinC, BrahicM, TangyF. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J Infect Dis2005; 191:207 - 14; http://dx.doi.org/10.1086/426824; PMID: 15609230
- WangZ, HangartnerL, CornuTI, MartinLR, ZunigaA, BilleterMA, NaimHY. Recombinant measles viruses expressing heterologous antigens of mumps and simian immunodeficiency viruses. Vaccine2001; 19:2329 - 36; http://dx.doi.org/10.1016/S0264-410X(00)00523-5; PMID: 11257357
- LinigerM, ZunigaA, TaminA, Azzouz-MorinTN, KnuchelM, MartyRR, WiegandM, WeibelS, KelvinD, RotaPA, et al. Induction of neutralising antibodies and cellular immune responses against SARS coronavirus by recombinant measles viruses. Vaccine2008; 26:2164 - 74; PMID: 18346823
- LiS, LockeE, BruderJ, ClarkeD, DoolanDL, HavengaMJ, HillAV, LiljestromP, MonathTP, NaimHY, et al. Viral vectors for malaria vaccine development. Vaccine2007; 25:2567 - 74; http://dx.doi.org/10.1016/j.vaccine.2006.07.035; PMID: 16914237
- CantarellaG, LinigerM, ZunigaA, SchillerJT, BilleterM, NaimHY, GlueckR. Recombinant measles virus-HPV vaccine candidates for prevention of cervical carcinoma. Vaccine2009; 27:3385 - 90; http://dx.doi.org/10.1016/j.vaccine.2009.01.061; PMID: 19200837
- BilleterMA, NaimHY, UdemSA. Reverse genetics of measles virus and resulting multivalent recombinant vaccines: applications of recombinant measles viruses. Curr Top Microbiol Immunol2009; 329:129 - 62; http://dx.doi.org/10.1007/978-3-540-70523-9_7; PMID: 19198565
- MrkicB, PavlovicJ, RülickeT, VolpeP, BuchholzCJ, HourcadeD, AtkinsonJP, AguzziA, CattaneoR. Measles virus spread and pathogenesis in genetically modified mice. J Virol1998; 72:7420 - 7; PMID: 9696838
- van BinnendijkRS, van der HeijdenRW, OsterhausAD. Monkeys in measles research. Curr Top Microbiol Immunol1995; 191:135 - 48; http://dx.doi.org/10.1007/978-3-642-78621-1_9; PMID: 7789157
- DörigRE, MarcilA, ChopraA, RichardsonCD. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell1993; 75:295 - 305; http://dx.doi.org/10.1016/0092-8674(93)80071-L; PMID: 8402913
- BaccalaR, KonoDH, TheofilopoulosAN. Interferons as pathogenic effectors in autoimmunity. Immunol Rev2005; 204:9 - 26; http://dx.doi.org/10.1111/j.0105-2896.2005.00252.x; PMID: 15790347
- SadlerAJ, WilliamsBR. Interferon-inducible antiviral effectors. Nat Rev Immunol2008; 8:559 - 68; http://dx.doi.org/10.1038/nri2314; PMID: 18575461
- Le BonA, ToughDF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol2002; 14:432 - 6; http://dx.doi.org/10.1016/S0952-7915(02)00354-0; PMID: 12088676
- Havenar-DaughtonC, KolumamGA, Murali-KrishnaK. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol2006; 176:3315 - 9; http://dx.doi.org/10.4049/jimmunol.176.6.3315; PMID: 16517698
- RallGF, ManchesterM, DanielsLR, CallahanEM, BelmanAR, OldstoneMB. A transgenic mouse model for measles virus infection of the brain. Proc Natl Acad Sci U S A1997; 94:4659 - 63; http://dx.doi.org/10.1073/pnas.94.9.4659; PMID: 9114047
- OhnoS, OnoN, SekiF, TakedaM, KuraS, TsuzukiT, YanagiY. Measles virus infection of SLAM (CD150) knockin mice reproduces tropism and immunosuppression in human infection. J Virol2007; 81:1650 - 9; http://dx.doi.org/10.1128/JVI.02134-06; PMID: 17135325
- YanagiY, HuHL, SeyaT, YoshikuraH. Measles virus infects mouse fibroblast cell lines, but its multiplication is severely restricted in the absence of CD46. Arch Virol1994; 138:39 - 53; http://dx.doi.org/10.1007/BF01310037; PMID: 7980010
- KemperC, LeungM, StephensenCB, PinkertCA, LiszewskiMK, CattaneoR, AtkinsonJP. Membrane cofactor protein (MCP; CD46) expression in transgenic mice. Clin Exp Immunol2001; 124:180 - 9; http://dx.doi.org/10.1046/j.1365-2249.2001.01458.x; PMID: 11422193
- ShingaiM, InoueN, OkunoT, OkabeM, AkazawaT, MiyamotoY, AyataM, HondaK, Kurita-TaniguchiM, MatsumotoM, et al. Wild-type measles virus infection in human CD46/CD150-transgenic mice: CD11c-positive dendritic cells establish systemic viral infection. J Immunol2005; 175:3252 - 61; http://dx.doi.org/10.4049/jimmunol.175.5.3252; PMID: 16116216
- ManchesterM, EtoDS, ValsamakisA, LitonPB, Fernandez-MuñozR, RotaPA, BelliniWJ, ForthalDN, OldstoneMB. Clinical isolates of measles virus use CD46 as a cellular receptor. J Virol2000; 74:3967 - 74; http://dx.doi.org/10.1128/JVI.74.9.3967-3974.2000; PMID: 10756008
- TatsuoH, OnoN, TanakaK, YanagiY. SLAM (CDw150) is a cellular receptor for measles virus. Nature2000; 406:893 - 7; http://dx.doi.org/10.1038/35022579; PMID: 10972291
- HorvatB, RivaillerP, Varior-KrishnanG, CardosoA, GerlierD, Rabourdin-CombeC. Transgenic mice expressing human measles virus (MV) receptor CD46 provide cells exhibiting different permissivities to MV infections. J Virol1996; 70:6673 - 81; PMID: 8794303
- YannoutsosN, IjzermansJN, HarkesC, BonthuisF, ZhouCY, WhiteD, MarquetRL, GrosveldF. A membrane cofactor protein transgenic mouse model for the study of discordant xenograft rejection. Genes Cells1996; 1:409 - 19; http://dx.doi.org/10.1046/j.1365-2443.1996.d01-244.x; PMID: 9135084
- OldstoneMB, LewickiH, ThomasD, TishonA, DalesS, PattersonJ, ManchesterM, HomannD, NanicheD, HolzA. Measles virus infection in a transgenic model: virus-induced immunosuppression and central nervous system disease. Cell1999; 98:629 - 40; http://dx.doi.org/10.1016/S0092-8674(00)80050-1; PMID: 10490102
- YanagiY, TakedaM, OhnoS. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol2006; 87:2767 - 79; http://dx.doi.org/10.1099/vir.0.82221-0; PMID: 16963735
- OnoN, TatsuoH, TanakaK, MinagawaH, YanagiY. V domain of human SLAM (CDw150) is essential for its function as a measles virus receptor. J Virol2001; 75:1594 - 600; http://dx.doi.org/10.1128/JVI.75.4.1594-1600.2001; PMID: 11160657
- VerhaaghS, de JongE, GoudsmitJ, LecollinetS, GillissenG, de VriesM, van LeuvenK, QueI, OuwehandK, MintardjoR, et al. Human CD46-transgenic mice in studies involving replication-incompetent adenoviral type 35 vectors. J Gen Virol2006; 87:255 - 65; http://dx.doi.org/10.1099/vir.0.81293-0; PMID: 16432010
- NanicheD, Varior-KrishnanG, CervoniF, WildTF, RossiB, Rabourdin-CombeC, GerlierD. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol1993; 67:6025 - 32; PMID: 8371352
- LinigerM, ZunigaA, MorinTN, CombardiereB, MartyR, WiegandM, IlterO, KnuchelM, NaimHY. Recombinant measles viruses expressing single or multiple antigens of human immunodeficiency virus (HIV-1) induce cellular and humoral immune responses. Vaccine2009; 27:3299 - 305; http://dx.doi.org/10.1016/j.vaccine.2009.01.057; PMID: 19200842
- ZunigaA, WangZ, LinigerM, HangartnerL, CaballeroM, PavlovicJ, WildP, ViretJF, GlueckR, BilleterMA, et al. Attenuated measles virus as a vaccine vector. Vaccine2007; 25:2974 - 83; http://dx.doi.org/10.1016/j.vaccine.2007.01.064; PMID: 17303293
- MüllerU, SteinhoffU, ReisLF, HemmiS, PavlovicJ, ZinkernagelRM, AguetM. Functional role of type I and type II interferons in antiviral defense. Science1994; 264:1918 - 21; http://dx.doi.org/10.1126/science.8009221; PMID: 8009221
- ZhangSY, JouanguyE, Sancho-ShimizuV, von BernuthH, YangK, AbelL, PicardC, PuelA, CasanovaJL. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev2007; 220:225 - 36; http://dx.doi.org/10.1111/j.1600-065X.2007.00564.x; PMID: 17979850
- NossalGJ. Vaccines of the future. Vaccine2011; 29:Suppl 4D111 - 5; http://dx.doi.org/10.1016/j.vaccine.2011.06.089; PMID: 22185835
- NomuraLE, EmuB, HohR, HaalandP, DeeksSG, MartinJN, McCuneJM, NixonDF, MaeckerHT. IL-2 production correlates with effector cell differentiation in HIV-specific CD8+ T cells. AIDS Res Ther2006; 3:18; http://dx.doi.org/10.1186/1742-6405-3-18; PMID: 16859558
- Combadiere B. Understanding Mechanism of Skin Vaccination and Tailored Immunity to Vaccines. Ninth World Congress on Vaccines, Immunisation and Immunotherapy 2014;abstract PS1-5.
- KnuchelMC, MartyRR, MorinTN, IlterO, ZunigaA, NaimHY. Relevance of a pre-existing measles immunity prior immunization with a recombinant measles virus vector. Hum Vaccin Immunother2013; 9:599 - 606; http://dx.doi.org/10.4161/hv.23241; PMID: 23324399
- RadeckeF, SpielhoferP, SchneiderH, KaelinK, HuberM, DötschC, ChristiansenG, BilleterMA. Rescue of measles viruses from cloned DNA. EMBO J1995; 14:5773 - 84; PMID: 8846771
- SchadeckEB, PartidosCD, FooksAR, ObeidOE, WilkinsonGW, StephensonJR, StewardMW. CTL epitopes identified with a defective recombinant adenovirus expressing measles virus nucleoprotein and evaluation of their protective capacity in mice. Virus Res1999; 65:75 - 86; http://dx.doi.org/10.1016/S0168-1702(99)00103-3; PMID: 10564754