Abstract
The H1N1 influenza pandemic of 2009 stimulated interest in developing safe and effective subunit influenza vaccines using rapid and cost-effective recombinant technologies that can avoid dependence on hens’ eggs supply and live viruses for production. Among alternative approaches to subunit vaccine development, virus-like particles (VLPs) represent an attractive strategy due to their safety and immunogenicity. Previously, we have produced a recombinant monomeric hemagglutinin (HA) protein derived from the A/California/04/09 (H1N1) strain of influenza virus in a plant-based transient expression system and demonstrated immunogenicity and safety of this monomeric HA in animal models and human volunteers. In an effort to produce higher potency influenza vaccine in plants, we have designed and generated enveloped VLPs using the ectodomain of HA from the A/California/04/09 strain and heterologous sequences. The resulting H1 HA VLPs (HAC-VLPs) elicited robust hemagglutination inhibition antibody responses in mice at doses lower than 1 µg in the presence or absence of Alhydrogel adjuvant. These results suggest enhanced immunogenicity of recombinant HA in the form of an enveloped VLP over soluble antigen.
Introduction
Influenza poses one of the most significant threats to human populations, and its prevention is one of the world’s greatest public health challenges because of antigenic variation of influenza viruses caused by mutations in hemagglutinin (HA).Citation1 From 1976–2007 in the US, the estimated annual average of excess deaths resulting from respiratory and circulatory causes associated with seasonal influenza was about 23 600 (range 3300 to 48 600).Citation2 The recent emergence of H1N1 swine influenzaCitation3-Citation5 is testimony to the unpredictability and threat posed by influenza viruses and underscores the need for rapid and robust influenza vaccine production methods to mitigate and prevent the consequences of potential pandemics. The increased worldwide awareness of seasonal and pandemic influenza has stimulated interest in the development of platforms utilizing recombinant DNA technologies for large-scale production of safe and effective subunit vaccines. Progress in recombinant DNA technology allows for rapid cloning of genes, expression of correctly folded and biologically active antigens in eukaryotic systems, and high levels of recombinant antigen production. Additionally, this approach eliminates dependence on complex hens’ eggs-based manufacturing and avoids concerns caused by changes due to the egg adaptation to vaccine strain that might lead to the loss of vaccine efficacy.
Influenza HA is one of the major viral glycoproteins present as a homotrimeric type I transmembrane protein on the surface of virions which plays a major role in the virus entry into host cells through binding cell surface receptors.Citation6 Thus, HA plays the key role in viral infectivity and pathogenicity. A large body of evidence suggests that protection provided by influenza vaccines is primarily mediated by anti-HA antibodies.Citation7 Therefore, HA has been a major target antigen for influenza vaccine development. Recombinant HA expressed in a baculovirus-insect cell system (FluBlok® trivalent seasonal influenza vaccine; Protein Sciences Corp), which was shown to be well tolerated and about 44.6 percent effective against all circulating influenza strains, has been approved for the prevention of seasonal influenza in people 18 through 49 y of age.Citation8
Recently, plant production systems have emerged as a promising alternative for manufacturing of vaccine antigens. Plants possess the eukaryotic post-translational protein modification machinery broadly similar to that of mammals, do not harbor human pathogens, and can be rapidly scaled up as economic biomass generators.Citation9-Citation11 Previously, we reported on recombinant monomeric HA (HAC1) derived from the A/California/04/09 (H1N1) strain of influenza virus produced using transient expression vectors,Citation12 manufactured HAC1 in compliance with current Good Manufacturing Practices,Citation13 and demonstrated immunogenicity and safety of HAC1 in a Phase 1 clinical trial.Citation14 Subsequently, to mimic the HA structure presented on the virus surfaceCitation15 and to enhance immunogenicity of the HA antigen, we generated trimeric HA (tHA-BC) from the A/California/04/09 (H1N1) strain by introducing a trimerization motif from a heterologous protein into the HA sequence, and demonstrated the induction of serum hemagglutination inhibition (HI) antibodies and protective efficacy against a lethal viral challenge in mice, at levels comparable with a licensed egg-based H1N1 influenza vaccine. In addition, immunogenic and protective doses of tHA-BC were shown to be much lower than those of monomeric HAC1.Citation16
Virus-like particles (VLPs) represent a further step towards enhancing immunogenicity of vaccine antigens. VLPs lack infectious genetic material but mimic parental viruses by structure and display of multiple copies of target antigens, thereby inducing enhanced protective immunity.Citation17,Citation18 VLP-based vaccines against numerous pathogens have been produced in various expression systems, including plants.Citation19 Here, we have engineered, produced in plants, purified and characterized H1 HA enveloped VLPs (HAC-VLPs) from the A/California/04/09 strain of influenza virus, and evaluated the immunogenicity of this influenza vaccine candidate in mice.
Results
Engineering, production in plants and characterization of plant-derived HAC-VLPs
To produce H1 HA-based VLPs (HAC-VLPs) in plants, we first engineered a construct pGRD4-CA-HAC-TMcT containing the signal peptide of calreticulin (CA) from Nicotiana tabacum fused to full-length HA (lacking its native signal peptide) from the A/California/04/09 strain (HAC). Subsequently, the transmembrane (TM) and cytosolic tail (CT) domains of HAC were replaced by heterologous sequences, resulting in pGRD4-CA-HAC-TMhT that expressed target protein in Nicotiana benthamiana at higher levels than pGRD4-CA-HAC-TMcT (data not shown).
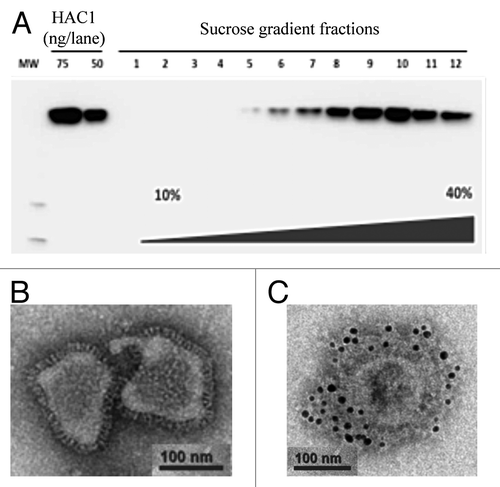
Plant-produced HAC-VLPs were fractionated over a sucrose density gradient, and their presence in different fractions was assessed by western blot analysis () using an anti-HAC monoclonal antibody (mAb). Fractions #7–10, shown to contain the majority of HAC-VLPs, were combined and used as a single preparation for further characterization. Electron microscopy (EM) analysis using negative staining showed closely packed protein spikes on the surface of particles, resembling influenza A viruses by morphology (). To confirm that these protein spikes represent the HAC antigen, immunogold labeling using an anti-HAC mAb was performed, demonstrating that VLPs were extensively decorated with HAC ().
Immunogenicity of plant-derived HAC-VLPs in mice
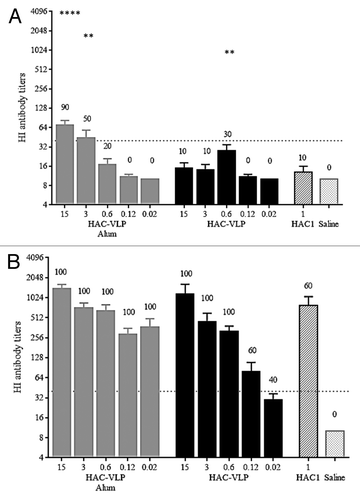
The immunogenicity of plant-produced HAC-VLPs was evaluated in a set of mouse experiments using a prime/boost regimen. In the first study, groups of mice were immunized twice with HAC-VLPs at doses ranging from 15 to 0.02 µg with or without Alhydrogel. Control groups received monomeric HAC1 or saline plus Alhydrogel. Serum was collected post prime (study day 21) and post boost (study day 42) and analyzed by a HI assay. The results of the HI assay demonstrated that a single administration of HAC-VLPs at 15 or 3 μg in the presence of Alhydrogel elicited significant HI antibody titers with HI titers of ≥1:40 in 90% and 50% of animals, respectively (). At doses below 3 μg (0.6, 0.12, or 0.02 μg), a single administration of HAC-VLPs plus Alhydrogel elicited either undetectable HI titers or titers just above the detection limit. In the absence of Alhydrogel, levels of HI antibody titers after a single dose of HAC-VLPs were either undetectable or just above the detection limit, except for 3 animals in the 0.6 μg group (). After the second administration of HAC-VLPs, either in the presence or absence of Alhydrogel, on study day 42, HI titers were significantly enhanced (). Furthermore, 100% of animals in all adjuvanted groups and in the groups immunized with 15, 3, or 0.6 μg of HAC-VLPs without Alhydrogel had HI titers of ≥1:40. Although HI titers from animals in the groups that received 0.12 or 0.02 μg of HAC-VLPs were lower by comparison, HI titers of ≥1:40 were still observed in 60% and 40% of animals, respectively, and there was no statistically significant difference in HI titers when compared with the group that received monomeric HAC1 plus Alhydrogel (). Two immunizations with HAC1 plus Alhydrogel elicited HI antibody titers of ≥1:40 in 60% of the animals ().
Figure 2. Serum HI antibody titers in mice immunized with HAC-VLPs and the percent responders per group. Data are shown as the average HI antibody titer per group plus SEM. The numbers on the top of each bar indicate the percent responders per group, meaning the percent of mice per group generating a HI titer of ≥1:40. (A): Post primary immunization (study day 21). (B): Post boost immunization (study day 42). Statistical analysis was performed to compare HI antibody titers in HAC-VLP immunized vs. HAC1 immunized groups by the Mann-Whitney testing using GraphPad Prism ver. 6.02. **, P < 0.01; ****, P < 0.0001; no asterisk, P > 0.05.
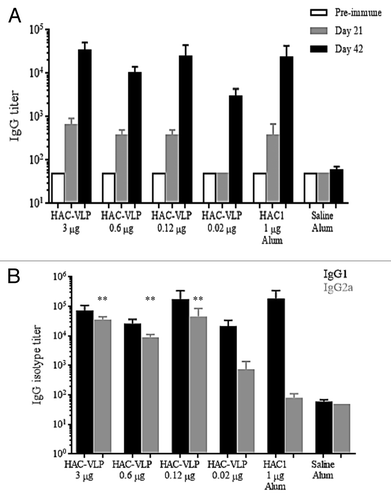
To further characterize antibody responses in mice elicited by HAC-VLPs themselves, groups of mice were immunized twice with HAC-VLPs at doses ranging from 3–0.02 µg in the absence of Alhydrogel. Control groups received monomeric HAC1 or saline with Alhydrogel. Serum total IgG titers of samples collected on study days 0, 21, and 42, and IgG1 and IgG2a titers of samples collected on study day 42 were assessed by enzyme-linked immunosorbent assays (ELISA) and compared with those elicited by immunization with monomeric HAC1 plus Alhydrogel. Total IgG responses after the prime or boost immunization with HAC-VLPs alone, at all doses tested, were not significantly different (P > 0.05) compared with the group that received 1 µg of HAC1 plus Alhydrogel (). The analysis of IgG1 and IgG2a responses after the boost immunization revealed that two intramuscular immunizations with HAC-VLPs in the absence of Alhydrogel elicited similar titers of IgG1 and IgG2a at the 3, 0.6, and 0.12 µg doses (). When IgG1 titers elicited by HAC-VLPs alone were compared with those generated by 1 µg of HAC1 with Alhydrogel, no statistically significant difference (P > 0.05) was observed. In contrast, IgG2a titers from animals in the groups that received 0.12–3 µg of HAC-VLPs alone were significantly higher (P < 0.01) than those in the group that received 1 µg of HAC1 plus Alhydrogel (). No statistically significant differences were observed in IgG2a titers between the groups receiving 0.02 µg of HAC-VLPs and 1 µg of HAC1 plus Alhydrogel (P > 0.05; ).
Figure 3. Total IgG (A) and IgG1/IgG2a (B) responses after immunization. Data are shown as the mean titer per group plus SEM. Statistical analysis was performed to compare IgG1 and IgG2a responses in HAC-VLP immunized vs. HAC1 immunized groups by the Mann-Whitney test using GraphPad Prism ver. 6.02. **, P < 0.01; no asterisk, P > 0.05.
Discussion
VLPs have emerged as a promising vaccine platform due to their repetitive presentation of target antigen(s) that resemble the morphology of parental virus particles and efficiently trigger host immunity. To date, recombinant VLPs have been shown to be the most immunogenic subunit vaccines and several VLP vaccines, such as those against hepatitis B and human papillomavirus, have been licensed.Citation20-Citation22 The emergence of a novel A/California/04/09 strain of influenza A virus in 2009 stimulated the development of new economic platforms for rapid, large-scale production of subunit influenza vaccines. Most of the influenza VLPs produced in mammalian or insect cell expression systems contain recombinant HA as well as at least one more viral protein such as NA and/or MCitation23-Citation25 to accelerate budding or facilitate particle formation. D’Aoust et al.Citation26,Citation27 reported that influenza H5 and H1 VLPs based on A/Indonesia/05/05 and A/California/04/09 strains, respectively, can be transiently produced in N. benthamiana plants by expressing HA alone, and safety and immunogenicity of these VLPs were demonstrated in Phase 1 and 2 clinical trials.Citation28-Citation30
In this study, we have utilized the launch vector-based transient expression technology to produce enveloped VLPs containing recombinant HA of the A/California/04/09 strain of influenza A virus in N. benthamiana. The resulting HAC-VLPs represented particles comprising HA alone and resembling influenza A viruses by morphology. Although the mechanism of HAC-VLP formation is currently unknown, replacing of the TM and CT domains of the H1 HA molecule (A/California/04/09) with heterologous sequences resulted in higher levels of VLP expression in plants, presumably due to preferred interactions between the heterologous TM domain and the plant cell membrane or more efficient VLP budding. Further studies are required to elucidate the exact mechanisms of plant-produced HAC-VLP assembly and budding.
Immunization of mice with HAC-VLPs in the presence or absence of aluminum adjuvant demonstrated superior immunogenicity of these VLPs over monomeric HA (HAC1), inducing robust HI antibody responses at lower antigen doses even in the absence of adjuvant. In a previous study, we demonstrated enhanced immunogenicity of plant-produced trimeric HA (tHA-BC) compared with monomeric HA (HAC1).Citation16 However, administration of tHA-BC with Alhydrogel was still required to induce protective immune responses. Of note, in the current study, HAC-VLPs elicited IgG1 and IgG2a responses of equal magnitude in the absence of adjuvant, suggesting that these VLPs induced balanced Th1/Th2 responses, in contrast to HAC1 monomer. The superior immunogenicity of HAC-VLPs demonstrated in this study can be partly explained by the results of Link et al.Citation31 who demonstrated in mice efficient transportation of particulate, but not soluble protein antigen to germinal center follicular dendritic cells by components of innate immunity in the absence of prior immunity. The ability of the launch vector-based plant expression system to produce three forms of influenza HA – monomer, trimer and VLP – should facilitate the investigation of the mechanism of enhanced immunogenicity of VLPs over soluble antigen.
In conclusion, we have engineered and produced in plants recombinant HA from the A/California/04/09 strain of influenza A virus in the form of enveloped VLPs (HAC-VLPs). Although further studies such as challenge infection in animal models are required to demonstrate protective efficacy, immunization of mice with HAC-VLPs, in the presence or absence of Alhydrogel adjuvant, elicited robust HI antibody responses that were superior to those elicited by the subunit plant-produced HA vaccine candidate.
Materials and Methods
Construction and production of influenza HAC-VLPs in plants
The HA nucleotide sequence of the A/California/04/09 (H1N1) influenza virus strain (NCBI accession number ACQ76318.1) was synthesized by GENEART AG and inserted into the TMV-based launch vector pGRD4.Citation12,Citation32 During the synthesis, sequence encoding the signal peptide of HA (a.a. 1–17) was replaced by the sequence encoding the signal peptide of CA from N. tabacum. To obtain the construct pGRD4-CA-HAC-TMhT, the TM and CT domains were replaced by heterologous sequences. The entire target sequence was optimized for codon usage of TMV. The resulting construct was transformed into A. tumefaciens strain GV3101 that was then introduced into hydroponically grown N. benthamiana by vacuum infiltration, as described previously.Citation12,Citation32
At 6 d post infiltration, leaf tissue of infiltrated plants was harvested, homogenized and centrifuged to remove solids. The microsomal fraction obtained from the crude clarified extract was fractionated over a 10–40% linear sucrose density gradient. The distribution of HAC-VLPs was assessed by western blot analysis as described. Peak fractions were collected and used for immunological evaluation.
Western blot analysis, EM and immunogold labeling
To characterize plant-produced HAC-VLPs, particles were separated on a 10% SDS-PAGE gel, transferred onto a polyvinylidene difluoride membrane, and probed with a mouse anti-HAC mAb. Particle formation and HA incorporation into VLPs were evaluated by EM using both negative staining and immunogold labeling. HAC-VLPs were absorbed onto grids and negatively stained with 1% phosphotungstic acid. To detect the HAC protein, the particles were incubated with an anti-HAC mAb followed by 6 nm gold-conjugated goat anti-mouse IgG antibody. The images of the negatively and immunogold stained particles were captured using a Zeiss LIBRA 120 transmission electron microscope.
Evaluation of HAC-VLP immunogenicity in mice
Groups of BALB/c mice, 10 animals per group, were intramuscularly immunized on study days 0 and 21 with HAC-VLPs at doses of 15, 3, 0.6, 0.12, or 0.02 μg, either in the presence or absence of Alhydrogel. Animals in the negative control group received saline plus Alhydrogel. To further evaluate target-specific IgG responses elicited by HAC-VLPs themselves, a second mouse study was conducted in which groups of BALB/c mice, 5 animals per group, were intramuscularly immunized on study days 0 and 21 with HAC-VLPs at doses of 3, 0.6, 0.12, or 0.02 μg without Alhydrogel. Monomeric HAC1 plus Alhydrogel was included in both studies at the dose of 1 μg in order to compare antibody responses elicited by HAC-VLPs vs. soluble protein. Serum samples for analysis of HI and IgG immune responses were collected prior to each immunization on study days 0 (pre-immune) and 21 (post prime) and 3 wk after the final immunization (study day 42, post boost).
HI assay
The HI assay was conducted as previously described.Citation16 HI antibody responses were assessed per group as HI geometric mean titers (GMTs) with a standard error of the mean (SEM) along with the percentage of animals with HI titers ≥1:40 (% responders).
Evaluation of IgG responses by ELISA
HA-specific serum IgG responses were evaluated by ELISA using the inactivated A/California/04/09 virus as a coating antigen as described previously.Citation33 Briefly, serum samples were tested in series of 4-fold dilutions and antigen-specific IgG was detected using horseradish peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratory Inc), IgG1 or IgG2a (Southern Biotechnology Associates Inc) antibodies and o-phenylenediamine dihydrochloride (SigmaFast OPD, Sigma) as a substrate. Reciprocal highest serum dilutions that produced mean optical density values three times greater than those from pre-immune sera at a 1:100 dilution were determined as endpoint titers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
The authors would like to thank Rebecca Snow for technical support and Natasha Kushnir for editorial assistance. The authors would like to thank Shannon Modla of the Delaware Biotechnology Institute Bio-Imaging Center at the University of Delaware (Newark, DE) for assistance with transmission electron microscopy. This project was supported by a grant from the Defense Advanced Research Projects Agency. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency.
References
- Gerhard W, Yewdell J, Frankel ME, Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature 1981; 290:713 - 7; http://dx.doi.org/10.1038/290713a0; PMID: 6163993
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057 - 62; PMID: 20798667
- Centers for Disease Control and Prevention (CDC). Swine-origin influenza A (H1N1) virus infections in a school - New York City, April 2009. MMWR Morb Mortal Wkly Rep 2009; 58:470 - 2; PMID: 19444151
- Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009; 302:1880 - 7; http://dx.doi.org/10.1001/jama.2009.1536; PMID: 19822626
- Centers for Disease Control and Prevention. Updated estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April 2009 – April 10, 2010. May 14, 2010. http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm (accessed 30 May 2014).
- Wilson IA, Cox NJ. Structural basis of immune recognition of influenza virus hemagglutinin. Annu Rev Immunol 1990; 8:737 - 71; http://dx.doi.org/10.1146/annurev.iy.08.040190.003513; PMID: 2188678
- Han T, Marasco WA. Structural basis of influenza virus neutralization. Ann N Y Acad Sci 2011; 1217:178 - 90; http://dx.doi.org/10.1111/j.1749-6632.2010.05829.x; PMID: 21251008
- U.S. Food and Drug Administration. News Release 01/16/2013. FDA approves new seasonal influenza vaccine made using novel technology. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm335891.htm (accessed 02 June 2014).
- Chichester JA, Yusibov V. Plants as alternative systems for production of vaccines. Hum Vaccin 2007; 3:146 - 8; http://dx.doi.org/10.4161/hv.3.4.4148; PMID: 17643065
- Mett V, Farrance CE, Green BJ, Yusibov V. Plants as biofactories. Biologicals 2008; 36:354 - 8; http://dx.doi.org/10.1016/j.biologicals.2008.09.001; PMID: 18938088
- Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnol J 2010; 8:620 - 37; http://dx.doi.org/10.1111/j.1467-7652.2010.00507.x; PMID: 20233333
- Musiychuk K, Stephenson N, Bi H, Farrance CE, Orozovic G, Brodelius M, Brodelius P, Horsey A, Ugulava N, Shamloul AM, et al. A launch vector for the production of vaccine antigens in plants. Influenza Other Respir Viruses 2007; 1:19 - 25; http://dx.doi.org/10.1111/j.1750-2659.2006.00005.x; PMID: 19453476
- Shoji Y, Chichester JA, Jones M, Manceva SD, Damon E, Mett V, Musiychuk K, Bi H, Farrance C, Shamloul M, et al. Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum Vaccin 2011; 7:Suppl 41 - 50; http://dx.doi.org/10.4161/hv.7.0.14561; PMID: 21266846
- Cummings JF, Guerrero ML, Moon JE, Waterman P, Nielsen RK, Jefferson S, Gross FL, Hancock K, Katz JM, Yusibov V, Fraunhofer USA Center for Molecular Biotechnology Study Group. Safety and immunogenicity of a plant-produced recombinant monomer hemagglutinin-based influenza vaccine derived from influenza A (H1N1)pdm09 virus: a Phase 1 dose-escalation study in healthy adults. Vaccine 2014; 32:2251 - 9; http://dx.doi.org/10.1016/j.vaccine.2013.10.017; PMID: 24126211
- Vanlandschoot P, Beirnaert E, Neirynck S, Saelens X, Jou WM, Fiers W. Molecular and immunological characterization of soluble aggregated A/Victoria/3/75 (H3N2) influenza haemagglutinin expressed in insect cells. Arch Virol 1996; 141:1715 - 26; http://dx.doi.org/10.1007/BF01718294; PMID: 8893793
- Shoji Y, Jones RM, Mett V, Chichester JA, Musiychuk K, Sun X, Tumpey TM, Green BJ, Shamloul M, Norikane J, et al. A plant-produced H1N1 trimeric hemagglutinin protects mice from a lethal influenza virus challenge. Hum Vaccin Immunother 2013; 9:553 - 60; http://dx.doi.org/10.4161/hv.23234; PMID: 23296194
- Grgacic EV, Anderson DA. Virus-like particles: passport to immune recognition. Methods 2006; 40:60 - 5; http://dx.doi.org/10.1016/j.ymeth.2006.07.018; PMID: 16997714
- Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines 2007; 6:381 - 90; http://dx.doi.org/10.1586/14760584.6.3.381; PMID: 17542753
- Kushnir N, Streatfield SJ, Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine 2012; 31:58 - 83; http://dx.doi.org/10.1016/j.vaccine.2012.10.083; PMID: 23142589
- Recombivax HB®. Prescribing information. Merck; July 2011
- Gardasil®. Prescribing information. Merck; April 2011
- Cervarix®. Prescribing information. GlaxoSmithKline; July 2011
- Latham T, Galarza JM. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J Virol 2001; 75:6154 - 65; http://dx.doi.org/10.1128/JVI.75.13.6154-6165.2001; PMID: 11390617
- Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 2005; 23:5751 - 9; http://dx.doi.org/10.1016/j.vaccine.2005.07.098; PMID: 16143432
- Chen BJ, Leser GP, Morita E, Lamb RA. Influenza virus hemagglutinin and neuraminidase, but not the matrix protein, are required for assembly and budding of plasmid-derived virus-like particles. J Virol 2007; 81:7111 - 23; http://dx.doi.org/10.1128/JVI.00361-07; PMID: 17475660
- D’Aoust MA, Lavoie PO, Couture MM, Trépanier S, Guay JM, Dargis M, Mongrand S, Landry N, Ward BJ, Vézina LP. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol J 2008; 6:930 - 40; http://dx.doi.org/10.1111/j.1467-7652.2008.00384.x; PMID: 19076615
- D’Aoust MA, Couture MM, Charland N, Trépanier S, Landry N, Ors F, Vézina LP. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J 2010; 8:607 - 19; http://dx.doi.org/10.1111/j.1467-7652.2009.00496.x; PMID: 20199612
- Landry N, Ward BJ, Trépanier S, Montomoli E, Dargis M, Lapini G, Vézina LP. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS One 2010; 5:e15559; http://dx.doi.org/10.1371/journal.pone.0015559; PMID: 21203523
- The Business Journals. Medicago Inc. Press Release June 8, 2011. Medicago reports positive U.S. clinical trial results for its H1N1/seasonal influenza vaccine. http://www.bizjournals.com/prnewswire/press_releases/2011/06/08/TO211 (accessed 30 May 2014).
- The Business Journals. Medicago Inc. Press Release June 30, 2011. Medicago reports positive Phase II final results for its avian flu pandemic vaccine. http://www.bizjournals.com/prnewswire/press_releases/2011/06/30/TO669 (accessed 30 May 2014).
- Link A, Zabel F, Schnetzler Y, Titz A, Brombacher F, Bachmann MF. Innate immunity mediates follicular transport of particulate but not soluble protein antigen. J Immunol 2012; 188:3724 - 33; http://dx.doi.org/10.4049/jimmunol.1103312; PMID: 22427639
- Shoji Y, Bi H, Musiychuk K, Rhee A, Horsey A, Roy G, Green B, Shamloul M, Farrance CE, Taggart B, et al. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine 2009; 27:1087 - 92; http://dx.doi.org/10.1016/j.vaccine.2008.11.108; PMID: 19100806
- Shoji Y, Chichester JA, Bi H, Musiychuk K, de la Rosa P, Goldschmidt L, Horsey A, Ugulava N, Palmer GA, Mett V, et al. Plant-expressed HA as a seasonal influenza vaccine candidate. Vaccine 2008; 26:2930 - 4; http://dx.doi.org/10.1016/j.vaccine.2008.03.045; PMID: 18440103