Abstract
Post licensure surveillance of adverse events following immunization (AEFI) is a fundamental activity to improve safety and maintain public confidence in vaccines.
Since 2011, the Liguria Region has been involved in the inter-regional project of post-marketing surveillance of AEFI, coordinated by the Italian Medicine Agency and the Veneto region. The main objectives of the project are: (1) to coordinate the surveillance activities in the 8 Italian Regions included in the project; (2) to encourage the signal of AEFI by healthcare workers and patients; (3) to organize education activities addressed to health care workers, and, finally; (4) to establish vaccination counseling services in each Region.
In particular, the Ligurian multidisciplinary team, composed by physicians expert in the field of vaccination and pharmacists, is involved in the causality assessment between vaccines and all adverse events signaled within the Liguria Region and in the analysis of all adverse events signaled in Italy as possibly related to influenza vaccines. During 2013, the team has organized 4 courses, addressed to healthcare personnel of vaccination outpatient clinics, focused on European and Italian legislation on pharmaco-vigilance and vaccine-vigilance and aimed at promoting signal of AEFI. Since October 2013, the Liguria Region has been participating to the inter-regional project of active surveillance of adverse events aimed at promoting the signal of AEFI by parents of vaccinated infants.
After two years of implementation of the project both the number of reported AEFI and the reporting rate per 100 000 administered doses of vaccine increased. The activities need to be consolidated in the next years in order to guarantee high standard of vaccine safety, maintain the confidence in current immunization programs and reach optimal vaccination coverage rate.
Introduction
Vaccines represent one of the most cost-effective public health tool in preventing and controlling infectious diseases. According to World Health Organization (WHO), every year 2–3 million deaths are globally prevented through the administration of vaccines.Citation1
Although the incredible results obtained with vaccination, vaccines have become a victim of its own success: as the incidence of diseases preventable by vaccination steadily decreases, the advances made sometimes could be undermined by vaccine safety concerns.Citation2 Immunization safety fears have existed since the introduction of the first vaccine against cowpox by Jenner and these concerns could derailed mass immunization campaigns and even well-established immunization programs.Citation3
In this view, preventing, controlling and monitoring the adverse events following immunization (AEFI) remain a keystone to improve safety and maintain public confidence in vaccines.Citation4 In particular, post-licensure surveillance of AEFI, defined as the continuous monitoring of vaccine safety in the general population after licensure, is a fundamental activity to identify and evaluate risk for rare adverse events or adverse events with delayed onset, that pre-licensure trials usually do not have the ability to detect, and to confirm the optimal safety profile of the millions doses of vaccines administered worldwide.Citation5 Indeed, although phase III clinical trials include up to tens of thousands of subjects, the power to detect most rare and AEFI during the clinical development of vaccines remains low. Actually, the basic pillar of post-licensure vaccine surveillance is spontaneous and passive reporting of AEFI by physicians, other healthcare workers or patients.Citation3
In Italy, AEFI surveillance is mandatory and spontaneous reports of AEFI are collected by the National Network of Pharmaco-vigilance which includes the Italian Medicine Agency (AIFA), the 20 Regions and Autonomous Provinces of Trento and Bolzano, 204 Local Health Unit, 112 hospitals, 38 Research Institutes, and 561 pharmaceutical industries.
Since 2011, the Liguria Region has been involved in the inter-regional project of post-marketing surveillance of AEFI, coordinated by the AIFA and the Veneto Region. The main objectives of the project are: (1) to coordinate the surveillance activities in the 8 Italian Regions included in the project; (2) to encourage the signal of AEFI by healthcare workers and patients; (3) to organize education activities addressed to health care workers, and, finally; (4) to establish vaccination counseling services in each Region. This paper summarizes the two-years’ experience of the Liguria Region in routine surveillance of AEFI and the main results obtained after the implementation of strategies to improve the signal of AEFIs.
The experience of Liguria Region
Analysis of influenza vaccine related signals
A Ligurian multidisciplinary team, composed by physicians expert in the field of vaccination and pharmacists, have been instituted since May 2011 and officially established since November 2012. The multidisciplinary team is involved in the biannual analysis of all AEFI signaled in Italy as possibly related to influenza vaccines. Since October 2011, 6 reports about signals related to influenza vaccines have been transmitted to the AIFA.
Causality assessment of AEFI signaled from Liguria Region
The Ligurian multidisciplinary team, according to Italian Legislation, perform the causality relationship assessment between vaccines and all severe AEFI signaled within the Liguria Region according to the WHO causality assessment criteria.Citation6
Since 2012, the causality relationship between four severe AEFI and vaccination have been assessed by the multidisciplinary team. The first case concerned the occurrence of intestinal intussusception in a 8-mo-old male 38 d after the administration of the second dose of the oral vaccine against rotavirus. This AEFI was evaluated as unlikely related to vaccination according to the WHO criteria.Citation6 The second case was a growth retardation signaled in a 6-mo-old male after the administration of the second dose of hexavalent and 13-valent conjugate pneumococcal vaccines. This AEFI was considered as unrelated to vaccination according to the WHO criteria.Citation6 The third case concerned a West syndrome signaled in a 5-mo-old female ten days after the administration of the second dose of hexavalent and 13-valent conjugate pneumococcal vaccines. This AEFI was evaluated as unlikely related to vaccination according to the WHO criteria.Citation6 The last case concerned the occurrence of lymphadenopathy, arthro-myalgia and swelling at injection site in 38-y-old female 13 d after the administration of meals-mumps-rubella vaccine. This AEFI was considered as possibly related to vaccination according to the WHO criteria.Citation6 For each severe AEFI the multidisciplinary team furnished regular feedback data to healthcare personnel that signaled the adverse event.
The Ligurian multidisciplinary team together with the neurologists of the University Hospital of Genoa have also followed up a patient affected by acute disseminated encephalomyelitis occurred after the administration of virosomal influenza vaccine,Citation7 and contributed to the Report on post-marketing surveillance of vaccines in Italy during 2012 by AIFA with a case study on influenza vaccine and hemolytic anemia.Citation8
Education
During 2013, the team has organized 4 courses, addressed to healthcare personnel that worked in the vaccination outpatient clinics of the five Local Health Units of the Liguria Region. The courses have been focused on European and Italian legislation on pharmaco-vigilance and vaccine-vigilance, the organization of the Italian Network of pharmaco-vigilance, and the spontaneous signal of AEFI according to Italian legislation. Almost 150 healthcare workers attended the 4 courses, primarily aimed at promoting signal of AEFI by the personnel of outpatient clinics.
The active surveillance project
Since the recent European legislation on pharmaco-vigilance encourages the involvement of patients in the reporting activities,Citation9 the Liguria Region has been participating to the inter-regional project of active surveillance of adverse events aimed at promoting the signal of AEFI by parents of vaccinated infants.
A 3-mo pilot phase of the project was conducted in the Local Health Unit of Genoa. Overall, from October to December 2013, 782 vaccine diaries were delivered to the parents of infants vaccinated in the outpatient clinic of the Local Health Unit of Genoa. Within 31st march 2014, 492 (62.9%) diaries returned to the Local Health Unit and 196 (39.8%) diaries reported the occurrence of one or more mild adverse events following immunization. None severe adverse events have been reported.
Since March 2014 the project has been extended to the remaining four Local Health Unit of Liguria Region.
Results and discussion
In the period 2002–2013, 499 AEFI have been signaled within Liguria Region. The mean number of AEFI signaled each year was 41.6 and the median number was 25.5. In was reported the number of AEFI reported each year during the past 14 y.
Figure 1. Number of adverse events following immunization reported within Liguria Region, Italy, in the period 2002–2014.
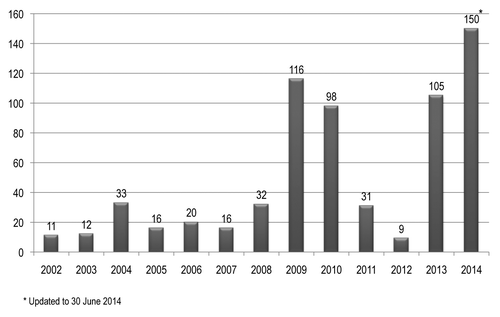
With the exception of the years 2009 and 2010, during which a project of active vaccine-surveillance related to the pandemic influenza vaccine was active in a Local Health Unit of the Liguria Region, the mean number of signaled AEFI, before the implementation of educational activities and the active surveillance project in 2013, was 20 and the median number was 16.
In terms of reported AEFI rates for 100 000 administered doses of vaccine (), in the period 2009–2012 the mean rate in the Liguria Region was 1.7 reported AEFI for 100 000 administered doses of vaccine while the mean Italian reporting rate was 5.5. After the implementation of educational activities and the active surveillance project in 2013, the number of reported AEFIs increased to 105 and the reporting rate was estimated to increase to approximately 14.0 for 100 000 administered doses. In the first six months of 2014 the number of reported AEFIs was 150, thus confirming the improvement in the number of reported adverse event.
Figure 2. Adverse events following immunization signal rate per 100 000 administered doses of vaccine in Italy and Liguria Region in the period 2009–2012.
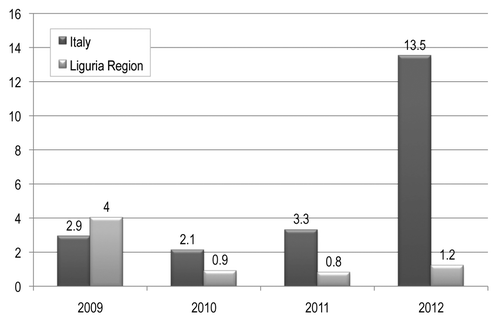
The efficacy in increase the AEFI reporting rate of national or regional vaccine-surveillance programs has been demonstrated by other authors.
In the first 3 y (2007–2010) of operation of the Surveillance of Adverse Events Following Vaccination in the Community (SAEFVIC), established in 2007 in the Australian Region of Victoria, the number of reports received increased annually over the 3-y-period and the reporting rates rose from 2.6 per 100 000 in 2003 to 13.5 per 100 000 in 2009.Citation10
In the Italian Region of Veneto, the Green Channel Centre has been established in 1992 to offer a consultancy activity for individuals at risk of adverse events and to ensure a more efficient AEFI surveillance system with regular feedback data for vaccine personnel. During a 16 y surveillance period, the Green Channel received and evaluated 5006 AEFI report forms, resulting in a mean reporting rate of 23 per 100 000 administered doses per year.Citation11
The reporting rate of Green Channel Centre was greater than the mean Italian reporting rate in the same period and, interestingly, also superior to the reporting rate of Vaccine Adverse Event Reporting System (VAERS), the largest database of AEFI reports established in the United States, that received annually nearly 28 000 reports of AEFI, corresponding to a AEFI reporting rate of 12.7 for 100 000 administered doses.Citation5,Citation12
In conclusion, preliminary results of the two-years’ experience of the Liguria Region in post marketing surveillance of vaccine safety and in improving the signal of AEFI, by education of healthcare personnel and involvement of patients, seems to confirm the satisfactory results obtained by similar projects.
These activities need to be consolidated in the next years in order to guarantee high standard of vaccine safety, maintain the confidence in current immunization programs and reach optimal vaccination coverage rate.
| Abbreviations, | ||
| WHO | = | World Health Organization |
| AEFI | = | adverse events following immunization |
| AIFA | = | Italian Medicine Agency |
Disclosure of Potential Conflicts of Interest
All authors declare that they have no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
The work was not funded.
References
- DuclosP, Okwo-BeleJM, Gacic-DoboM, CherianT. Global immunization: status, progress, challenges and future. BMC Int Health Hum Rights2009; 9:Suppl 1S2; http://dx.doi.org/10.1186/1472-698X-9-S1-S2; PMID: 19828060
- LankinenKS, PastilaS, KilpiT, NohynekH, MäkeläPH, OlinP. Vaccinovigilance in Europe--need for timeliness, standardization and resources. Bull World Health Organ2004; 82:828 - 35; PMID: 15640918
- BonhoefferJ, BlackS, IzurietaH, ZuberP, SturkenboomM. Current status and future directions of post-marketing vaccine safety monitoring with focus on USA and Europe. Biologicals2012; 40:393 - 7; http://dx.doi.org/10.1016/j.biologicals.2012.07.007; PMID: 22902972
- ZanoniG, BerraP, LucchiI, FerroA, O’FlanaganD, Levy-BruhlD, SalmasoS, TridenteG. Vaccine adverse event monitoring systems across the European Union countries: time for unifying efforts. Vaccine2009; 27:3376 - 84; http://dx.doi.org/10.1016/j.vaccine.2009.01.059; PMID: 19200851
- Miller ER, Haber P, Hibbs B, Broder K. Surveillance for Adverse Events Following Immunization Using the Vaccine Adverse Event Reporting System (VAERS). In Manual for the Surveillance of Vaccine-Preventable Diseases. Centers for Disease Control and Prevention, Atlanta, GA, 2008.
- ColletJP, MacDonaldN, CashmanN, PlessR, Advisory Committee on Causality Assessment. Monitoring signals for vaccine safety: the assessment of individual adverse event reports by an expert advisory committee. Bull World Health Organ2000; 78:178 - 85; PMID: 10743282
- AlicinoC, InfanteMT, GandogliaI, MioloN, MancardiGL, ZappettiniS, CapelloE, OrsiA, TamburiniT, GrandisM. Acute disseminated encephalomyelitis with severe neurological outcomes following virosomal seasonal influenza vaccine. Hum Vaccin Immunother2014; 10:10; http://dx.doi.org/10.4161/hv.28961; PMID: 24785431
- Agenzia Italiana del Farmaco. Report on post-markeing surveillance of vaccines in Italy during 2012. Rome 2013.
- REGULATION. (EU) No 1235/2010 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, and Regulation (EC) No 1394/2007 on advanced therapy medicinal products. Available from https://eudravigilance.ema.europa.eu/human/docs/Regulations/reg_2010_1235_en.pdf.
- ClothierHJ, CrawfordNW, KempeA, ButteryJP. Surveillance of adverse events following immunisation: the model of SAEFVIC, Victoria. Commun Dis Intell Q Rep2011; 35:294 - 8; PMID: 22624490
- MichelettiF, MorettiU, TridenteG, ZanoniG. Consultancy and surveillance of post-immunisation adverse events in the Veneto region of Italy for 1992-2008. Hum Vaccin2011; 7:Suppl234 - 9; http://dx.doi.org/10.4161/hv.7.0.14613; PMID: 21301223
- LoughlinAM, MarchantCD, AdamsW, BarnettE, BaxterR, BlackS, CaseyC, DekkerC, EdwardsKM, KleinJ, et al. Causality assessment of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine2012; 30:7253 - 9; http://dx.doi.org/10.1016/j.vaccine.2012.09.074; PMID: 23063829
